Abstract
Objective
To evaluate the status of iodine nutrition among pregnant women presenting for routine antenatal care in Toronto, Canada, as determined by the median urine iodine concentration (UIC) of this population.
Methods
A cross-sectional, observational study was conducted involving 142 pregnant women recruited from four low-risk antenatal outpatient clinics in Toronto, Canada. Subjects completed a questionnaire and provided a spot urine sample for the measurement of iodine concentration.
Results
Mean maternal age was 33.8 ± 4.3 years. Mean gestational age was 29.3 ± 7.8 weeks. The median UIC was 221 μg/L (interquartile range, 142 to 397 μg/L). Six women (4.2%) had urine iodine levels <50 μg/L, and 36 women (25.4%) had levels between 50 and 150 μg/L.
Conclusion
This cohort of primarily Caucasian, well-educated, and relatively affluent pregnant women in Toronto, Canada, are iodine sufficient, perhaps due to universal salt iodization and/or other dietary and lifestyle factors.
INTRODUCTION
Normal thyroid function depends on sufficient dietary iodine intake. The fetal thyroid does not begin to concentrate iodine until the 10th to 12th weeks of gestation, and the synthesis and secretion of thyroid hormone, which is controlled by fetal pituitary thyroid stimulating hormone, begins at approximately 20 weeks of gestation (1). As such, the developing fetus depends heavily upon adequate maternal iodine nutrition and thyroid hormone levels, particularly during early gestation.
The consequences of iodine deficiency have been recognized for many decades (2), and include endemic goiter, cretinism, intellectual impairments, growth retardation, neonatal hypothyroidism, and increased rates of pregnancy loss and infant mortality (3). Insufficient maternal iodine intake during pregnancy and the immediate postpartum period results in neurological and psychological deficits in the offspring (4,5). Thyroid hormone is required for oligodendrocyte differentiation and myelin distribution (6), and animal studies have demonstrated that low thyroid hormone levels in early pregnancy up to midgestation impair radial migration of neurons to the cortex and hippocampus and are associated with behavior changes in the offspring (7).
Dietary iodine requirements are higher for pregnant women than for nonpregnant adults due to increased thyroid hormone production, increased renal iodine losses, and fetal iodine requirements (8). The recommended daily dietary iodine intake is higher for pregnant women than for nonpregnant, nonlactating adolescents and adults (Table 1).
Table 1.
Recommendations for Median Daily Dietary Iodine Intake
| Nonpregnant, nonlactating adolescents and adults | Pregnant women | |
|---|---|---|
| Institute of Medicine (26) | 150 μg | 220 μg |
| WHO, UNICEF, ICCIDD (11) | 150 μg | 250 μg |
| Endocrine Society (27) | ---- | 250 μg |
Abbreviations: WHO = World Health Organization; UNICEF = United Nations Children’s Fund; ICCIDD = International Council for the Control of Iodine Deficiency Disorders.
Several accepted methods are available for monitoring population iodine sufficiency, including measurement of thyroid volume, serum thyroglobulin level, and median urine iodine concentration (9). The median urine iodine concentration (as a biomarker for dietary iodine intake) reflects recent iodine ingestion, and can be assessed from both spot and 24-hour urine collections. Thresholds for iodine sufficiency based on median spot urine iodine concentrations have been identified for populations, but not for individuals, due to significant day-to-day variation in iodine intake (10). The population iodine sufficiency threshold in pregnant women is defined as a median urine iodine concentration ≥150 μg/L (11).
Universal salt iodization has been present in Canada since the 1920s. Although historically considered an overall iodine sufficient country (12), the World Health Organization (WHO) has no official data on the status of iodine nutrition in Canada (13). The current study assessed the iodine status of pregnant women, the subpopulation most vulnerable to the effects of iodine deficiency, by determining the iodine concentration in spot urine samples collected from pregnant women in Toronto, Canada.
METHODS
Patients
We recruited 250 pregnant women presenting for routine obstetric care at an outpatient hospital clinic in Toronto, Canada, between December 2009 and February 2011. All women were in their second or third trimester of a singleton pregnancy. Exclusion criteria included a known history of thyroid disease in the nonpregnant state, and previous participation in the study. A spot urine sample was collected for the determination of iodine concentration.
Study participants completed a questionnaire to provide information regarding their age, gestational age, race, country of origin, highest educational level, income level, cigarette smoking status, and use of prenatal and/or multivitamins prior to and during pregnancy. Participants also completed a food frequency questionnaire to provide information on their dietary iodine intake, including use of iodized salt. The Mount Sinai Hospital Ethics Board approved the protocol.
Laboratory Measurements
The concentration of iodine in spot urine samples was measured spectrophotometrically using a Technicon Autoanalyzer (Technicon Instrument Inc., Tarrytown, NY), according to a modification of the method of Benotti et al (14). Analyses were conducted at the Boston Medical Center Iodine Research Laboratory. The total iodine concentration of each sample was determined at least twice. In cases where the initial two measurements were not within 15% of each other, a third or a fourth measurement was obtained and the average of all measurements was then reported. The interassay coefficient of variation for this assay in our laboratory ranged from 2.7 to 7%.
Statistical Analysis
Descriptive statistics are reported as the mean (SD) and median (interquartile range), and frequencies are reported as percentages. Median urine iodine concentration (UIC) data were compared to recent WHO guidelines (11). The Fisher exact test was used to assess associations between UIC (stratified as <150 μg/L and ≥150 μg/L) and the following characteristics: frequency of adding salt to prepared food, frequency of adding salt during cooking, use of sea salt, use of kosher salt, and use of prenatal vitamins. Statistical analyses were performed using R software version 2.14 (Development Core Team [2012]; R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org).
RESULTS
Of the original cohort of 250 pregnant women that were recruited for the study, the urine samples collected from the first 100 women enrolled and 8 of the later-enrolled women were thought to be contaminated by urine dipstick testing (15) at the clinic prior to measurement of urine iodine. Data pertaining to these samples and the women that provided them were thus omitted from all analyses, and the reported results are those of the remaining 142 pregnant women.
Subject demographics are presented in Table 2. Mothers were primarily Caucasian, well-educated, non-smokers, and relatively affluent. Only 7 women (4.9%) reported never adding salt during cooking or to their prepared food, and 86 women (60.6%) reported using salt on food or during cooking at least four to six times a week, with 65 women (45.8%) reporting daily or more frequent salt use. A total of 101 women (71.1%) reported taking a multivitamin prior to pregnancy, and 70 of these women indicated that the vitamins were specifically labeled as prenatal. A total of 129 women (90.8%) reported taking a multivitamin during pregnancy, and 124 of these women indicated that the vitamins were specifically labeled as prenatal.
Table 2.
Subject Demographics
| Age (years) | 33.8 ± 4.3 |
| Gestational age (weeks) | 29.3 ± 7.8 |
| Ethnicity | |
| Caucasian | 95 (66.9%) |
| Asian | 28 (19.7%) |
| Black | 4 (2.8%) |
| Other/Unknown | 15 (10.6%) |
| Canadian-born | 92 (64.8%) |
| Highest level of education | |
| High school | 6 (4.2%) |
| College/University | 86 (60.6%) |
| Post graduate | 50 (35.2%) |
| Annual income >$100,000 | 90 (63.3%) |
| Cigarette smokers | 4 (2.8%) |
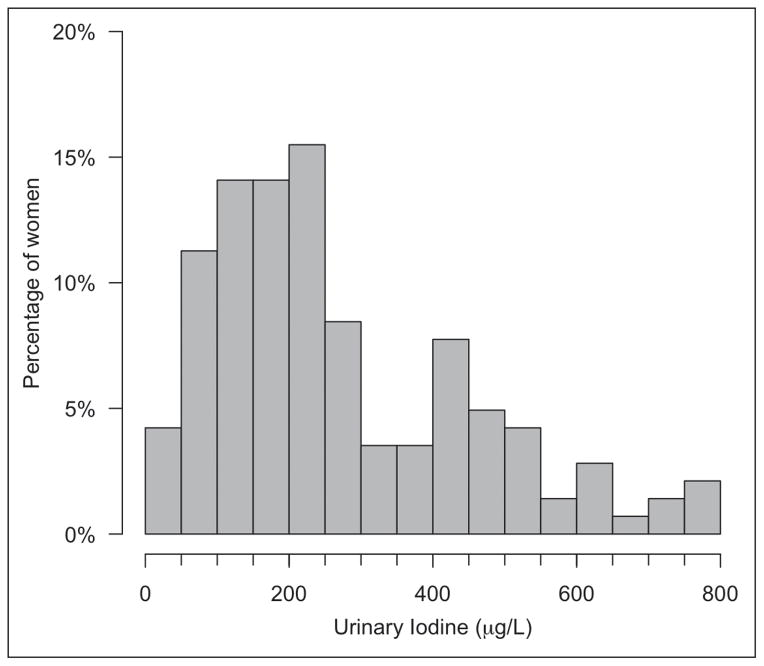
Figure 1 shows the distribution of UIC values. The median UIC was 221.3 μg/L (interquartile range, 142 to 397 μg/L). Six women (4.2%) had a UIC <50 μg/L, and 36 women (25.4%) had a UIC between 50 and 150 μg/L. Self-reported infrequent addition of salt during cooking was significantly associated with a UIC ≥150 μg/L (Table 3).
Fig. 1.
Distribution of urine iodine concentration (UIC) values in the study population.
Table 3.
Self-reported Use of Iodine-containing Vitamin and Food Items and UIC
| UIC <150 (n = 42) | UIC ≥150 (n = 100) | P value | |
|---|---|---|---|
| Preconception multivitamin use | |||
| No | 12 (28.6.0%) | 29 (30%) | .836 |
| Yes – not labeled prenatal | 8 (19.0%) | 23 (23%) | |
| Yes – labeled prenatal | 22 (52.4%) | 48 (48%) | |
| Multivitamin use during pregnancy | |||
| No | 4 (9.5%) | 7 (7%) | .434 |
| Yes – not labeled prenatal | 0 (0.0%) | 5 (5%) | |
| Yes – labeled prenatal | 38 (90.5%) | 88 (88%) | |
| Add salt to food | |||
| Never | 14 (33.3%) | 32 (32%) | .873 |
| 1–3 times/week | 14 (33.3%) | 38 (38%) | |
| 4–7 times/week | 8 (19.0%) | 20 (20%) | |
| >1 time/day | 6 (14.3%) | 10 (10%) | |
| Add salt to cooking | |||
| Never | 0 (0.0%) | 9 (9%) | .0218 |
| 1–3 times/week | 10 (23.8%) | 40 (40%) | |
| 4–7 times/week | 21 (50.0%) | 35 (35%) | |
| >1 time/day | 11 (26.2%) | 16 (16%) | |
| Use kosher salt (contains no iodine) | |||
| Yes | 9 (21.4%) | 16 (16%) | .473 |
| No | 33 (78.6%) | 84 (84%) | |
| Use sea salt (contains no iodine) | |||
| Yes | 23 (54.8%) | 47 (47%) | .465 |
| No | 19 (45.2%) | 53 (53%) | |
DISCUSSION
The major concerns regarding the global burden of iodine deficiency are related to goiter, neurocognitive impairments, and, in cases of severe deficiency, hypothyroidism resulting in cretinism. Although efforts by the WHO, the International Council for the Control of Iodine Deficiency Disorders (11,16), and the Canada-based Network for the Sustained Elimination of Iodine Deficiency (17) have been successful in alleviating iodine deficiency worldwide in recent decades, the WHO estimates that 2 billion people remain iodine deficient (18).
Given the crucial importance of adequate iodine nutrition for thyroid hormone synthesis and normal neurodevelopment, pregnant and lactating women and their offspring are the groups most vulnerable to even mild iodine deficiency. The American Thyroid Association has recommended that women in the U.S. and Canada receive dietary supplements containing 150 μg of iodine daily during pregnancy and lactation, and that all prenatal vitamins contain 150 μg of iodine (19). However, only 20.3% of pregnant and 14.5% of lactating women in the U.S. take a supplement containing iodine, according to data from the National Health and Nutrition Examination Survey (20). Currently, only 114 of 223 (51%) brands of prescription and nonprescription prenatal multivitamins marketed in the U.S. list iodine as a constituent, and many of those that do contain iodine do not contain the labeled amount, especially when kelp is the iodine source (21). The frequency of prenatal vitamin or supplement use in Canada is unknown. One Canadian study conducted in the early 1990s reported iodine concentrations ranging from 87 to 1304 μg/L in samples of whole (mean iodine concentration of 177 ± 35 μg/L) and 2% fat (mean iodine concentration of 456 ± 151 μg/L) commercially available retail milk brands (22).
Historically, iodine supplementation has been implemented using a public health approach. Iodine supplementation has largely been implemented through universal salt iodization (USI) programs in a number of countries, including Canada, where USI has been mandatory since the 1920s. Salt iodization in Canada and the U.S. is achieved by supplementing salt with 100 ppm of potassium iodide (77 μg of iodine/g of salt), a concentration that is higher than that used in most other programs (10 to 40 ppm) (12). Unfortunately, data on median urine iodine concentrations in Canada are limited. A small observational study of 11 adults in Montreal reported a mean 24-hour urine iodine level of 446 ± 164 μg (23). A multicenter study reported a median UIC of 148 μg/L among 81 Canadian newborn infants, with 11.9% of samples having a median UIC <50 μg/L (24). The only large national assessment of median UIC, based on previously unpublished data collected between 2007 and 2009, was recently reported by Andersson and colleagues (25), who found a median UIC of 174 μg/L among 847 school-age Canadian boys, with 26% having a concentration <100 μg/L.
The results of the present study of a cohort of primarily Caucasian, well-educated, and affluent pregnant women in Canada indicate that iodine intake is adequate in this population. The results are reassuring and contribute to the limited data regarding the state of iodine nutrition in Canada. We acknowledge that these findings may not reflect the status of iodine nutrition in pregnant women in other regions of the country, and suggest that a national assessment of population median UIC be carried out in Canada.
CONCLUSION
Primarily Caucasian, well-educated, relatively affluent pregnant women during the second and third trimesters in Toronto, Canada are iodine sufficient, perhaps due to universal salt iodization or other dietary and lifestyle factors.
Acknowledgments
This work was supported in part by National Institutes of Health grant 5K23HD068552-02 (AML), the Mount Sinai Hospital Department of Medicine Research Fund (PGW), and the Mount Sinai Hospital Foundation Da Vinci Gala Fundraiser (PGW).
Abbreviations
- UIC
urine iodine concentration
- WHO
World Health Organization
Footnotes
To purchase reprints of this article, please visit: www.aace.com/reprints.
DISCLOSURE
The authors have no multiplicity of interest to disclose.
References
- 1.Brown RS. Minireview: developmental regulation of thyrotropin receptor gene expression in the fetal and newborn thyroid. Endocrinology. 2004;145:4058–4061. doi: 10.1210/en.2004-0458. [DOI] [PubMed] [Google Scholar]
- 2.Pharoah PO, Buttfield IH, Hetzel BS. Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. Lancet. 1971;1:308–310. doi: 10.1016/s0140-6736(71)91040-3. [DOI] [PubMed] [Google Scholar]
- 3.Boyages SC. Clinical review 49: Iodine deficiency disorders. J Clin Endocrinol Metab. 1993;77:587–591. doi: 10.1210/jcem.77.3.8370679. [DOI] [PubMed] [Google Scholar]
- 4.Cao XY, Jiang XM, Dou ZH, et al. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med. 1994;331:1739–1744. doi: 10.1056/NEJM199412293312603. [DOI] [PubMed] [Google Scholar]
- 5.Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–818. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
- 6.Younes-Rapozo V, Berendonk J, Savignon T, Manhães AC, Barradas PC. Thyroid hormone deficiency changes the distribution of oligodendrocyte/myelin markers during oligodendroglial differentiation in vitro. Int J Dev Neurosci. 2006;24:445–453. doi: 10.1016/j.ijdevneu.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 7.de Escobar GM, Obregón MJ, del Rey FE. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 2007;10:1554–1570. doi: 10.1017/S1368980007360928. [DOI] [PubMed] [Google Scholar]
- 8.Glinoer D. Pregnancy and iodine. Thyroid. 2001;11:471–481. doi: 10.1089/105072501300176426. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30:376–408. doi: 10.1210/er.2009-0011. [DOI] [PubMed] [Google Scholar]
- 10.König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr. 2011;141:2049–2054. doi: 10.3945/jn.111.144071. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization, United Nations Children’s Fund (UNICEF), and International Council for the Control of Iodine Deficiency Disorders (ICCIDD) Assessment of the iodine deficiency disorders and monitoring their elimination. 2007 WHO/NHD/01.1. [Google Scholar]
- 12.International Council for the Control of Iodine Deficiency Disorders (ICCIDD) [Accessed August 22, 2012]; Available at: http://www.iccidd.org.
- 13.World Health Organization Vitamin and Mineral Nutritional Information System (VMNIS) [Accessed August 22, 2012];Database on Iodine Sufficiency. 2012 Available at: http://www.who.int/vmnis/database/iodine/en/index.html.
- 14.Benotti J, Benotti N, Pino S, Gardyna H. Determination of total iodine in urine, stool, diets, and tissue. Clin Chem. 1965;11:932–936. [PubMed] [Google Scholar]
- 15.Pearce EN, Lazarus JH, Smyth PP, et al. Urine test strips as a source of iodine contamination. Thyroid. 2009;19:919. doi: 10.1089/thy.2009.0120. [DOI] [PubMed] [Google Scholar]
- 16.Hetzel BS. The development of a global program for the elimination of brain damage due to iodine deficiency. Asia Pac J Clin Nutr. 2012;21:164–170. [PubMed] [Google Scholar]
- 17.Network for Sustained Elimination of Iodine Deficiency. [Accessed April 18, 2012]; Available at: http://www.iodinenetwork.net.
- 18.Andersson M, Takkouche B, Egli I, Allen HE, de Benoist B. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull World Health Organ. 2005;83:518–525. [PMC free article] [PubMed] [Google Scholar]
- 19.Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregory CO, Serdula MK, Sullivan KM. Use of supplements with and without iodine in women of childbearing age in the United States. Thyroid. 2009;19:1019–1020. doi: 10.1089/thy.2009.0166. [DOI] [PubMed] [Google Scholar]
- 21.Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. N Engl J Med. 2009;360:939–940. doi: 10.1056/NEJMc0807851. [DOI] [PubMed] [Google Scholar]
- 22.Fischer PWF, Giroux A. Iodine content of Canadian retail milk samples II. After the ethylenediamine dihydroiodide ban. Food Res Int. 1993;26:277–281. [Google Scholar]
- 23.Bastomsky CH, Banovac K, Skreb F, Sekso M. Similar serum concentrations of thyroid hormones in two geographically separate populations on disparate iodine intake. Horm Metab Res. 1979;11:301–304. [PubMed] [Google Scholar]
- 24.Delange F, Heidemann P, Bourdoux P, et al. Regional variations of iodine nutrition and thyroid function during the neonatal period in Europe. Biol Neonate. 1986;49:322–330. doi: 10.1159/000242547. [DOI] [PubMed] [Google Scholar]
- 25.Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr. 2012;142:744–750. doi: 10.3945/jn.111.149393. [DOI] [PubMed] [Google Scholar]
- 26.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 27.Abalovich M, Amino N, Barbour LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S1–S47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]