Abstract
Objectives.
Brain reserve theory, typically discussed in relation to dementia, was examined with regard to late-life depression symptomatology and cerebrovascular burden (CVB) in older-old women.
Method.
It was predicted that in a 6-year longitudinal sample (Health and Retirement Study) of 1,355 stroke-free women aged 80 years and older, higher levels of depressive symptomatology (8-item Center for Epidemiologic Studies-Depression score) would be predicted by high CVB, less educational attainment, and the education × CVB interaction after controlling for age and cognitive functioning (Telephone Interview for Cognitive Status). A latent growth curve model was used to identify differences in depression symptomatology at baseline and over time. Logistic regression analyses were used to predict clinically significant depressive symptomatology at each wave based on CVB, education, and the education × CVB interaction.
Results.
Results indicate that among older women, greater educational attainment predicted fewer depression symptoms at baseline, but this advantage was partially eroded over time. The education × CVB interaction predicted clinically significant depressive symptoms at baseline when the benefits of education were most robust.
Discussion.
Brain reserve, characterized by educational attainment, may counterbalance the effect of high CVB with respect to depressive symptoms, thereby preserving mood in late life. These findings support the application of brain reserve theory to late-life depression.
Key Words: Cerebrovascular burden, Education, Mood.
Depression is both common and medically relevant in late life. One recent study of 335 community-dwelling older adults reported a normative increase in depression symptoms after the age of 77 (Teachman, 2006). Depression was identified as the leading cause of disability worldwide and depressive symptoms in late life predict cognitive decline (González, Bowen, & Fisher, 2008), higher levels of disability, and mortality (Blazer, 2003). By comparison to men, older women have higher rates of depression, longer life expectancies, and experience more years of disability (von Strauss, Aguero-Torres, Kareholt, Winblad, & Fratiglioni, 2003). Women aged 80 years and older, in particular, represent a rapidly growing demographic group (National Center for Health Statistics, 2010) and are the fastest growing target group for medical and behavioral interventions to enhance longevity and reduce morbidity. Thus, research examining the risk factors, such as high cerebrovascular burden (CVB; Alexopoulos et al., 1997b), and protective factors, such as high levels of education, of depression among older women may inform interventions designed to reduce dependence, need for health and social services, and medical care costs in later life (Blazer, 2003). The goals of this 6-year longitudinal study are to examine CVB and education as indicators of brain reserve and predictors of depression in a sample of community-dwelling women aged 80 years and older.
One attempt to better understand late-life depression has been the vascular depression hypothesis (Alexopoulos et al., 1997a). Vascular depression theory suggests that CVB, characterized by factors such as hypertension, diabetes, cardiac disease, and smoking, may predispose, precipitate, and perpetuate symptoms of depression in late life (Alexopoulos et al., 1997b). CVB was conceptualized in our study as the cumulative burden associated with cerebrovascular risk factors. High CVB contributes to diffuse deterioration in the central nervous system (Wilson, Finch, & Cohen, 2002) and prefrontal white matter hyperintensities (WMHs) in particular (Raz, Rodrigue, & Acker, 2003). Presence of prefrontal WMHs, and particularly those affecting frontostriatal white matter tracks, is predictive of late-onset depression symptoms (Krishnan, Hays, & Blazer, 1997) and executive functioning deficits (Cahana-Amitay et al., in press; Raz et al., 2003). As predicted by vascular depression theory, WMHs predated late-onset depression (Nebes et al., 2002) but not depression among middle-aged individuals (Salloway et al., 1996). Our research group found that clinically defined vascular depression was best described as a threshold effect, whereby elders with two or more cerebrovascular risk factors (CVB group) have more depressive symptoms than those with zero or one risk factor (Mast, Yochim, MacNeill, & Lichtenberg, 2004). Most studies of vascular depression have mixed middle-aged, younger-old (65–79 years), and older-old (80 years and older) individuals together and may mask unique vascular depression patterns in the oldest old. Importantly, older adults with both depression symptoms and cognitive deficits have a three times higher mortality risk than their counterparts (Blazer, 2003; Mehta et al., 2003), making cognitive functioning an important control variable in studies of this kind. Like other geriatric disorders characterized by neurological decline, vulnerability to vascular depression may be predicted by individual characteristics such as brain reserve.
Brain reserve theory has been proposed as an explanation for why considerable interindividual variation exits with regard to behavioral response to brain changes. Experiences in early life, such as education, are associated with greater synaptic density, described as brain reserve capacity (Stern, 2002). As a result, well-educated individuals are better able to cope with cognitive decline secondary to neurological change. Cognitive change can be measured as a continuous variable, with diagnostic categories reflecting an extreme point of a continuum where disordered behavior interrupts an individual’s capacity for adaptive functioning (American Psychiatric Association, 2000). Brain reserve theory (Stern, 2002) posits that although neurological change results in cognitive decline for all individuals (e.g., age-related cognitive decline, dementia), those with high brain reserve are less likely to exhibit behavior that can be characterized by a diagnostic code such as Alzheimer’s disease. This model is based on syndromal disorders, which are triggered when the accumulation of symptoms crosses a particular threshold, as opposed to a dimensional perspective where symptoms are simply measured across a continuum. The effect with respect to clinical presentation, that brain reserve ultimately delays the emergence of clinically relevant cognitive deficits and corresponding diagnosis, has been described for dementia (Stern, 2009), epilepsy (Jokeit & Ebner, 1999), and delirium (Jones et al., 2010). Paralleling the study of cognition and dementia, mood can be evaluated as both a continuous and categorical variable in community samples using a semicontinuous measure with recommended clinical cutoffs, reflecting clinically significant depressive symptomatology. Understanding both incidence of mood disorders and more subtle, subacute dysphoric states are especially important when working with older adults, as even subclinical depression suggests a guarded prognosis for older adults characterized by lower quality of life (Chachamovich, Fleck, Laidlaw, & Power, 2008), worse memory performance (Murphy & O’Leary, 2010), and shortened remaining life span (Paulson, Bowen, & Lichtenberg, 2011).
Given the theorized neurological basis of vascular depression, it may be valuable to investigate the brain reserve hypothesis in a geriatric sample. Brain reserve theory predicts that in contrast to older adults with relatively little education, well-educated individuals may have fewer depression symptoms in late life, even in the face of CVB. Consistent with this hypothesis, a large, multinational cross-sectional study reported a relationship between educational attainment and depression in later life, independent of socioeconomic conditions (Ladin, 2008) such that participants with greater education had lower rates of depression. This study did not examine, however, the influence of either CVB or education on the development of depression symptoms over time and did not control for cognitive functioning. Similarly, Schoevers and colleagues (2003) found that in a sample aged 65–84 years, having 8 or fewer years of education is related to greater chronic depression for men but not for women. Less attention has been devoted to these relationships among the older old.
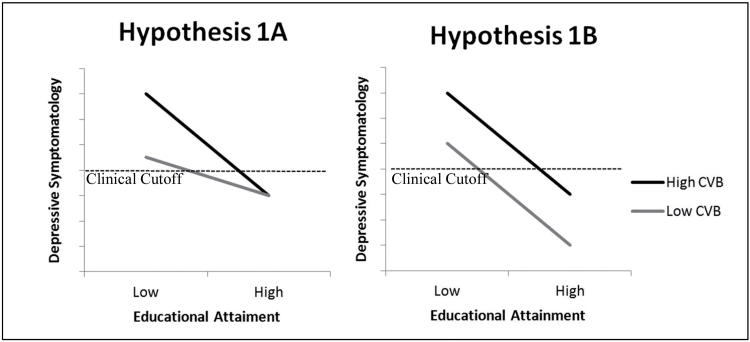
The primary goal of this study was to describe the relationship between CVB, education, and mood over time in a sample of women aged 80 years and older. Brain reserve theory suggests two competing hypotheses for the relationship between CVB, education, and depressive symptomatology. The first competing hypothesis (1A) describes a scenario in which education delays or prevents the development of depressive symptoms. In this scenario, level of depressive symptomatology at baseline and rate of change over time would be predicted by the CVB × education interaction. Within this interaction, CVB would predict higher levels of depressive symptomatology, but this relationship would be attenuated by greater educational attainment. That is, education would modify the effect of CVB on depressive symptomatology. The second competing hypothesis (1B) describes a scenario in which fewer years of educational attainment and high CVB confer independently greater risk for depressive symptomatology, and the effects of CVB and education are essentially additive with respect to degree of depressive symptomatology. In this scenario, main effects for CVB and education would emerge without a significant CVB × education interaction. Figure 1 is theoretically analogous to figures from Stern’s (2002) conceptualization of brain reserve and represents results at intercept that would be supportive of either Hypothesis 1A or 1B, respectively.
Figure 1.
Lines representing Hypotheses 1A, specifying an interaction between cerebrovascular burden (CVB) and education in the prediction of depressive symptoms, and 1B depicting an additive relationship. Both represent cross-sectional data.
The second goal of this study is to examine how CVB and education predict risk of clinically significant depressive symptomatology at each wave. As illustrated in Figure 1, risk for clinically significant depressive symptomatology appears greater for respondents with high CVB than for those with low CVB, regardless of which hypothesis, 1A or 1B, is supported. In both scenarios, this trend is exaggerated for respondents with relatively fewer years of education, and as illustrated in the figure, the hypothesized result is a disproportionately larger percentage of less-educated individuals with high CVB meeting criteria for clinically significant depressive symptomatology. Based on this hypothesized outcome, drawn from Stern’s conceptualization of brain reserve, Hypothesis 2 states that years of education will modify the risk of clinically significant depressive symptomatology associated with high CVB. Specifically, brain reserve theory predicts that clinically significant depressive symptomatology will be significantly predicted by the interaction of CVB and years of education. If neither Hypothesis 1A nor 1B is supported, however, no certain predictions can be made regarding Hypothesis 2.
Method
Sample
The Health and Retirement Study (HRS) is a prospective cohort study conducted by the University of Michigan with support from the National Institute on Aging. The first wave of the HRS occurred in 1992 with a 51-to-61 year-old cohort and was merged with the older (70 years and older) Asset and Health Dynamics of the Oldest Old (AHEAD) study cohort in 1998. Two additional cohorts were added in 1998 to fill in the gaps between these two groups. Briefly, the HRS is a multistage probability cohort sample of U.S. households. Further details on the HRS design and methods have been previously published (Heeringa & Conner, 1995). Participants in the HRS were not explicitly screened for dementia, but all participants were community dwelling when first enrolled in the study. In the event that participants were unable to independently respond to questionnaires, data such as the Center for Epidemiologic Studies-Depression (CES-D) score and Telephone Interview for Cognitive Status (TICS) were not collected and data such as health and residential status were collected from a “proxy” respondent such as a family member or close friend. Complete data are available for all respondents in this study at baseline (1998).
Four waves of data (1998–2004) from the HRS and the RAND Center for the Study of Aging were analyzed in this study (RAND HRS Data, 2006). We analyzed data from 1,355 community-dwelling older women who were 80 years or older in 1998. Respondents were excluded if they were unable to complete testing without assistance (proxy). Because stroke is associated with subsequent depression symptoms (Bour et al., 2009), mania (Santos, Caeiro, Ferro, & Figueira, 2011), and misapprehension of emotional changes (Paradiso, Vaidya, Tranel, Kosier, & Robinson, 2008; Rush et al., 2010), respondents were excluded from this study if they reported a history of stroke. Over the course of this study (years 1998–2004), the combined year response rate was 37.9% and wave-to-wave reinterview response rates ranged from 73.0% (2000) to 74.4% (2002). Of the 1,355 individuals with complete baseline data, 514 completed the CES-D scale across all waves of this study. Attrition rates in the HRS are comparable to that of other panel surveys (Groves & Couper, 1998) and sample attrition has not significantly influenced the representativeness of the remaining sample based on demographic, economic, and health measures (Cao & Hill, 2005). However, women aged 80 years and older have higher mortality rates than the general population. Respondents missing by the end of the study (year 2004) were older (t = −11.654, p < .001), with fewer years of education (t = 4.593, p < .001), more likely to have high CVB (χ2(1, n = 1,355) = 15.987, p < .001) and depression scores (U = 18,7997.5, p < .001), and lower TICS scores (t = 9.84, p < .001) than their counterparts. Of the 808 respondents lost between baseline and 2004, 605 were identified as deceased and the vast majority of the remaining 202 respondents responded by proxy, suggesting incapacity.
Measures
Depression symptomatology.
The dependent variable, depression symptoms, was measured using an abbreviated eight-item version of the CES-D scale (Radloff, 1977). Respondents were asked if they were depressed, if everything was an effort, if their sleep was restless, if they were happy (reverse coded), if they were lonely, if they enjoyed life (reverse coded), if they felt sad, and if they could not get going (yes/no within the week prior to the interview). Responses were coded either 0 (nondepressive response) or 1 (depressive response) and CES-D score was calculated by summing across these eight items. Higher CES-D scores reflected more depressive symptoms and a CES-D score greater than or equal to 3 was used to indicate clinical depression (Steffick, 2000). The eight-item CES-D has similar symptom dimensions as the 20-item CES-D and past research suggests high internal consistency (α = 0.77) and validity of the CES-D as implemented in the HRS (Steffick, 2000). The eight-item CES-D demonstrated good internal consistency in the subsample represented in this study (α = 0.72).
Cerebrovascular burden.
CVB was based on smoking history and physician diagnosed self-reports of hypertension, heart disease, and diabetes. When respondents were first enrolled in the study, they were asked if they were “a current smoker, [a] former smoker, or someone who has never smoked.” Respondents who endorsed either “current smoker” or “former smoker” status were assigned a value of 1 and those who had never smoked were assigned a value of 0. When first enrolled, they were also asked if physician had ever told them that they had these conditions (i.e., measure of prevalent conditions including hypertension, diabetes, and/or heart disease). In later survey waves, respondents were asked if they had been told by a physician (since the prior interview) that they had developed any of these conditions. Values of 1 were assigned for each of these three reported comorbidities. Number of cerebrovascular items reflected the number of endorsed items (range = 0–4). Reasonable concordance values between self-reports of disease and medical chart reviews have been reported (Psaty et al., 1995). In this sample, 53.7% reported hypertension, 11.3% reported diabetes, 30.9% reported cardiac disease, and 33.7% reported history of smoking. Past work on CVB and depressive symptoms (Mast, MacNeill, & Lichtenberg, 2004; Mast, Neufeld, MacNeill, & Lichtenberg, 2004; Mast, Yochim, et al., 2004) has consistently shown that maximal difference on number of symptoms is observed between participants with zero or one risk factor and those with two or more. Consistent with past work, respondents in this study were categorized into low and high CVB groups based on the report of either low (0–1) or high (2–4) CVB risk factors.
Demographic variables.
Age was calculated based on reported birth date. Education was assessed as self-reported years of formal schooling. Both age and education were incorporated into statistical models as continuous variables.
Cognitive functioning.
Cognitive functioning was measured by the modified TICS included in the AHEAD/HRS data (Ofstedal, Fisher, & Herzog, 2005). This test is based on the older TICS measure published by Brandt, Spencer, and Folstein (1988) and is a brief standardized test developed for remote screening of cognitive disorders. Because cognitive functioning has been identified as a confounding variable in the relationship between CVB and depressive symptoms (Mast, Yochim, et al., 2004), it was controlled for using this brief cognitive screen. TICS measures orientation, concentration, short-term memory, mathematical skills, praxis, and language with the maximum score of 35 reflecting higher cognitive functioning. The TICS has high test–retest reliability and is generally sensitive to cognitive impairment (Desmond, Tatemichi, & Hanzawa, 1994). Scores on the TICS were used as a semicontinuous variable.
Auxiliary variables.
Self-rated health was assessed using the question, “Would you say your health is excellent, very good, good, fair, or poor?” Self-rated health has been identified as a leading indicator of mortality across studies, including the HRS/AHEAD sample (Siegel, Bradley, & Kasl, 2003). The activities of daily living (ADLs) scale assessed self-reported difficulty walking across a room, getting in and out of bed, dressing, bathing, and eating and produced a score ranging from 0 to 5. Instrumental activities of daily living (IADLs) were measured using a three-question instrument including items assessing difficulty with using the telephone, taking medication, and handling money and produced a score ranging from 0 to 3. Cumulatively, these variables accurately identified 79.4% of the attrited participants and all three variables significantly predicted attrition (p ≤ .001).
Statistical methodology.
Longitudinal research with the older old is complicated by attrition reflecting the extremely high mortality risk and threat of incapacity. It has been argued that in cognitive aging research, disregarding participants with missing data resulting from death or dropout results produces a systematic underestimation of disorder severity and only the most robust elders are represented (Rabbitt, Lunn, & Wong, 2008). McArdle and Hamagami’s (1991) seminal work used maximum likelihood estimation to analyze longitudinal data sets with significant missing data. More recently, Graham (2003) has built on this methodology by including auxiliary variables in the imputation of missing data. Like cognitive decline, depression predicts mortality and incapacity in late life. For this reason, it is appropriate to utilize an analytical strategy that permits inclusion of baseline respondents who attrite over the course of the study.
Data were analyzed using a latent growth curve (LGC) modeling approach specifically designed to account for missing data (Enders, 2010). This aim was achieved within the full maximum likelihood (FIML; Arbuckle, 1996) framework with informative covariates. To this end, use of suitably chosen auxiliary variables was made (Graham, 2003), which represents a modern principled method for dealing with missing data that are implemented in the popular latent variable modeling software Mplus (Muthen & Muthen, 2007a). Specifically, variables that were judged on substantive grounds to be related to the missing values were utilized as auxiliaries in all models fitted to address the research questions pursued in this article. These variables were self-rated health, ADLs, and IADLs. In this way, data from all 1,355 participants in the available sample were used, regardless of number of repeated measures of depression that each one of them provided. In addition, through the inclusion of the earlier auxiliary variables, important information about the missing values—as contained in those variables—was incorporated into the model fitting and parameter estimation process (Little & Rubin, 2002). For all LGC models reported in this article, we used the robust maximum likelihood method of estimation (Muthen & Muthen, 2007b) to handle some marked deviations from normality in their dependent variables.
In order to develop the (unconditional) LGC model, we examined the patterns of temporal change in depression symptomatology using the intercept-and-slope model (IS model; Raykov & Marcoulides, 2008). This unconditional model does not include predictors and correlates of change patterns, whereas describing the individual development over time in depression symptomatology as following a linear regression with assessment occasion playing the role of predictor variable. Specifically, this two-level model is based on the subject-level (Level 1) equation:
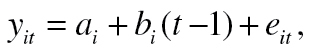 |
(1) |
where y it is the depression score of the ith elderly person at tth assessment occasion (t = 1, 2, 3, 4), a i and b i are his or her starting position (initial depression) and rate (slope, gradient) of change in depression over time, and e it is the associated error term (use of t − 1 as a formal predictor allows interpretation of the intercept a i as a starting position in depression, that is, initial depression [Raudenbush & Bryk, 2002], where i = 1, . . . , n, with n = 1,355 denoting sample size).
The Level 2 model specified fixed and random effects for baseline CVB (dichotomized), baseline cognitive functioning (dichotomized), education (continuous), baseline age (continuous), and the interaction of baseline CVB and education. To address multicollinearity associated with interaction terms, the dichotomized CVB and continuous education terms were mean centered. The CVB × education interaction term was calculated using these mean-centered variables. Then, a LGC was used to assess how these variables predicted change in depression over time.
Logistic regression analyses were used to test Hypothesis 2, that the CVB × education interaction will predict clinically significant depressive symptomatology. Consistent with the LGC model, CVB was dichotomized so that respondents with two or more risk factors were identified as high CVB group and those with zero or one risk factor were identified as low CVB group. Education was used as continuous variable. Based on the recommended clinical cutoff for this instrument (Steffick, 2000), respondents with CES-D score of 3 or more were identified as having clinically significant depressive symptomatology. Four stepwise logistic regression analyses were completed to evaluate how high CVB and education predict clinically significant depressive symptomatology. As in the IS model, both education and CVB were mean centered to reduce multicollinearity. The first step of each logistic regression included CVB and education. A term representing the interaction of CVB and education was included in the second step.
Results
Complete data were available for all 1,355 participants at baseline (1998). Of this baseline sample, 990 participants completed survey materials in 2000, 737 provided information in 2002, and 547 completed the survey in 2004. The baseline characteristics of the sample are displayed in Table 1. Of 5,420 possible CES-D data points (1,355 at each of four waves), CES-D data were available for 3,629 data points. Overall, 1,791 data points, or 33%, had missing values. As described, the FIML procedure estimated parameters using all available data used in the model in addition to the baseline supplementary variables, that is, ADL, IADL, and self-rated health.
Table 1.
Demographic, Independent, and Dependent Statistics for 1,355 Participants at Baseline (1998)
| Mean (SD) | |
|---|---|
| Age | 84.8 (4.1) |
| Years of education | 10.86 (3.52) |
| Ethnic distribution | |
| White | 79.5% |
| Black | 14.3% |
| Hispanic | 5.2% |
| Other | 1% |
| CVB (total count: 0–4) | 1.31 (0.92) |
| TICS | 18.14 (6.16) |
| CES-D | 2.12 (2.00) |
| CES-D score: 0–2 | 876 (64.6%) |
| CES-D score: 3–4 | 281 (20.7%) |
| CES-D score 5+ | 198 (14.6%) |
Notes. CVB = cerebrovascular burden; SD = standard deviation; TICS = Telephone Interview for Cognitive Status; CES-D score= Center for Epidemiologic Studies-Depression score.
Level 1 Model
The IS model defined in Equation 1 was then fitted to the data from the four repeated assessments of depression and found to be associated with the following fit indexes (Raykov & Marcoulides, 2006): chi square (χ 2(5, N = 1,355)) = 3.998, associated p value of .550, and root mean square error of approximation (RMSEA) = 0, with a 90% confidence interval (CI; 0, 0.034). Goodness of fit (GF) indexes indicated a tenable model, and the linear change model adequately described the four waves of depression data. Although this linear model fits very well, some have described late-life changes in depressive symptomatology using nonlinear terms (Teachman, 2006). In the interests of methodological rigor, nonlinear models were also explored, but it was found that the linear equation (as opposed to quadratic) was a better fit to the data.
Next, we examined the estimates of the means and variances of the random effects (intercept and slope, i.e., starting depression and rate of its change) in this model. The estimate of mean starting CES-D score (mean intercept) was 2.121, with a standard error (SE) of 0.053 and associated p value of <.001, indicating significant mean initial depression level as could be expected. The mean slope was estimated at 0.046, SE = 0.027, and p = .091. This result indicated that the average slope (rate of change over time in depression averaged across all participants) was not significant in the sampled population.
Level 2 Model
To gain further insight into these findings, we examined the individual differences in starting position and rate of change in depression, as reflected in the variances of these two random effects (intercept and slope). Specifically, the variance of initial depression was estimated at 2.369, SE = 0.194, and p < .001 (two tailed), indicating that there were significant individual differences in starting depression. The variance in the rate of temporal change in depression symptomatology was estimated at 0.138, SE = 0.046, and p = .003 (two tailed), which is also significant. This finding indicated that although the average slope of depression symptomatology change over time was not significant, there were considerable individual differences in the rate of change in depression symptomatology—for some participants the rate increased, whereas for others the rate of change in depression symptomatology decreased. Thereby, the correlation between starting position and rate of change in depression symptomatology was estimated at −0.155, SE = 0.082, and p = 0.060. This result suggested a trend that lower starting levels of depression symptomatology were associated with higher rates of changes in CES-D score over time.
Conditional Latent Growth
Although the unconditional IS model examined the patterns of change in depression symptomatology over time, it did not explain individual differences in these patterns in terms of other variables. To address this question, we fitted next the conditional IS model that explained individual differences in starting depression symptomatology and rate of change in it in terms of individual differences of CVB (dichotomous; denoted hereafter x 1), cognitive functioning (continuous; x 2), education (continuous; x 3), age (continuous; x 4), and the interaction of CVB with education (x 5). Specifically, this conditional model was defined by the following equations (Raykov & Marcoulides, 2008):
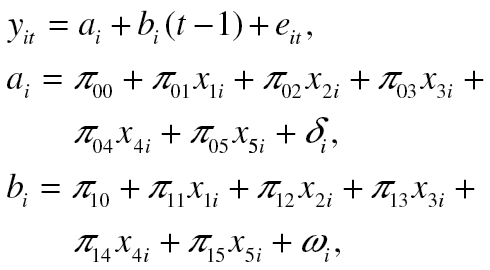 |
(2) |
where (i = 1, . . . , n). When fitted to the data, this two-level model was found to be associated with the following fit indexes: χ 2(15, N = 1,355) = 7.356, p = .947, RMSEA = 0, with a 90% CI (0, 0.003). These GF measures indicate a tenable model. Its parameter estimates of particular interest for this article are presented in Table 2.
Table 2.
Parameter Estimates, Standard Errors (SE), t Values, and p Values Associated With the Conditional Latent Growth Curve (LGC) Model (see Equation 2)
| Predictors | Estimate | SE | t Values | p Values |
|---|---|---|---|---|
| Regression of initial depression on | ||||
| Age | 0.018 | 0.013 | 1.365 | .172 |
| CF | −0.018 | 0.110 | −1.755 | .079 |
| CVB | 0.480 | 0.106 | 4.515 | <.001 |
| Education | −0.111 | 0.017 | −6.546 | <.001 |
| CVB × Education | 0.027 | 0.031 | 0.882 | .378 |
| Regression of rate of change in depression on | ||||
| Age | −0.010 | 0.007 | −1.375 | .169 |
| CF | −0.005 | 0.005 | −0.844 | .399 |
| CVB | 0.097 | 0.055 | 1.748 | .080 |
| Education | 0.020 | 0.009 | 2.151 | .032 |
| CVB × Education | −0.017 | 0.017 | −0.957 | .338 |
Notes: CF = cognitive functioning measured using the Telephone Interview for Cognitive Status; CVB = cerebrovascular burden; CVB × Education = interaction of centered CVB and education.
The top panel results in Table 2 suggest that once controlling for age and cognitive functioning, CVB and education explain a significant portion of individual differences in initial depression symptomatology. Thereby, for respondents of the same age and cognitive functioning level, and CVB, those with higher education had lower starting levels of depression symptomatology. Similarly, CVB is uniquely significant after controlling for age, education, and cognitive functioning. That is, older adults with higher CVB show higher level of depression symptomatology at start of study. Both age and cognitive functioning, however, are not uniquely significant. For otherwise similar respondents, neither age nor cognitive functioning is associated with higher or lower initial depression symptomatology. The interaction of CVB and education also did not significantly predict initial depression symptomatology.
The lower panel of Table 2 indicates that only education has unique predictive power with respect to rate of change in depression symptomatology. Specifically, for participants of the same age, with similar CVB and cognitive functioning, higher education is associated with higher rates of change in depression symptomatology. We stress, however, that respondents with higher education tend to be among those with lower starting position on depression symptomatology, as indicated in the top panel of Table 2 and mentioned previously. We also note that this finding is consistent with the earlier indicated result of a trend (p = .09) and negative correlation between starting position and rate of temporal change in depression symptomatology, which was obtained with the unconditional IS model fitted and reported first in this section.
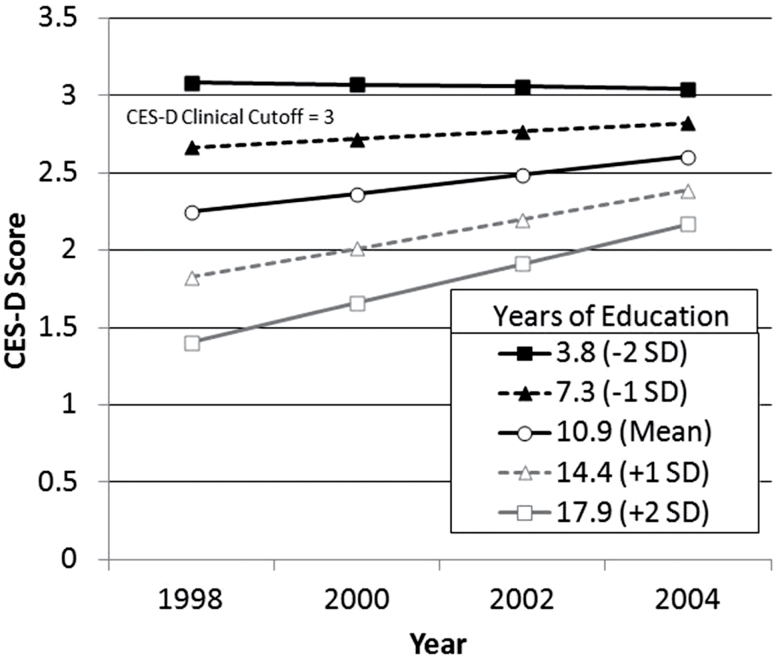
Last but not least, the lower panel of Table 2 also indicates that none of the remaining three predictors is associated with unique explanatory power with regard to individual differences in rate of change in depression symptomatology. That is, age, CVB, and cognitive functioning do not explain differences in rate of change in depression symptomatology once accounting for the remaining three predictors of this quadruple of explanatory variables. Figure 2 represents change in mood over time between respondents of varying education levels represented in this study.
Figure 2.
Plotted growth curves depicting Center for Epidemiologic Studies-Depression (CES-D) scores over time for respondents with mean cerebrovascular burden (CVB) and varying levels of education.
In the interests of thoroughly evaluating the relationship between CVB, education, and depression symptomatology, a post hoc model was completed wherein the CVB variable was modeled as a semicontinuous variable. Results of this post hoc analysis were not substantively different from those using a dichotomized CVB variable. Higher CES-D scores on the intercept term were significantly predicted by more cerebrovascular risk factors (β = 0.28, SE = 0.05, p < .001) and less education (β = −0.11, SE = 0.02, p < .001). As in the previous model, having more years of education significantly predicted a higher rate of change over time (β = 0.02, SE = 0.01, p = .03). As in the previous model in which CVB was dichotomized, the interaction between CVB (as a semicontinuous variable) and education did not significantly predict depressive symptomatology at intercept or change in depressive symptomatology over time.
Significant Depressive Symptomatology Analysis
Clinically significant depressive symptomatology was identified based on a cut-score of 3 on the CES-D. Using these criteria, the overall base rate for clinically significant depressive symptomatology ranged from was identified as 33.5% at baseline to 32.8%, 32.7%, and 33.5% at following waves, respectively. Four logistic regression analyses (shown in Table 3) were completed to identify how CVB and education at baseline predicted prevalence of clinically significant depressive symptomatology (CES-D score ≥ 3) at each wave. Both CVB and years of education significantly predicted clinically significant depressive symptomatology at all four waves. Notably, the odds ratio for education indicating risk of clinically significant depressive symptomatology was smaller at baseline (indicating a greater protective effect) and systematically approached 1.0 at subsequent waves, indicating that the benefits of education with respect to depressive symptomatology were gradually lost over time. The CVB × education interaction was statistically significant only at the 1998 wave.
Table 3.
Results of Four Stepwise Logistic Regression Analyses Predicting Clinically Significant Depressive Symptomatology at 1998, 2000, 2002, and 2004 Based on Dichotomized Cardiovascular Burden (CVB), Years of Education, and the Interaction of These Variables
| Step 1 | Step 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Year | β | SE | Wald χ2 | Odds ratio, OR (Range) | β | SE | Wald χ2 | OR (Range) |
| 1998 | ||||||||
| Dichotomized CVB | 0.44 | 0.12 | 13.93* | 1.56 (1.23–1.96) | 0.46 | 0.12 | 15.30* | 1.59 (1.26–2.01) |
| Years of education | −0.12 | 0.02 | 47.95* | 0.89 (0.86–0.92) | −0.12 | 0.02 | 50.22* | −0.89 (0.86–0.92) |
| CVB × Education | 0.07 | 0.03 | 3.91† | 1.07 (1.00–1.14) | ||||
| Constant | −0.63 | 0.06 | 115.95* | −0.63 | 0.06 | 115.61* | ||
| 2000 | ||||||||
| Dichotomized CVB | 0.58 | 0.14 | 17.13* | 1.79 (1.36–2.36) | 0.58 | 0.14 | 17.01* | 1.79 (1.36–2.35) |
| Years of education | −0.09 | 0.02 | 20.94* | 0.91 (0.88–0.95) | −0.09 | 0.02 | 20.15* | 0.91 (0.88–0.95) |
| CVB × Education | −0.01 | 0.04 | 0.02 | 0.94 (0.92–1.08) | ||||
| Constant | −0.72 | 0.07 | 108.66* | −0.72 | 0.07 | 108.64* | ||
| 2002 | ||||||||
| Dichotomized CVB | 0.50 | 0.16 | 9.42† | 1.66 (1.20–2.28) | 0.49 | 0.16 | 9.20† | 1.65 (1.19–2.27) |
| Years of education | −0.08 | 0.02 | 11.79* | 0.92 (0.88–0.97) | −0.08 | 0.02 | 12.09* | 0.92 (0.88–0.96) |
| CVB × Education | 0.03 | 0.05 | 0.35 | 1.03 (0.94–1.13) | ||||
| Constant | −0.68 | 0.08 | 73.11* | −0.68 | 0.08 | 72.89* | ||
| 2004 | ||||||||
| Dichotomized CVB | 0.74 | 0.19 | 14.88* | 2.11 (1.44–3.07) | 0.76 | 0.20 | 15.14* | 2.14 (1.46–3.13) |
| Years of education | −0.08 | 0.03 | 8.69† | 0.92 (0.87–0.97) | −0.09 | 0.03 | 8.84† | 0.92 (0.87–0.97) |
| CVB × Education | −0.04 | 0.06 | 0.39 | 0.96 (0.85–1.09) | ||||
| Constant | −0.62 | 0.09 | 44.56* | −0.62 | 0.09 | 44.07* | ||
Notes: CVB = cerebrovascular burden.
*p < .001. † p < .05.
Discussion
The objectives of this article were to examine whether brain reserve, as measured by education and CVB, was related to depressive symptomatology in older-old women. Consistent with Stern’s (2002) brain reserve analyses with cognitive function and dysfunction, we examined these relationships by measuring depressive symptomatology as both a continuous variable and by a clinical threshold level (i.e., dichotomous). The first goal was to test two competing hypotheses presenting contrasting perspectives on brain reserve. The first finding of this article is that CVB and education confer independent, “counterbalancing” effects on the development of mood over time. This finding supports Hypothesis 1A. Consistent with past research (Mast, Azar, & Murrell, 2005), high CVB predicted more depressive symptomatology at baseline. We also found that educational attainment was inversely related to depressive symptomatology at baseline, but for more than 6 years, poorly educated respondents reported a steady level of depressive symptoms and better-educated respondents developed more depressive symptoms, indicating that the benefits conferred by abundant brain reserve diminished over time. Change in depressive symptomatology over time was not significantly predicted by CVB. Overall, we found that when measuring depressive symptomatology as a continuous variable, CVB and brain reserve have an additive relationship. That is, greater brain reserve confers similar benefits for older women with high CVB and low CVB alike and these benefits are eroded over time regardless of CVB level.
The second goal of this study is to examine how CVB and education predict clinically significant depressive symptomatology, based on a cut-score on the CES-D. At baseline, respondents with both high CVB and less educational attainment were at increased risk for clinically significant depressive symptomatology. At later waves, additive main effects for CVB and education, similar to those for the IS model, were found. These results suggest that, by comparison to older women with fewer years of education, those with greater educational attainment and high CVB may have reduced risk for developing clinically significant depressive symptomatology. Respondents with more years of education had fewer depression symptoms at baseline, and greater rates of change over the course of the study, “catching up” to respondents with less education. This pattern is consistent with brain reserve idea of delaying, not preventing, clinical syndromes. This finding partially supports the hypothesis that brain reserve modifies the risk of clinically significant depressive symptomatology associated with high CVB. The absence of a significant interaction between education and CVB at later waves may indicate that the diminishing benefits of education are no longer robust to the relative risk of clinically significant depressive symptomatology conferred by high CVB as women approach age 90. This hypothesis should be explored in future research. When considered in light of declining benefits of brain reserve, these results support the application of brain reserve to vascular depression.
These findings are also generally consistent with the vascular depression hypothesis (Alexopoulos et al., 1997a) and previous findings (Mast, Yochim, et al., 2004; Mehta et al., 2003) relating CVB and late-life depression. The finding that threshold levels of CVBs predict both the number of depression symptoms and clinically significant depressive symptomatology has important implications for clinicians. Although research on the mechanisms of vascular depression will continue to evolve, primary care practitioners can use this simple method of calculating CVB as a guide to heightened risk of depression in older patients. The longitudinal data suggest limits for brain reserve. This study found that those with higher vascular burden reported more depression symptoms at baseline, and this difference persisted over time, regardless of the level of education.
Overall, this effect is analogous to Stern’s (2002) depiction of how brain reserve capacity counterbalances the effects of dementing disorders. For instance, although neuropathological characteristic of Alzheimer’s disease undermines cognition (measured as a continuous variable) for all older adults, those with less brain reserve are at higher risk for developing clinically defined Alzheimer’s disease (a categorical variable). A very recent study (Murray et al., 2013) found that fluid intelligence may “counterbalance” depressive symptomatology based on a cross-sectional sample. This study similarly suggests that brain reserve may counterbalance the effects of high CVB with respect to depressive symptomatology. Our study builds on this recent work with longitudinal results. Specifically, the benefit of educational experience with respect to depressive symptoms was slowly lost over the course of the study, suggesting that education delays but does not eliminate the risk of depression, particularly among elders with high CVB who had more depressive symptomatology at baseline. Clearly, more research is required to clearly define the relationships between vascular disease, brain reserve, and mood disorders across the life span and in late life.
Missing data in this study present a potential confound. The use of supplementary variables in the estimation of missing data is an empirically supported strategy to reduce bias while using all available data in longitudinal modeling. Although latent growth models can accommodate missing values, our inclusion of auxiliary variables substantively improves the accuracy of parameter estimates (Graham, 2003). Of note, a post hoc latent growth model was completed excluding auxiliary variables but otherwise similar to the model presented in this study (data not shown). This post hoc model produced very similar results obtained using auxiliary variables. Attrition is an unfortunate reality of longitudinal research, particularly with older adults. In this sample of women living in the community, attrition reflects the high mortality rate for this demographic. The alternative, listwise deletion, would exclude a significant portion of the sample in these longitudinal analyses of depression. Past work (Rabbitt et al., 2008) illustrates how listwise deletion can cause systematic underrepresentation of medical and psychological comorbidities and their risk factors, particularly when these disorders significantly predict attrition. As a result, listwise deletion compromises external validity. In contrast, the use of empirically supported longitudinal modeling is a strength of this study.
These results suggest several opportunities for further research. Specifically, it is known that educational quality relates to neuropsychological test performance (Manly, Jacobs, Touradji, & Small, 2002); however, there are no measures of educational quality in the HRS data. Quality of education may better predict depression (and in fact, cognitive functioning) in late life than years of education. Finally, as the HRS lacks information on the severity of CVB indicators, this study could not determine the effect of disease severity on the development of depression symptoms over time. Despite these limitations, the findings from this longitudinal study of older women suggest that CVB and education are associated with depression in late life. Future work may develop on these findings to examine how CVB interacts with other variables such as depression symptoms to predict the incidence of, or time to, death among the older old.
Funding
This work was generously supported by the Blue Cross Blue Shield of Michigan Foundation (1680.SAP); and by the T32 grant-supported NIH Pre-Doctoral Training Program in Aging and Urban Health at the Institute of Gerontology (T-32 AG00275-06).
Acknowledgments
We also thank professors Tenko Raykov and Wassim Tarraf for their assistance in developing the statistical methodology and presentation of results used in this article.
References
- Alexopoulos G. S., Meyers B. S., Young R. C., Kakuma T., Silbersweig D., Charlson M. (1997a). Clinically defined vascular depression. American Journal of Psychiatry, 154, 562–565 [DOI] [PubMed] [Google Scholar]
- Alexopoulos G. S., Meyers B. S., Young R. C., Campbell S., Silbersweig D., Charlson M. (1997b). ‘Vascular depression’ hypothesis. Archives of General Psychiatry, 54, 915–922. 10.1001/archpsyc.1997.01830220033006 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders (4th ed., text revision ed.). Washington, DC: Author [Google Scholar]
- Arbuckle J. L. (1996). Full information estimation in the presence of incomplete dataIn Marcoulides G. A., Schumacker R. E. (Eds.), Advanced structural equation modeling: Issues and techniques (pp. 243–277). Hillsdale, NJ: Erlbaum [Google Scholar]
- Blazer D. G. (2003). Depression in late life: Review and commentary. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 58, 249–265. 10.1093/gerona/58.3.M249 [DOI] [PubMed] [Google Scholar]
- Bour A., Rasquin S., Aben I., Strik J., Boreas A., Crijns H. … Verhey F. (2009). The symptomatology of post-stroke depression: Comparison of stroke and myocardial infarction patients. International Journal of Geriatric Psychiatry, 24, 1134–1142. 10.1002/gps.2236 [DOI] [PubMed] [Google Scholar]
- Brandt J., Spencer M., Folstein M. (1988). The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 1, 111–117 [Google Scholar]
- Cahana-Amitay D., Albert M. L., Ojo E. A., Sayers J., Goral M., Obler L. K., Spiro A. (in press). Effects of hypertension and diabetes on sentence comprehension in aging. The Journals of Gerontology, Series B, Psychological Sciences and Social Sciences. 10.1093/geronb/gbs085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Hill D. H. (2005). Active versus passive sample attrition: The Health and Retirement Study. Ann Arbor, MI: University of Michigan [Google Scholar]
- Chachamovich E., Fleck M., Laidlaw K., Power M. (2008). Impact of major depression and subsyndromal symptoms on quality of life and attitudes toward aging in an international sample of older adults. The Gerontologist, 48, 593–602. 10.1093/geront/48.5.593 [DOI] [PubMed] [Google Scholar]
- Desmond D. W., Tatemichi T. K., Hanzawa L. (1994). The telephone interview for cognitive status (tics): Reliability and validity in a stroke sample. International Journal of Geriatric Psychiatry, 9, 803–807. 10.1002/gps.930091006 [Google Scholar]
- Enders C. K. (2010). Applied missing data analysis (1st ed.). New York: Guilford Press [Google Scholar]
- González H. M., Bowen M. E., Fisher G. G. (2008). Memory decline and depressive symptoms in a nationally representative sample of older adults: The Health and Retirement Study (1998-2004). Dementia and Geriatric Cognitive Disorders, 25, 266–271. 10.1159/000115976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. W. (2003). Adding missing-data relevant variables to FIML-based structural equation models. Structural Equation Modeling, 10, 80–100. 10.1207/S15328007SEM1001_4 [Google Scholar]
- Groves R. M., Couper M. P. (1998). Nonresponse in household interview surveys. New York, NY: John Wiley and Sons [Google Scholar]
- Heeringa S. G., Conner J. (1995). Technical description of the Health and Retirement Study sample design: Hrs/ahead documentation report dr-002. Ann Arbor, MI: University of Michigan [Google Scholar]
- Jokeit H., Ebner A. (1999). Long term effects of refractory temporal lobe epilepsy on cognitive abilities: A cross sectional study. Journal of Neurology, Neurosurgery, and Psychiatry, 67, 44–50. 10.1136/jnnp.67.1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Fong T. G., Metzger E., Tulebaev S., Yang F. M., Alsop D. C. … Inouye S. K. (2010). Aging, brain disease, and reserve: Implications for delirium. American Journal of Geriatric Psychiatry, 18, 117–127. 10.1097/JGP.0b013e3181b972e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K. R., Hays J. C., Blazer D. G. (1997). MRI-defined vascular depression. American Journal of Psychiatry, 154, 497–501 [DOI] [PubMed] [Google Scholar]
- Ladin K. (2008). Risk of late-life depression across 10 European Union countries: Deconstructing the education effect. Journal of Aging and Health, 20, 653–670. 10.1177/0898264308321002 [DOI] [PubMed] [Google Scholar]
- Little R. J. A., Rubin D. B. (2002). Statistical analysis with missing data (2nd ed.). New York, NY: John Wiley [Google Scholar]
- Manly J. J., Jacobs D. M., Touradji P., Small S. A., Stern Y. (2002). Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society: JINS, 8, 341–348. 10.1017/S1355617702813157 [DOI] [PubMed] [Google Scholar]
- Mast B. T., Azar A. R., Murrell S. A. (2005). The vascular depression hypothesis: The influence of age on the relationship between cerebrovascular risk factors and depressive symptoms in community dwelling elders. Aging & Mental Health, 9, 146–152. PG68278202518457 [pii] 10.1080/13607860412331336832 [DOI] [PubMed] [Google Scholar]
- Mast B. T., MacNeill S. E., Lichtenberg P. A. (2004). Post-stroke and clinically-defined vascular depression in geriatric rehabilitation patients. American Journal of Geriatric Psychiatry, 12, 84–92 [PubMed] [Google Scholar]
- Mast B. T., Neufeld S., MacNeill S. E., Lichtenberg P. A. (2004). Longitudinal support for the relationship between vascular risk factors and late-life depressive symptoms. American Journal of Geriatric Psychiatry, 12, 93–101 [PubMed] [Google Scholar]
- Mast B. T., Yochim B., MacNeill S. E., Lichtenberg P. A. (2004). Risk factors for geriatric depression: The importance of executive functioning within the vascular depression hypothesis. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 59, 1290–1294. 10.1093/gerona/59.12.1290 [DOI] [PubMed] [Google Scholar]
- McArdle J. J., Hamagami F. (1991). Modeling incomplete longitudinal and cross-sectional data using latent growth structural models. Experimental Aging Research, 18, 145–166. 10.1080/ 03610739208253917 [DOI] [PubMed] [Google Scholar]
- Mehta K. M., Yaffe K., Langa K. M., Sands L., Whooley M. A., Covinsky K. E. (2003). Additive effects of cognitive function and depressive symptoms on mortality in elderly community-living adults. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 58, M461–M467. 10.1093/gerona/ 58.5.M461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M., O’Leary E. (2010). Depression, cognitive reserve and memory performance in older adults. International Journal of Geriatric Psychiatry, 25, 665–671. 10.1002/gps.2404 [DOI] [PubMed] [Google Scholar]
- Murray A. D., Staff R. T., McNeil C. J., Salarirad S., Phillips L. H., Starr J. … Whalley L. J. (2013). Depressive symptoms in late life and cerebrovascular disease: The importance of intelligence and lesion location. Depression and Anxiety, 30, 77–84. 10.1002/da.22022 [DOI] [PubMed] [Google Scholar]
- Muthen L. K., Muthen B. O. (2007a). Mplus (Version 5.0). Los Angeles, CA: Muthen & Muthen [Google Scholar]
- Muthen L. K., Muthen B. O. (2007b). Mplus; statistical analysis with latent variables (5th ed.). Los Angeles, CA: Muthen & Muthen [Google Scholar]
- National Center for Health Statistics (2010). Health, united states, 2010: With special feature on death and dying. Hyattsville, MD: Author; [PubMed] [Google Scholar]
- Nebes R. D., Reynolds C. F., 3rd, Boada F., Meltzer C. C., Fukui M. B., Saxton J. … DeKosky S. T. (2002). Longitudinal increase in the volume of white matter hyperintensities in late-onset depression. International Journal of Geriatric Psychiatry, 16, 526–530. 10.1002/gps.635 [DOI] [PubMed] [Google Scholar]
- Ofstedal M. B., Fisher G. G., Herzog A. R. (2005). Documentation of cognitive functioning measures in the Health and Retirement Study. Ann Arbor, MI: University of Michigan [Google Scholar]
- Paradiso S., Vaidya J., Tranel D., Kosier T., Robinson R. G. (2008). Nondysphoric depression following stroke. Journal of Neuropsychiatry and Clinical Neurosciences, 20, 52–61. 10.1176/ appi.neuropsych.20.1.52 [DOI] [PubMed] [Google Scholar]
- Paulson D., Bowen M. E., Lichtenberg P. A. (2011). Successful aging and longevity in older old women: the role of depression and cognition. Journal of Aging Research, 2011, 912680. 10.4061/ 2011/912680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaty B. M., Kuller L. H., Bild D., Burke G. L., Kittner S. J., Mittelmark M. … Robbins J. (1995). Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Annals of Epidemiology, 5, 255–335. 10.1016/1047–2797(94)00092-8 [DOI] [PubMed] [Google Scholar]
- Rabbitt P., Lunn M., Wong D. (2008). Death, dropout, and longitudinal measurements of cognitive change in old age. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 63B, P271–P278. 10.1093/geronb/63.5.P271 [DOI] [PubMed] [Google Scholar]
- Radloff L. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. 10.1177/014662167700100306 [Google Scholar]
- RAND HRS Data, Version F (2006). Center for the study of aging with funding from the national institute on aging and the social security administration. Santa Monica, CA: National Institute on Aging and the Social Security Administration [Google Scholar]
- Raudenbush S. W., Bryk A. S. (2002). Hierarchical linear models (2nd ed.). Thousand Oaks, CA: Sage Publications [Google Scholar]
- Raykov T., Marcoulides G. A. (2006). A first course in structural equation modeling. Mahwah, NY: Lawrence Erlbaum Assoc., Inc [Google Scholar]
- Raykov T., Marcoulides G. A. (2008). An introduction to applied multivariate analysis. New York, NY: Routledge [Google Scholar]
- Raz N., Rodrigue K. M., Acker J. D. (2003). Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience, 117, 1169–1180. 10.1037/ 0735-7044.117.6.1169 [DOI] [PubMed] [Google Scholar]
- Rush B. K., McNeil R. B., Gamble D. M., Luke S. H., Richie A. N., Albers C. S. … Meschia J. F. (2010). Behavioral symptoms in long-term survivors of ischemic stroke. Journal of Stroke and Cerebrovascular Diseases, 19, 326–332. 10.1016/j.jstrokecerebrovasdis.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Salloway S., Malloy P., Kohn R., Gillard E., Duffy J., Rogg J. … Westlake R. (1996). MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology, 46, 1567–1574. 10.1212/WNL.46.6.1567 [DOI] [PubMed] [Google Scholar]
- Santos C. O., Caeiro L., Ferro J. M., Figueira M. L. (2011). Mania and stroke: A systematic review. Cerebrovascular Diseases, 32, 11–21. 10.1212/WNL.46.6.1567 [DOI] [PubMed] [Google Scholar]
- Schoevers R. A., Beekman A. T. F., Deeg D. J. H., Hooijer C., Jonker C., van Tilburg W. (2003). The natural history of late-life depression: Results from the amsterdam study of the elderly (amstel). Journal of Affective Disorders, 76, 5–14. 10.1016/S0165-0327(02)00060-5 [DOI] [PubMed] [Google Scholar]
- Siegel M., Bradley E. H., Kasl S. V. (2003). Self-rated life expectancy as a predictor of mortality: Evidence from the HRS and AHEAD surveys. Gerontology, 49, 265–271. 10.1159/000070409 [DOI] [PubMed] [Google Scholar]
- Steffick D. E. (2000). Documentation of affective functioning measures in the Health and Retirement Study. Hrs documentation report dr-005. Ann Arbor, MI: Survey Research Center at the Institute for Social Research [Google Scholar]
- Stern Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 9, 448–460. 10.1017/S1355617702813248 [PubMed] [Google Scholar]
- Stern Y. (2009). Cognitive reserve. Neuropsychologia, 47, 2015–2028. 10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachman B. A. (2006). Aging and negative affect: The rise and fall and rise of anxiety and depression symptoms. Psychology and Aging, 21, 201–207. 10.1037/0882-7974.21.1.201 [DOI] [PubMed] [Google Scholar]
- von Strauss E., Agüero-Torres H., Kåreholt I., Winblad B., Fratiglioni L. (2003). Women are more disabled in basic activities of daily living than men only in very advanced ages: A study on disability, morbidity, and mortality from the Kungsholmen Project. Journal of Clinical Epidemiology, 56, 669–677. 10.1016/S0895-4356(03)00089-1 [DOI] [PubMed] [Google Scholar]
- Wilson C. J., Finch C. E., Cohen H. J. (2002). Cytokines and cognition—The case for a head-to-toe inflammatory paradigm. Journal of the American Geriatrics Society, 50, 2041–2056. 10.1046/ j.1532-5415.2002.50619.x [DOI] [PubMed] [Google Scholar]