Abstract
The outcome of liver resection (LR) for elderly hepatocellular carcinoma (HCC) patients with portal hypertension (PHT) who may be excluded as liver transplantation candidates has not been fully evaluated. One hundred ninety-five patients who underwent initial curative LR for HCC with PHT were divided into 2 groups: age <70 years (n = 131) and age ≥70 years (n = 64). Clinicopathologic data and postoperative complications were compared. Preoperative characteristics and postoperative complications were similar in both groups. However, in-hospital mortality was significantly more frequent in elderly than in younger patients (11% versus 1%, P = 0.002). No significant intergroup differences were observed in the 5-year disease-free survival rate or recurrence rate (19.7% versus 17.2%; P = 0.338, 63% versus 56%; P = 0.339). Although LR for elderly HCC patients with PHT can be performed with curative intent and gives results comparable with those in younger patients, it is associated with higher in-hospital mortality.
Key words: Liver resection, Recurrence, Portal hypertension, Liver transplantation, Liver failure
Recently, the number of elderly patients with hepatocellular carcinoma (HCC) has been increasing with the increased proportion of the geriatric population in Japan.1,2 Thanks to recent advances in surgical techniques, perioperative management, and anesthesia, the indications for surgical treatment modalities such as liver resection (LR) or liver transplantation (LT) in elderly patients have expanded.3,4 Thus, age itself is no longer a contraindication for liver surgery.
When considering the treatment of HCC, most patients already have existing liver dysfunction due to chronic hepatitis or liver cirrhosis, and portal hypertension (PHT) may be present at the time of diagnosis.5,6 The American Association for the Study of Liver Diseases (AASLD)/Barcelona Clinic for Liver Cancer (BCLC) Guidelines have been widely utilized for the management of HCC in Western countries.7,8 They recommend that only LT can be regarded as curative treatment for early-stage HCC (single nodule or up to 3 nodules measuring ≤3 cm) with PHT. However, LT for all HCC patients with PHT is impossible because of donor organ shortage, especially in Asian counties.9 In addition, expanding the indications of LT for elderly patients is still controversial. A previous study suggested that patients with PHT who underwent LR showed the same incidence of postoperative complications and survival rate as patients without PHT.10 In other words, LR still plays an important role as the mainstay of curative treatment for HCC patients with PHT, even if they are elderly. There have been several reports on the safety and feasibility of LR for elderly HCC patients, but there is little information on the outcome of elderly patients with PHT, who are considered to be at extremely high risk.11–16 Against this background, the aim of the present study was to examine the short- and long-term outcomes of LR in both elderly (age ≥70 years) and younger (age <70 years) HCC patients with PHT at a single center over a 12-year period.
Patients and Methods
The database available to us comprised 435 patients who had undergone initial curative LR for HCC, without extrahepatic metastasis, at Dokkyo Medical University Hospital between April 2000 and March 2012. Among them, 195 patients who had PHT were included in the present study. Because the hepatic venous pressure gradient was not measured, PHT was defined as the presence of esophageal varices, a platelet count of <100,000/μL with splenomegaly, or both, in accordance with the BCLC group criteria.17 Based on this definition, 143 patients were diagnosed as having esophageal varices, 133 had a platelet count of <100,000/μL, and 81 had both conditions. We classified the patients into 2 age groups, <70 years (n = 131) and ≥70 years (n = 64), according to their age at the time of LR. Preoperative upper-gastrointestinal endoscopy was performed in all patients to evaluate the presence of esophageal varices. If patients had esophageal varices with a red color sign, they were preoperatively treated by endoscopic variceal ligation or sclerotherapy to prevent variceal rupture. Clinicopathologic parameters such as preoperative and postoperative liver function data, surgical data, perioperative data, pathologic data, and postoperative complications were compared between the 2 groups.
The indications for LR and surgical procedures in our department were based on the Makuuchi criteria.18,19 The type of LR was classified according to the Brisbane 2000 Nomenclature of Hepatic Anatomy and Resections.20 Major hepatectomy included hemihepatectomy, sectionectomy, and bisegmentectomy, while minor hepatectomy included segmentectomy and wedge resection with a sufficient margin. Splenectomy before or simultaneously with hepatectomy was additionally performed to prevent hemorrhagic complications in patients with a platelet count of <50,000/μL, based on our departmental policy.
Postoperative complications were evaluated according to the Clavien grading systems21 (i.e., grade-I and -II complications were classified as minor problems that did not require invasive intervention). Complications worse than grade III were considered to be major problems requiring invasive intervention: grade III required surgical, endoscopic, or radiologic intervention; grade-IV complications were life-threatening, requiring intermediate care/intensive care unit management; and grade V represented the death of a patient. The definition and severity grading of posthepatectomy liver failure formulated by the International Study Group of Liver Surgery was used.22
Patients visited the hospital once a month for the initial 12 months and at 3-month intervals after surgery. The tumor markers alpha-fetoprotein (AFP) and protein induced by vitamin K antagonism-II (PIVKA-II) were examined at each visit, and ultrasonography was performed. Patients were monitored using contrast-enhanced computed tomography (CT) of the abdomen and noncontrast CT of the chest at 3-month intervals for the initial 12 months and at 6-month intervals thereafter.23
Data are presented as mean ± SD. The χ2test, Fisher's test, and Mann-Whitney U test were used for comparisons of categoric and continuous data between the 2 groups. Survival curves were calculated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazards model. Statistical analysis was performed using SPSS software Version 17.0 (SPSS Inc, Chicago, IL). Statistical significance was defined as P < 0.05.
Results
Preoperative characteristics in the patients aged <70 years (younger) and ≥70 years (elderly) are shown in Table 1. There were no significant differences between the 2 groups in terms of body mass index (BMI), hepatitis C virus positivity, Child-Pugh class A, total bilirubin, albumin, indocyanine green retention rate at 15 minutes (ICG-R15%), platelet count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, AFP, PIVKA-II, presence or absence of esophageal varices, and follow-up period. However, with regard to comorbidity, hypertension was more frequent in the elderly patients, while diabetes mellitus was more frequent in the younger patients. Males accounted for a higher proportion of the younger patients. Table 2 summarizes the surgical and perioperative data in both groups. The type of LR employed, such as major or minor hepatectomy, splenectomy, operation time, blood loss, Pringle time, and hospital stay, did not differ significantly between the 2 groups. Postoperative liver function parameters such as ALT, total bilirubin, prothrombin time, and the amount of ascites showed no significant intergroup differences. Pathologic findings in the 2 groups are listed in Table 3. Maximum tumor size was significantly greater in elderly than in younger patients. However, no significant intergroup differences were found in tumor number, percentage of patients within the Milan criteria (MC), histologic grade, and presence of intrahepatic metastasis, vascular invasion, and liver cirrhosis. Postoperative complications according to the Clavien grading system are noted in Table 4. No significant differences were observed between the 2 groups in terms of grade-II, -III, and -IV complications. However, the frequency of grade-V complication (in-hospital mortality) was significantly higher in elderly than in younger patients, being mainly attributable to liver failure. The overall postoperative mortality and recurrence rates in the 2 groups are shown in Table 5. Eighty-three (63%) of the 131 younger patients developed recurrence, whereas 36 (56%) of the 64 elderly patients did so. No significant intergroup difference was observed in the time to recurrence (13 months versus 14 months, P = 0.339). The main cause of death in younger patients was HCC recurrence, whereas that in elderly patients was both HCC recurrence and liver failure. The results of univariate and multivariate analyses for overall survival (OS) of elderly patients are shown in Table 6. Univariate analysis showed that Child-Pugh class B or C (P = 0.034), PIVKA-II >100 mAU/mL (P = 0.005), tumor size >5 cm (P < 0.001), multiple tumors (P = 0.044), operation time >250 minutes (P = 0.002), and blood loss >400 mL (P = 0.001) were significant risk factors for OS. Multivariate analysis revealed that 3 independent risk factors: tumor size >5 cm (P = 0.030), multiple tumors (P = 0.010), and blood loss >400 mL (P = 0.007) were significant.
Table 1.
Preoperative characteristics of hepatocellular carcinoma patients with portal hypertension
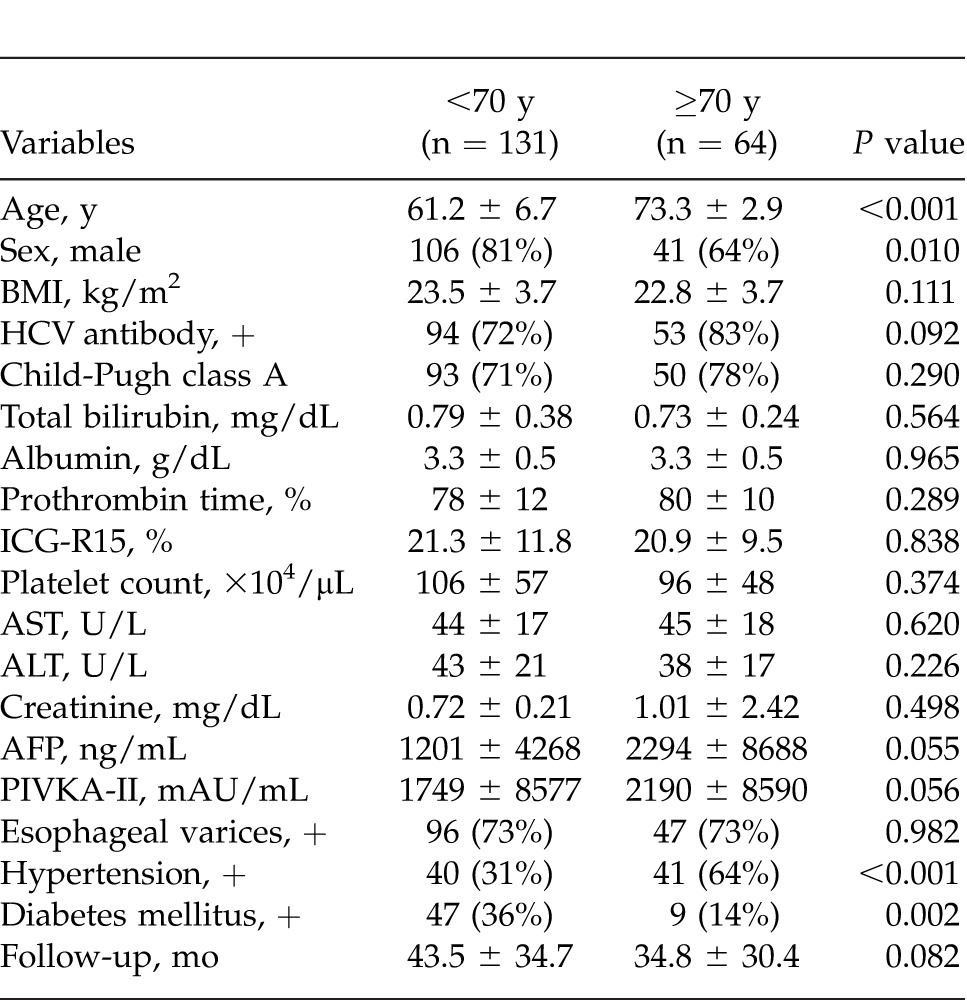
Table 2.
Surgical and perioperative data of hepatocellular carcinoma patients with portal hypertension
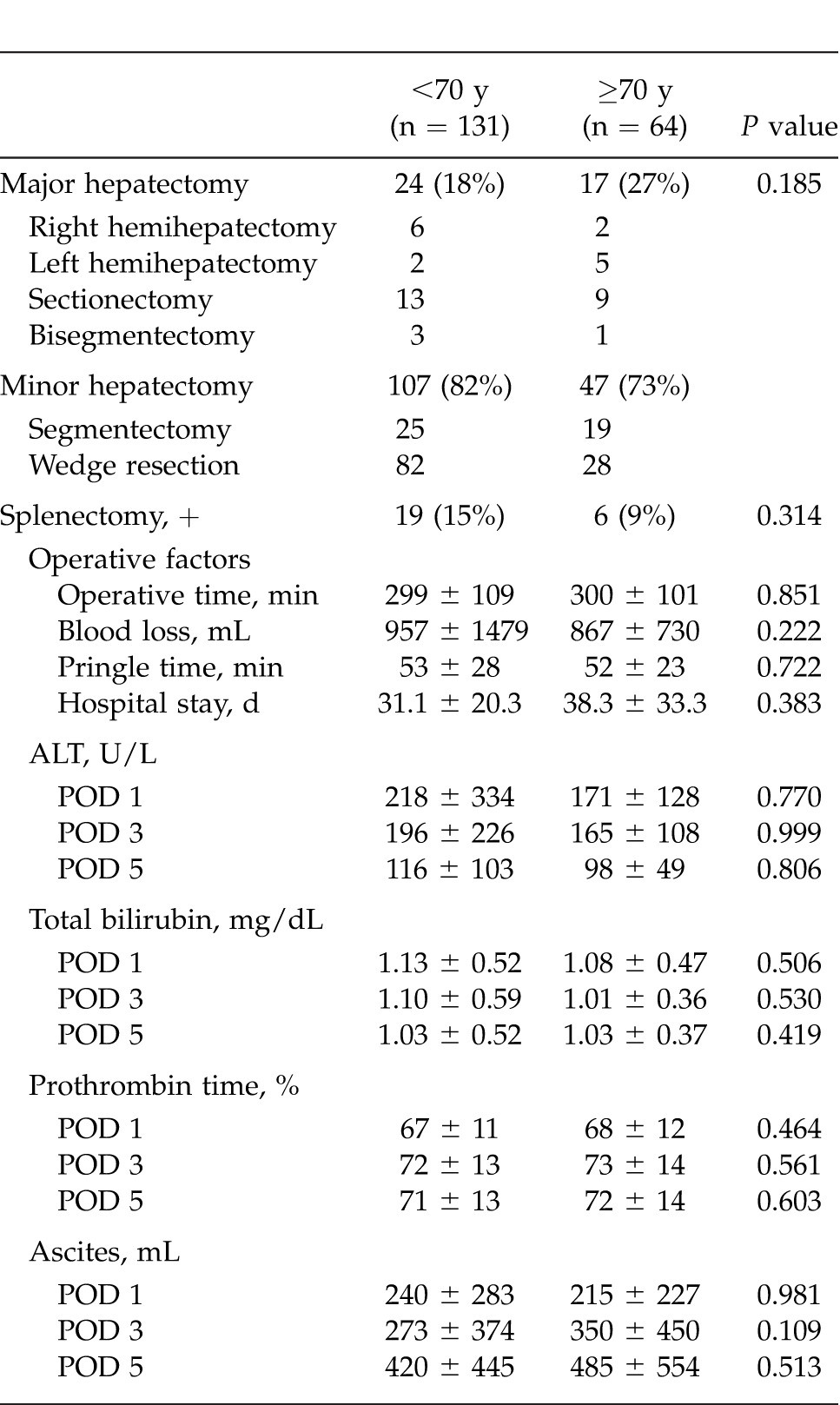
Table 3.
Pathologic findings of hepatocellular carcinoma patients with portal hypertension
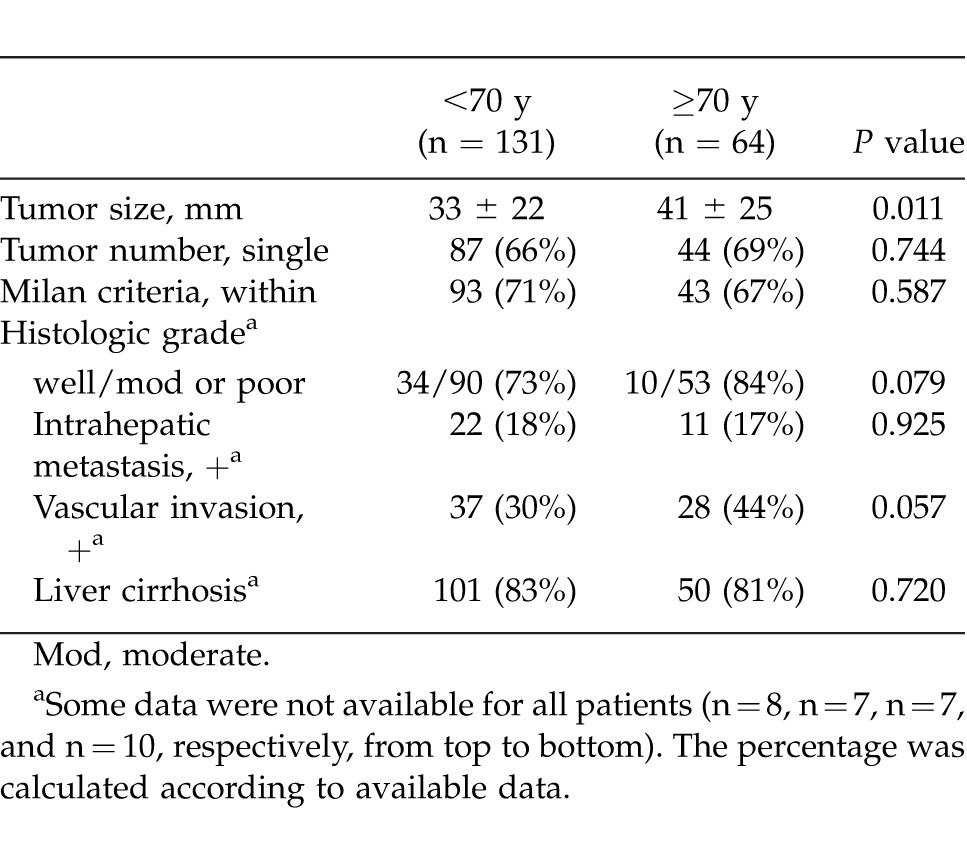
Table 4.
Postoperative complications according to the Clavien grading systems
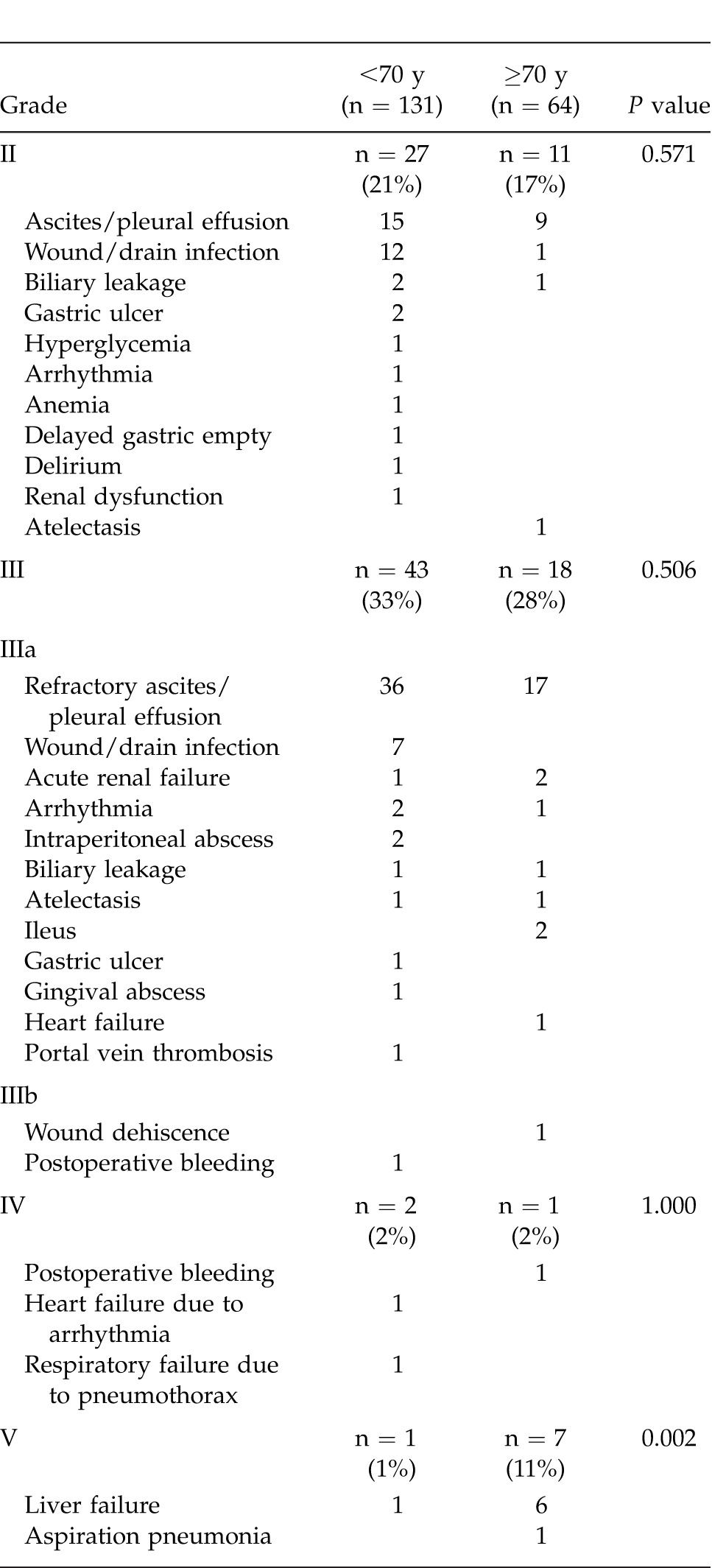
Table 5.
Postoperative recurrence and mortality of hepatocellular carcinoma patients with portal hypertension
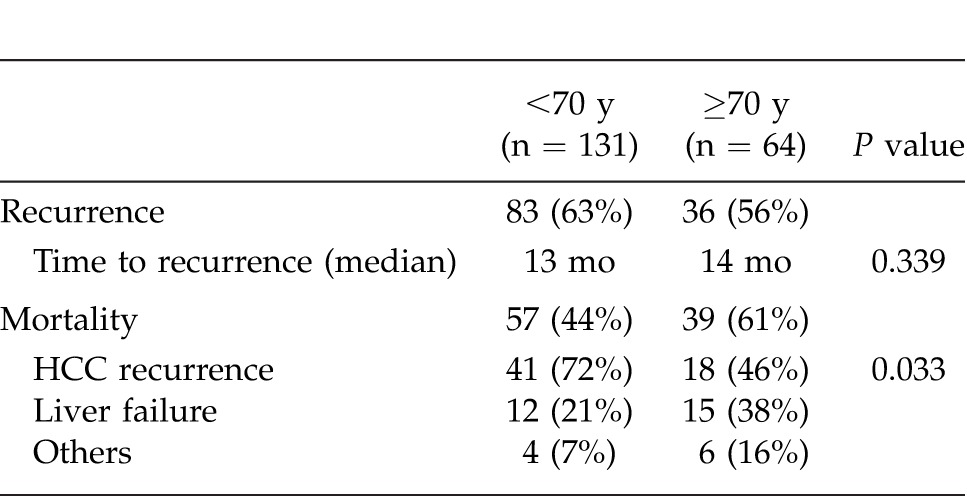
Table 6.
Univariate and multivariate analyses for overall survival of age ≥70 years group in hepatocellular carcinoma patients with portal hypertension
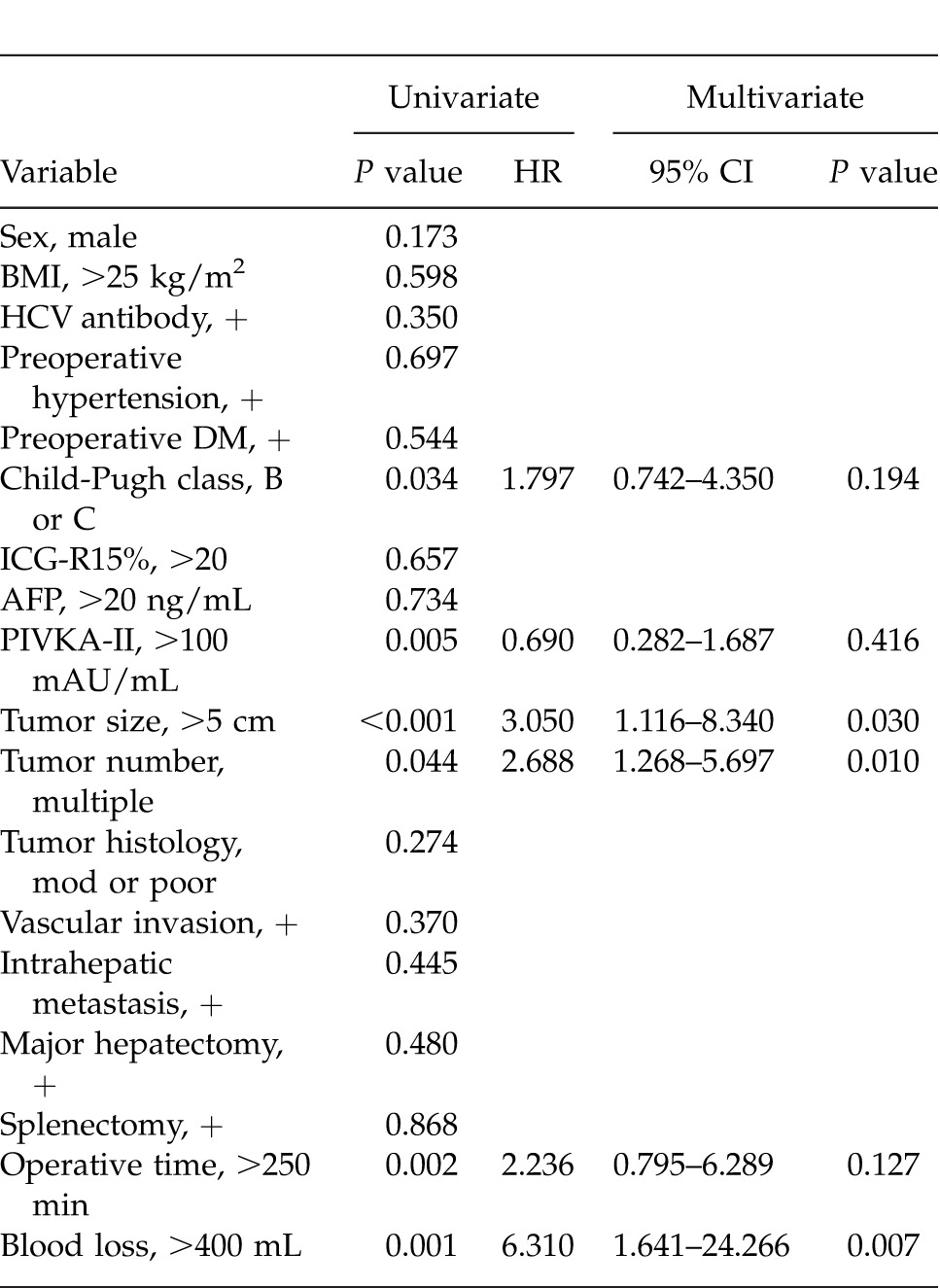
Figure 1 shows the OS and disease-free survival (DFS) curves for the 2 groups. Elderly patients showed a poorer 5-year OS than younger patients (36.0% versus 56.3%, P = 0.010). However, the difference in the 5-year DFS did not reach significance (19.7% versus 17.2%, P = 0.338).
Fig. 1.
The 5-year overall survival was significantly higher in the <70 years age group than in the ≥70 years age group (56.3% versus 36.0%, P = 0.010) of HCC patients with portal hypertension. However, the difference in disease-free survival between the groups did not reach significance (19.7% versus 17.2%, P = 0.338).
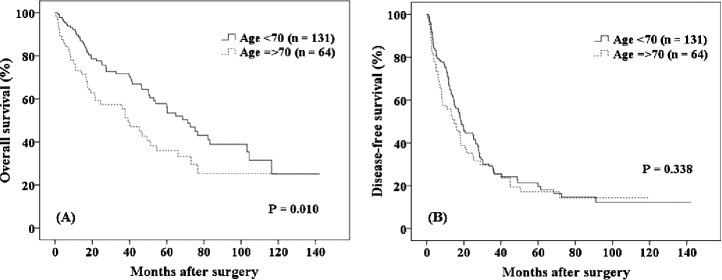
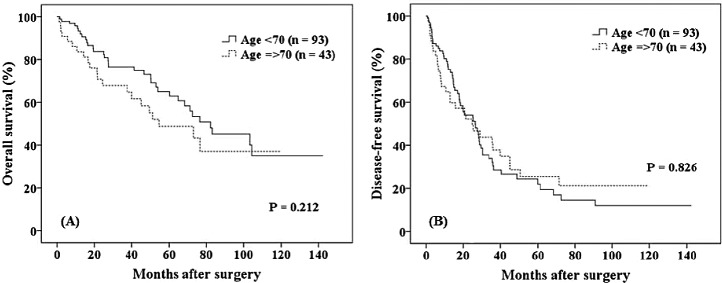
The OS and DFS rates for younger patients and elderly patients within the MC are shown in Fig. 2. No significant intergroup differences were evident (65% versus 49%, respectively, P = 0.212; 22% versus 25%, respectively, P = 0.826). For patients outside the MC (Fig. 3), the 5-year OS and 1-year DFS rates were significantly higher in younger than in elderly patients (37% versus 9%, respectively, P = 0.002; 46% versus 37%, respectively, P = 0.050).
Fig. 2.
For patients within the Milan criteria, no significant differences in the 5-year overall survival and disease-free survival rates were observed between younger and elderly patients (65% versus 49%, respectively, P = 0.212; 22% versus 25%, respectively, P = 0.826).
Fig. 3.
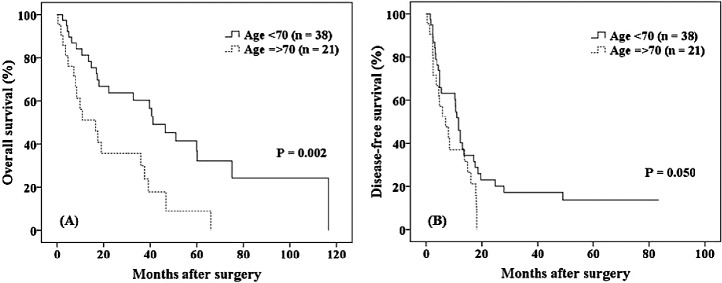
For patients outside the Milan criteria, the 5-year overall survival and 1-year disease-free survival rates were significantly higher for younger than for elderly patients (37% versus 9%, respectively, P = 0.002; 46% versus 37%, respectively, P = 0.050). CI, confidence interval; DFS, disease-free survival; DM, diabetes mellitus; HCV, hepatitis C virus; HR, hazard ratio; POD, postoperative day.
Discussion
There is no doubt that LT is a better therapeutic approach for HCC patients with a cirrhotic liver, as it can provide potential cure of both the cancer and underlying liver disease at the same time. In the United States and Europe, the AASLD/BCLC Guidelines have been widely utilized for the management of HCC.7,8 They recommend that LT can be applied for HCC patients with PHT, and LR can be applied for HCC patients without PHT who have a single nodule with or without cirrhosis, with preservation of liver function. Although LT has recently been used in an increasing number of patients older than 65 years,4,24 it remains debatable whether the indications for LT in elderly patients should be expanded. In Japan, low availability of donated organs is a serious limitation to the use of LT as an initial treatment for patients with HCC,9,25 and therefore implementation of LR for HCC has been developed as the preferred initial treatment. LR still plays a crucial role as one of the curative treatment modalities in Japan for elderly HCC patients, irrespective of the presence of PHT. Clarification of the outcome of LR for elderly HCC patients with PHT may help to better define the indications for LT in such patients. However, to our knowledge, no reports have described the outcome of LR for elderly patients with PHT, who are considered to be at extremely high risk. Accordingly, in the present study, we compared the OS, DFS, recurrence rate, and postoperative complications between elderly patients and younger patients.
In the present study, values of preoperative liver function parameters such as total bilirubin, albumin, prothrombin time, and ICG-R15% in the 2 groups were similar. Operation time, blood loss, and Pringle time in elderly patients were comparable with those in younger patients, irrespective of whether major or minor hepatectomy was performed. Values of postoperative liver function parameters such as ALT, total bilirubin, and prothrombin time, the amount of drained ascites up to postoperative day 5, and the duration of hospitalization were generally equivalent between the 2 groups. Furthermore, there were no significant intergroup differences in the recurrence rate and time to recurrence after surgery. These results indicate that LR can be performed safely and curatively based on adequate selection criteria even in elderly patients with PHT. The feasibility of LR for elderly patients reflects the results of previous studies that show operative outcomes similar to those for younger patients in terms of the type of hepatectomy, operation time, blood loss, requirement for blood transfusion, and hospital stay.11–16
Our study demonstrated that the incidence of grade-II, -III, and -IV complications did not differ between the 2 age groups. Ascites, pleural effusion, wound infection, and drain infection frequently developed in both. Patients with cirrhosis who have PHT may have intestinal circulatory disturbance, causing bacterial translocation and thus increasing the levels of inflammatory cytokines.26,27 These background factors may contribute to such complications. Furthermore, it has been reported that platelets may play an important role in antimicrobial host defenses against bacterial and fungal pathogens.28 Therefore, patients with thrombocytopenia due to PHT may be more susceptible to various infections than patients without thrombocytopenia. Elderly patients exhibited a higher incidence of grade-V complication (i.e., in-hospital mortality) than younger patients (7 cases versus 1 case, P = 0.002). Among these 7 grade-V cases, 6 were due to postoperative liver failure. These results suggest that despite the similarity of preoperative values of liver function parameters between the 2 groups, the liver functional capacity of some elderly patients might not allow them to tolerate the metabolic demands after LR.
Both HCC recurrence and liver failure due to progression of underlying liver disease were often observed as part of the background contributing to overall mortality in elderly patients (Table 5). Wakabayashi et al demonstrated a discrepancy between the liver volume estimated by CT and actual functional hepatocyte volume examined using 99mTc-galactosyl-human serum albumin liver scintigraphy in elderly patients with liver tumors, who were awaiting surgery, and considered that this might have a critical impact on preoperative liver functional reserve prior to hepatic resection.29 In the field of LT, donor age is a well-known risk factor affecting graft failure and patient survival. Grafts from older donors are associated with slow recovery of liver function, a higher incidence of primary nonfunction, and a higher risk of early and long-term mortality.30–32 These findings suggest that liver functional capacity declines with aging, finally resulting in a difference of OS, as was observed between the 2 groups in this series.
Although the 5-year OS rate was significantly lower in elderly than in younger patients, it did not differ significantly between the groups for patients who were within the MC. In contrast, for patients outside the MC, the 5-year OS and 1-year DFS rates were significantly lower in the elderly. These results indicate that LR for elderly HCC patients with PHT is obviously preferable for patients within the MC.
In conclusion, although LR for elderly HCC patients with PHT can be performed safely and curatively, allowing outcomes comparable with those in younger HCC patients, it is associated with a higher in-hospital mortality rate and a lower 5-year OS rate. Therefore, LR should be performed for patients within the MC while ensuring careful postoperative management.
References
- 1.Kiyosawa K, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Gad A, et al. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127(5):S17–S26. doi: 10.1053/j.gastro.2004.09.012. suppl 1. [DOI] [PubMed] [Google Scholar]
- 2.Umemura T, Ichijo T, Yoshizawa K, Tanaka E, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol. 2009;44((suppl 19)):102–107. doi: 10.1007/s00535-008-2251-0. [DOI] [PubMed] [Google Scholar]
- 3.Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, et al. Report of the 17th nationwide follow-up of primary liver cancer in Japan. Hepatol Res. 2007;37(9):676–691. doi: 10.1111/j.1872-034X.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 4.Abecassis M, Bridges ND, Clancy CJ, Dew MA, Eldadah B, Englesbe MJ, et al. Solid-organ transplantation in older adults: current status and future research. Am J Transplant. 2012;12(10):2608–2622. doi: 10.1111/j.1600-6143.2012.04245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santambrogio R, Kluger MD, Costa M, Belli A, Barabino M, Laurent A, et al. Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh's A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford) 2013;15(1):78–84. doi: 10.1111/j.1477-2574.2012.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliante F, Ardito F, Pinna AD, Sarno G, Giulini SM, Ercolani G, et al. Liver resection for hepatocellular carcinoma ≤3 cm: results of an Italian multicenter study on 588 patients. J Am Coll Surg. 2012;215(2):244–254. doi: 10.1016/j.jamcollsurg.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373(9664):614–616. doi: 10.1016/S0140-6736(09)60381-0. [DOI] [PubMed] [Google Scholar]
- 8.Chow PK. Resection for hepatocellular carcinoma: is it justifiable to restrict this to the American Association for the Study of the Liver/Barcelona Clinic for Liver Cancer criteria? J Gastroenterol Hepatol. 2012;27(3):452–457. doi: 10.1111/j.1440-1746.2011.07034.x. [DOI] [PubMed] [Google Scholar]
- 9.de Villa VH, Lo CM, Chen CL. Ethics and rationale of living-donor liver transplantation in Asia. Transplantation. 2003;75(3):S2–S5. doi: 10.1097/01.TP.0000046532.44975.57. suppl. [DOI] [PubMed] [Google Scholar]
- 10.Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, et al. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250(6):922–928. doi: 10.1097/SLA.0b013e3181b977a5. [DOI] [PubMed] [Google Scholar]
- 11.Tsujita E, Utsunomiya T, Ohta M, Tagawa T, Matsuyama A, Okazaki J, et al. Outcome of repeat hepatectomy in patients with hepatocellular carcinoma aged 75 years and older. Surgery. 2010;147(5):696–703. doi: 10.1016/j.surg.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 12.Kondo K, Chijiiwa K, Funagayama M, Kai M, Otani K, Ohuchida J. Hepatic resection is justified for elderly patients with hepatocellular carcinoma. World J Surg. 2008;32(10):2223–2229. doi: 10.1007/s00268-008-9688-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee CR, Lim JH, Kim SH, Ahn SH, Park YN, Choi GH, et al. A comparative analysis of hepatocellular carcinoma after hepatic resection in young versus elderly patients. J Gastrointest Surg. 2012;16(9):1736–1743. doi: 10.1007/s11605-012-1966-7. [DOI] [PubMed] [Google Scholar]
- 14.Yamada S, Shimada M, Miyake H, Utsunomiya T, Morine Y, Imura S, et al. Outcome of hepatectomy in super-elderly patients with hepatocellular carcinoma. Hepatol Res. 2012;42(5):454–458. doi: 10.1111/j.1872-034X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 15.Zacharias T, Jaeck D, Oussoultzoglou E, Bachellier P, Weber JC. First and repeat resection of colorectal liver metastasis in elderly patients. Ann Surg. 2004;240(5):858–865. doi: 10.1097/01.sla.0000143272.52505.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oishi K, Itamoto T, Tsuyoshi K, Oshita A, Amano H, Ohdan H, et al. Hepatectomy for hepatocellular carcinoma in elderly patients aged 75 years or more. J Gastrointest Surg. 2009;13(4):695–701. doi: 10.1007/s11605-008-0758-6. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 18.Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9(4):298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- 19.Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134(9):984–992. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 20.Strasberg SM, Phillips C. Use and dissemination of the Brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2013;257(3):377–382. doi: 10.1097/SLA.0b013e31825a01f6. [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149(5):713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Sawada T, Kubota K, Kita J, Kato M, Shiraki T, Park K, et al. Clinical outcome of hepatectomy for hepatocellular carcinomas ≤2 cm. World J Surg. 2011;35(2):377–385. doi: 10.1007/s00268-010-0851-3. [DOI] [PubMed] [Google Scholar]
- 24.Secunda K, Gordon EJ, Sohn MW, Shinkunas LA, Kaldjian LC, Voigt MD, et al. National survey of provider opinions on controversial characteristics of liver transplant candidates. Liver Transpl. 2013;19(4):395–403. doi: 10.1002/lt.23581. [DOI] [PubMed] [Google Scholar]
- 25.Umeshita K, Fujiwara K, Kiyosawa K, Makuuchi M, Satomi S, Sugimachi K, et al. Operative morbidity of living liver donors in Japan. Lancet. 2003;362(9385):687–690. doi: 10.1016/S0140-6736(03)14230-4. [DOI] [PubMed] [Google Scholar]
- 26.Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34(1):32–37. doi: 10.1016/s0168-8278(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 27.Riordan SM, Williams R. The intestinal flora and bacterial infection in cirrhosis. J Hepatol. 2006;45(5):744–757. doi: 10.1016/j.jhep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Chang FY, Singh N, Gayowski T, Wagener MM, Mietzner SM, Stout JE, et al. Thrombocytopenia in liver transplantation recipients: predictors, impact on fungal infections, and role of endogenous thrombopoietin. Transplantation. 2000;69(1):70–75. doi: 10.1097/00007890-200001150-00014. [DOI] [PubMed] [Google Scholar]
- 29.Wakabayashi H, Nishiyama Y, Ushiyama T, Maeba T, Maeta H. Evaluation of the effect of age on functioning hepatocyte mass and liver blood flow using liver scintigraphy in preoperative estimations for surgical patients: comparison with CT volumetry. J Surg Res. 2002;106(2):246–253. doi: 10.1006/jsre.2002.6462. [DOI] [PubMed] [Google Scholar]
- 30.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 31.Yersiz H, Shaked A, Olthoff K, Imagawa D, Shackleton C, Martin P, et al. Correlation between donor age and the pattern of liver graft recovery after transplantation. Transplantation. 1995;60(8):790–794. [PubMed] [Google Scholar]
- 32.Busquets J, Xiol X, Figueras J, Jaurrieta E, Torras J, Ramos E, et al. The impact of donor age on liver transplantation: influence of donor age on early liver function and on subsequent patient and graft survival. Transplantation. 2001;71(12):1765–1771. doi: 10.1097/00007890-200106270-00011. [DOI] [PubMed] [Google Scholar]


