Abstract
Oxytocinergic neurotransmission during lactation contributes to reduction of anxiety levels and fear. However, our knowledge of where oxytocin acts in the brain to achieve this effect, particularly to an unconditioned fear stimulus, is incomplete. We used blood oxygenation level dependent (BOLD) fMRI to test whether central administration of oxytocin 45–60 minutes before fMRI scanning alters maternal brain activation in response to a predator scent (TMT, trimethylthiazoline). Comparison behavioral experiments that examined maternal responses to this unconditioned fear -inducing odor were carried out in a separate cohort of lactating rats given similar treatments. Behavioral experiments confirmed the effectiveness of oxytocin at reducing freezing behavior as compared to vehicle controls. Our fMRI findings indicate that oxytocin modulated both positive and negative BOLD responses across several olfactory and forebrain nuclei. Significantly greater percent increases in BOLD signal in response to TMT were observed in the anterior cingulate, bed nucleus of stria terminalis and perirhinal area of oxytocin pretreated rats. These animals also showed significantly larger percent decreases in BOLD in mammillary bodies, secondary motor cortex, gustatory cortex, prelimbic prefrontal cortex, orbital cortex, and the anterior olfactory nucleus. The observed pattern of brain activity suggests that oxytocin enhances neural processing in emotion and cognition driven brain areas such as the cingulate cortex, while dramatically reducing activity in areas also controlling autonomic, visceromotor and skeletomotor responses. The present data contribute to the growing literature suggesting the oxytocin modulate the integration of emotional and cognitive information through myriad brain regions to facilitate decreases in anxiety (even to an unconditioned stimulus) while potentially promoting pair-bonding, social memory and parental care.
Keywords: Fox scent, TMT, trimethylthiazoline, innate fear, unconditioned fear, predatory, Predator, behavior, lactation, maternal behavior, maternal anxiety, fMRI, awake rats, BOLD signal
1. INTRODUCTION
The ability to perceive and respond to threats is vital during the postpartum period. Lactating rats are more aggressive towards conspecifics and less fearful than virgin rats. Postpartum rats will rapidly confront and attack nest intruders to protect their litters (Erskine et al., 1978). Mothers are also less fearful in general. For example, time spent in fear associated freezing postures in response to an aversive auditory tone is lower in maternal rats than in virgins (Hansen and Ferreira, 1986a; Hansen and Ferreira, 1986b; Hard and Hansen, 1985). Moreover, lactating rats spend more time in open arms of an elevated plus maze (Ferreira et al., 2002) and show less signs of anxiety and fear in open field tests (Wartella et al., 2003).
The central release of oxytocin and vasopressin during lactation may contribute to the observed changes in offensive aggression and reduced fearfulness in females (Bosch and Neumann, 2008; Francis et al., 2000; Bale et al., 2001; Windle et al., 2004; Blume et al., 2008). These neuropeptides are synthesized by neurons in the paraventricular (PVN) and supraoptic nuclei (SON) of the hypothalamus and are released in both brain and periphery. In the case of oxytocin, it is released in the PVN and central amygdala during lactation (Neumann et al., 1993) and nest defense (Bosch et al., 2005), respectively. Oxytocin acts through receptors distributed throughout the brain including in regions associated with olfaction, emotion and memory (Ostrowski et al., 1994; Vaccari et al., 1998; Veinante and Freund-Mercier, 1997; Yoshimura et al., 1993). Blocking oxytocin receptors reduces lactation-related behaviors (Bosch and Neumann, 2008; Pedersen and Boccia, 2003), increases anxiety related behavior in an elevated plus maze (Bosch and Neumann, 2008) and affects maternal offensive and defensive behaviors (Bosch et al., 2005). The actions of oxytocin on aggression and fear are perhaps exerted through PVN, central amygdala and septum (Bosch et al., 2005; Heinrichs et al., 2009); however, it is quite possible that an ample circuitry involving additional limbic and forebrain sites also contribute to oxytocin’s role in general and specifically during the postpartum period. The present study investigated whether oxytocin modulates maternal brain activity in response to a stressful and fear-evoking predator scent that is extracted from fox feces, trimethylthiazoline (TMT).
TMT has been shown to evoke fear-associated immobility, or freezing behavior (Wallace and Rosen, 2001), and it also has been observed to elevate plasma levels of corticosterone (Day et al., 2004; Morrow et al., 2002; Shields and King, 2008). TMT-associated increases in corticosterone in lactating rats are enhanced when pups are in the nest (Deschamps et al., 2003). The physiological and behavioral signs of stress and fear in response to TMT are distinguishable from responses to novel acrid odors (Morrow et al., 2002; Staples et al., 2008) and considered innate since laboratory rodents have no prior exposure to predator odors. However, behavioral patterns and neuronal activity evoked by TMT across the brain have had some controversy (Blanchard et al., 2003; Staples et al., 2008). Although researchers report that fox scent does not produce conditioned fear responses, as observed with actual predator encounters (Blanchard et al., 2003), others have shown that TMT-induced expression of conditioned fear is highly context dependent (Endres and Fendt, 2007). Neuronal activation patterns that are observed in studies employing assays for protein markers c-fos and erg-1 are arguably reminiscent of stress, aversion, or avoidance and not just fear (Rosen et al., 2005; Staples et al., 2008). Indeed, patterns of neuronal activity as measured by these cellular markers also vary with the predator species (Staples et al., 2008), and are localized to brain structures that may be distinct from areas involved in conditioned fear (Wallace and Rosen, 2001). Herein, we employ fMRI to resolve immediate and real-time brain activity patterns in response to TMT in lactating rats given synthetic oxytocin centrally. Recent fMRI findings indicate that brain BOLD activation in female rats vary across the estrous cycle (Chen et al., 2009). We further extend those findings in females with the present experiment by showing here that oxytocin given centrally modulates maternal brain BOLD activation patterns in response to TMT.
2. RESULTS
2.1 Effect ICV oxytocin on behavioral response to TMT
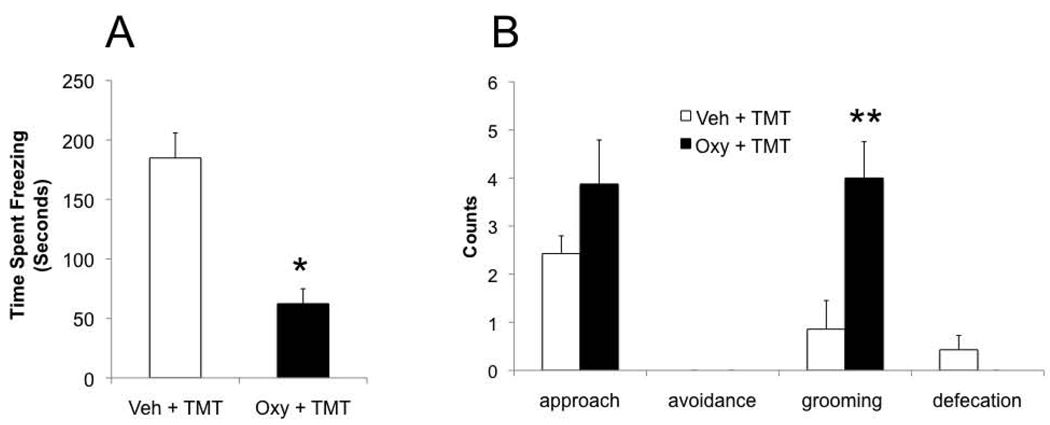
We carried out behavioral tests to examine the effects of intracerebral oxytocin administration on innate, unconditioned fear responses in the presence of TMT. Student’s t-test analysis revealed a significant effect of oxytocin on freezing behavior and grooming in the presence of TMT. Oxytocin treated dams spent significantly less time freezing than vehicle controls (t13= 5.2, p < 0.001) and more time grooming (t13= −3.2, p = 0.007). There was only a non-significant trend for less avoidance of the novel odor in oxytocin treated rats. No effect of oxytocin treatment was observed for approach responses and number of defecation boli (Figure 1).
Figure 1.
Behavioral response to TMT in postpartum dams pretreated with vehicle or oxytocin. A) Time spent freezing during TMT presentation in vehicle and oxytocin pretreated rats. B) Number of times (in counts) dams avoided or approached the novel fox TMT odor, number of grooming and defecation bouts during a 10 minute test. All data presented as mean ± standard error. Symbols above error bars denotes significantly different from vehicle. * p < 0.05; **p < 0.01.
2.2 Effect of ICV oxytocin on maternal BOLD response to TMT
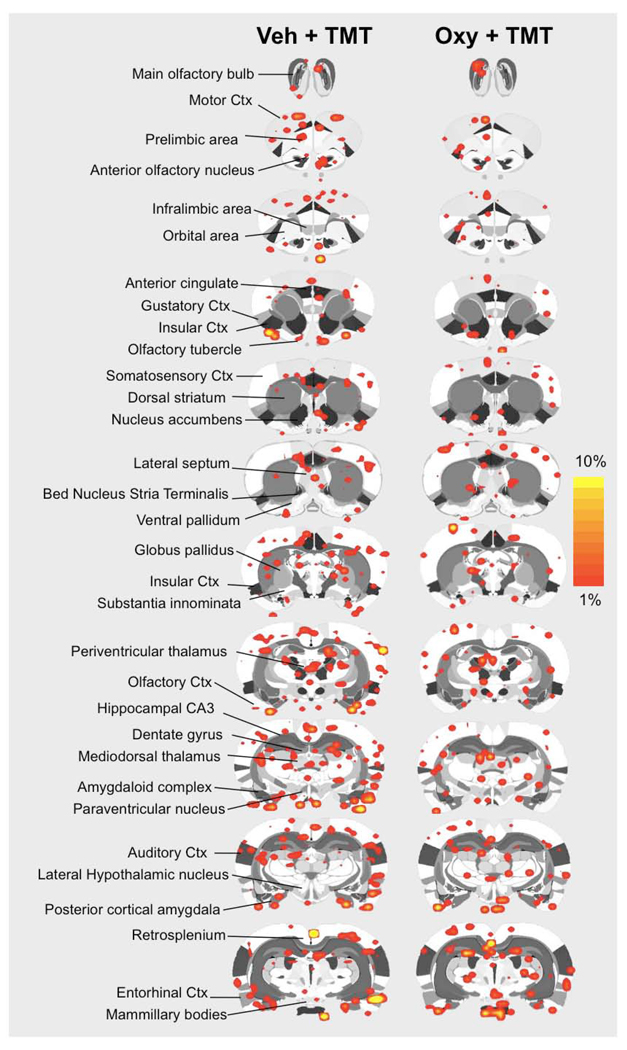
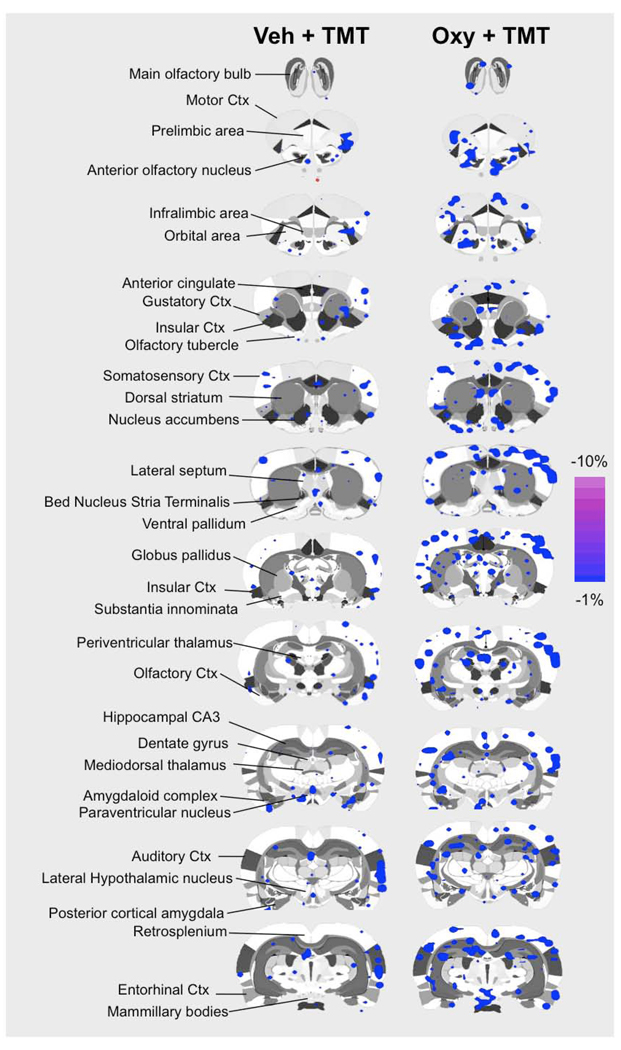
Functional MRI studies in awake postpartum rats were done to test the effects of intracerebral oxytocin administration on brain BOLD activation in response to TMT odor presentation. Vehicle or oxytocin was administered 45–60 minutes before fMRI scanning. Sixty-nine regions of interest spanning the cortex, limbic system, hypothalamus, basal forebrain, and midbrain were analyzed using a t-test analysis (corrected for multiple comparisons) for changes in BOLD signal intensity upon odor stimulus presentation. Volumes of activation (reported as number of voxels) and magnitude BOLD response (reported as percent change in BOLD signal in response to TMT odor) were analyzed. Data for all regions of interest are summarized online in Table 1 and Table 2 (see Online Supplementary Data). BOLD signal increases and decreases were observed in response to TMT presentation in lactating rats pretreated with vehicle or oxytocin 45–60 minutes before scanning. Pups were not present during fMRI scanning. Data for positive BOLD voxels are summarized in the 2D composite maps shown in Figure 2. Negative BOLD voxel data is shown in Figure 3. Significant BOLD activation was observed in both treatment groups. Importantly, areas of the olfactory system, including the main olfactory bulbs, showed significant BOLD activity in response to TMT. Both groups of animals (vehicle and oxytocin pretreated) also showed BOLD responses in areas such as the prelimbic prefrontal cortex, posterior cortical amygdala, piriform region, hypothalamus and striatum (Figure 2 and Figure 3).
Figure 2.
Composite coronal brain maps showing positive BOLD signal changes in response to TMT in vehicle and oxytocin pretreated postpartum dams. TMT was used as a stimulus during functional MRI scanning 45–60 after vehicle or oxytocin pretreatment intracerebroventricularly. Red-to-yellow pixels on 2D maps indicate areas that showed significant increases in BOLD signal with TMT stimulus presentation (t-test p < 0.05 corrected for multiple comparisons). Scale bar hue (red-to-yellow) indicates percent increase in BOLD signal intensity with a lower threshold cut-off of 1%. Various regions of interest are highlighted to the left of the figure as a guide.
Figure 3.
Composite coronal brain maps showing negative BOLD signal changes in vehicle and oxytocin treated postpartum dams presented with TMT. TMT was used as a stimulus during functional MRI scanning 45–60 after vehicle or oxytocin pretreatment intracerebroventricularly. Blue-to-purple pixels in 2D maps indicate areas that showed significant increases in BOLD signal with TMT stimulus presentation (t-test p < 0.05 corrected for multiple comparisons). Scale bar hue (blue-to-purple) indicates percent decrease in BOLD signal intensity with a higher threshold cut-off of −1%. Various regions of interest are highlighted to the left of the figure as a guide.
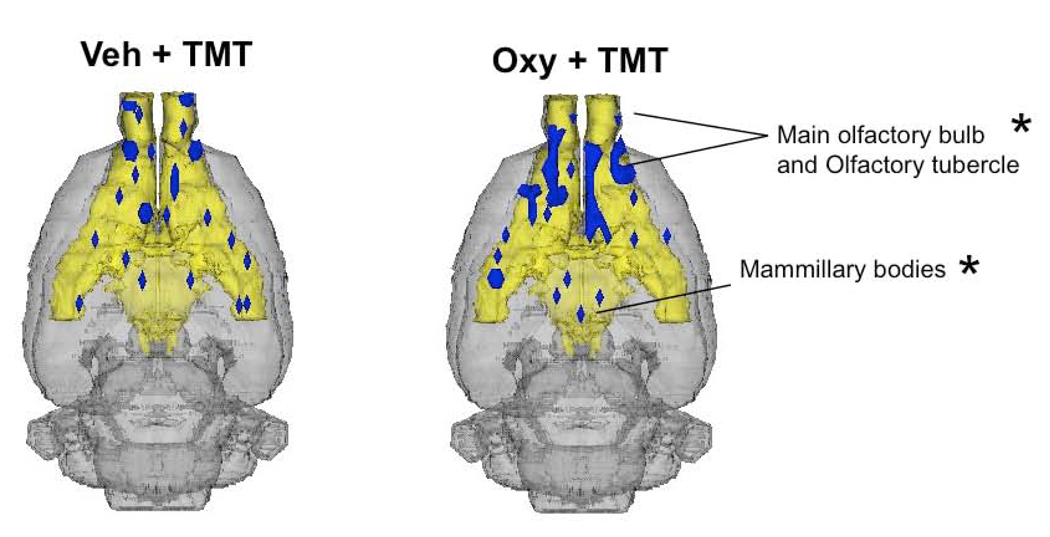
Functional MRI data was analyzed as previously reported (Ferris et al., 2005), focusing on both volumes of activation or deactivation and percent changes in BOLD in response to TMT. The effect of oxytocin pretreatment on the number of BOLD voxels in response to TMT showed little significant effects. No significant differences were observed in positive BOLD voxels (Figure 2 and Table 1 - Online). Differences in the number of negative BOLD voxels (Figure 3 and Figure 4) were observed in mammillary bodies (t13 = −2.6, p = 0.012), olfactory tubercle (t13 = −2.3, p = 0.03) and main olfactory bulb (t13 = 2.2, p = 0.03); oxytocin dams showing more negative BOLD voxels in the olfactory tubercle and less in main olfactory bulb (Figure 4; Online Table 2). Greater effects of oxytocin pretreatment on the BOLD response to TMT were observed for percent changes in BOLD signal intensity. Relative changes in BOLD signal intensity, presumably representing the magnitude of underlying neuronal activity, have been correlated with multi-unit activity and local field potentials (Logothetis et al., 2001). Percent changes in BOLD signal for several limbic cortical areas are shown in Figure 5 and are summarized in Table 1 and 2 (Online Supplementary Data). Significantly larger increases in BOLD were observed in the perirhinal area (t13 = −2.1, p = 0.05), anterior cingulate cortex (t13 = −2.3, p = 0.03), bed nucleus of stria terminalis (t13 = −2.2, p = 0.03). Significantly larger decreases in BOLD were observed in visual cortex (t13 = 2.2 p = 0.03), raphe (t13 = 2.3, p = 0.03), mammillary bodies (t13 = 2.3, p = 0.03), secondary motor cortex (t13 = 2.3, p = 0.03), gustatory cortex (t13 = 2.4, p = 0.02), prelimbic medial prefrontal cortex (t13 = 2.3, p = 0.03), orbital cortex (t13 = 2.3, p = 0.03), and the anterior olfactory nucleus (t13 = 3.7, p = 0.002).
Figure 4.
Olfactory and hypothalamic negative BOLD response in vehicle and oxytocin pretreated postpartum dams presented with TMT. Composite 3D brain volume maps showing negative BOLD volumes signal changes in olfactory structures, hypothalamus and mammillary bodies. Yellow filled areas of the 3D rat brain volume denote the olfactory system (from olfactory bulbs to piriform cortex) and the hypothalamus, including the mammillary bodies. Blue filled areas indicate regions of the olfactory system and hypothalamus that showed BOLD signal decreases. Asterisks denote significant differences in volume of negative BOLD activity between vehicle and oxytocin treated dams (p < 0.05).
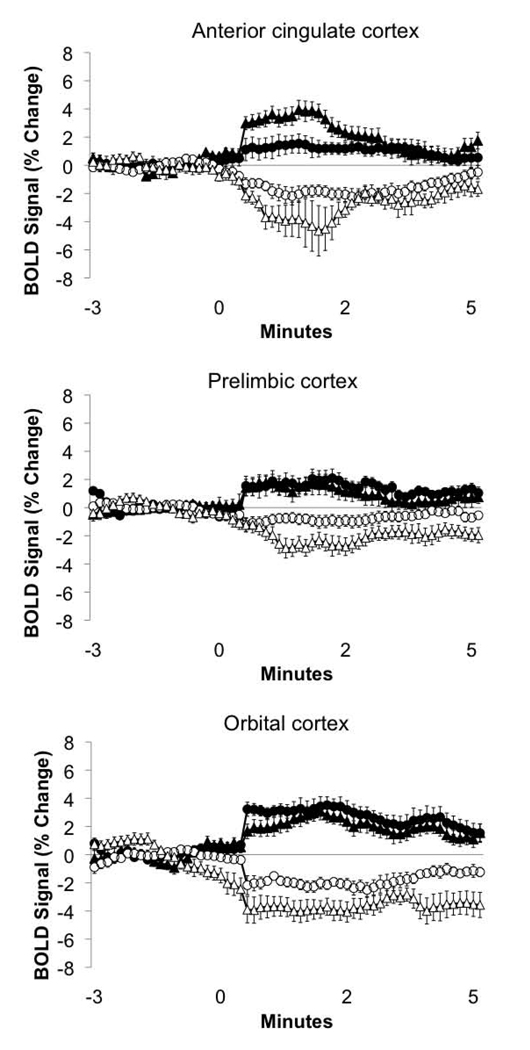
Figure 5.
BOLD signal time courses for the anterior cingulate, prelimbic and orbital regions of the prefrontal cortex of vehicle and oxytocin pretreated postpartum dams presented with TMT. Positive and negative BOLD signals are plotted for voxels within each region. Data are presented as percent change from baseline signal (mean ± standard error). Negative BOLD voxels plotted as empty symbols: circles (○), vehicle pretreated; triangles (Δ), oxytocin pretreated. Positive BOLD voxels plotted as filled symbols: circles (●), vehicle pretreated; triangles (▲), oxytocin pretreated. Statistical comparisons were made between vehicle and oxytocin pretreated data. Results of statistics are summarized in text and in Table 1 and Table 2 Online.
3. DISCUSSION
The current study explored the impact of the neuropeptide oxytocin on behavior and neuronal activation accompanying TMT-induced unconditioned fear responses in dams. The results support the notion that oxytocin decreases anxiety in lactating dams by dampening, freezing, a prototypic fear behavior, in the presence of TMT odor. In addition, we observed that oxytocin treatment modulated both BOLD signal increases and decreases in response to TMT in lactating rats. Given the important role of oxytocin in stress reduction, social bonding, parenting and affiliation, the behaviors noted in the lactating dams are not unexpected. However the brain regions engaged in this phenomenon are less apparent. Oxytocin enhanced TMT-induced positive BOLD signal changes in the anterior cingulate, a brain region involved in emotional expression and fear conditioning. While the BNST, which was previously shown to be responsive to TMT (Day et al., 2004) and also constitute part of the neural circuitry mediating behavioral responses to this predator scent (Fendt et al., 2003; Fendt et al., 2005), also showed greater BOLD signal as compared to vehicle controls. Interestingly, a wider array of structures showed negative BOLD signal changes in response to TMT following oxytocin. The anterior olfactory nucleus, which serves as an initial relay site for olfactory-processing, showed primarily signal decreases. This was observed along with signal decreases in the prelimbic prefrontal cortex, orbital cortex, mammillary bodies, secondary motor cortex and the gustatory cortex, among other regions. The pattern of brain activity observed in the present work underscores the importance of oxytocin in regulating fear and anxiogenic responses during the lactation period.
3.1 Physiological basis of BOLD signal response to TMT
Based on the current knowledge on fMRI, we can interpret the present findings as evidence that the observed changes in BOLD signal arose from immediate real-time changes in neuronal activity upon presentation of a predator odor. This is important to point out when comparing with or trying to differentiate from rather slower temporal scales provided by assays detecting neuronal activity markers such as c-fos. fMRI allows the indirect measure of neuronal activity through complex interplay of vascular and metabolic responses. More specifically, microvascular magnetic field gradients that are sensitive to changes in deoxy- and oxy-hemoglobin concentrations near brain areas of high or reduced neuronal metabolism are the likely source of the detected fMRI signal (Ogawa et al., 1990). Changes in neuronal activity and the accompanying compensatory adjustments in blood flow, blood volume and cerebral metabolic rate for oxygen thus give origin to the BOLD signal observed in the present, and many other studies (Davis et al., 1998). There is an ever-growing literature providing stronger evidence that this is the case and that also provide a strong link between BOLD and particular aspects of neuronal computing (Logothetis et al., 2001; Logothetis, 2008).
3.2 Behavioral and Neural Effects of TMT
The behavioral effects of TMT and the neural circuits responsive to this predator scent have been studied using molecular techniques. Using in situ hydribization to detect c-fos mRNA expression, Day et al. (Day et al., 2004) demonstrated induced c-fos in hippocampus, amygdala, septum, BNST, hypothalamus along with the supramammillary region, and midbrain nuclei. This selective pattern of c-fos induction was distinguished from butyrate, a novel and acrid odor. More recently, Staples et al. (Staples et al., 2008) used c-Fos immunocytochemistry to detect protein induction and demonstrated selective increases in only in the main olfactory bulb and the central amygdala. Additional structures showed Fos induction to both TMT and cat odor, these included the ventral orbital cortex, piriform and anterior cortical amygdala. Animals in the above studies were euthanized 30 and 60 minutes after TMT exposure, respectively. Using fMRI, Chen and co-workers (Chen et al., 2009) showed a brain activation patterns occurring within seconds of TMT exposure that was similar to Day et al. (Day et al., 2004) and very consistent with the present results. TMT-induced BOLD signal changes were dependent on phase of the estrous cycle in female rats (Chen et al., 2009). Both the cellular expression of neuronal activity markers and BOLD fMRI seem to suggest a neurophysiological response to an unconditioned stressor and not learned fear. This is consistent with behavioral paradigms showing that TMT or cat odor fails to produce conditioned fear responses (Blanchard et al., 2003). Indeed, the lateral amygdala, which is activated by conditioned fear stimuli, is not by unconditioned predator odors (Wallace and Rosen, 2001).
3.3 Oxytocin modulates the neural response to TMT in lactating rats
Our present results are in agreement with a previous study looking at the BOLD response to oxytocin administration in lactating rats (Febo et al., 2005) and also with more recent work (Nephew et. al., 2009). In the Febo et al. (2005) study, ICV oxytocin administration was compared to vehicle and was observed to selectively activate the anterior cingulate, parietal cortex, somatosensory, temporal cortices, dorsal striatum, preoptic area and ventral tegmental area. The resultant actions of oxytocin administration on BOLD responses within these regions may hold importance to emotional reactivity, particularly to innate fear reactions. For instance, the anterior cingulate is involved in the initiation of goal directed behaviors (Devinsky et al., 1995), as well as the emotional control of visceral, skeletal, and endocrine functions (Vogt et al., 1992). In the Nephew et al. study (2009), presentation of a male nest intruder to lactating rats, in the presence of pups, evoked robust BOLD response in the anterior cingulate as well. The pups themselves, which represent a rewarding stimulus for the lactating rat, did not significantly activate the cingulate (Nephew et al., 2009).
Oxytocin receptor binding sites and its mRNA have been detected in the BNST, lateral septum, posterior cortical amygdala, anterior olfactory nucleus, piriform cortex, main olfactory bulbs, central amygdala, subiculum of the hippocampus and dispersed cortical regions (Veinante and Freund-Mercier, 1997; Yoshimura et al., 1993). TMT induced BOLD responses observed in the present study were in partial accordance with areas of the rat brain that express oxytocin receptors. The BNST is particularly interesting. We observed a greater volume of activation in this area with oxytocin pretreatment, suggesting a greater neuronal activity in response to TMT. Recent report shows that TMT exposure elevates extracellular norepinephrine levels in the BNST and blocking norepinephrine receptors with clonidine results in less time spent freezing in response to TMT (Fendt et al., 2005). Although electrolytic lesions of the lateral amygdala block conditioned fear, but not innate fear (Wallace and Rosen, 2001), the opposite has been shown for the BNST (Fendt et al., 2003), suggesting an essential role of the BNST in unconditioned fear response. However, it should be noted that the BNST also appears to be important in behavior and endocrine responses to contextual fear stimuli (Sullivan et al., 2004). Therefore the BNST may be an important brain area where oxytocin may exert its effect on TMT-induced freezing in lactating rats (Figure 1). Although there is no evidence showing oxytocin receptors in the anterior cingulate, this region shares synaptic communication with the septum and amygdala, which might indirectly have regulated TMT-induced BOLD activation. Indeed, previous reports indicate that it plays a role in memory of emotional and fear inducing events (Malin and McGaugh, 2006; Malin et al., 2007).
Several brain areas showed negative BOLD signal changes in response to TMT following oxytocin pretreatment and this might have significance for the neural effects of TMT. Negative BOLD signal changes were enhanced in the AON, mammillary, prelimbic, and orbital cortex with oxytocin administration. It is tempting to speculate on whether this represents an underlying physiological mechanism where activity in the anterior cingulate during exposure to an innate fear stimulus occurs in parallel with a lack of neuronal activity or inhibition in other adjacent limbic cortical regions controlling approach behavior and autonomic responses. Negative BOLD has, however, less support from the literature on its role in neuronal processing than positive BOLD (Logothetis, 2002). Although there are strong indications that negative BOLD correlates with reductions in synaptic activity (Shmuel et al., 2006). Interestingly, we observed both positive and negative BOLD signal changes in several regions of interest, for instance in prefrontal cortical areas shown in Figure 5. The co-existence of separate groups of voxels that show opposing changes in BOLD signal within a localized region of the brain is not unforeseen and has been proposed to arise as a consequence of the vascular steal effect, where blood is delivered to foci of high activity from surrounding silent regions, or from an actual reduction in neuronal activity that is segregated from areas showing increases. This has been experimentally tested in the primary visual cortex of primates (Shmuel et al., 2006). Both the meaning of this phenomenon and its relevance to neural processing is the focus of intense investigation.
3.4 Concluding remarks
Finally, it is important to note several caveats in the present study design. First, a pre-existent anxiogenic state may have occurred during fMRI scanning, as a result of the acute ICV surgery for injections, that could have affected TMT evoked BOLD responses. One may speculate that in the absence of this surgical procedure, the evoked BOLD response to TMT may have been somewhat different or the fear inducing effects of TMT might be worse. The surgical procedure, however, was necessary due to the current inability of implanting permanent cannulas that are stable under the overlying surface coil placed on top of the animals’ head (see Experimental Methods). Although control scans for ICV injections and odor presentation was not included to measure non-specific BOLD responses, these have been included in our previous work (Febo et al., 2004; Chen et al., 2009). Indeed, the methods used in the present study have proven to be effective for imaging awake animals following acute ICV treatments. Secondly, it is quite possible that oxytocin may be exerting nonspecific effects on arousal (Lancel et al., 2003) instead of specific effects on innate fear responsiveness. We have addressed this issue in the past and have observed that, at least for the suckling stimulus, the effects of oxytocin receptor blockade appeared to be specific (Febo et al., 2005). However, given that TMT as well as suckling in previous work (Febo et al., 2005) are sensory stimuli, it is not unlikely that arousal places a prominent role. What’s important is how the maternal brain interprets the sensory arousing stimulus in the presence or absence of oxytocin.
Vasopressin V1a receptor mRNA (Szot et al., 1994) and vasopressin peptide (Hernando et al., 2001), as well as oxytocin receptors (Veinante and Freund-Mercier, 1997; Yoshimura et al., 1993), have been detected in the rat AON. It is clear that olfaction plays a significant role in maternal aggression, as surgically removing the olfactory bulb (Kolunie and Stern, 1995) or olfactory epithelium decreases maternal aggression and increases fear (Ferreira et al., 1987). The prelimbic area of the medial prefrontal cortex has previously reported to show elevated dopamine metabolism in response to TMT (Morrow et al., 2002). It has also been shown to connect with the AON, orbital prefrontal cortex and supramammilary nucleus (Vertes, 2006). Therefore, it is possible that the set of structures showing negative BOLD may be part of a neural network responsive to anxiogenic stimuli and that is modulated by oxytocin during lactation. The present work adds to the body of literature seeking to understand the neural circuitry of innate, unconditioned fear. Furthermore, it demonstrates that oxytocin pretreatment can modulate the neural processing of olfactory information related to innate fear in lactating dams. We speculate that these studies may also add to our conceptualization of the role of oxytocin in modulating phobias (Bartz and Hollander, 2006) and its potential use as a therapeutic option in certain anxiety related disorders.
4. EXPERIMENTAL PROCEDURES
4.1 Subjects
Virgin Sprague-Dawley rats, 80–90 days old, were obtained from Charles River Laboratories (MA) and pair housed in 48 × 25 × 20 cm Plexiglas cages before breeding. Animals were maintained on a 12 hr light-dark cycle with lights on at 0700 hr and provided food and water ad libitum. Prior to breeding, animals were acclimated to the rodent restrainer and the imaging protocol as described below. All dams were primiparous with a minimum litter size of 10 pups. Rats were acquired and cared for in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 85-23, Revised 1985) and adhere to the National Institutes of Health and the American Association for Laboratory Animal Science guidelines. The Institutional Animal Care and Use Committee at the University of Massachusetts Medical School approved the protocols used for this study.
4.2 Acclimatization procedures and preparations for imaging
All imaging experiments were done in fully awake, unanesthetized primiparous postpartum day 4 to day 8 (P4 to P8) dams at ca. 45–60 minutes after ICV injections (see below for surgical procedures). Anesthesia (2– 4% isoflurane) was used during rat setup immediately preceding the acclimatization procedures, surgical procedure, and MRI scanning. Rats were imaged while fully awake. In order to minimize physiological response and gross motion during MR scanning, all rats were acclimatized to a head-restraining unit and MRI sounds on days P2 to P4 (King et al., 2005). Before MR scanning, dams were again anesthetized with 2–4 % isoflurane. Details of the setup procedure have been previously reported (Ferris et al., 2005). Briefly, a topical anesthetic of 5–10 % lidocaine cream was applied to the skin and soft tissue around the ear canals and over the bridge of the nose before the animal is placed inside a dual coil radiofrequency system under restraint (Ferris et al., 2005). This procedure took 5–6 min, after which gaseous anesthesia flow was turned off and the entire unit was placed through the bore of the magnet for imaging. After the entire unit was placed in the magnet, scanning preparations controlled by Paravision 4.0 typically took 15 minutes and thereafter the entire imaging session including 1 anatomical scan (ca. 6–8 minutes) and 1 functional scan (ca. 10–12 minutes total) lasted about 30–35 minutes. Thus, the entire experiment per animal in an unanesthetized state lasted 40–45 minutes.
4.3 Intracerebroventricular oxytocin administration
Female rats were pretreated with oxytocin (Sigma-Aldrich, St Louis, MO; Dose: 1 ug in 10 ul of artificial cerebrospinal fluid) before imaging the BOLD response to predator odor. The dose of oxytocin has been previously shown to modulate the BOLD response to suckling in P4 – P8 rats (Febo et al., 2005). Oxytocin does not cross the blood brain barrier and therefore must be given centrally in the lateral cerebral ventricles (Pedersen and Prange, 1979). We used previous published methods to deliver an acute dose of oxytocin before fMRI (Febo et al., 2005). Forty five to 60 minutes prior to scanning, rats were anesthetized with 2–4 % isoflurane, ten percent lidocaine cream was applied to the scalp, the skull surface was exposed and the landmark suture Bregma located. A 26-gauge cannula of polyethylene tubing (PE-10: inner diameter 0.28 mm, outer diameter 0.61 mm) was implanted into the lateral cerebral ventricle (1 mm caudal to Bregma, 2 mm lateral to the mid-sagittal sinus, and 4 mm ventral to dura) and secured to the skull with surgical glue. Correct cannula placement was always verified by running a pilot anatomical MRI-scan.
4.4 Imaging the neural response to TMT (predator odor)
Trimethylthiazoline (TMT; Phero Tech Inc., British Columbia, Canada) is a chemical constituent of fox feces and has been used previously to evoke both fear and stress reactions in several rat strains (Rosen et al., 2006). Twenty-four primiparous rats were imaged for their neural response to TMT following ICV injection of oxytocin or artificial cerebrospinal fluid (aCSF contents in mM: 145 NaCl, 2.7 KCl, 10 MgCl2, 12 CaCl2, 20 Na2HPO4•7H20, pH 7.4; initial n = 12 per group). Pups were not present during fMRI scanning. To deliver the TMT during fMRI, flexible tubing was set near the rats face while inside the dual-coil Rf system (see above). The tubing was connected at the other end to a ‘mock’ plastic connector. The empty connector was attached at other end to additional tubing that allowed continuous airflow, generated by an air pump, across the entire system. During scanning, the empty connector was easily accessed through the front outer end of the magnet bore and was switched out for another connector containing the TMT odor. Inside the connector, a patch of gauze pad had approximately 10–20 uL of TMT. All tubing and the bore of the magnet were thoroughly cleansed after imaging sessions by using ethanol (95%). For TMT presentations, 72 repetitions (8.3 seconds per repetition for a total 600 seconds) were collected and the stimulus was presented at repetition 35–36 (approx. 290 seconds).
4.5 Magnetic resonance imaging scanning parameters
Experiments were conducted in a Bruker Biospec 4.7-T/40-cm horizontal magnet (Oxford Instrument, Oxford, U.K.) equipped with a Biospec Bruker console (Bruker, Billerica, MA U.S.A) and a 20-G/cm magnetic field gradient insert (ID = 12 cm) capable of a 120-µs rise time (Bruker). Radiofrequency signals were sent and received with the dual coil electronics built into the animal restrainer (Ludwig et al., 2004). The volume coil for sending RF signal features an 8-element microstrip line configuration in conjunction with an outer copper shield. The arch-shaped geometry of the receiving surface coil provides excellent coverage and high signal-to-noise. To prevent mutual coil interference, the volume and surface coils were actively tuned and detuned. Functional images were acquired using a multi-slice fast spin echo sequence. A single data acquisition acquired fourteen, 1.2 mm slices in 8.3 sec (FOV 3.0 cm; data matrix 64 × 64; TR 1.4 sec TE 7 msec; NEX 1). This sequence was repeated 72 times in an 10 min imaging session consisting of 5 min of baseline data followed by 5 min of stimulation data. At the end of each imaging session a high-resolution anatomical data set was collected using the RARE pulse sequence (14 slice; 1.2 mm; FOV 3.0 cm; 256 × 256; TR 2.1 sec; TE 12.4 msec; NEX 6; 6 min acquisition time).
4.6 Behavioral testing of TMT reactivity in lactating rats
In a separate set of studies, groups of vehicle and oxytocin treated rats were tested for fear responses in the presence of TMT. Pups were present in the cage during behavioral testing. Rats were prepared as described above for functional MRI studies, and the same dose and route of administration of oxytocin was used for behavioral testing. Behavior tests were carried out during a 10-minute session that was used to generate digital recordings. Digital videos were taken from a side view of the cage therefore allowing the discernment of freezing from non-specific immobility. Litters were present during testing. A trained observer scored behavior. During experiments a small plastic eppendorf vial containing a small gauze pad with 10–20 uL of TMT was placed in a corner of the home cage. Behaviors included: time approaching vial, time avoiding vial (time spent on the opposite half of cage), self-grooming, freezing, and defecation bouts. Behavioral testing was carried out in 7 aCSF and 8 oxytocin treated dams. Statistical comparisons were made using Students t-test (two-tailed, significant p < 0.05).
4.7 MRI Statistical analysis
Full details for the MRI data analysis using in house software has been previously reported (Ferris et al., 2005; Ferris et al., 2008). Animals showing an average displacement exceeding 25% of the total in plane (X–Y) pixel resolution (>117 µm out of 468 µm) or slice (Z) direction (>300 µm out of 1200 µm slice thickness) were excluded (n = 6). This cutoff criterion was pre-established by stimulated studies showing false positive BOLD activation with movements corresponding to 6/10 of a single voxel (Ferris et al., 2008). Also, scans with linear baseline drifts over 0.5% were corrected using in house software (Ferris et al., 2008). Following preprocessing steps, groups consisted of 9 oxytocin and 9 aCSF treated dams.
ROI-based statistical analysis was done using Medical Image Visualization and Analysis (MIVA) software (Ferris et al., 2005). Each subject was registered to a fully segmented electronic rat brain atlas (Paxinos and Watson, 1997; Swanson, 1999). Statistical t tests were performed on each subject within the original coordinate system. The baseline period used was 20 repetitions immediately preceding stimulus (TMT) presentation and the stimulation window was 20 repetitions. Statistical t tests used a 95 % confidence level, two-tailed distribution, and heteroscedastic variance assumptions. In order to provide a conservative estimate of significance, a false-positive detection-controlling algorithm is introduced into the analysis (Genovese et al., 2002). This ensures that the false-positive detection rate is below our confidence level of 5 % (Ferris et al., 2005). Statistically significant pixels were assigned their percentage change values (stimulus mean minus control mean). Activated voxel numbers and percent changes in BOLD signal were exported to SPSS for statistical comparisons between groups. The number of voxels per region of interest and their corresponding average percent change values were statistically evaluated between groups using Student’s t-test (two-tailed, significant p < 0.05).
Supplementary Material
ACKNOWLEDGEMENTS
Support for this research was provided by an NIH grants NIMH MH067096 (JAK), NIDA DA019946 (MF) and NIDA DA13517 (CFF). Its contents are solely the responsibility of the authors and do not represent the official views of the NIMH nor NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Markham C, Yang M, Hubbard D, Madarang E, Blanchard RJ. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav Neurosci. 2003;117:360–368. doi: 10.1037/0735-7044.117.2.360. [DOI] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur. J Neurosci. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams' trait anxiety. Proc Natl Acad Sci U S A. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Shields J, Huang W, King JA. Female fear: influence of estrus cycle on behavioral response and neuronal activation. Behav Brain Res. 2009;201:8–13. doi: 10.1016/j.bbr.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingluate cortex to behaviour. Brain. 2005;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Deschamps S, Woodside B, Walker CD. Pups presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. J Neuroendocrinol. 2003;15:486–497. doi: 10.1046/j.1365-2826.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- Endres T, Fendt M. Conditioned behavioral responses to a context paired with the predator odor trimenthylthiazoline. Behav Neurosci. 2007;121(3):594–601. doi: 10.1037/0735-7044.121.3.594. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behav Biol. 1978;23:206–218. doi: 10.1016/s0091-6773(78)91814-x. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Segarra AC, Tenney JR, Brevard ME, Duong TQ, Ferris CF. Imaging cocaine-induced changes in the mesocorticolimbic dopaminergic system of conscious rats. J Neurosci. Meth. 2004;139:167–176. doi: 10.1016/j.jneumeth.2004.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Dahlof LG, Hansen S. Olfactory mechanisms in the control of maternal aggression, appetite, and fearfulness: effects of lesions to olfactory receptors, mediodorsal thalamic nucleus, and insular prefrontal cortex. Behav Neurosci. 1987;101:709–717. doi: 10.1037//0735-7044.101.5.709. 746. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Pereira M, Agrati D, Uriarte N, Fernandez-Guasti A. Role of maternal behavior on aggression, fear and anxiety. Physiol Behav. 2002;77:197–204. doi: 10.1016/s0031-9384(02)00845-4. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr., Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hansen S, Ferreira A. Effects of bicuculline infusions in the ventromedial hypothalamus and amygdaloid complex on food intake and affective behavior in mother rats. Behav Neurosci. 1986a;100:410–415. doi: 10.1037//0735-7044.100.3.410. [DOI] [PubMed] [Google Scholar]
- Hansen S, Ferreira A. Food intake, aggression, and fear behavior in the mother rat: control by neural systems concerned with milk ejection and maternal behavior. Behav Neurosci. 1986b;100:64–70. doi: 10.1037//0735-7044.100.1.64. [DOI] [PubMed] [Google Scholar]
- Hard E, Hansen S. Reduced fearfulness in the lactating rat. Physiol Behav. 1985;35:641–643. doi: 10.1016/0031-9384(85)90155-6. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Frontiers in Neuroendocrinology. 2009;10:1016. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hernando F, Schoots O, Lolait SJ, Burbach JP. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142:1659–1668. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolunie JM, Stern JM. Maternal aggression in rats: effects of olfactory bulbectomy, ZnSO4-induced anosmia, and vomeronasal organ removal. Horm Behav. 1995;29:492–518. doi: 10.1006/hbeh.1995.1285. [DOI] [PubMed] [Google Scholar]
- Lancel M, Krömer S, Neumann ID. Intracerebral oxytocin modulates sleep-wake behaviour in male rats. Regulatory Peptides. 2003;114:145–152. doi: 10.1016/s0167-0115(03)00118-6. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Ludwig R, Bodgdanov G, King J, Allard A, Ferris CF. A dual RF resonator system for high-field functional magnetic resonance imaging of small animals. J Neurosci Methods. 2004;132:125–135. doi: 10.1016/j.jneumeth.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proc Natl Acad Sci U S A. 2006;103:1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin EL, Ibrahim DY, Tu JW, McGaugh JL. Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: interaction with the basolateral amygdala. Neurobiol Learn Mem. 2007;87:295–302. doi: 10.1016/j.nlm.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Fear-like biochemical and behavioral responses in rats to the predator odor, TMT, are dependent on the exposure environment. Synapse. 2002;46:11–18. doi: 10.1002/syn.10109. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Caffrey MK, Felix-Ortiz AC, Ferris CF, Febo M. Blood oxygen level dependent signal responses in corticolimbic ‘emotions’ circuitry of lactating rats facing nest intruder threat to pups. Eur J Neurosci. 2009;30:934–945. doi: 10.1111/j.1460-9568.2009.06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski NL, Lolait SJ, Young WS., 3rd Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994;135:1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Stereotaxic Coordinates. Boston: Academic Press; 1997. The Rat Brain. [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin antagonism alters rat dams' oral grooming and upright posturing over pups. Physiol Behav. 2003;80:233–241. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Adamec RE, Thompson BL. Expression of egr-1 (zif268) mRNA in select fear-related brain regions following exposure to a predator. Behav Brain Res. 2005;162:279–288. doi: 10.1016/j.bbr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Rosen JB, West EA, Donley MP. Not all rat strains are equal: differential unconditioned fear responses to the synthetic fox odor 2,4,5-trimethylthiazoline in three outbred rat strains. Behav Neurosci. 2006;120:290–297. doi: 10.1037/0735-7044.120.2.290. [DOI] [PubMed] [Google Scholar]
- Shields J, King JA. The role of 5-HT1A receptors in the behavioral responses associated with innate fear. Behav Neurosci. 2008;122:611–617. doi: 10.1037/0735-7044.122.3.611. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008;151:937–947. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, LeDoux JE. Lesions of the bed nucleus of stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Boston: Elsevier Science; 1999. [Google Scholar]
- Szot P, Bale TL, Dorsa DM. Distribution of messenger RNA for the vasopressin V1a receptor in the CNS of male and female rats. Brain Res Mol Brain Res. 1994;24:1–10. doi: 10.1016/0169-328x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139:5015–5033. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb. Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. J Neurosci. 2001;21:3619–3627. doi: 10.1523/JNEUROSCI.21-10-03619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartella J, Amory E, Lomas LM, Macbeth A, McNamara I, Stevens L, Lambert KG, Kinsley CH. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiol Behav. 2003;79:373–381. doi: 10.1016/s0031-9384(03)00150-1. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura R, Kiyama H, Kimura T, Araki T, Maeno H, Tanizawa O, Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993;133:1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.