Summary
Leukemia stem cells (LSC) play a pivotal role in chronic myeloid leukemia (CML) tyrosine kinase inhibitor (TKI) resistance and progression to blast crisis (BC), in part, through alternative splicing of self-renewal and survival genes. To elucidate splice isoform regulators of human BC LSC maintenance, we performed whole transcriptome RNA sequencing; splice isoform-specific qRT-PCR, nanoproteomics, stromal co-culture and BC LSC xenotransplantation analyses. Cumulatively, these studies show that alternative splicing of multiple pro-survival BCL2 family genes promotes malignant transformation of myeloid progenitors into BC LSC that are quiescent in the marrow niche and contribute to therapeutic resistance. Notably, a novel pan-BCL2 inhibitor, sabutoclax, renders marrow niche-resident BC LSC sensitive to TKIs at doses that spare normal progenitors. These findings underscore the importance of alternative BCL2 family splice isoform expression in BC LSC maintenance and suggest that combinatorial inhibition of pro-survival BCL2 family proteins and BCR-ABL may eliminate dormant LSC and obviate resistance.
Introduction
Human leukemia stem cells (LSC), first described in acute myeloid leukemia (AML) (Lapidot et al., 1994), subvert stem cell properties, such as quiescence, enhanced self-renewal and survival, which render them resistant to conventional therapy (Guzman et al., 2002; Visvader, 2011). Chronic myeloid leukemia (CML) represents an important paradigm for dissecting the molecular evolution of LSC during leukemic progression and the role of LSC in therapeutic resistance because CML was the first malignancy to be targeted with therapy that selectively inhibits the aberrant kinase responsible for CML initiation (Druker et al., 2001). Although BCR-ABL-targeted tyrosine kinase inhibitors (TKIs) eradicate the bulk of BCR-ABL1 expressing cells, they frequently fail to eliminate quiescent, niche-resident LSC that drive relapse (Abe et al., 2008; Barnes and Melo, 2006; Chomel et al., 2011; Corbin et al., 2011) and blast crisis (BC) transformation following TKI discontinuation (Chomel and Turhan, 2011; Cortes et al., 2004; Deininger, 2008; Stuart et al., 2009). Despite improved overall survival (Druker et al., 2006), no curative pharmacologic therapy for CML exists, in part, because the genetic and epigenetic drivers of human BC LSC generation remain to be elucidated.
In human BC CML and in many cases of AML, LSC are enriched within the CD34+CD38+Lin− compartment, which is composed predominantly of granulocyte-macrophage progenitors (GMP) (Eppert et al., 2011; Goardon et al., 2011; Jamieson et al., 2004) with aberrant self-renewal capacity. Serial transplantation experiments show that as few as 1,000 GMP serially transplant human BC CML (Abrahamsson et al., 2009). Moreover, GMP LSC have been identified in transgenic mouse models of both BC CML (Jaiswal et al., 2003) and of AML (Krivtsov et al., 2006) suggesting that malignant transformation of progenitors into LSC, through aberrant acquisition of stem cell properties, is a key driver of leukemic progression.
Evidence from primary patient samples demonstrates that chronic phase (CP) CML is a clonal disorder (Martin et al., 1980) that originates from BCR-ABL (Daley et al., 1990) expressing hematopoietic stem cells (HSC) (Jamieson et al., 2004). Although necessary for CP initiation, BCR-ABL expression is not sufficient to drive BC transformation (Radich et al., 2006). Both mouse transgenic model and xenotransplantation data show that activation of stem cell signaling pathways, including WNT/β-catenin (Abrahamsson et al., 2009; Jamieson et al., 2004; McWeeney et al., 2009; Zhao et al., 2007), Hedgehog (Zhao et al., 2009) and the intrinsic apoptotic pathway regulated by the BCL2 gene family (Jaiswal et al., 2003), promote BC transformation. Malignant transformation of BCR-ABL1 expressing GMP into self-renewing BC LSC (CD34+CD38+Lin−) occurs, in some cases, as a consequence of alternative splicing of GSK3β, a negative regulator of Wnt/β-catenin, Hedgehog signaling and MCL1 (Abrahamsson et al., 2009; Ding et al., 2007). While recent reports reveal that mutations in splicing genes promote progression of myeloid malignancies to acute leukemia (Yoshida et al., 2011), alternative splicing-mediated alterations in the transcriptome may also enable BC transformation in a malignant microenvironment.
Because CML becomes increasingly refractory to TKIs during progression to BC (Karbasian Esfahani et al., 2006; Sawyers et al., 2002), understanding the epigenetic mechanisms that drive BC LSC maintenance and contribute to therapeutic resistance is essential. In addition, several studies suggest that LSC quiescence induction by the stem cell niche is a major component of therapeutic resistance (Barnes and Melo, 2006; Corbin et al., 2011; Forsberg et al., 2010; Holyoake et al., 1999; Saito et al., 2010). Although, recent evidence shows that increased expression of BCL2 family members contributes to CML pathogenesis (Aichberger et al., 2005; Dai et al., 2004; Tauchi et al., 2003), the precise nature of BCL2 splice isoform usage had not been examined even though a number of isoforms have antithetical functions (Akgul et al., 2004).
Pro-survival BCL2 family genes contribute to leukemogenesis (Beverly and Varmus, 2009), CML progression (Jaiswal et al., 2003), TKI resistance (Aichberger et al., 2005; Horita et al., 2000; Jaiswal et al., 2003; Konopleva et al., 2002; Sanchez-Garcia and Grutz, 1995) and hematopoietic stem and progenitor cell survival (Domen and Weissman, 2003; Milyavsky et al., 2010) by direct inhibition of MOMP. Expression of BCL2 family genes has also been linked to bone marrow niche-dependent TKI resistance in vitro (Bewry et al., 2008). However, whether pro-survival BCL2 family gene splice isoform expression promotes human BC LSC maintenance has not been elucidated. Moreover, the role of niche-dependent BCL2 family gene expression has not been delineated in the context of BC LSC quiescence induction and TKI resistance in vivo. Thus we compared BCL2 family expression in FACS-purified CML progenitors from normal, CP and BC patients and in BC LSC engrafted in different hematopoietic niches. We also investigated whether BC LSC could be targeted with a novel pan-BCL2 inhibitor, sabutoclax, capable of inhibiting BCL2, MCL1, BFL1, and BCLXL. Finally, the capacity of pan-BCL2 inhibition to overcome niche-dependent TKI resistance was assessed both in vitro and in BC LSC xenograft models as a paradigm for understanding the potential utility of sabutoclax in the sensitization of quiescent CSCs to anti-proliferative agents in a broad array of malignancies.
Results
Pro-survival BCL2 Isoform Expression Increases During BC Transformation
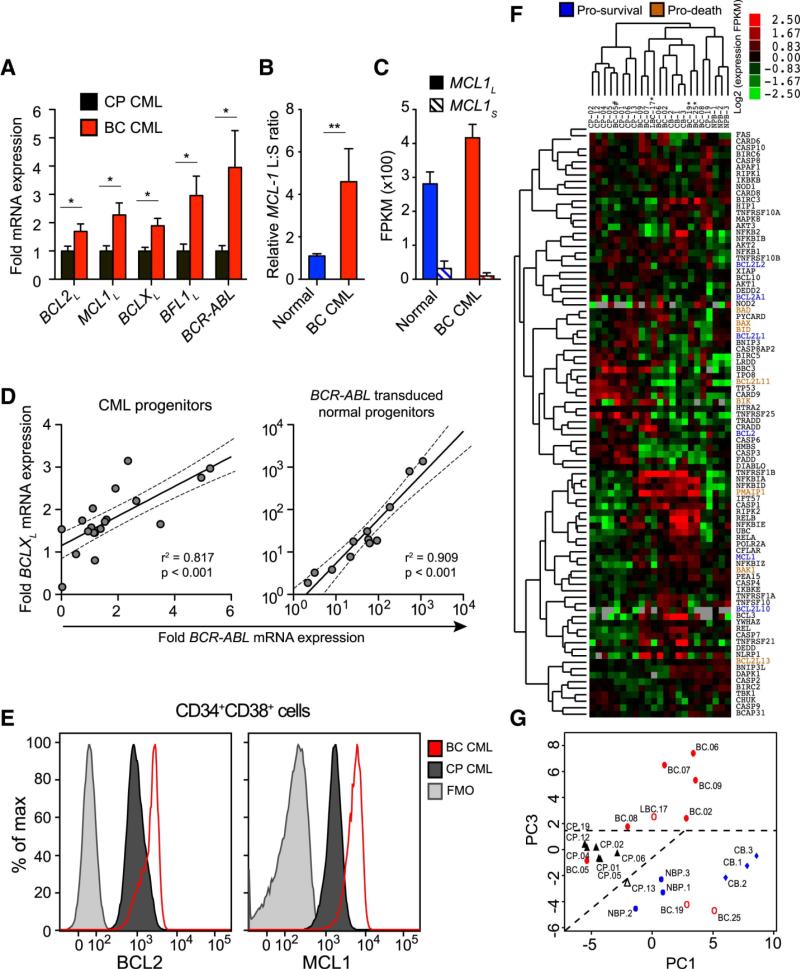
Although several studies have linked BCL2 gene upregulation with CML progression, most have focused on BCR-ABL-expressing cell lines (Amarante-Mendes et al., 1998; Gesbert and Griffin, 2000; Sanchez-Garcia and Grutz, 1995) or bulk CD34+ cells (Aichberger et al., 2005; Horita et al., 2000; Radich et al., 2006) rather than self-renewing human BC LSC (CD34+CD38+Lin−) that promote BC transformation. While many BCL2 family genes encode splice variants with both pro-apoptotic and anti-apoptotic functions (Moore et al., 2010), relatively little is known about the pattern of BCL2 family gene isoform expression in human BC LSC. Therefore, we utilized splice-isoform specific qRT-PCR and whole transcriptome RNA sequencing (RNASeq) to analyze BCL2 family isoform expression in FACS-purified progenitors from primary human normal (n=6), CP (n=8) and BC (n=9) samples (Supplementary Table 1). Notably, BC LSC expressed significantly higher levels of BCR-ABL and pro-survival BCL2L, MCL1L, BCLXL and BFL1L splice isoforms than CP progenitors (Fig. 1a), as well as higher BCL2L, BCLXL and BFL1L than normal progenitors (Supplementary Fig. 1a-b). Both qRT-PCR and RNASeq revealed a relative abundance of anti-apoptotic MCL1-long compared with pro-apoptotic short isoforms in BC LSC (Fig. 1b-c and Supplementary Fig. 1a-b). These data suggest that pro-survival BCL2 family gene isoforms are globally upregulated during CML BC transformation.
Figure 1.
see also Figure S1 and Table S1 and S2
a) qRT-PCR of pro-survival (long isoforms) BCL2, MCL1, BCLX, BFL1 and BCR-ABL mRNAs in primary CP (black, n=13) and BC (red, n=11) progenitors. Values are normalized to human HPRT mRNA expression. Graphs show mean +/− SEM; * p<0.05 by unpaired t-test. b) Relative ratio of MCL1 long to short isoforms in normal (n=7) and BC (n=8) progenitors. Graph shows mean +/− SEM; ** p<0.01 by unpaired t-test. c) Whole transcriptome sequencing of FACS-sorted progenitor cells from a normal and a BC sample showing quantification of MCL1L and MCL1S isoforms in each sample. Graph shows fragments per kilobase of exon per million fragments mapped (FPKM) +/− 95% confidence interval. Graphs show mean +/− SEM d) Correlation between BCR-ABL mRNA expression and BCLXL mRNA expression in progenitors. Left: Primary CP and BC samples (n=20). Right: BCR-ABL transduced normal progenitor colonies (n=12). Both graphs depict best-fit line and 95% confidence intervals by Pearson correlation analysis. e) Representative FACS histograms of BCL2 and MCL1 protein expression in CP progenitors (black) versus BC progenitors (red). Fluorescence minus one (FMO) controls are shown in grey. f) Full transcriptome RNA sequencing analysis of survival-pathway genes in FACS-sorted (CD45+CD34+CD38+LIN−) progenitors from 3 normal cord blood (CB), 3 normal adult peripheral blood (NP), 8 CP, 1 lymphoid BC (LBC) and 8 BC samples. Heat map depicts log2 fold FPKM. BCL2 family genes are highlighted and their functions are indicated by blue (pro-survival) or orange (pro-death) coloring. g) Unsupervised principal component analyses of the survival pathway genes for FACS-sorted CD45+CD34+CD38+LIN− progenitors from 3 normal cord blood (CB), 3 normal adult peripheral blood (NP), 8 CP, 1 lymphoid BC (LBC) and 8 BC samples.
Because BCR-ABL induces BCL2 family gene expression in CML cell lines (Aichberger et al., 2005; Horita et al., 2000; Sanchez-Garcia and Grutz, 1995), we examined whether pro-survival BCL2 family overexpression coincided with BCR-ABL amplification in sorted CML progenitors. A striking correlation was observed between BCR-ABL and BCLXL levels in CML progenitors, which was confirmed in lentiviral BCR-ABL-transduced progenitors (Fig. 1d), suggesting that increased BCLXL expression is driven by BCR-ABL amplification in BC LSC, as previously reported (Aichberger et al., 2005; Horita et al., 2000; Sanchez-Garcia and Grutz, 1995). Expression of other pro-survival BCL2 family gene isoforms did not correlate with BCR-ABL, indicating that upregulation occurs through BCR-ABL-independent mechanisms. Consistent with qRT-PCR results, an increase in BCL2 and MCL1 proteins was detected by FACS analysis in BC LSC compared with CP progenitors (Fig. 1e and Supplementary Fig. 1c). Notably, BCL2 protein expression was higher in serially transplantable CD34+CD38+Lin− BC LSC than normal or CP CD34+CD38−Lin− and CD34+CD38+Lin− cells (Supplementary Fig. 1d). Moreover, increased expression of both BCL2 transcript levels and protein correlated with expansion of CD123+ GMP BC LSC (Supplementary Fig. 1e-f) suggesting that BCL2 overexpression portends CML progression. In addition to the increased pro-survival BCL2 family gene expression detected by RNA Seq (Supplementary Fig. 1g), an apoptosis qRT-PCR array demonstrated that BC LSC harbored distinct expression patterns of pro-death BCL2 family genes as well as TP53 and TNF superfamily receptors, such as FAS and other components of the extrinsic apoptotic machinery compared with normal progenitors (Supplementary Table 2). To gain further insight into the role of survival regulators in BC transformation, RNASeq analysis was performed on FACS-purified CD34+CD38+Lin− normal, CP and BC samples (Supplementary Table 1). Both heat map (Fig. 1f) and unsupervised principal component (Fig. 1g) analyses revealed that survival-related gene expression distinguished BC LSC from CP as well as TKI-treated and normal progenitor samples. Together these data suggest that a distinct survival gene signature predicts LSC generation and BC transformation.
Quiescent BC LSC Engraft the Bone Marrow Niche and are TKI Resistant
Previous research demonstrated a link between BCL2 family member expression and arrest of cells in G0/G1 of the cell cycle (Zinkel et al., 2006) (Vairo et al., 1996). In T and B cells of BCL2 transgenic mice, higher BCL2 expression correlated with a higher G0/G1 fraction, lower S phase fraction and decreased BrdU incorporation (O'Reilly et al., 1997a; O'Reilly et al., 1997b; O'Reilly et al., 1996). Moreover, enforced BCL2 expression was recently shown to restore quiescence of progenitors in a mouse model of myelodysplastic syndrome (Slape et al., 2012). Seminal studies also show that quiescent LSC are TKI resistant (Barnes and Melo, 2006; Bewry et al., 2008; Holyoake et al., 1999; Saito et al., 2010).
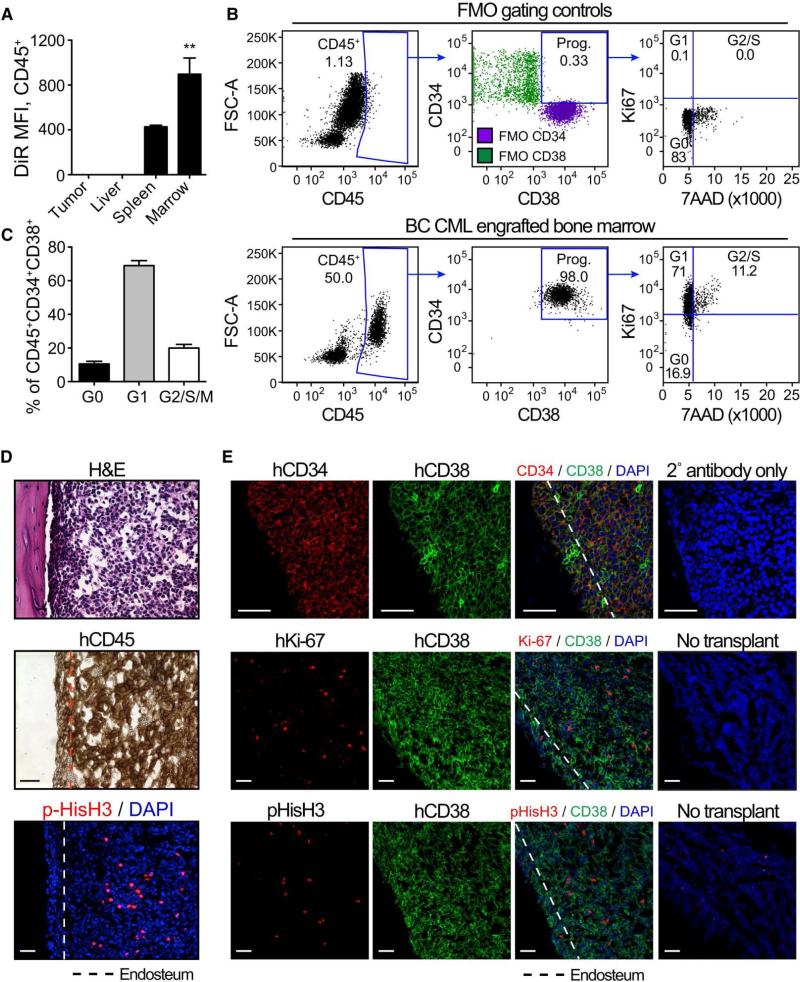
To analyze the capacity of various hematopoietic niches to maintain dormant LSC, human BC CD34+ cells, labeled with a membrane-bound fluorescent dye, DiR, which is retained by non-dividing cells, were transplanted into neonatal RAG2−/−γ −/−c mice (Abrahamsson et al., 2009). Within 10 weeks, transplanted mice developed BC CML typified by myeloid sarcoma formation as well as robust liver, spleen, blood and bone marrow engraftment (Supplementary Fig. 2 and Supplementary Table 3). Notably, FACS analysis revealed that marrow-engrafted BC LSC harbored higher levels of DiR fluorescence than those in other niches (Fig. 2a), corresponding to a distinct population of G0 (Ki67low7-AADlow) progenitors (Fig. 2b-c) in the marrow. Confocal fluorescence microscopic and immunohistochemical analyses revealed dormant pHis-H3−Ki-67low human CD45+CD34+CD38+ cells adjacent to the marrow endosteal region (Fig. 2d-e and Supplementary Fig. 3a-b), as previously reported in AML LSC xenograft models (Saito et al., 2010). Moreover, FACS analysis revealed that CD34+CD38+CD123+CD45RA+Lin− (granulocyte-macrophage progenitor; GMP) BC LSC, previously shown to harbor the greatest serial transplantation potential, were more prevalent in the marrow than other hematopoietic niches (Supplementary Fig. 3c). In addition, cell cycle FACS analysis revealed that proportion of quiescent BC LSC was enriched in the marrow compared to the splenic niche (Supplementary Fig. 3d-e).
Figure 2.
see also Figure S2 and S3 and Table S3
a) Retained DiR fluorescence of BC progenitors (CD45+CD34+CD38+LIN−) engrafted in tumor (n=4), liver (n=4), spleen (n=3), and bone marrow (n=2) 18 weeks after DiR surface staining and transplant. Graph shows mean +/− SEM; *** p<0.001 by ANOVA and Tukey post-hoc analysis. b) Representative FACS plots showing gating and cell cycle analysis of live (PI−), bone marrow-engrafted BC progenitors (CD45+CD34+CD38+LIN−). FMO gating controls are shown in the top row, while engrafted bone marrow is shown in the bottom row. c) Quantification of BC progenitors in untreated marrow in the different phases of the cell cycle. N=10 engrafted bones. Graph shows mean +/− SEM. d-e) Histological analysis of engrafted bone marrow showing H&E, human CD34, CD38 and Ki67, and pHis-H3 staining. The dotted lines delineate the endosteum (~50μm from the bone edge). All scale bars equal 50μm.
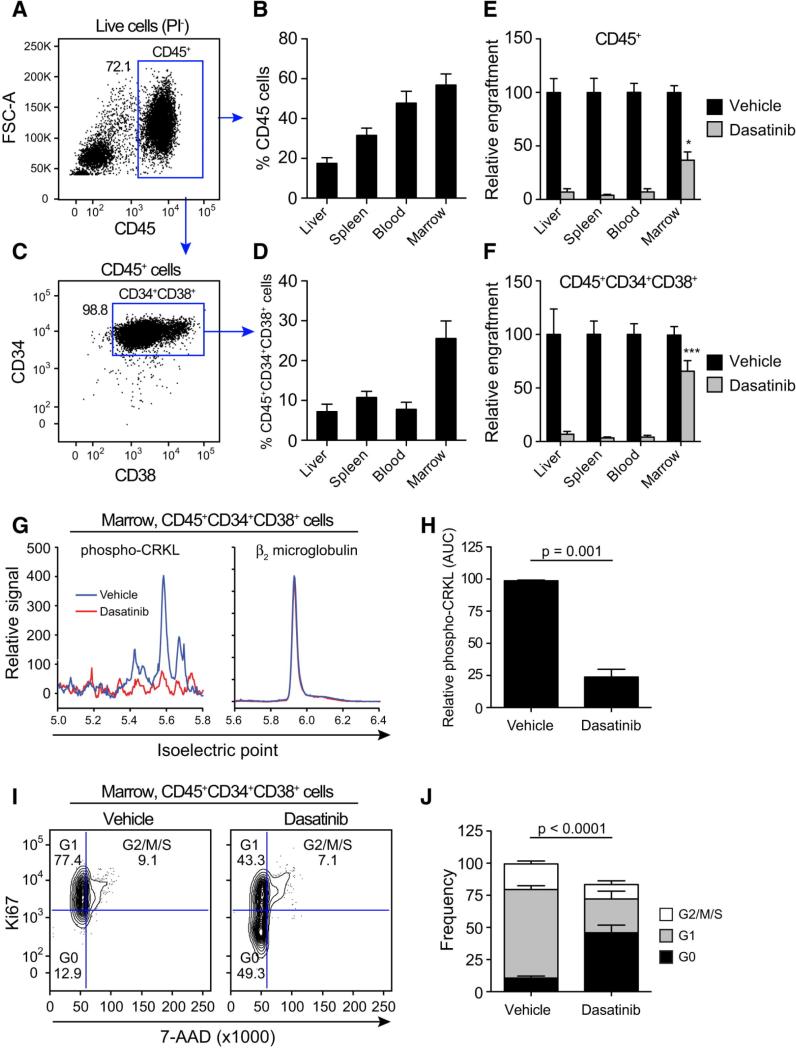
To examine the capacity of TKIs to eliminate quiescent self-renewing BC LSC, RAG2−/−γc−/− mice were transplanted with human BC CD34+ cells and treated orally with dasatinib, a potent BCR-ABL-targeted TKI (Supplementary Fig. 4a). Transplantation resulted in robust engraftment of human CD45+ (Fig. 3a-b and Supplementary Fig. 2a-b) and BC LSC (CD34+CD38+Lin−) cells in medullary and extramedullary microenvironments (Fig. 3b-d and Supplementary Fig. 3). Although dasatinib treatment (50 mg/kg) significantly reduced the CD45+ leukemic burden compared with vehicle treated controls (Fig. 3e and Supplementary Fig. 4b-d), a dasatinib-resistant BC LSC population persisted in the marrow (Fig. 3c, 3f and Supplementary Fig. 4e). Following dasatinib treatment, nanoproteomic analysis of FACS-purified marrow-derived BC LSC revealed a significant reduction in phosphorylation of CRKL, a direct substrate of the BCR-ABL kinase (Fig. 3g-h) (Goldman and Brender, 2000), indicative of adequate BCR-ABL kinase inhibition. However, cell cycle FACS analysis demonstrated an increase in quiescence (Fig. 3i-j) suggesting that quiescent BC LSC are resistant to BCR-ABL kinase inhibition and enriched in the marrow niche thereby providing a reservoir for relapse.
Figure 3.
see also Figure S4
a) Representative FACS plots (Live cells PI−) showing human CD45 engraftment in mouse bone marrow. Percentages shown are based on parental population as indicated. b) Total human CD45 engraftment in liver, spleen, blood, and bone marrow of BC transplanted mice (n=31) for 3 different CML BC patients samples. Graphs show mean +/− SEM. c) Representative FACS plots showing human BC progenitors (CD45+CD34+CD38+LIN−) engraftment in mouse bone marrow. d) Total engraftment of progenitors in the hematopoietic organs of BC transplanted mice (n=31) for 3 different CML BC patients samples. Values are a back gated calculation, based on the percentage of CD45+ cells = (% of CD45+ cells) x (% of CD34+CD38+ cells). Graphs show mean +/− SEM. e) Relative engraftment (% of vehicle treated controls) of human CD45 cells in the hematopoietic organs following treatment with vehicle (n=13) or dasatinib (50mg/kg, n=14). Statistical analysis is shown comparing the residual engraftment in each tissue (grey bars) and depicts the results of ANOVA with Tukey post-hoc comparisons; * p<0.05. Graphs show mean +/− SEM. f) Relative engraftment (% of vehicle treated controls) of progenitors in the hematopoietic organs following treatment with vehicle (n=19) or dasatinib (n=19). Statistical analysis is shown comparing the residual engraftment in each tissue (grey bars) and depicts the results of ANOVA with Tukey post-hoc comparisons; *** p<0.001. Graphs show mean +/− SEM. g) Representative proteomics plots showing analysis of phosphorylated-CRKL (left) and β2 microglobulin (right) in BC progenitors sorted from engrafted mouse bone marrow and after treatment with either vehicle or dasatinib. h) Quantification of total area under the curve (AUC) of phosphorylated-CRKL peaks in vehicle (n=5) and dasatinib (n=5) treated samples. All values are normalized β2 microglobulin protein expression in the same sample. Graphs show mean +/− SEM. i) Representative FACS-cell cycle plots of live, CD45+ BC progenitors engrafted in marrow following vehicle and dasatinib treatment. j) Cell cycle status of live, CD45+ BC progenitors sorted from the marrow and following treatment with vehicle (n=10) or dasatinib (n=10). All graphs in figure 3 show mean +/− SEM. All statistical analyses are by unpaired t-test.
Marrow Niche Engrafted BC LSC have a Pro-survival Gene Signature
Because BCL2 overexpression has been linked, in mouse transgenic models and cell lines, to apoptosis and TKI-resistance (Amarante-Mendes et al., 1998; Domen and Weissman, 2003; Konopleva et al., 2002), we hypothesized that pro-survival BCL2 family gene expression is enhanced in marrow engrafted BC LSC and that they harbor greater TKI resistance than those in other niches. Comparative apoptosis qRT-PCR array analysis performed on FACS-purified CD45+CD34+CD38+Lin− cells revealed that while BCLX, BFL1 and BCLW were not differentially expressed, BCL2 was significantly upregulated in marrow compared with spleen (Fig. 4a-b) as was the expression of the pro-survival isoforms of MCL1 and BFL1 (Supplementary Fig. 5a-b), thereby favoring BC LSC survival. Similarly, RNA Seq revealed increased BCL2 and decreased BIM expression in marrow-engrafted BC LSC compared to BC LSC before transplantation (Supplementary Fig. 5c). To further support these findings, gene set enrichment analysis (GSEA) of RNA Seq data demonstrated that cell cycle checkpoint and cell cycle arrest genes were upregulated in FACS purified BC LSC compared with their normal counterparts (Supplementary Figure 5d). Finally, BCL2 protein expression was significantly higher in marrow engrafted BC LSC than in non-LSC (human CD45+CD34− cells) in the same niche, and correlated with a decreased sensitivity to dasatinib treatment (Supplementary Fig. 5e-f). Thus, marrow niche-resident BC LSC express high levels of pro-survival BCL2 family gene isoform expression leading to enhanced TKI resistance.
Figure 4.
see also Figure S5
a) BCL2 family RT2 Profiler PCR Array System data of FACS-sorted progenitors from engrafted mice (n=3). The graph depicts fold expression in marrow-engrafted progenitors relative to spleen-engrafted progenitors, which are set at 1. Graphs show mean +/− SEM. p<0.05 by unpaired t-test b) BCL2L mRNA isoform expression and BCL2 protein expression in marrow versus spleen-engrafted BC progenitors. c) Representative immunohistochemical analysis of gross (top) and endosteal (bottom) engraftment of human CD34+, BCL2+ and MCL1+ cells in mouse bone marrow. Scale bars equal 1mm in low-magnification images and 100μm in high-magnification images. d) Representative images showing immunofluorescence analysis of hCD38+ and hBCL2+ cells in BC CML engrafted marrow compared to no-transplant control marrow. All scale bars equal 50μm. The boxed area shows a close-up of the endosteal region.
Both immunohistochemical and confocal fluorescence microscopic analysis demonstrated that human BCL2 and MCL1 protein expression (Fig. 4c) co-localized with human CD34 and CD38 expressing cells in the marrow endosteal niche (Fig. 4d). Interestingly, BCL2 and MCL1 expressing human BC CD34+ cells were enriched in the femoral epiphysis, a preferential site for homing, proliferation and survival of human leukemia cells following xenotransplantation (Supplementary Fig. 5g) (Ninomiya et al., 2007). Dasatinib treatment increased BCL2 and MCL1 expression and reduced Ki67 (Supplementary Fig. 5h-i), consistent with FACS analyses showing an increase in the proportion of quiescent BC LSC following TKI treatment (Fig. 3i-j). Although TKIs effectively eliminate LSC in extramedullary microenvironments, they fail to eradicate quiescent, BCL2- and MCL1-expressing BC LSC from the marrow niche.
Sabutoclax Inhibits BC LSC Survival
Detection of increased pro-survival BCL2 isoforms in primary BC samples together with enhanced BCL2 and MCL1 expression in marrow-engrafted BC LSC, particularly following dasatinib treatment (Supplementary Fig. 4), provided the impetus for testing the LSC inhibitory capacity of an optically pure novel derivative of apogossypol, sabutoclax, which inhibits all pro-survival BCL2 family proteins (Wei et al., 2009; Wei et al., 2010) (Fig. 5a). Sabutoclax treatment increased apoptosis of BC LSC in a dose-dependent manner in vitro, as measured by cleaved capase-3 and propidium iodide staining (Fig. 5b). Because BC LSC were TKI-resistant in the marrow niche, the anti-LSC efficacy of sabutoclax was tested in a genetically engineered SL/M2 stromal co-culture system that secretes human SCF, IL-3 and G-CSF and supports long-term survival of self-renewing BC LSC (Hogge et al., 1996) (Supplementary Fig. 6a). Despite induction of pro-survival BCL2 family gene expression in BC LSC supportive stromal co-cultures (Supplementary Fig. 6b), sabutoclax reduced LSC survival and LSC colony forming capacity (Fig. 5c-d and Supplementary Fig. 6a) at doses that spared normal progenitors (Fig. 5c-d and Supplementary Table 4). Moreover, lentiviral-mediated short-hairpin RNA knockdown of BCL2 reduced colony-forming capacity of BC LSC but not normal progenitors (Fig. 5e-f). However, BCL2 knockdown did not completely abrogate BC LSC colony formation suggesting that inhibition of multiple BCL2 family proteins, including MCL1, is required to eradicate BC LSC in supportive niches.
Figure 5.
see also Figure S6 and Table S4 and S5
a) In vitro experimental design used in the present studies. b) FACS analysis of activated caspase-3+ CML progenitors following in vitro culture with BI-97C1 (sabutoclax). Graph shows mean +/− SEM from 3 independent experiments. c) FACS analysis of normal (blue) and BC (red) progenitors cultured on SL/M2 bone marrow stroma in the presence of sabutoclax. All values are normalized to vehicle treated control. Graph shows mean +/− SEM from 5 different normal and BC samples. d) Total colonies formed by normal (blue) and BC (red) progenitors following sabutcolax treatment. All values are normalized to vehicle treated control. Graph shows mean +/− SEM from 3 different normal and 3 BC samples. Panel c and d show best-fit lines and statistical comparisons by non-linear regression analysis. e) Left: Representative colonies from control or sh-BCL2 transduced progenitors. Right: qRT-PCR of BCL2L mRNA in normal or BC cells following transduction with control and sh-BCL2 lentivirus (n=3 colonies for each sample type). Graph shows mean +/− SEM. f) Number of colonies formed by FACS-sorted normal (blue) or BC (red) progenitors following transduction with control or sh-BCL2 lentivirus. Graph shows average colonies per well for 4 different normal and BC samples and statistical analysis by paired t-test.
To further assess the role of BCL2 in BC LSC survival, ABT-737, a potent BCL2 and BCLXL inhibitor, was utilized in parallel stromal co-culture experiments. Fluorescence polarization assays (FPA) demonstrated that sabutoclax and ABT-737 dissociate a BIM-peptide from BCL2 and BCLXL at nanomolar concentrations. However, only sabutoclax effectively displaces BIM from MCL1 and BFL1 (Supplementary Fig. 6c-d and Supplementary Table 4). Because ABT-737 resistance is associated with increased MCL1 and BFL1 expression (Vogler et al., 2009a; Yecies et al., 2010) and both qRT-PCR and transcriptome data showed that BC LSC express multiple BCL2 family members, including MCL1 and BFL1 (Supplementary Fig. 5a-b), the anti-LSC efficacy of sabutoclax and ABT-737 were compared. Sabutoclax reduced BC LSC survival more than ABT-737 at all doses tested in stromal co-cultures (Supplementary Fig. 6f-g and Supplementary Table 4), even though activity looked comparable in stroma-independent K562 cells (Supplementary Fig. 6h), thereby underscoring the importance of the niche in BCL2 family member induction. Hence, eradication of niche-dependent BC LSC is predicated on inhibition of multiple BCL2 family proteins, including MCL1 and BFL1.
Sabutoclax Sensitizes Marrow-niche Engrafted BC LSC to Dasatinib
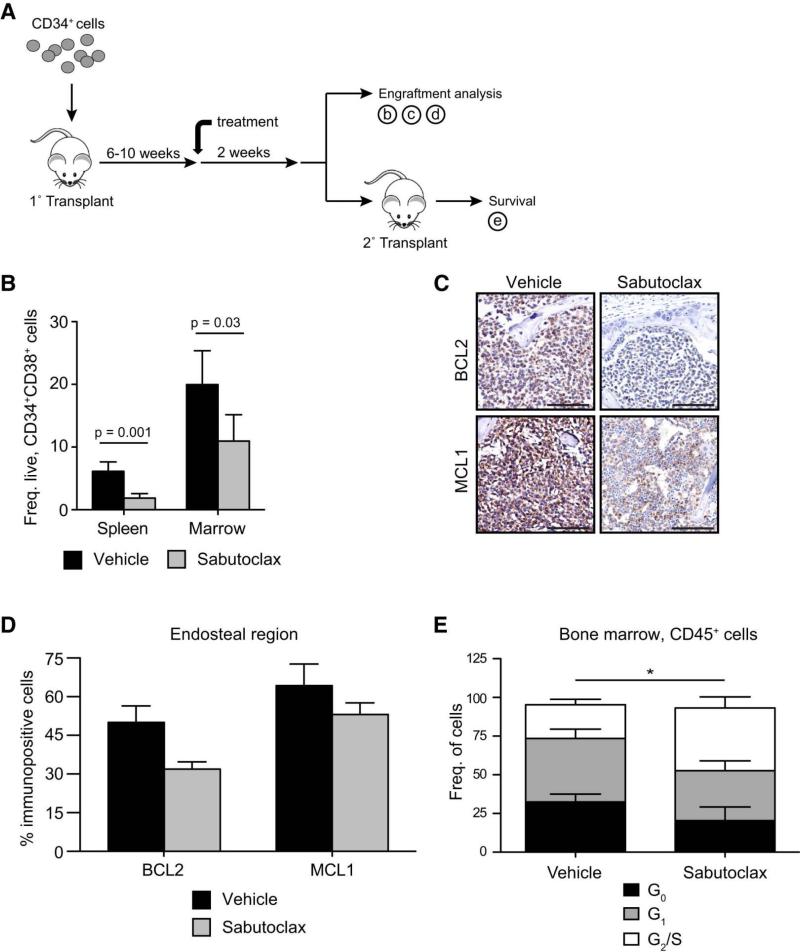
To examine the necessity of pro-survival BCL2 family expression for BC LSC maintenance, we tested the efficacy of sabutoclax at inhibiting BC LSC survival in the marrow compared with the splenic niche (Fig. 6a). In BC CD34+ cell engrafted mice, FACS analysis revealed that sabutoclax (5 mg/kg) reduced LSC burden (Fig. 6b and Supplementary Table 5) commensurate with a decrement in human BCL2 and MCL1 expressing cells in the marrow (Fig. 6c-d). Moreover, sabutoclax treatment increased G2/S (Fig. 6e) and TUNEL+ apoptotic cells (Fig. 7a), indicative of both cell cycle and apoptosis induction. Consistent with in vitro results, no significant reduction was observed in normal progenitor engraftment in the marrow following sabutoclax treatment (Supplementary Fig. 7b-d) suggesting that a reasonable therapeutic index exists between BC LSC and normal HSC.
Figure 6.
see also Figure S7 and Table S6
a) In vivo experimental design used in the present studies. b) Engraftment of BC progenitors in spleen and bone marrow following vehicle (n=27) and sabutoclax treatment (5mg/kg, n=26). Graph shows mean +/− SEM for 3 different BC patient samples and statistical analysis by Mann-Whitney test. c) Representative immunohistochemical analysis of BCL2 and MCL1 staining in engrafted bone marrow following vehicle and sabutoclax treatment. Scale bars equal 100μm. d) Quantification of BCL2+ and MCL1+ cells in the endosteum of engrafted bone marrow following vehicle (n=3) and sabutoclax (n=3) treatment. Graph shows mean +/− SEM. e) FACS-cell cycle analysis of CD45+ bone marrow engrafted BC cells in vehicle (n=6) and sabutoclax (n=5) treated mice. Graph shows mean +/− SEM. *p<0.05 by unpaired t-test.
Figure 7.
a) Representative TUNEL staining of BC-engrafted bone marrow following treatment with vehicle or sabutoclax. b) Relative engraftment of normal and BC progenitors in bone marrow following vehicle and sabutoclax treatment (5mg/kg). Normal: vehicle n=6, sabutoclax n=6. BC: vehicle n=26, sabutoclax n=27. Graph shows mean +/− SEM and statistical analysis by Mann-Whitney test. c) Fold change of normal stem cell in the bone marrow after treatment with Vehicle (n=8); Sabutoclax 10 mg/kg IV, biweekly for 4 doses (n=8); Dasatinib 50 mg/kg, daily oral gavage, 14 doses total (n=11); combination of Sabutoclax and Dasatinib (n=10). Graph shows mean +/− SEM. d) Fold change of normal stem cell in the spleen after treatment with Vehicle (n=8); Sabutoclax 10 mg/kg IV, biweekly for 4 doses (n=8); Dasatinib 50 mg/kg, daily oral gavage, 14 doses total (n=11); combination of Sabutoclax and Dasatinib (n=10). Graph shows mean +/− SEM. e) Relative engraftment of BC progenitors in spleen, blood and bone marrow following treatment with vehicle (n=9), sabutoclax (1.25mg/kg, n=9), dasatinib (25mg/kg, n=9) and sabutoclax in combination with dasatinib (n=11). Graph shows mean +/− SEM. * p<0.05, ** p<0.01, *** p<0.001 by Kruskal-Wallis test with Dunn's post-hoc analysis. f) Survival of mice following serial transplanted with vehicle-(n=11), sabutoclax- (n=9), dasatinib- (n=9), and combination- (n=13) treated whole bone marrow. Statistical analysis is by log-rank test.
To quantify the TKI-sensitizing effects of sabutoclax in the presence of human BC LSC-supportive cytokines not present in mouse marrow, human BC LSC from sabutoclax or vehicle treated mice were FACS-sorted into SL/M2 stromal co-cultures in the presence of dasatinib (Supplementary Fig. 7a). In this ex vivo assay, sabutoclax pre-treated progenitors were more sensitive to dasatinib than vehicle pre-treated controls (Supplementary Fig. 7b). To further examine the synergistic effects of sabutoclax and dasatinib, BC LSC-engrafted mice were treated with lower dose sabutoclax, dasatinib or the combination followed by FACS-mediated LSC analysis. While lower dose dasatinib and sabutoclax alone had no significant effect on marrow BC LSC engraftment, combination treatment significantly reduced marrow LSC survival (Fig. 7e and Supplementary Table 6). These results suggest that sabutoclax sensitizes quiescent, BCL2 and MCL1-expressing BC LSC to dasatinib-mediated cell death. Finally, the capacity of combined treatment to eradicate self-renewing BC LSC was assessed by transplanting treated marrow into secondary recipients and monitoring survival time. Mice transplanted with combination-treated marrow had a significant survival advantage compared to those that received dasatinib-treated marrow (Fig. 7f). Sabutoclax-mediated TKI sensitization was dose (Supplementary Table 6) and route of administration dependent, with greater bioavailability provided by intravenous dosing as shown by pharmacokinetic studies (Supplementary Fig. 7c). More clinically applicable intravenous dosing resulted in a significant reduction in BC LSC following combination sabutoclax and dasatinib (Supplementary Fig. 7d) at doses that spared normal hematopoietic progenitors (Fig. 7 c-d). Overall, our data demonstrate that dasatinib alone, while effective at reducing bulk leukemic cell burden, does not eradicate marrow niche-resident BC LSC. In contrast, combined dasatinib and sabutoclax therapy significantly inhibits both primary and serial LSC engraftment (graphical summary), indicative of abrogation of both TKI-resistance and BC LSC self-renewal.
Discussion
Malignant transformation of human myeloid progenitors into BC LSC through alternative splicing represents a novel molecular mechanism driving CML BC transformation and therapeutic resistance. By analyzing FACS-sorted serially transplantable CD34+CD38+lin− cells from primary patient samples, we show that BC LSC harbor increased expression of multiple pro-survival BCL2 family genes compared to both CP and normal progenitors. This pro-survival gene expression is further upregulated upon co-culture with human LSC supportive cytokine-secreting bone marrow stroma and upon engraftment in the bone marrow niche. These data are consistent with previous reports demonstrating increased BCL2 family expression in CML cells (Aichberger et al., 2005; Horita et al., 2000; Sanchez-Garcia and Grutz, 1995) and upregulation via niche-dependent signals (Bewry et al., 2008). However, our study is unique in that we show pro survival-BCL2 family splice isoform upregulation in self-renewing BC LSC and that niche-dependent BCL2 family expression is associated with TKI resistance in vivo. This study represents the first whole transcriptome and splice isoform specific qRT-PCR-based elucidation of isoform-specific BCL2 family gene expression signatures in CML LSC, which is important given that the BCL2 family is spliced into variants with antithetical functions (Akgul et al., 2004; Bingle et al., 2000) and has potential clinical significance with regard to predicting leukemic progression.
In a robust RAG2−/−γc−/− xenograft model of human BC CML, we demonstrate that BC LSC are protected from TKI-mediated cell death when engrafted in the marrow microenvironment compared with extramedullary hematopoietic niches suggesting that LSC are subject to marrow-specific cytoprotection (graphical summary) independent of BCR-ABL as demonstrated by nanoproteomic phospho-CRKL analysis. Although dasatinib treatment effectively reduces leukemic burden in engrafted mice, it does not fully eliminate BC LSC as evidenced by the fact that mice serially transplanted with dasatinib-treated bone marrow quickly develop BC CML. These data add to previous findings that CML BC LSC also depend on BCR-ABL-independent survival mechanisms (Corbin et al., 2011). Our findings expand on this concept by identifying pro-survival BCL2 family isoform expression as an important niche-specific survival mechanism and molecular target for CML BC LSC sensitization to TKI therapy. While lentiviral BCR-ABL transduction experiments suggest that BCLXL expression is BCR-ABL dependent, our in vivo studies suggest that marrow microenvironmental cues promote splice isoform switching that favors expression of multiple pro-survival BCL2 family splice isoforms in BC LSC thereby providing the impetus for elucidating these extrinsic factors in future studies.
Both cell cycle and immunofluorescence analysis, demonstrate that quiescent CML BC LSC engraft the marrow niche and are enriched in the endosteal region, consistent with previous AML xenograft studies (Saito et al., 2010). Moreover, immunohistochemical analyses show that endosteal niche resident BC LSC express pro-survival BCL2 and MCL1. Strikingly, dasatinib treatment does not eliminate quiescent bone marrow BC LSC. These quiescent BC LSC harbor enhanced engraftment potential (Barnes and Melo, 2006), which may explain why mice serially transplanted with dasatinib-treated marrow still develop BC CML.
Notably, BC LSC in stromal co-culture and in the marrow are sensitive to a novel pan-BCL2 inhibitor, sabutoclax, in a dose dependent manner (Wei et al., 2009; Wei et al., 2010). Sabutoclax also sensitizes marrow-niche BC LSC to TKI treatment suggesting that marrow-specific TKI protection is predicated, at least in part, on BCL2 family expression in the niche and can be overcome with a pan-inhibitor. Also, unlike dasatinib, sabutoclax targets quiescent self-renewing LSC. This is further evidenced by our observation that sabutoclax combined with dasatinib significantly improves survival of serially transplanted mice.
While BCL2 inhibition has been previously explored in CML, most studies have focused on CML cell lines (Kuroda et al., 2006; Meng et al., 2007) or CD34+ cells grown in culture (Mak et al., 2011) rather than self-renewing CML BC LSC in selective niches. Moreover, published reports do not address the potential antithetical roles of BCL2 family splice isoforms or the role of the microenvironment in promoting LSC survival. Treatment with ABT-737 (Kuroda et al., 2006; Mak et al., 2011), a potent BCL2 and BCLXL inhibitor, does not inhibit MCL1L or BFL1(Oltersdorf et al., 2005; Wei et al., 2010), which accelerate leukemogenesis (Beverly and Varmus, 2009), mediate resistance (Chen et al., 2007; Vogler et al., 2009a; Yecies et al., 2010) and are upregulated in CML progenitors during progression from CP to BC. Because inhibition of both subfamilies of pro-survival BCL2 family proteins is necessary for apoptosis initiation (Vogler et al., 2009b), inhibition strategies that include MCL1 would be expected to be more successful than those that target BCL2 alone (Placzek et al., 2010). Recently, paired-end DNA sequencing analysis revealed an intronic deletion polymorphism in the pro-apoptotic gene BIM (BCL2-like 11), which generated a splice isoform lacking the BH3 domain and preventing BIM-induced apoptosis in response to TKI therapy (Ng et al., 2012). Thus, pan-BCL2 inhibition may prove to be more effective at targeting TKI resistant BC LSC that naturally express multiple BCL2 family proteins in response to niche-dependent stimuli in vivo.
BCL2 family genes are regulated in a wide variety of hematologic malignancies (Beverly and Varmus, 2009; Reed, 2008) and solid tumors (Placzek et al., 2010). Moreover, CSC identified in several tumor types (Hermann et al., 2010), could conceivably rely on expression of multiple pro-survival BCL2 family isoforms, making them candidates for panBCL2 inhibition as a vital addition to combination CSC eradication therapy. Our findings may also have relevance for the elimination of therapeutically recalcitrant solid tumor CSC where metastasis and survival in the metastatic niche are mediated by pro-survival BCL2 family expression (Mehlen and Puisieux, 2006). Thus, panBCL2 inhibition with sabutoclax could provide an important new component of combination therapies that target a broad array of CSC residing in protective niches.
Experimental Procedures
Additional details and methods can be found in the supplementary experimental procedures section.
Patient sample preparation and FACS sorting
Normal cord blood and adult peripheral blood samples were purchased from All Cells. CML samples were obtained from consenting patients at the University of California San Diego, Stanford University, the University of Toronto Health Network, MD Anderson and the University of Bologna according to Institutional Review Board approved protocols. CD34+ cells were initially purified by magnetic bead separation (MACS; Miltenyi, Bergisch Gladbach, Germany) followed by FACS progenitor purification using human-specific CD34 and CD38 antibodies as previously described(Abrahamsson et al., 2009; Jamieson et al., 2004). Peripheral blood mononuclear cells (PBMC) were extracted from peripheral blood following Ficoll density centrifugation, CD34+ selected, stained with fluorescent conjugated antibodies, and analyzed and purified using a FACS Aria and Flowjo software as described previously(Abrahamsson et al., 2009; Jamieson et al., 2004).
BCL2 family gene splice isoform analysis
Normal or CML CD34+ cells were stained with mouse anti-human BCL2 (Dako) monoclonal antibody and analyzed by FACS. Quantitative RT-PCR to detect BCL2, MCL1, BCLX and BFL1 isoforms in FACS-sorted normal versus CML progenitors was performed with SYBR GreenER two-step qRT-PCR Kit (Invitrogen). Quantitative BCL2 isoform and apoptosis gene analysis was also performed in FACS-sorted normal and CML progenitors by whole transcriptome RNA sequencing.
BCL2 genes were also analyzed in engrafted CML cells. Briefly, 20,000-50,000 CD34+CD38+lin− cells were FACS-sorted from engrafted tissues and analyzed using isoform-specific qRT-PCR as above or using an RT-PCR apoptosis-pathway OpenArray “nanoplate” (Invitrogen). BCL2 protein was also measured in engrafted tissue cells as described above.
Quantitative RT-PCR
20,000-50,000 hematopoietic progenitor cells were sorted from the indicated cell populations using FACS, total RNA was isolated and cDNA was synthesized as described previously(Abrahamsson et al., 2009; Jamieson et al., 2004). Quantitative PCR (qRT-PCR) was performed in duplicate on an iCycler using SYBR GreenER Super Mix (Invitrogen, Carlsbad, California), 5ng of template mRNA, and 0.4mM of each forward and reverse primer. Splice isoform-specific primers were designed for BCL2, MCL1, BCLX, and BFL1 and isoform specificity was confirmed by sequencing of each PCR product. mRNA levels for each transcript were normalized to HPRT and compared using the delta-delta CT method.
SL/M2 co-culture and in vitro drug treatment
Normal or CML CD34+ cells were selected and plated on confluent, mitomycin-C treated SL/M2 cells along with different doses of BI-97C1 (sabutoclax). After 1 week of culture, human progenitor cells were quantified by FACS and cells were plated in methylcellulose for colony forming assays. Colonies were scored after 2 additional weeks in culture.
Lentivirus transduction
BCL2 mRNA expression was silenced using shBCL2 encoding SMARTVector 2.0 lentiviral particles (Thermo-Dharmacon, #SK-003307). Efficiency of shBCL2 and control lentiviral vectors was tested by transduction of 293T and K562 cell-lines. Knockdown of ~50% of BCL2 transcripts was confirmed by qRT-PCR. Cells transduced with lentiviral shBCL2 and shControl were FACS-sorted into Methocult media (20-50 cells per well of a 96-well plate, 5-10 wells per condition), total colonies were counted for each condition after 2 weeks of culture and BCL2 knockdown was measured in the colonies.
Statistical analysis
Statistical analyses were performed with the aid of Microsoft Excel, SAS version 9.2 and Graphpad Prism software as indicated in the figure legends.
Supplementary Material
Acknowledgements
We thank Dennis Carson for his continuous advice and mentorship, Dennis Young for expert assistance on all FACS experiments, Ida Deichaite for excellent assistance with material transfer and industry relations, Jennifer Black and Rusty Wall for their help with sample preparation and mouse experiments, Isabel Newton for advice and help with DiR studies, Derrick Duarte for assistance with IHC studies, Jerry Wu for assistance with FACS cell cycle experiments and Kimberly Wilson for assistance with grant and manuscript preparation and submission. This work was generously supported by the Ratner Family Fund and by California Institute for Regenerative Medicine (CIRM) grants (#RN2-00910-1; #RS1-00228-1; #TR2-01789; #DR1-01430). DJG was supported by the CIRM UCSD Stem Cell Training Grant II and the UCSD Cancer Training Grant. This work was also funded by the National Cancer Institute (NCI, #CA-55164) and National Institute of Health (NIH, #CA-149668), and supported by the Ontario Institute for Cancer Research, through generous support from the Ontario Ministry of Research and Innovation and the Cancer Stem Cell Consortium with funding from the Government of Canada through Genome Canada and the Ontario Genomics Institute (OGI-047), and through the Canadian Institute of Health Research (CSC-105367). Drs. Reed, Wei and Pellecchia are co-inventors on Sabutoclax and related compounds, licensed by the SBMRI to Oncothyreon (Seattle).
References
- Abe A, Minami Y, Hayakawa F, Kitamura K, Nomura Y, Murata M, Katsumi A, Kiyoi H, Jamieson CH, Wang JY, et al. Retention but significant reduction of BCR-ABL transcript in hematopoietic stem cells in chronic myelogenous leukemia after imatinib therapy. Int J Hematol. 2008;88:471–475. doi: 10.1007/s12185-008-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsson AE, Geron I, Gotlib J, Dao KH, Barroga CF, Newton IG, Giles FJ, Durocher J, Creusot RS, Karimi M, et al. Glycogen synthase kinase 3beta missplicing contributes to leukemia stem cell generation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3925–3929. doi: 10.1073/pnas.0900189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aichberger KJ, Mayerhofer M, Krauth MT, Skvara H, Florian S, Sonneck K, Akgul C, Derdak S, Pickl WF, Wacheck V, et al. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood. 2005;105:3303–3311. doi: 10.1182/blood-2004-02-0749. [DOI] [PubMed] [Google Scholar]
- Akgul C, Moulding DA, Edwards SW. Alternative splicing of Bcl-2-related genes: functional consequences and potential therapeutic applications. Cell Mol Life Sci. 2004;61:2189–2199. doi: 10.1007/s00018-004-4001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarante-Mendes GP, McGahon AJ, Nishioka WK, Afar DE, Witte ON, Green DR. Bcl-2-independent Bcr-Abl-mediated resistance to apoptosis: protection is correlated with up regulation of Bcl-xL. Oncogene. 1998;16:1383–1390. doi: 10.1038/sj.onc.1201664. [DOI] [PubMed] [Google Scholar]
- Barnes DJ, Melo JV. Primitive, quiescent and difficult to kill: the role of non-proliferating stem cells in chronic myeloid leukemia. Cell Cycle. 2006;5:2862–2866. doi: 10.4161/cc.5.24.3573. [DOI] [PubMed] [Google Scholar]
- Beverly LJ, Varmus HE. MYC-induced myeloid leukemogenesis is accelerated by all six members of the antiapoptotic BCL family. Oncogene. 2009;28:1274–1279. doi: 10.1038/onc.2008.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewry NN, Nair RR, Emmons MF, Boulware D, Pinilla-Ibarz J, Hazlehurst LA. Stat3 contributes to resistance toward BCR-ABL inhibitors in a bone marrow microenvironment model of drug resistance. Mol Cancer Ther. 2008;7:3169–3175. doi: 10.1158/1535-7163.MCT-08-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle CD, Craig RW, Swales BM, Singleton V, Zhou P, Whyte MK. Exon skipping in Mcl-1 results in a bcl-2 homology domain 3 only gene product that promotes cell death. The Journal of biological chemistry. 2000;275:22136–22146. doi: 10.1074/jbc.M909572199. [DOI] [PubMed] [Google Scholar]
- Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- Chomel JC, Bonnet ML, Sorel N, Bertrand A, Meunier MC, Fichelson S, Melkus M, Bennaceur-Griscelli A, Guilhot F, Turhan AG. Leukemic stem cell persistence in chronic myeloid leukemia patients with sustained undetectable molecular residual disease. Blood. 2011;118:3657–3660. doi: 10.1182/blood-2011-02-335497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel JC, Turhan AG. Chronic myeloid leukemia stem cells in the era of targeted therapies: resistance, persistence and long-term dormancy. Oncotarget. 2011;2:713–727. doi: 10.18632/oncotarget.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCRABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J, O'Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood. 2004;104:2204–2205. doi: 10.1182/blood-2004-04-1335. [DOI] [PubMed] [Google Scholar]
- Dai Y, Rahmani M, Corey SJ, Dent P, Grant S. A Bcr/Abl-independent, Lyn-dependent form of imatinib mesylate (STI-571) resistance is associated with altered expression of Bcl-2. The Journal of biological chemistry. 2004;279:34227–34239. doi: 10.1074/jbc.M402290200. [DOI] [PubMed] [Google Scholar]
- Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Deininger M. Resistance and relapse with imatinib in CML: causes and consequences. J Natl Compr Canc Netw 6 Suppl. 2008;2:S11–S21. [PubMed] [Google Scholar]
- Ding Q, He X, Xia W, Hsu JM, Chen CT, Li LY, Lee DF, Yang JY, Xie X, Liu JC, et al. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3beta activity and associates with poor prognosis in human breast cancer. Cancer Res. 2007;67:4564–4571. doi: 10.1158/0008-5472.CAN-06-1788. [DOI] [PubMed] [Google Scholar]
- Domen J, Weissman IL. Hematopoietic stem cells and other hematopoietic cells show broad resistance to chemotherapeutic agents in vivo when overexpressing bcl-2. Exp Hematol. 2003;31:631–639. doi: 10.1016/s0301-472x(03)00084-5. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, Metzeler KH, Poeppl A, Ling V, Beyene J, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nature medicine. 2011;17:1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- Forsberg EC, Passegue E, Prohaska SS, Wagers AJ, Koeva M, Stuart JM, Weissman IL. Molecular signatures of quiescent, mobilized and leukemia-initiating hematopoietic stem cells. PLoS One. 2010;5:e8785. doi: 10.1371/journal.pone.0008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesbert F, Griffin JD. Bcr/Abl activates transcription of the Bcl-X gene through STAT5. Blood. 2000;96:2269–2276. [PubMed] [Google Scholar]
- Goardon N, Marchi E, Atzberger A, Quek L, Schuh A, Soneji S, Woll P, Mead A, Alford KA, Rout R, et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer cell. 2011;19:138–152. doi: 10.1016/j.ccr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Goldman DA, Brender JD. Are standardized mortality ratios valid for public health data analysis? Statistics in medicine. 2000;19:1081–1088. doi: 10.1002/(sici)1097-0258(20000430)19:8<1081::aid-sim406>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, Jordan CT. Preferential induction of apoptosis for primary human leukemic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann PC, Bhaskar S, Cioffi M, Heeschen C. Cancer stem cells in solid tumors. Semin Cancer Biol. 2010;20:77–84. doi: 10.1016/j.semcancer.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Hogge DE, Lansdorp PM, Reid D, Gerhard B, Eaves CJ. Enhanced detection, maintenance, and differentiation of primitive human hematopoietic cells in cultures containing murine fibroblasts engineered to produce human steel factor, interleukin-3, and granulocyte colony-stimulating factor. Blood. 1996;88:3765–3773. [PubMed] [Google Scholar]
- Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- Horita M, Andreu EJ, Benito A, Arbona C, Sanz C, Benet I, Prosper F, Fernandez-Luna JL. Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. The Journal of experimental medicine. 2000;191:977–984. doi: 10.1084/jem.191.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Traver D, Miyamoto T, Akashi K, Lagasse E, Weissman IL. Expression of BCR/ABL and BCL-2 in myeloid progenitors leads to myeloid leukemias. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10002–10007. doi: 10.1073/pnas.1633833100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Karbasian Esfahani M, Morris EL, Dutcher JP, Wiernik PH. Blastic phase of chronic myelogenous leukemia. Curr Treat Options Oncol. 2006;7:189–199. doi: 10.1007/s11864-006-0012-y. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Zhao S, Hu W, Jiang S, Snell V, Weidner D, Jackson CE, Zhang X, Champlin R, Estey E, et al. The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol. 2002;118:521–534. doi: 10.1046/j.1365-2141.2002.03637.x. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC, Kimura S, Ottmann OG, Druker BJ, Villunger A, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14907–14912. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Mak DH, Wang RY, Schober WD, Konopleva M, Cortes J, Kantarjian H, Andreeff M, Carter BZ. Activation of apoptosis signaling eliminates CD34(+) progenitor cells in blast crisis CML independent of response to tyrosine kinase inhibitors. Leukemia. 2011 doi: 10.1038/leu.2011.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PJ, Najfeld V, Hansen JA, Penfold GK, Jacobson RJ, Fialkow PJ. Involvement of the B-lymphoid system in chronic myelogenous leukaemia. Nature. 1980;287:49–50. doi: 10.1038/287049a0. [DOI] [PubMed] [Google Scholar]
- McWeeney SK, Pemberton LC, Loriaux MM, Vartanian K, Willis SG, Yochum G, Wilmot B, Turpaz Y, Pillai R, Druker BJ, et al. A gene expression signature of CD34+ cells to predict major cytogenetic response in chronic phase chronic myeloid leukemia patients treated with imatinib. Blood. 2009 doi: 10.1182/blood-2009-03-210732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- Meng Y, Li Y, Li J, Li H, Fu J, Liu Y, Liu H, Chen X. (−)Gossypol and its combination with imatinib induce apoptosis in human chronic myeloid leukemic cells. Leuk Lymphoma. 2007;48:2204–2212. doi: 10.1080/10428190701583991. [DOI] [PubMed] [Google Scholar]
- Milyavsky M, Gan OI, Trottier M, Komosa M, Tabach O, Notta F, Lechman E, Hermans KG, Eppert K, Konovalova Z, et al. A distinctive DNA damage response in human hematopoietic stem cells reveals an apoptosis-independent role for p53 in self-renewal. Cell Stem Cell. 2010;7:186–197. doi: 10.1016/j.stem.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Wang Q, Kennedy CJ, Silver PA. An alternative splicing network links cell-cycle control to apoptosis. Cell. 2010;142:625–636. doi: 10.1016/j.cell.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KP, Hillmer AM, Chuah CT, Juan WC, Ko TK, Teo AS, Ariyaratne PN, Takahashi N, Sawada K, Fei Y, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nature medicine. 2012;18:521–528. doi: 10.1038/nm.2713. [DOI] [PubMed] [Google Scholar]
- Ninomiya M, Abe A, Katsumi A, Xu J, Ito M, Arai F, Suda T, Kiyoi H, Kinoshita T, Naoe T. Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia. 2007;21:136–142. doi: 10.1038/sj.leu.2404432. [DOI] [PubMed] [Google Scholar]
- O'Reilly LA, Harris AW, Strasser A. bcl-2 transgene expression promotes survival and reduces proliferation of CD3-CD4-CD8- T cell progenitors. International immunology. 1997a;9:1291–1301. doi: 10.1093/intimm/9.9.1291. [DOI] [PubMed] [Google Scholar]
- O'Reilly LA, Harris AW, Tarlinton DM, Corcoran LM, Strasser A. Expression of a bcl-2 transgene reduces proliferation and slows turnover of developing B lymphocytes in vivo. J Immunol. 1997b;159:2301–2311. [PubMed] [Google Scholar]
- O'Reilly LA, Huang DC, Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. The EMBO journal. 1996;15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Placzek WJ, Wei J, Kitada S, Zhai D, Reed JC, Pellecchia M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell death & disease. 2010;1:e40. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radich JP, Dai H, Mao M, Oehler V, Schelter J, Druker B, Sawyers C, Shah N, Stock W, Willman CL, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood. 2008;111:3322–3330. doi: 10.1182/blood-2007-09-078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, Najima Y, Takagi S, Aoki Y, Wake A, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010;28:275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Garcia I, Grutz G. Tumorigenic activity of the BCR-ABL oncogenes is mediated by BCL2. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5287–5291. doi: 10.1073/pnas.92.12.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, Schiffer CA, Talpaz M, Guilhot F, Deininger MW, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99:3530–3539. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- Slape CI, Saw J, Jowett JB, Aplan PD, Strasser A, Jane SM, Curtis DJ. Inhibition of apoptosis by BCL2 prevents leukemic transformation of a murine myelodysplastic syndrome. Blood. 2012 doi: 10.1182/blood-2012-05-430736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SA, Minami Y, Wang JY. The CML stem cell: evolution of the progenitor. Cell Cycle. 2009;8:1338–1343. doi: 10.4161/cc.8.9.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi T, Sumi M, Nakajima A, Sashida G, Shimamoto T, Ohyashiki K. BCL-2 antisense oligonucleotide genasense is active against imatinib-resistant BCR-ABL-positive cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:4267–4273. [PubMed] [Google Scholar]
- Vairo G, Innes KM, Adams JM. Bcl-2 has a cell cycle inhibitory function separable from its enhancement of cell survival. Oncogene. 1996;13:1511–1519. [PubMed] [Google Scholar]
- Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, Cohen GM. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009a;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell death and differentiation. 2009b;16:360–367. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- Wei J, Kitada S, Rega MF, Stebbins JL, Zhai D, Cellitti J, Yuan H, Emdadi A, Dahl R, Zhang Z, et al. Apogossypol derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2009;52:4511–4523. doi: 10.1021/jm900472s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Stebbins JL, Kitada S, Dash R, Placzek W, Rega MF, Wu B, Cellitti J, Zhai D, Yang L, et al. BI-97C1, an optically pure Apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2010;53:4166–4176. doi: 10.1021/jm1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, Sato Y, Sato-Otsubo A, Kon A, Nagasaki M, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009 doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell death and differentiation. 2006;13:1351–1359. doi: 10.1038/sj.cdd.4401987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.