Abstract
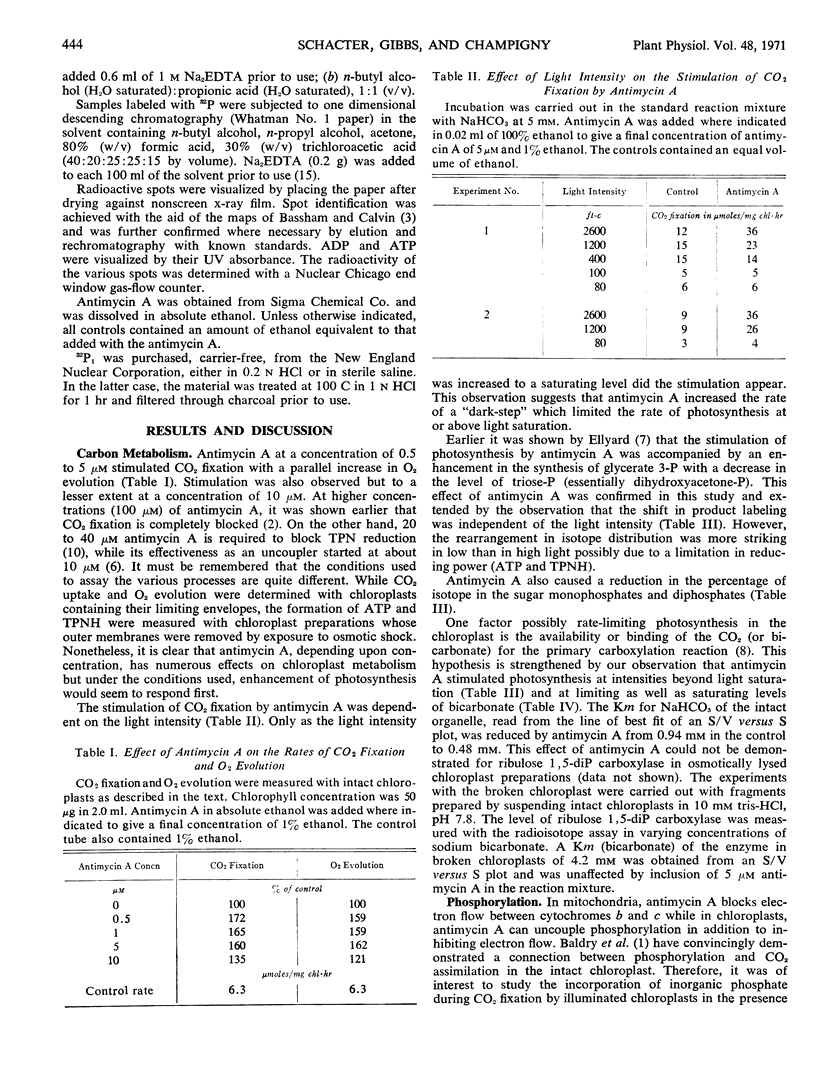
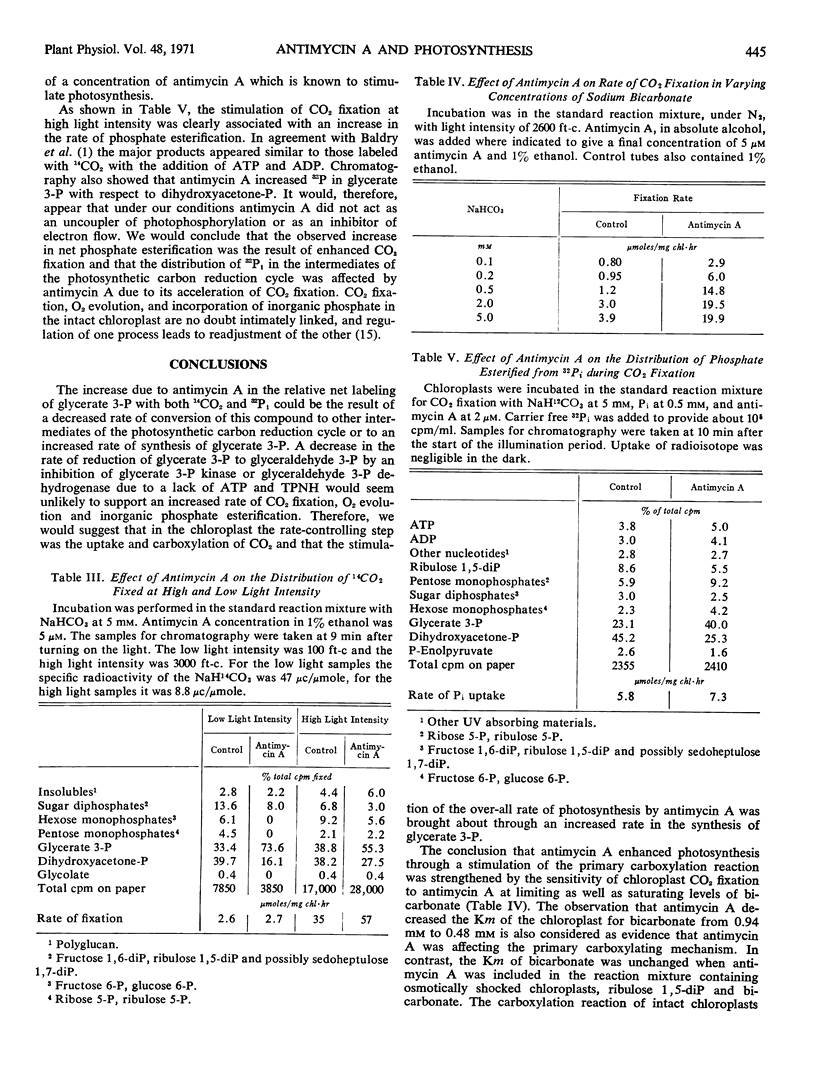
Low concentrations (0.5-10 μm) of antimycin A were shown to increase the rate of CO2 fixation, O2 evolution and inorganic phosphate esterification in intact spinach (Spinacia oleracea) chloroplasts. The increase was highest when the light intensity was saturating. Stimulation was independent of the bicarbonate concentration and was accompanied by an enhancement in the synthesis of glycerate 3-phosphate with a decrease in dihydroxyacetone phosphate. The antibiotic decreased the Michaelis constant of the chloroplast but not of ribulose 1,5-diphosphate carboxylase for bicarbonate. It was suggested that antimycin A is affecting that portion (outer envelope) of the intact chloroplast which contains the enzyme mechanism for controlling the pace of CO2 fixation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger E. S., Black C. C., Fewson C. A., Gibbs M. Inhibitor Studies on Carbon Dioxide Fixation, Adenosine Triphosphate Formation, & Triphosphopyridine Nucleotide Reduction by Spinach Chloroplasts. Plant Physiol. 1963 Jul;38(4):483–487. doi: 10.1104/pp.38.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Filmer D. The active species of "CO2" utilized by ribulose diphosphate carboxylase. J Biol Chem. 1969 Feb 10;244(3):1081–1083. [PubMed] [Google Scholar]
- Drechsler Z., Nelson N., Neumann J. Antimycin A as an uncoupler and electron transport inhibitor in photoreactions of chloroplasts. Biochim Biophys Acta. 1969 Sep 16;189(1):65–73. doi: 10.1016/0005-2728(69)90226-6. [DOI] [PubMed] [Google Scholar]
- Hind G. Light-induced changes in cytochrome b-559 in spinach chloroplasts. Photochem Photobiol. 1968 Apr;7(4):369–375. doi: 10.1111/j.1751-1097.1968.tb08025.x. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Nakayama N., Akazawa T. Structure and function of chloroplast proteins. V. Homotropic effect of bicarbonate in RuDP carboxylase reaction and the mechanism of activation by magnesium ions. Arch Biochem Biophys. 1968 Sep 10;126(3):737–745. doi: 10.1016/0003-9861(68)90465-7. [DOI] [PubMed] [Google Scholar]
- TAGAWA K., TSUJIMOTO H. Y., ARNON D. I. SEPARATION BY MONOCHROMATIC LIGHT OF PHOTOSYNTHETIC PHOSPHORYLATION FROM OXYGEN EVOLUTION. Proc Natl Acad Sci U S A. 1963 Sep;50:544–549. doi: 10.1073/pnas.50.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Crofts A. R. Photosynthesis. Annu Rev Biochem. 1970;39:389–428. doi: 10.1146/annurev.bi.39.070170.002133. [DOI] [PubMed] [Google Scholar]


