Abstract
The aim of this study was to analyze the association of potential risk factors such as positive family cleft history, smoking, use of drugs during pregnancy, and parental age with oral clefts in offspring within the Kosovo population. We conducted a population-based case-control study of live births in Kosovo from 1996 to 2005. Using a logistic regression model, 244 oral cleft cases were compared with 488 controls. We have excluded all syndromic clefts. Heredity increases the risk of clefts in newborns [odds ratio (OR) = 8.25, 95% confidence interval (CI) 3.12–23.52]. Clefts were also associated with smoking (OR = 1.87, 95% CI 0.75–4.08), use of drugs during pregnancy (OR = 2.25, 95% CI 0.82–5.12), increasing maternal age (OR = 1.83, 95% CI 1.42–2.49), and increasing paternal age (OR = 1.3, 95% CI 1.2– 1.4). We found heredity to be the most important factor for cleft occurrence in Kosovar newborns. Another significant potential risk factor for occurrence of clefts is the parental age. We found the use of drugs and smoking during pregnancy to be less significant.
Key words: Oral clefts, Cleft lip with or without cleft palate, Cleft palate only, Potential risk factors, Logistic regression model, Dummy variables
Cleft lip with or without cleft palate and isolated cleft palate are the most common facial birth defects. The etiology involves complex interactions between genetic and environmental factors.1 Genetic factors appear to create the most susceptibility for clefts. When environmental factors (i.e., triggers) interact with a genetically susceptible genotype, a cleft develops during an early stage of development.2 The aim of this study was to analyze the association of potential risk factors such as positive family cleft history, smoking, use of drugs during pregnancy, and parental age with oral clefts in offspring in Kosovo. Risk factors vary among populations, and our ultimate goal was to provide data that may show new potential risk factors influencing the occurrence of clefts in Kosovo. With this new information, we hope to increase public awareness of such risks and thereby help reduce the incidence of cleft in Kosovo.
Methods
We conducted a population-based case-control study. A total of 304,629 births from 1996 to 2005 in Kosovo were screened for oral clefts. We found 244 cases with nonsyndromic oral clefts, [62 cases of cleft lip (CL), 101 cases of cleft lip and cleft palate (CL/P), and 81 cases with cleft palate only (CP)]; the overall prevalence was 0.8 per 1000. Clinical and laboratory examinations conducted by neonatologists and pediatricians served as the basis for differentiating between syndromic and nonsyndromic clefts, although we have only included the nonsyndromic clefts.
To analyze the relation between cleft occurrence and potential risk factors, we used a logistic regression model: 244 oral cleft cases were compared with 488 controls. Listing of variables was done through their transformation into the so-called dummy variables, by making use of the reference coding (RC).
We analyzed the following potential risk factors: (1) positive family history, such as the presence of clefts in the mother's or father's family (compared with a negative family history, i.e., no family member with a cleft as a constant); (2) smoking during the first trimester of pregnancy (compared with nonsmokers); (3) the use of various drugs (which could cause clefts) during the first trimester of pregnancy (compared with no drug history); and (4) maternal and paternal age. In particular, we analyzed the risk for cleft occurrence, according to different age groups of parents (compared with parents over age 35). For this purpose, we applied the model of multiple logistic regression.
Results
The overall prevalence of cleft lip with or without cleft palate in Kosovo between 1996 and 2005 was 0.8 per 1000 live births. Positive family cleft-related history increased the risk for cleft occurrence in newborns compared with cases with a negative family history (P < 0.001).The mother's smoking history (P = 0.22) and the mother's use of drugs during pregnancy (P = 0.19), as potential risk factors, had little influence on the prevalence of clefts. The risk for clefts in a newborn rose with an increase in the maternal and paternal age. The risk for cleft occurrence increased 1.83 times for every subsequent year of maternal age (P < 0.001) and 1.3 times for every subsequent year of paternal age (Fig. 1).
Fig. 1.
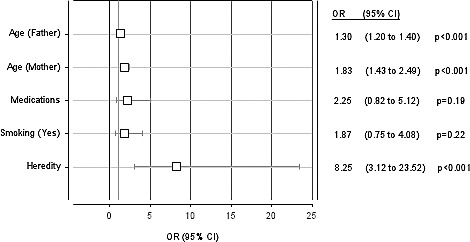
Logistic regression analysis. The risk for cleft lip and palate according to risk factors; OR (95% CI).
By comparing the risk for cleft occurrence in mothers in the age groups 20 to 25, 26 to 35, and age >35 compared with the age-group ≤19, we found the highest risk was in mothers with age >35 (P = 0.002). In all other groups of maternal age, the risk for cleft occurrence compared with the age >35 was reduced (Fig. 2).
Fig. 2.
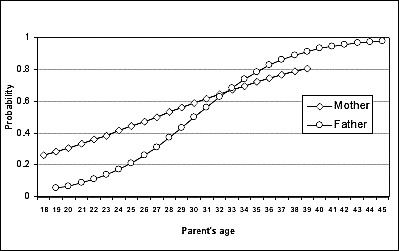
Multiple logistic regression analysis. Probability for oral clefts according to parent's age (n = 244).
According to our results, the highest risk for cleft occurrence was with paternal age >35 (P < 0.001). With all other groups of paternal age, the risk for cleft occurrence was reduced (Fig. 3).
Fig. 3.
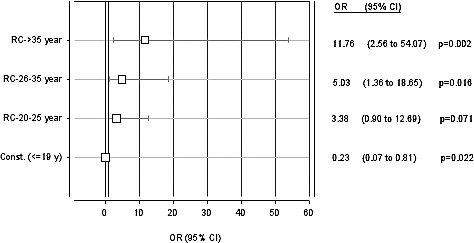
Multiple logistic regression analysis. Mother's age as a risk factor for CL/P; OR (95% CI).
Discussion
Factors affecting the incidence of facial clefts are considered to be a positive family history, the use of drugs during the first trimester of pregnancy, smoking while pregnant, stress, avitaminosis, radiologic examination, infections during the first trimester, intoxication with different substances, marriage with close relatives (consanguinity), along with others.3
Our findings confirmed that heredity (i.e., a positive family cleft history) increased the odds of having a child with cleft lip and cleft palate [CL/P: OR = 8.25, 95% confidence interval (CI) 3.12–23.52, P < 0.001] compared with those cases with a negative family history. Leite and Koifman, in a case-control study, found that the history of oral clefts either in the father's (CL/P: OR = 16, 95% CI 5.6–69.2; CP: OR = 6.6, 95% CI 1.5–33.7) or in the mother's family (CL/P: OR = 5, 95% CI 2.3–10.9, CP: OR = 12.4, 95% CI 1.3–294.9) is strongly associated with both types of clefts, but parental consanguinity was associated only with cleft lip and cleft palate (CL/P: OR = 3.8, 95% CI 1.3–12.2).4
Zarante et al found that a positive family history of another craniofacial malformation (OR = 3.1, CI 95% 2.2–4.3) and cleft lip with or without cleft palate (CL ± P) (OR = 2.5, CI 95% 1.1–5.8) are important risk factors for orofacial clefting.5
In Korea, a positive family history is found in 7% of cleft cases, with the most common type being a CL (10.8%) followed by CL ± P (6.7%), and the rarest type being a CP (3.7%).6 Natsume et al found that 15.4% (47 of 306) of cases of cleft in Japan had a positive family history for CL ± P, whereas in the control group only 1.6% (5 of 306) of cases had a positive family history for clefting.7
Smoking while pregnant is also considered to be a risk factor. In our study, 31 women smoked during their pregnancy. Smoking during pregnancy increased odds of having a child with cleft lip/palate compared with nonsmokers, but the OR (1.87, 95% CI 0.75–4.08) was not statistically significant (P = 0.22).
Leite and Koifman, in a case-control study, investigated the possible associations between smoking during the first trimester of pregnancy and nonsyndromic orofacial cleft (CL/P and CP).4 The prevalence of maternal smoking during the first trimester of pregnancy was higher among cases, but the OR (1.13, 95% CI 0.81–1.57) was not statistically significant.
Honein et al, in a study on maternal smoking and environmental tobacco smoke exposure and the risk of orofacial clefts, found that periconceptional smoking is associated with cleft lip and palate (OR = 1.3; 95% CI 1.0–1.6) and more strongly associated with bilateral clefting (OR = 1.7; 95% CI 1.2–2.6), with a weaker association observed for isolated cleft palate. Heavy maternal smoking (≥25 cigarettes/d) is associated with cleft lip and palate (OR = 1.8; 95% CI 1.0–3.2) and bilateral CL/P (OR = 4.2; 95% CI 1.7–10.3) .8
Little et al found a positive association between maternal smoking during the first trimester of pregnancy with both cleft lip with or without cleft palate (OR = 1.9, 95% CI 1.1–3.1) and isolated cleft palate (OR = 2.3, 95% CI 1.3–4.1) in the United Kingdom.9
Chung et al reported the largest study to test the association between maternal cigarette smoking during pregnancy and having a newborn with cleft lip and/or palate.10 To determine the dose-response of cigarette smoking during pregnancy, the cigarette consumption per day was divided into 4 groups: (1) no smoking; (2) 1 to 10; (3) 11 to 20; (4) and 21 or more. A dose-response relationship was found when comparing each smoking category with the no-smoking reference group: OR = 1.5 (1.28, 1.76), 1.55 (95% CI 1.23, 1.95), and 1.78 (95% CI 1.22, 2.59), respectively. This means that increased cigarette smoking during pregnancy results in increased odds of having a child with cleft lip and/or palate.
Another factor to be considered is the intake of drugs during the first trimester of pregnancy. In our study, a total of 27 mothers had taken drugs during their pregnancy, mainly analgesics, antiemetics, sedatives, corticosteroids, anticonvulsants, and drugs containing iron and folate. Our study confirmed that the intake of drugs during pregnancy as a potential risk factor has little influence on the prevalence of clefts (P = 0.19).
Puhó et al evaluated the possible association between all kinds of drug treatments during pregnancy and isolated cleft lip with or without cleft palate and posterior cleft palate in the offspring.11 They found that children born to mothers treated with amoxicillin, phenytoin, oxprenolol, and thiethylperazine during the second and third months of pregnancy have an increased risk for isolated CL/P.
Zarante et al found that medication use during pregnancy is an important risk factor for orofacial malformation (OR = 2.0, CI 95% 1.4–2.9).5 Wang et al found the use of analgesic and antipyretic drugs significantly increases the risk for oral clefts (adjusted OR 3.1, 95% CI 1.41–6.86).12
Chuangsuwanich et al found in Thailand that in 52.74% (578 of 1096) of cases of clefts, women used drugs during their first trimester of pregnancy.13 The most frequently used drugs were analgesics. Jonson found that mothers who had anticonvulsive therapy during their first trimester of pregnancy are 5% more likely to have newborns with clefts.14 Abrishamchian et al analyzed the level of risk for a facial cleft in pregnant women treated for epilepsy, claiming that the level of risk for CL ± P incidence is higher by a factor of 7.77, whereas for CP it is higher by a factor of 3.61.15 The use of polytherapy shows the highest increase of risk for facial cleft incidence (CL ± P; OR = 10.5, 95% CI 1.52–59.9). Antiemetics taken during the first trimester of pregnancy were found to increase the incidence of clefts, according to Niebyl.16
A deficiency of folic acid could be the consequence of a reaction with other drugs taken by the patient whose metabolism exhausts folic acid and may result in newborns with orofacial clefts.17 Various sedatives such as benzodiazepines have been associated with clefts in newborns. Laegreid et al reported on the role of benzodiazepines in causing different anomalies if taken in the first months of pregnancy.18 Of 8 newborns whose mothers used this medication, 2 were born with a cleft palate. In the literature, there are contradictory data, and different studies do not agree that benzodiazepines can cause congenital anomalies.19,20
Another important factor is the parental age. The older the parents, the higher the probability there is for a cleft to occur. In our study, we found that with an increase in the maternal age, there was an increased occurrence of clefts in the newborns (P < 0.001). Our conclusion was that the maternal age has a greater impact compared with that of the father. A 1 year increase in the maternal age increases the risk for clefts 1.83 times, while the same increase in the paternal age increases this risk 1.3 times (P < 0.001).
In reference to the effect of maternal age as a risk factor, Sipak et al confirmed that women older than 35 were prone to a higher risk for orofacial clefts.21 Whereas, Shaw et al concluded that the risk for women older than 39 of having children with orofacial clefts is 3 times higher compared with women aged 25 to 29.22
Conclusions
From the findings in our study, we concluded that heredity with a positive family cleft history is the most important factor for a risk of clefts in newborns. Another risk with a significant effect on the incidence of clefts was the maternal age followed by the paternal age. There was an increased risk for clefts when both the mother and father were above the age of 35 years. The use of drugs and smoking during pregnancy also increased the risk for cleft incidence but did not reach a significant level.
These findings are interesting as they confirm the fact that heredity is a strong risk factor for the development of clefts. However, our study shows that parental age seems to affect the Kosovo population more than other populations. Furthermore, environmental risk factors did not seem to be as important in the development of clefts as noted in other studies. Education of the Kosovo population with this new information will hopefully help to reduce the incidence of clefts in the country.
Fig. 4.
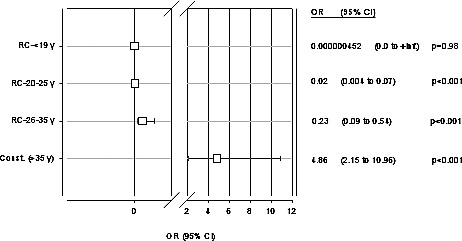
Multiple logistic regression analysis. Father's age as a risk factor for CL/P; OR (95% CI).
Acknowledgments
The authors would like to thank James D. Smith, MD, Professor Emeritus (Oregon Health & Science University), for his help in preparing this manuscript.
Footnotes
Reprints will not be available from the authors.
References
- 1.Lidral AC, Moreno LM, Bullard SA. Genetic factors and orofacial clefting. Semin Orthod. 2008;14(2):103–114. doi: 10.1053/j.sodo.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tolarova MM. Cleft lip and palate. Mar 23, 2009. http://emedicine.medscape.com/article/995535-overview. Accessed 05/20/2013. [Google Scholar]
- 3.Peterson LJ. Contemporary Oral and Maxillofacial Surgery. St Louis, MO:: Mosby; 1998. pp. 656–679. 3rd ed. [Google Scholar]
- 4.Leite IC, Koifman S. Oral clefts, consanguinity, parental tobacco and alcohol use: a case-control study in Rio de Janeiro, Brazil. Braz Oral Res. 2009;23(1):31–37. doi: 10.1590/s1806-83242009000100006. [DOI] [PubMed] [Google Scholar]
- 5.Zarante I, López MA, Caro A, García-Reyes JC, Ospina JC. Impact and risk factors of craniofacial malformations in a Colombian population. Int J Pediatr Otorhinolaryngol. 2009;73(10):1434–1437. doi: 10.1016/j.ijporl.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Sukwha K. Woo Jung K, Changhyun O, Jae-Chan K. Cleft lip and palate incidence among the live births in the Republic of Korea. J Korean Med Sci. 2002;17(1):49–52. doi: 10.3346/jkms.2002.17.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natsume N, Kawai T, Ogi N, Yoshida W. Maternal risk factors in cleft lip and palate: case control study. Br J Oral Maxillofac Surg. 2000;38(1):23–25. doi: 10.1054/bjom.1999.0133. [DOI] [PubMed] [Google Scholar]
- 8.Honein MA, Rasmussen SA, Reefhuis J, Romitti PA, Lammer EJ, Sun L, et al. Maternal smoking and environmental tobacco smoke exposure and the risk of orofacial clefts. Epidemiology. 2007;18(2):226–233. doi: 10.1097/01.ede.0000254430.61294.c0. [DOI] [PubMed] [Google Scholar]
- 9.Little J, Cardy A, Arslan MT, Gilmour M, Mossey PA. Smoking and orofacial clefts: a United Kingdom-based case-control study. Cleft Palate Craniofac J. 2004;41(4):381–386. doi: 10.1597/02-142.1. [DOI] [PubMed] [Google Scholar]
- 10.Chung KC, Kowalski CP, Kim HM, Buchman SR. Maternal cigarette smoking during pregnancy and the risk of having a child with cleft lip/palate. Plast Reconstr Surg. 2000;105(2):485–491. doi: 10.1097/00006534-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Puhó EH, Szunyogh M, Métneki J, Czeizel AE. Drug treatment during pregnancy and isolated orofacial clefts in Hungary. Cleft Palate Craniofac J. 2007;44(2):194–202. doi: 10.1597/05-208.1. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Guan P, Xu W, Zhou B. Risk factors for oral clefts: a population-based case-control study in Shenyang, China. Paediatr Perinat Epidemiol. 2009;23(4):310–320. doi: 10.1111/j.1365-3016.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- 13.Chuangsuwanich A, Aojanepong C, Muangsombut S, Tongpiew P. Epidemiology of cleft lip and palate in Thailand. Ann Plast Surg. 1998;41(1):7–10. doi: 10.1097/00000637-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Jonson K. Reevaluate anticonvulsant use in pregnant epilepsy patients. Fam Pract News. 2001;15(1):1–2. [Google Scholar]
- 15.Abrishamchian AR, Khoury MJ, Calle EE. The contribution of maternal epilepsy and its treatment to the etiology of oral clefts: a population based case-control study. Genet Epidemiol. 1994;11(4):343–351. doi: 10.1002/gepi.1370110404. [DOI] [PubMed] [Google Scholar]
- 16.Niebyl JR, Blake DA, Rocco LE, Baumgardner R, Mellits ED. Lack of metabolic, endocrine, and environmental influences in the etiology of cleft lip with or without palate. Cleft Palate J. 1985;22(1):20–28. [PubMed] [Google Scholar]
- 17.Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. New Engl J Med. 2000;343(22):1608–1614. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- 18.Laegreid L, Olegard R, Walstrom J, Conradi N. Teratogenic effects of benzodiazepine use during pregnancy. J Pediatr. 1989;114(1):126–131. doi: 10.1016/s0022-3476(89)80619-5. [DOI] [PubMed] [Google Scholar]
- 19.Czeizel AE, Erös E, Rockenbauer M, Sørensen HT, Olsen J. Short-term oral diazepam treatment during pregnancy: a population-based teratological case-control study. Clin Drug Invest. 2003;23(7):451–462. doi: 10.2165/00044011-200323070-00004. [DOI] [PubMed] [Google Scholar]
- 20.St. Clair SM, Schirmer RG. First trimester exposure to alprazolam. Obstet Gynecol. 1992;80(5):843–846. [PubMed] [Google Scholar]
- 21.Sipek A, Gregor V, Horacek J, Masatova D. Facial cleft from 1961–2000—incidence, prenatal diagnosis and prevalence by maternal age. Ceska Gynekol. 2002;67(5):260–267. [PubMed] [Google Scholar]
- 22.Shaw GM, Croen LA, Curry CJ. Isolated oral cleft malformations: associations with maternal and infant characteristics in a California population. Teratology. 1991;43(3):225–228. doi: 10.1002/tera.1420430306. [DOI] [PubMed] [Google Scholar]


