Abstract
The decision to undergo surgery for gastric cancer patients aged ≥85 years should be made carefully. We retrospectively reviewed the prognostic factors of gastrectomy for 64 patients aged ≥85 years who had undergone curative gastrectomy for gastric cancer. The effects of various clinical characteristics and surgical interventions on survival were retrospectively analyzed. Univariate analysis revealed that sex (male/female; P = 0.001), the extent of gastric resection (total/distal; P = 0.028), the extent of lymph node dissection (D2/<D2; P = 0.019), and blood loss (P = 0.005) were significant prognostic factors for overall survival. Multivariate analysis demonstrated that sex was the only independent prognostic factor. For pneumonia-specific survival, sex was also the only prognostic factor by multivariate analysis.Prognoses of males aged ≥85 years after gastrectomy were significantly worse than those of females, as they were more likely to die of pneumonia.
Key words: Gastric cancer, Aged, Gastrectomy, Prognosis, Pneumonia
The population of Japan is aging, with the average Japanese life expectancy being 85.9 years for females and 79.4 years for males in 2011.1 People aged 85 years or older are sometimes called the oldest old.2 The number of the oldest old patients with gastric cancer who receive gastrectomy has been increasing recently. Most postoperative courses are uneventful; however, patients will often experience early death due to complications such as pneumonia. Considering their decreased life expectancies and decreased tolerance to stress, the decision to perform surgery on the oldest old should be made carefully.
Previously, we retrospectively compared the prognoses of patients aged ≥85 years who underwent surgery and those who did not, and we demonstrated that overall survival (OS) for operable cases was significantly better in patients who underwent surgery.3 However, patients who underwent surgery had heterogeneous characteristics in terms of sex, age, cancer stage, and the extent of surgical intervention. In the current study, we retrospectively reviewed prognostic factors that have an influence on survival after surgery.
In addition, the cause of death after gastrectomy varies in the oldest old patients. A preliminary study showed that the leading cause of death after gastrectomy was pneumonia, and the second was gastric cancer.4 In the current study, survival analyses were made not only for OS, but also for gastric cancer–specific survival and pneumonia-specific survival. Criteria for deciding whether surgery would be beneficial for the oldest old patients with gastric cancer were evaluated from various aspects.
Materials and Methods
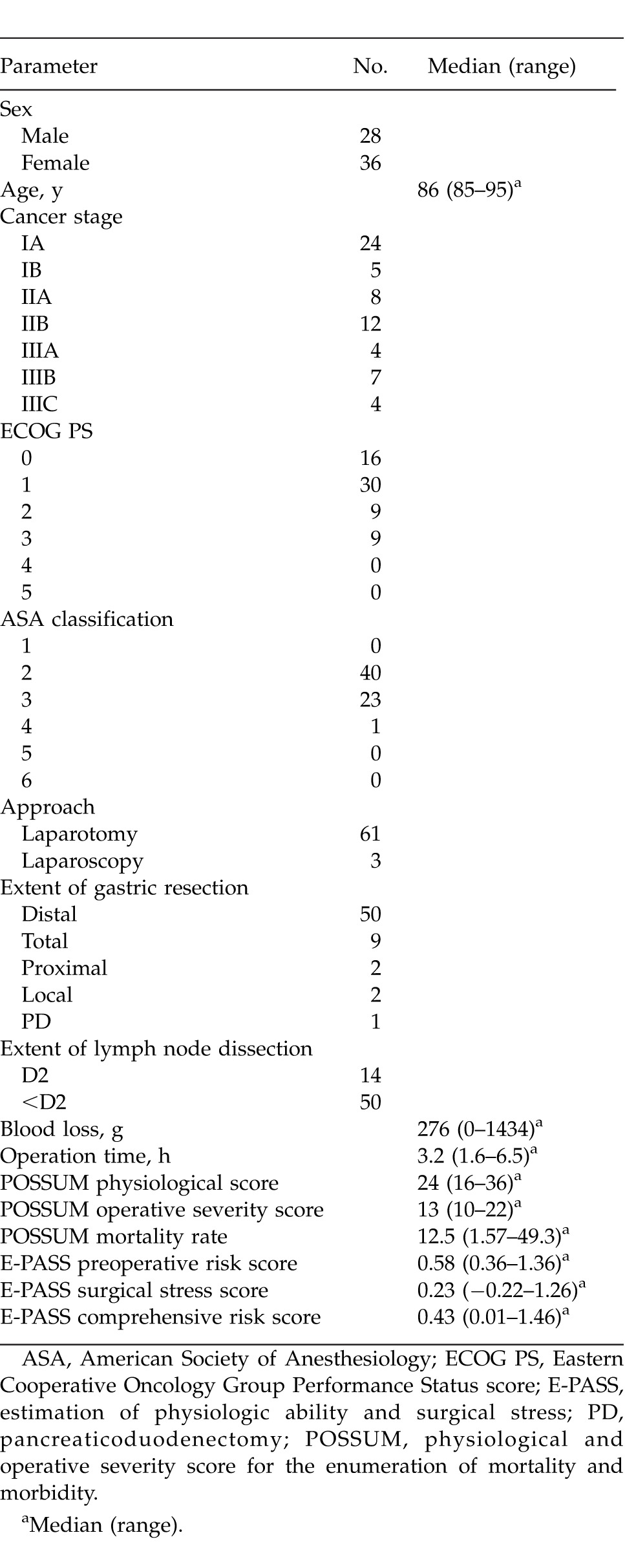
The cases for 64 gastric cancer patients aged ≥85 years who underwent curative gastrectomy were retrospectively reviewed and included 43 patients from the National Hospital Organization Kure Medical Center/Chugoku Cancer Center between 1996 and 2010 and 21 patients from Higashiosaka City General Hospital between 2000 and 2012. Their clinical and operative findings are shown in Table 1. The effects of the following clinical and surgical factors on survival were evaluated by univariate and multivariate analysis using the Cox proportional hazards model: sex, age, cancer stage according to the International Union Against Cancer (UICC) TNM classification5 and the Japanese Classification of Gastric Carcinoma (JCGC),6 Eastern Cooperative Oncology Group Performance Status (PS) score,7 American Society of Anesthesiology (ASA) physical status classification,8 Brinkman index (numbers of cigarettes smoked per day times smoking years), approach of surgical intervention, the extent of gastric resection, the extent of lymph node dissection, estimated blood loss, operation time, the physiological and operative severity score for the enumeration of mortality and morbidity (POSSUM) score,9 and estimation of physiologic ability and surgical stress (E-PASS) score.10
Table 1.
Clinical features and operative findings of patients aged ≥85 years who underwent curative gastrectomy
The PS score was defined as follows: grade 0, fully active, able to carry on all predisease activity without restriction; grade 1, restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature (e.g., light house work, office work); grade 2, ambulatory and capable of all self-care but unable to carry out any work activities, and up and about more than 50% of waking hours; grade 3, capable of only limited self-care, confined to bed or chair more than 50% of waking hours; grade 4, completely disabled, cannot carry on any self-care, and totally confined to bed or chair; grade 5, dead.
The ASA classes were defined as follows: class 1, a normally healthy patient; class 2, a patient with mild systemic disease; class 3, a patient with severe systemic disease; class 4, a patient with severe systemic disease that is a constant threat to life; class 5, a moribund patient who is not expected to survive without the operation; and class 6, a declared brain-dead patient whose organs are being removed for donor purposes.
The physiological score of POSSUM was calculated using age, cardiac signs, chest X-ray signs, respiratory history, systolic blood pressure, pulse rate, Glasgow coma score, hemoglobin level, white cell count, plasma urea level, plasma sodium level, plasma potassium level, and electrocardiography results. The operative severity score of POSSUM was calculated using operation grade, multiple procedures, blood loss, peritoneal soiling, malignancy, and mode of surgery. Each item was scored from 1 to 8 and summed. The POSSUM mortality rate (%) was expressed as follows: 100/{1 + exp[−0.13 × (Physiological score) − 0.16 × (Operative severity score) + 7.04]}
E-PASS was calculated as follows: preoperative risk score (PRS) = −0.0686 + 0.00345X1 + 0.323X2 + 0.205X3 + 0.153X4 + 0.148X5 + 0.0666X6 [X1, age; X2, presence (1) or absence (0) of severe heart disease; X3, presence (1) or absence (0) of severe pulmonary disease; X4, presence (1) or absence (0) of diabetes mellitus; X5, performance status index (0–4); X6, the ASA physiological status classification (1–5)]. Severe heart disease was defined as heart failure of New York Heart Association Class III or IV, or severe arrhythmia requiring mechanical support. Severe pulmonary disease was defined as any condition with a %vital capacity (VC) of less than 60% and/or a forced expiratory volume (FEV) 1.0% of less than 50%. The definition of diabetes mellitus was based on the criteria of the World Health Organization. Surgical stress score (SSS) = −0.342 + 0.0139X1 + 0.0392X2 + 0.352X3 [X1, blood loss/body weight (g/kg); X2, operation time (h); X3, the extent of skin incision (0: minor incisions for laparoscopic or thoracoscopic surgery, including scope-assisted surgery; 1: laparotomy or thoracotomy alone; 2: both laparotomy and thoracotomy)]. Comprehensive risk score (CRS) = −0.328 + 0.936PRS + 0.976SSS.
The Cox proportional hazards model was used to calculate hazard ratios (HRs) for death. OS was defined as the interval from the date of surgery to the date of death from any cause. Alive patients were censored at the date last known to be alive. Gastric cancer–specific survival was defined as the interval from the date of surgery to the date of death caused by gastric cancer. Patients who died of diseases other than gastric cancer were also censored at the date of death. Pneumonia-specific survival was defined as the interval from the date of surgery to the date of death caused by pneumonia. Patients who died of diseases other than pneumonia were also censored at the date of death. Survival was shown on Kaplan-Meier curves and was compared by the log-rank test. P < 0.05 was defined as significant. All analyses were carried out using StatView (version 5.0 for Macintosh, SAS Institute Inc, Cary, North Carolina).
Results
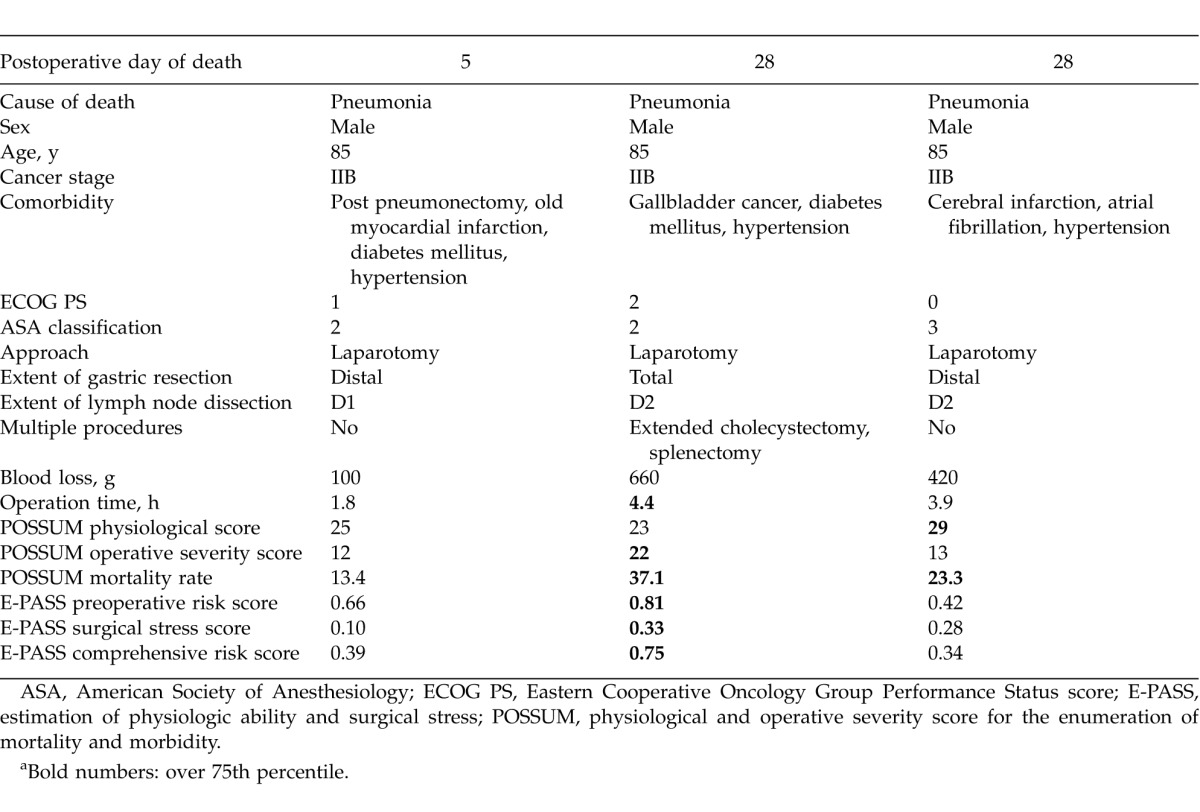
At the time of analysis, 36 patients (56%) had died, and the median follow-up period of the 29 surviving patients was 39 months. The 5-year OS rate was 38%. The cause of death was known in 28 cases: gastric carcinoma in 8 cases (29%), pneumonia in 10 cases (36%), other carcinoma in 3 cases, cardiac failure in 2 cases, cerebral hemorrhage in 1 case, pulmonary embolism in 1 case, trauma in 1 case, general poor condition in 1 case, and sudden death in 1 case. Within 90 days of surgery, 3 patients died of pneumonia; these patients were all males. The details of these 3 cases are shown in Table 2.
Table 2.
Summary of three cases of patients who died within 90 days of surgerya
In univariate analyses, sex, the extent of gastric resection, the extent of lymph node dissection, blood loss, and the POSSUM operative severity score were significantly correlated with OS. Multivariate analysis was conducted with sex, the extent of gastric resection, the extent of lymph node dissection, and blood loss. The POSSUM operative severity score was excluded from multivariate analysis because it is calculated on the basis of blood loss. Multivariate analysis demonstrated that sex was the only independent prognostic factor (Table 3). Figure 1 shows the OS curve after surgery for each sex. Baseline characteristics between male and female patients had no significant difference (Table 4).
Table 3.
Survival analysis of variables predicting risk of death for patients aged ≥85 years who underwent curative gastrectomya
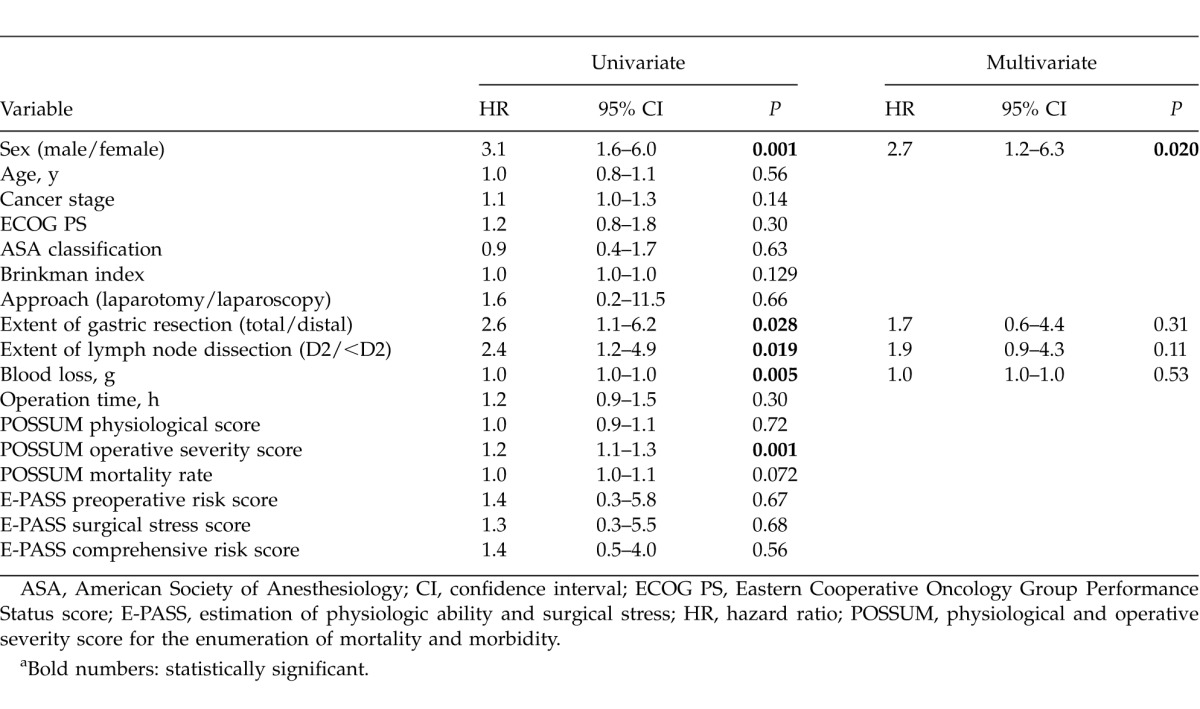
Fig. 1.
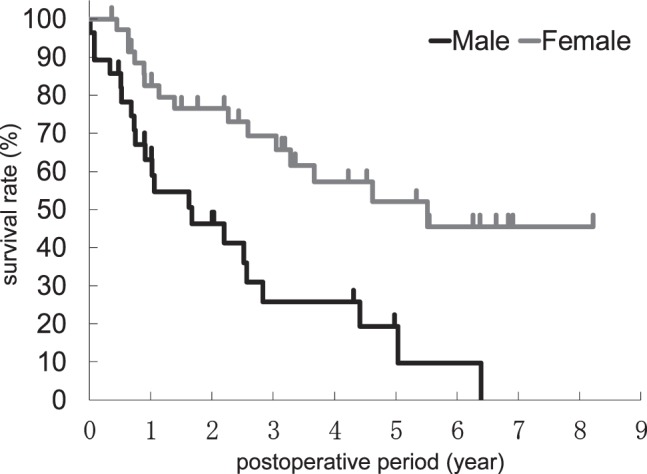
Kaplan-Meier OS curves for each sex. The log-rank test showed a significant difference between the sexes (P = 0.001).
Table 4.
Clinical features of male and female patients aged ≥85 years who underwent curative gastrectomy
For gastric cancer–specific survival, cancer stage, total gastrectomy, blood loss, and the POSSUM operative severity score were significant prognostic factors by univariate analyses. Multivariate analysis was conducted with cancer stage, total gastrectomy, and blood loss, and revealed that cancer stage was the only independent prognostic factor (Table 5).
Table 5.
Survival analysis of variables predicting risk of gastric cancer–specific death for patients aged ≥85 years who underwent curative gastrectomya
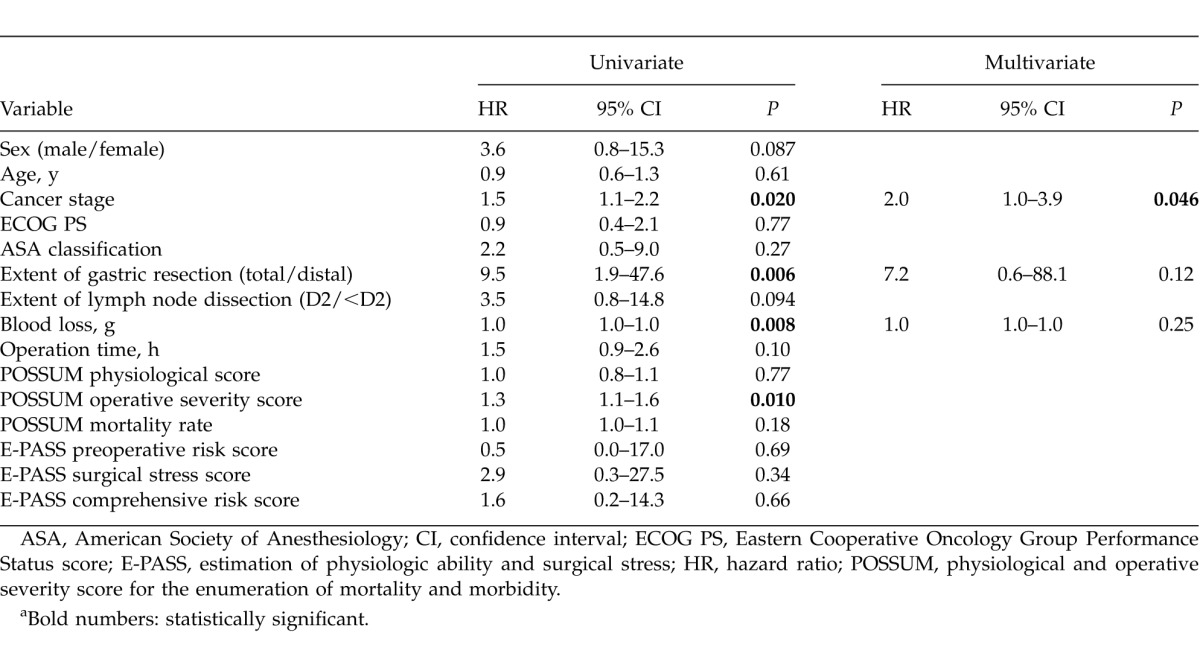
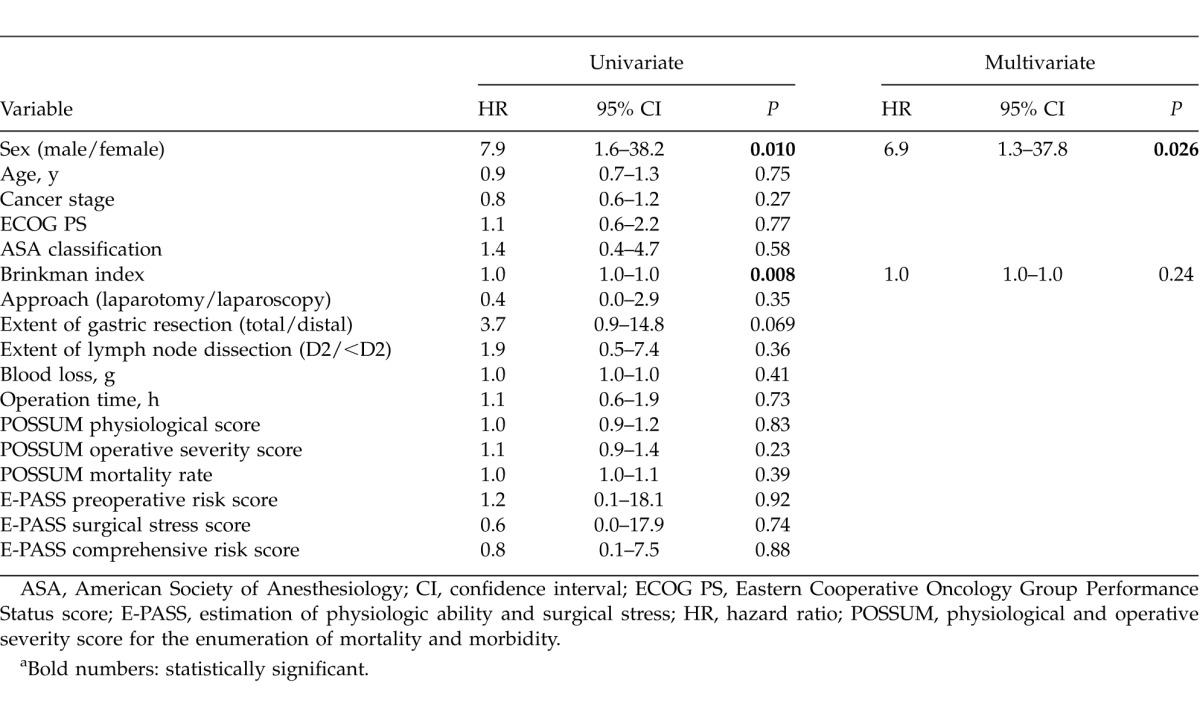
For pneumonia-specific survival, sex and Brinkman index were significant prognostic factors by univariate analyses. Multivariate analysis was conducted with sex and Brinkman index, and showed that sex was the only independent prognostic factor (Table 6).
Table 6.
Survival analysis of variables predicting risk of pneumonia-specific death for patients aged ≥85 years who underwent curative gastrectomya
Discussion
As the population in Japan ages, we more often encounter the oldest old patients with gastric cancer. Previously, we seldom performed surgery on these patients. Before 1996, no patient aged ≥85 years in our institutions underwent gastrectomy. However, as surgical and anesthesiological techniques, equipment, and management are becoming more advanced, surgery on elderly patients is not uncommon. In the current study, we performed univariate and multivariate analyses to clarify criteria that may worsen the prognosis after surgery in the oldest old patients.
For OS, male sex, total gastrectomy, D2 lymph node dissection, and more blood loss were identified as prognostic factors by univariate analysis. Katai et al11 reported that total gastrectomy and extended nodal dissection were both associated with a high operation-related death rate in patients with preoperative morbidity. However, multivariate analysis revealed that male sex was the only independent prognostic factor. As is well known, males have a shorter life expectancy than females. In the natural course, the death rates of 85- to 89-year-old Japanese people were 11.4% for males and 6.8% for females in 2009, with an HR of 1.7.12 In comparison with these findings, the HR for OS by sex (3.2 and 2.9, respectively) in the current univariate and multivariate study seems much higher. This may mean that male patients are less tolerant of surgical intervention than females.
For gastric cancer–specific survival, the cancer stage was the only independent prognostic factor by multivariate analysis, as expected.
For pneumonia-specific survival, sex was the only prognostic factor by multivariate analysis. Male patients were more likely to die of pneumonia, with an HR of 7.9 by univariate analysis and 6.9 by multivariate analysis. Several studies concerning pneumonia among older individuals suggest that men are more susceptible to infections, and once an infection occurs, they are more likely to die. Reade et al13 mentioned that biologic response to pneumonia was different among men and women, with higher pro (TNF, IL-6) and anti- (IL-10) inflammatory biomarkers, decreased circulating concentrations of coagulation factors, including antithrombin III and Factor IX, and increased D-dimer, suggesting greater fibrinolysis among men. There are also several explanations for gender difference, such as sex hormones, the pattern of immune response, and microbiologic factors.13 May et al14suggested that estradiol concentrations are higher in older women compared to men during critical illness.
Except for sex and Brinkman index, no factors showed statistically significant influence on pneumonia-specific survival by univariate analysis. As Marumo et al15 mentioned that aspiration of esophageal reflux contents was the most important risk factor of recurrent pneumonia in patients with total gastrectomy, it was thought that total gastrectomy had more influence on pneumonia because of possible induction of jejuno-esophageal reflux, but it had less influence than sex. Laparoscopic surgery is considered to provide a better respiratory function for gastrectomy patients than open surgery because it does less damage to the respiratory muscles.16,17 These days, safety of laparoscopic gastrectomy on extremely old patients has been reported.18 Thus, laparoscopic gastrectomy is expected to reduce the number of cases of postoperative pneumonia in the oldest old patients. In the current series, however, laparoscopic surgery was performed on only 3 patients. More cases will be needed to assess the effectiveness of laparoscopic surgery.
The National Comprehensive Cancer Network (NCCN) has published guidelines concerning senior adult oncology.2 According to the approach chart to decision making, patients with a moderate or high risk of dying of or suffering from cancer during their lifetime can be further evaluated to assess their functional dependency, decision-making capacity, overall goals, and desire for the proposed treatment. If the patient does not have risk factors for adverse outcomes from cancer treatment, including comorbidities, geriatric syndromes, and socioeconomic issues, he or she should be treated as recommended in the disease-specific treatment guidelines. If the patient has risk factors but they are modifiable and treatable, options may include surgery and other therapies. In general, age is not a primary consideration for surgical risk, although a physiologic status assessment using standard surgical evaluation tools is needed. The PS and comorbidities of the patient are more important factors than age.
In the current series, the POSSUM operative severity score was an effective predictor of OS and gastric cancer–specific survival. PS, ASA, the POSSUM physiological score, and the E-PASS scores were expected to be predictors, but failed statistically. One of the patients who died of pneumonia on the 28th postoperative day had the highest POSSUM operative severity score because of simultaneous resection of gallbladder cancer. The other patients who died of pneumonia within 90 days of surgery had comorbidities that might exacerbate postoperative pneumonia such as pneumonectomy and cerebral infarction, however, these comorbidities were not reflected in the predictive scores. After this, a comprehensive geriatric assessment will be needed for elderly patients, which should include not only functional status and comorbidities, but also nutritional status, cognitive function, psychological status, socioeconomic issues, and geriatric syndromes.2
In conclusion, we retrospectively analyzed the outcomes of patients aged ≥85 years with gastric cancer who had undergone curative surgical resection. The results of univariate analyses indicate that attempts to reduce surgical invasion, such as diminishing the extent of gastric resection, the extent of lymph node dissection, and blood loss seemed to be effective in acquiring longer OS. However, multivariate analyses revealed that sex was the only independent prognostic factor for OS. In particular, male patients were more likely to die of pneumonia after gastrectomy. Careful attention is required when performing gastrectomy in male patients aged ≥85 years. As this study was a retrospective analysis with a limited number of patients, more cases are needed for definitive conclusions.
Acknowledgments
The authors are grateful to the health information managers who investigated the outcomes of the patients: Ms. Fumiko Matsufuru from National Hospital Organization Kure Medical Center/Chugoku Cancer Center and Ms. Chiga Iitani from Higashiosaka City General Hospital.
References
- 1.Ministry of Health, Labour and Welfare. Abridged life tables for Japan 2011. 2012 Nov; Available at: http://www.mhlw.go.jp/english/database/db-hw/lifetb11/dl/lifetb11-05.pdf. Accessed. [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN clinical practical guidelines in oncology (NCCN Guidelines): senior adult oncology. 2012 Nov; Version 2. Available at: http://www.nccn.org/professionals/physician_gls/pdf/senior.pdf. Accessed. [Google Scholar]
- 3.Endo S, Yoshikawa Y, Hatanaka N, Tominaga H, Shimizu Y, Hiraoka K, et al. Treatment for gastric carcinoma in the oldest old patients. Gastric Cancer. 2011;14(2):139–143. doi: 10.1007/s10120-011-0022-8. [DOI] [PubMed] [Google Scholar]
- 4.Endo S, Dousei T, Yoshikawa Y, Hatanaka N, Kamiike W, Nishijima J. Prognosis of gastric carcinoma patients aged 85 years or older who underwent surgery or who received best supportive care only. Int J Clin Oncol. 2013;(6):1014–1019. doi: 10.1007/s10147-012-0482-9. 18. [DOI] [PubMed] [Google Scholar]
- 5.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. 7th ed. Oxford: Wiley-Blackwell; 2009. pp. 73–77. [Google Scholar]
- 6.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 7.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 8.American Society of Anesthesiologists. ASA physical status classification system. 2012 Nov; Available at: http://www.asahq.org/Home/For-Members/Clinical-Information/ASA-Physical-Status-Classification-System. Accessed. [Google Scholar]
- 9.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78(3):355–360. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 10.Haga Y, Ikei S, Ogawa M. Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. 1999;29(3):219–225. doi: 10.1007/BF02483010. [DOI] [PubMed] [Google Scholar]
- 11.Katai H, Sasako M, Sano T, Fukagawa T. Gastric cancer surgery in the elderly without operative mortality. Surg Oncol. 2004;13(4):235–238. doi: 10.1016/j.suronc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Statistics and Information Department, Minister's Secretariat, Ministry of Health, Labour and Welfare. Deaths and death rates by age (1985–2009. 2012 Nov; Available at: http://www.stat.go.jp/data/nenkan/zuhyou/y0226000.xls. Accessed. [Google Scholar]
- 13.Reade MC, Yende S, D'Angelo G, Kong L, Kellum JA, Barnato AE, et al. Differences in immune response may explain lower survival among older men with pneumonia. Crit Care Med. 2009;37(5):1655–1662. doi: 10.1097/CCM.0b013e31819da853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.May AK, Dossett LA, Norris PR, Hansen EN, Dorsett RC, Popovsky KA, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit Care Med. 2008;36(1):62–68. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marumo K, Homma S, Fukuchi Y. Postgastrectomy aspiration pneumonia. Chest. 1995;107(2):453–456. doi: 10.1378/chest.107.2.453. [DOI] [PubMed] [Google Scholar]
- 16.Tanimura S, Higashino M, Fukunaga Y, Kishida S, Ogata A, Fujiwara Y, et al. Respiratory function after laparoscopic distal gastrectomy—an index of minimally invasive surgery. World J Surg. 2006;30(7):1211–1215. doi: 10.1007/s00268-005-0115-9. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura H, Yokota R, Homma S, Kondo Y. Comparison of respiratory function recovery in the early phase after laparoscopy-assisted gastrectomy and open gastrectomy. Surg Endosc. 2010;24(11):2739–2742. doi: 10.1007/s00464-010-1037-7. [DOI] [PubMed] [Google Scholar]
- 18.Yamada H, Kojima K, Inokuchi M, Kawano T, Sugihara K. Laparoscopy-assisted gastrectomy in patients older than 80. J Surg Res. 2010;161(2):259–263. doi: 10.1016/j.jss.2009.01.032. [DOI] [PubMed] [Google Scholar]


