Abstract
This work examined the chondrogenic potential of chondrocyte and mesenchymal stem cell (MSC) coculture generated poly(ɛ-caprolactone) (PCL)/extracellular matrix (ECM) hybrid scaffolds. Five different ratios of chondrocytes and MSCs were cocultured to generate cartilage-like ECM within electrospun fibrous scaffolds for 7, 14, and 21 days. These constructs were then devitalized to isolate the chondrogenic effects of the ECM alone. Devitalization was successful at removing cellular matter from the scaffolds, yet did reduce the amount of matrix present in the scaffolds. Following devitalization, the PCL/ECM scaffolds were then cultured with MSCs in serum-free conditions with or without TGF-β3 treatment for 21 days. TGF-β3 supplemented culture caused an induction of chondrogenesis in each scaffold type, but also somewhat masked the subtle differences of the different ECM coatings. Without TGF-β3, the cartilaginous matrix generated by 1:1 cocultures of chondrocytes to MSCs for 14 days supported similar chondrogenic gene expression patterns of MSCs cultured on scaffolds generated by chondrocytes alone. These scaffold formulations had a positive chondrogenic effect on aggrecan, collagen type II, and collagen II/I expression when compared to PCL controls. This study demonstrates that it is possible to utilize cocultures of chondrocytes and MSCs to coat a polymer scaffold with cartilage-like ECM capable of supporting chondrogenic differentiation of MSCs.
Introduction
Articular cartilage presents a particular challenge due to its limited capacity for intrinsic repair rendering it an appropriate target for regenerative therapies. Optimal tissue engineering scaffolds are designed to not only provide the structure to support tissue growth, but also the biological signals necessary to recruit and direct cells to actively promote tissue regeneration.1 The function of cartilage is highly dependent on the composition of its dense and abundant extracellular matrix (ECM). Various studies have shown that the prevalent cartilage ECM components, glycosaminoglycans (GAGs) and collagen can stimulate stem cell chondrogenic differentiation.2–4 Thus, it follows that a scaffold composed of cartilage-like ECM may be beneficial for the direction of chondrogenesis in stem cells. Previous approaches have investigated the use of scaffolds formed from isolated ECM components, such as collagen or hyaluronic acid, decellularized native tissue, as well as materials engineered specifically to release growth factors or gene delivery vectors as scaffolds for cartilage tissue engineering.5–14 Whereas scaffolds formed completely of ECM components have the benefit of incorporating ligands for cell–matrix signaling, natural proteins generally have insufficient mechanical strength and poorly controlled degradation rates hindering their capacity to provide structural support during tissue regeneration.15 This work presents a possible solution, which is to fabricate a hybrid scaffold composed of a synthetic polymer for structural support coated with ECM to provide the biological cues necessary to direct chondrogenesis and tissue regeneration.
Polymer/ECM composite scaffolds represent a relatively new direction in cartilage tissue engineering. An added benefit of generating polymer/ECM hybrid scaffolds using cell cultures is that in addition to ECM proteins, growth factors and other morphogens may also be deposited within the composite scaffold,16,17 and subsequently contribute to cartilage regeneration upon implantation in vivo. A previous study demonstrated that polymer/ECM composite scaffolds generated by perfused cultures of chondrocytes on electrospun poly(ɛ-caprolactone) (PCL) had the potential to direct the chondrogenesis of mesenchymal stem cells (MSCs) subsequently seeded on the constructs.18 Although chondrocytes produce ample amounts of cartilage-like ECM, their application as a primary cell source for cartilage tissue engineering strategies is complicated by their scarcity in native cartilage tissue, as well as their tendency to dedifferentiate upon in vitro expansion.19,20 The limitations of chondrocytes as a cell source have motivated recent studies on cocultures of chondrocytes with MSCs, which have been shown to be a viable option for reducing the number of chondrocytes necessary for producing cartilage-like ECM within a polymer scaffold.21–24
Using PCL/ECM constructs produced by cocultures of MSCs and chondrocytes recently described by our group,21 this study investigates the potential of decellularized PCL/ECM hybrid scaffolds with varying ECM maturities to direct chondrogenesis. Since our initial studies of these PCL/ECM composite scaffolds showed a culture duration-dependent increase in the ECM content,21 we hypothesized that a more mature ECM would result in scaffolds with a greater amount of cartilage-like ECM remaining after devitalization as compared to a less mature ECM. This was assessed by first devitalizing coculture generated PCL/ECM constructs using a freeze–thaw method, and then analyzing the resulting acellular PCL/ECM scaffolds for DNA and ECM contents as well as spatial distributions of ECM components.
After examining the effects of devitalization on the scaffold composition, we additionally hypothesized that cartilage-like ECM deposited on the PCL scaffolds, either by chondrocytes or cocultures of chondrocytes and MSCs, would be chondroinductive in nature. To test this hypothesis, following devitalization, PCL/ECM scaffolds were reseeded with MSCs and cultured in serum-free conditions with or without TGF-β3 supplements for 21 days. The gene expression of the MSCs cultured on the PCL/ECM scaffolds was then analyzed using real-time reverse transcription–polymerase chain reaction (rt-PCR) focusing on specific chondrogenic markers to determine the chondroinductive capacity of the various ECM formulations that had been deposited on the scaffolds.
Materials and Methods
Experimental design
This study utilized a full factorial design with three factors: (1) coculture ratio for PCL/ECM composite scaffold generation (with five levels), (2) ECM maturity (with three levels), and (3) presence of TGF-β3 (with two levels). As depicted schematically in Figure 1, PCL/ECM composite scaffolds were generated by coculturing chondrocytes and MSCs. Two of the three factors were varied during PCL/ECM scaffold production, specifically, the coculture ratio and ECM maturity. Five coculture ratios of chondrocytes to MSCs were used: 1:0, 1:1, 1:3, 1:5, and 0:1. The constructs were cultured for 7, 14, or 21 days, yielding PCL/ECM composite scaffolds containing ECM of varying maturities. The resulting 15 different scaffold formulations were devitalized, reseeded with fresh rabbit MSCs (as shown in Fig. 1), and cultured for 21 days either in the presence or absence of TGF-β3.
FIG. 1.
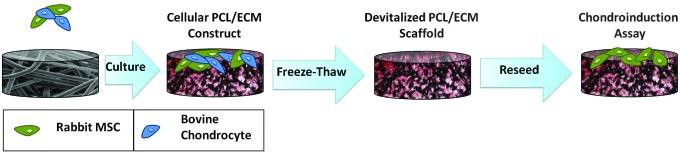
Experimental design schematic. Poly(ɛ-caprolactone) (PCL)/extracellular matrix (ECM) composite scaffolds were generated by coculturing chondrocytes and mesenchymal stem cells (MSCs). The PCL/ECM constructs were then devitalized by freeze–thaw, reseeded with fresh rabbit MSCs, and cultured for 21 days either in the presence or absence of TGF-β3.
Electrospinning
Nonwoven microfibrous PCL scaffolds (3 mm diameter discs) were die-cut from electrospun PCL meshes fabricated by electrospinning methods described previously.21 As in the previous study, scaffolds composed of fibers with an average diameter of 8.5 μm were used.
Cell isolation, expansion, and PCL/ECM construct generation
Bovine chondrocytes and rabbit MSCs were isolated and expanded using previously outlined methods.21 PCL/ECM constructs were then generated by coculturing chondrocytes and MSCs in five different ratios, at an initial total seeding density of 35,000 cells/scaffold, on electrospun PCL microfibrous scaffolds (thickness=1 mm; Ø=3 mm) for 7, 14, or 21 days of culture in the chondrocyte growth medium composed of the Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS), 10 mM HEPES buffer (Gibco, Grand Island, NY), 1% nonessential amino acids (Gibco), 0.28 mM ascorbic acid (Sigma, St Louis, MO), 0.4 mM l-proline (Sigma), and 1% penicillin–streptomycin–fungizone (Gibco) using established methods21 to achieve constructs with varying ECM maturities, as described in the Experimental Design section.
PCL/ECM construct devitalization
At the completion of all three predetermined culture durations, PCL/ECM constructs were rinsed with phosphate-buffered saline (PBS), then flash frozen in sterile ddH2O using liquid nitrogen for 10 min. Following freezing, constructs were subsequently thawed in a 37°C water bath for 10 min. This sequence of freeze/thaw was repeated three times. Following the third and final thaw stage, constructs were sonicated for 10 min and then rinsed with fresh sterile ddH2O to facilitate the removal of cellular debris. The resulting devitalized PCL/ECM (dPCL/ECM) scaffolds were then air-dried under laminar flow in a biosafety cabinet overnight. Following drying, the dPCL/ECM scaffolds were either prepared for biochemical and histological analysis or loaded into seeding cassettes, ethylene oxide sterilized, and stored at −20°C before characterization by the chondrogenic induction assay.
Biochemical assays
For each dPCL/ECM formulation at the three ECM maturities (n=4), two scaffolds were pooled in 500 μL of proteinase K solution consisting of 1 mg/mL proteinase K (Sigma), 185 μg/mL iodacetamide (Sigma), and 10 μg/mL pepstatin A (Sigma) dissolved in the aqueous Tris/EDTA buffer, which contained 6.055 mg/mL tris(hydroxymethyl aminomethane) (Sigma), and 372 μg/mL EDTA at pH 7.6. Samples were digested for 16 h at 56°C. The digested components of each biochemical sample were then further disrupted by three freeze/thaw/sonicate cycles (10 min each step). From the resulting proteinase K lysates, DNA, GAG, and collagen contents were quantified, respectively, using established PicoGreen, 1,9-dimethymethylene blue, and hydroxyproline assay protocols described previously.21
Histology
Histological staining was used to visualize the distribution of cells, GAGs, and collagen throughout the dPCL/ECM scaffolds. Days 7, 14, and 21 dPCL/ECM scaffolds (n=2) were prepared for histological analysis by formalin fixation, dehydration using an ascending ethanol gradient, and submersion in HistoPrep frozen tissue embedding media (Fisher, Fair Lawn, NJ), as detailed elsewhere.21 From each frozen block, 8-μm-thick sections were cut using a cryotome (CM1850; Leica Microsystems, Bannockburn, IL), mounted on glass Superfrost Plus microscope slides (VWR, Batavia, IL), and incubated at 45°C on a slide warmer for at least 7 days to ensure adhesion. Slides were subsequently stained with nuclear fast red, Safranin O, and Picrosirius red using previously described methods.21,25
Chondrogenic induction assay
The capacity of coculture generated dPCL/ECM scaffolds to induce chondrogenesis was evaluated by adapting an established cell culture-based chondroinductive assay.18 Plain PCL scaffolds without ECM were used as a control. The day before MSC seeding, untreated PCL scaffolds were prewet using a decreasing ethanol gradient (100%–35%), rinsed with PBS, and incubated in PBS overnight at 37°C.
Before cell seeding, dPCL/ECM scaffolds with three different ECM maturities (days 7, 14, and 21) were warmed from −20°C to ambient temperature. Next, plain PCL and dPCL/ECM scaffolds were incubated for 4 h with general media containing DMEM, 10% FBS, and 1% penicillin–streptomycin–fungizone (Gibco). All scaffolds had been press-fit into sterile polycarbonate seeding cassettes (inner diameter=2.94 mm, height=9.09 mm) and gas sterilized before this point. Passage 3 rabbit MSCs were then trypsinized and seeded onto the scaffolds at a density of 35,000 cells/scaffold. Seeded scaffolds were incubated at 37°C for a 2 h preattachment period, and then additional serum-free chondrogenic medium (DMEM, 1% ITS+premix; BD Biosciences, Bedford, MA, 10−7 M dexamethasone; Sigma, 50 mg/L ascorbic acid; Sigma, 1 mM sodium pyruvate; Sigma, and 1% penicillin–streptomycin–fungizone; Gibco) was added to the wells. The scaffolds were then cultured within the seeding cassettes for 24 h to allow for cell attachment.
After the 24-h attachment period, scaffolds were removed from the seeding cassettes using aseptic techniques and placed in ultra-low attachment 24-well plates (Costar, St Louis, MO). To ensure complete scaffold immersion within the small volume of culture media to be added to each well, scaffolds were pressed into sterile stainless steel rings ∼0.5 mm thick with a 2.9-mm inner diameter. Scaffolds were cultured with 650 μL of serum-free chondrogenic medium/well with or without 10 ng/mL TGF-β3 (PeproTech, Rocky Hill, NJ) with media changes every 3 days for 21 days. After 21 days, the scaffolds were rinsed with PBS and prepared for gene expression analysis by rt-PCR.
Real-time rt-PCR
Following cell culture, samples were prepared for rt-PCR by pooling two minced scaffolds in a 600 μL RLT lysis buffer (Qiagen, Valencia, CA) and storing at −20°C. Following lysis, the samples were first homogenized using a QIAshredder column (Qiagen). Subsequently, 600 μL of 70% ethanol was then added to the homogenized lysate. Total RNA was isolated and purified from the lysate using the RNeasy mini kit (Qiagen) using the manufacturer suggested protocol for isolation of RNA from animal cells. The protocol was modified slightly by adding two further RW1 washes (350 μL each; Qiagen) and an extra wash with 500 μL 70% ethanol after the final RPE rinse to improve the purity of the isolate, as described previously.18,26 Complementary DNA (cDNA) was then synthesized using 11 μL of the isolated RNA using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) together with Oligo(dT) primers (Promega, San Luis Obispo, CA). The synthesized cDNA was then analyzed by rt-PCR (7300 Real-Time PCR System; Applied Biosystems, Foster City, CA) with primers for collagen types I and II, aggrecan, and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primer sequences are listed in Table 1. Amplification and detection of the PCR product was achieved using Perfecta SYBR FastMix (Quanta BioSciences, Gaithersburg, MD). The results of rt-PCR were analyzed using the 2−ΔΔCt method.27 In this way, the presented results were first normalized to the housekeeping gene, GAPDH, and then converted to a fold change in the target gene expression over the initial expression of the reseeded MSCs at day 0. The ratio of collagen type II to I was calculated for each individual sample by dividing the collagen type II expression by the collagen type I expression for which the mean and standard deviations were calculated and presented herein.
Table 1.
Target Genes for rt-PCR Gene Expression Analysis and the Primer Sequences Utilized
| Target gene | Primer sequence |
|---|---|
| Aggrecan | 5′-GCTACGGAGACAAGGATGAGTTC-3′ |
| 5′-CGTAAAAGACCTCACCCTCCAT-3′ | |
| Collagen type I | 5′-CCCAGAATGGAGCAGTGGTTACT-3′ |
| 5′-AGCAGACGCATGAAGGCAAG-3′ | |
| Collagen type II | 5′-AACACTGCCAACGTCCAGAT-3′ |
| 5′-CTGCAGCACGGTATAGGTGA-3′ | |
| GAPDH | 5′-TCACCATCTTCCAGGAGCGA-3′ |
| 5′-CACAATGCCGAAGTGGTCGT-3′ |
rt-PCR, reverse transcription–polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
Biochemical assay results are displayed as the mean±standard deviation for (n=4) samples. For DNA, GAG, and collagen data, a three-way ANOVA was performed to determine the effects of coculture ratio, ECM maturity, and devitalization. If the ANOVA showed significant differences, then a Tukey's honestly significant difference test was utilized to perform multiple comparisons post hoc. To analyze rt-PCR data, the Kruskal–Wallis test was utilized followed by the Mann–Whitney U-test for multiple comparisons where appropriate. For statistical analyses, differences were considered significant if p<0.05.
Results
Cell and ECM distribution in devitalized constructs
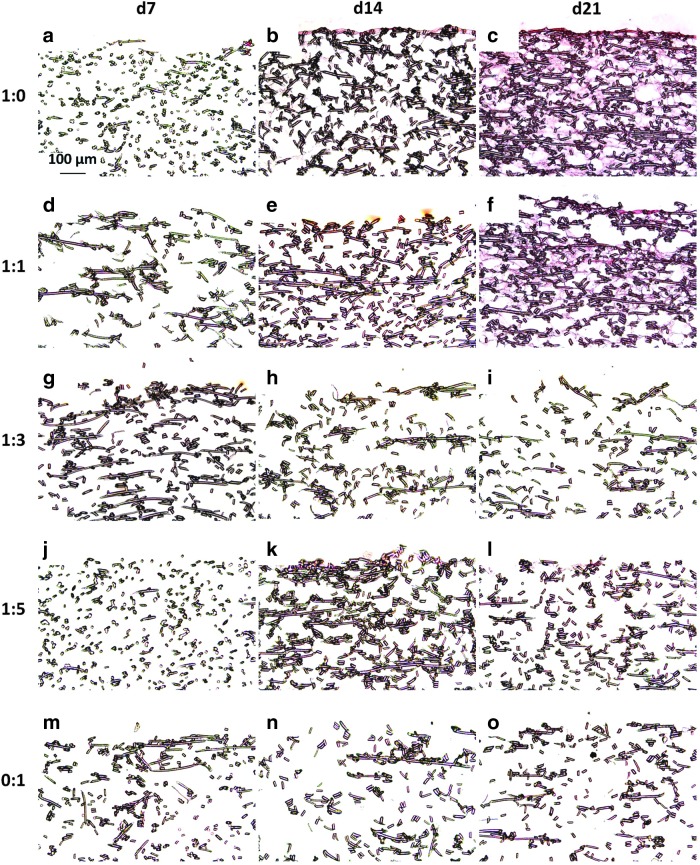
Safranin O histological staining to examine GAG localization is shown in Figure 2. The most intense GAG staining occurred in day 21 chondrocyte-only (1:0) generated scaffolds. GAG deposition shows a decreasing trend as the original concentration of chondrocytes decreased from 1:1 to 0:1. As shown in Figure 2o, there was limited staining for GAGs within MSC-only generated dPCL/ECM scaffolds. Comparison of these findings to previously presented Safranin O staining of cellular PCL/ECM scaffolds,21 which correspond to the dPCL/ECM scaffolds before devitalization, indicates that devitalization resulted in a marked reduction in the GAG content. Although dPCL/ECM scaffolds had reduced GAG content, GAG distribution was unaffected by devitalization and correlates to those previously observed in the cellular PCL/ECM constructs.21 Specifically, there was an increase in staining for GAGs with an increase in ECM maturity as determined by initial culture duration.
FIG. 2.
Safranin O stained sections of devitalized PCL/ECM (dPCL/ECM) scaffolds indicating the spatial arrangement of glycosaminoglycans (GAGs) within the acellular scaffolds. The images displayed here represent scaffolds generated with the following chondrocyte to MSC ratios: (a–c) 1:0, (d–f) 1:1, (g–i) 1:3, (j–l) 1:5, and (m–o) 0:1. ECM was deposited for (a, d, g, j, m) 7, (b, e, h, k, n) 14, or (c, f, i, l, o) 21 days before freeze–thaw devitalization. 10× magnification. Scale bar in (a) indicates 100 μm and applies to all images.
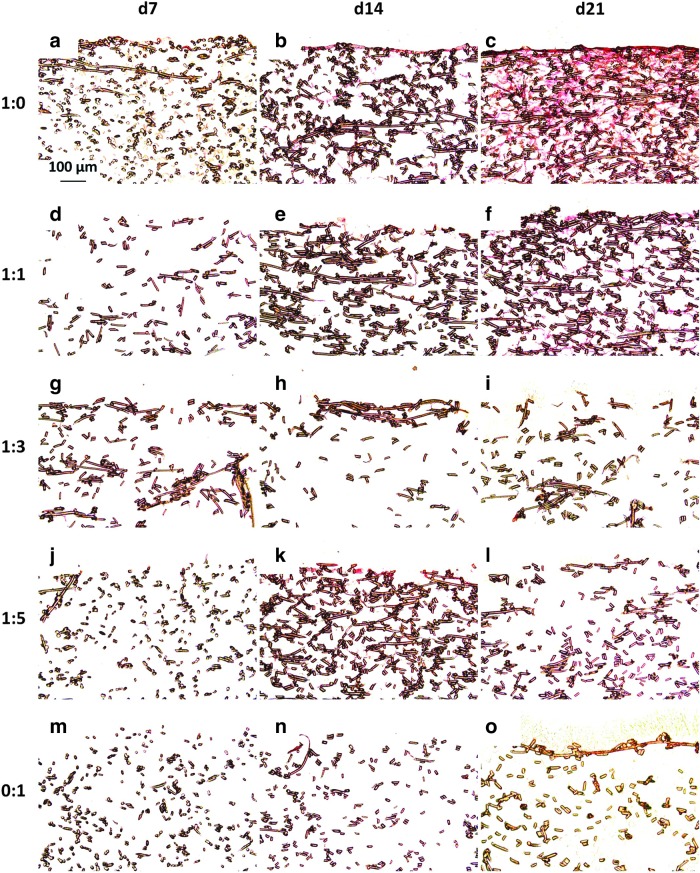
Picrosirius red staining on the dPCL/ECM scaffolds, shown in Figure 3, mimicked the intensity and localization patterns observed in the Safranin O stained sections in Figure 2. Furthermore, the staining patterns exhibited in Figure 3 mirror the staining localizations observed in the constructs before freeze–thaw,21 except that the dPCL/ECM scaffolds exhibited less intense staining for collagen than the PCL/ECM constructs before devitalization processing. Based on ECM localization as determined by staining for GAGs and collagen (Figs. 2 and 3), of the coculture generated groups, the 1:1 cocultured scaffolds most closely approximated the dPCL/ECM of the chondrocyte control group, 1:0.
FIG. 3.
Picrosirius red stained sections of dPCL/ECM scaffolds indicating the inclusion of collagen within the acellular scaffolds. The images displayed here represent scaffolds generated with the following chondrocyte to MSC ratios: (a–c) 1:0, (d–f) 1:1, (g–i) 1:3, (j–l) 1:5, and (m–o) 0:1. ECM was deposited for (a, d, g, j, m) 7, (b, e, h, k, n) 14, and (c, f, i, l, o) 21 days before freeze–thaw devitalization. 10× magnification. Scale bar in (a) indicates 100 μm and applies to all images.
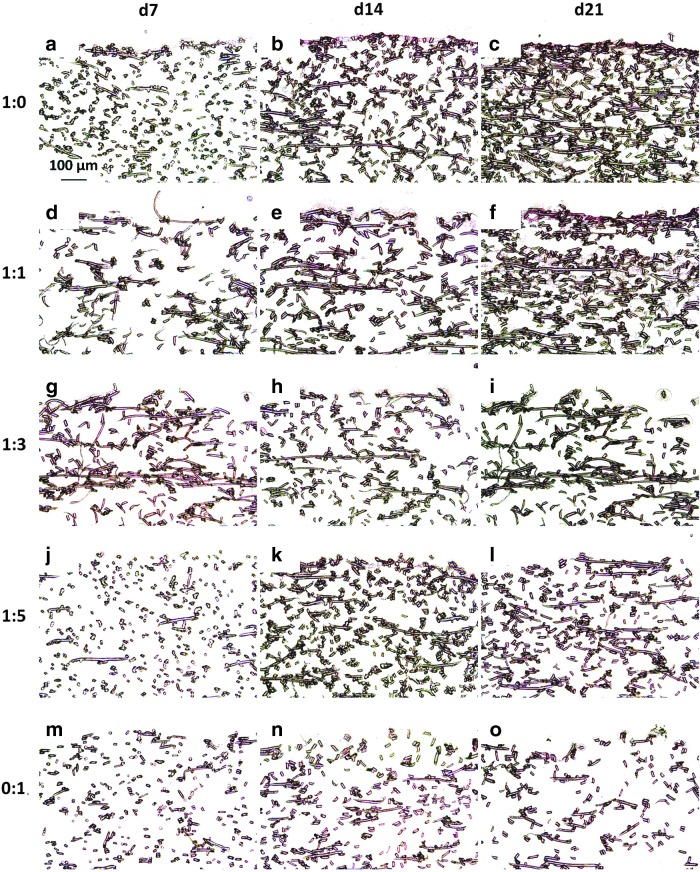
As shown in Figure 4, which depicts nuclear fast red staining of the dPCL/ECM scaffolds and depicts localization of cellular nuclei within the dPCL/ECM scaffolds, there was minimal nuclear fast red staining visible within the devitalized scaffolds. For the most part, any faint nuclear fast red stain was present near the surface of the scaffold, where Safranin O and Picrosirius red staining were most dense, indicating the presence of a dense coating of ECM. Compared to the cellular PCL/ECM constructs examined previously,21 there was significantly less staining for cellular components throughout all five dPCL/ECM scaffold groups at all three ECM maturity levels.
FIG. 4.
Nuclear fast red stained sections of dPCL/ECM scaffolds indicating the location of remaining cell nuclei within the devitalized scaffolds. The images displayed here represent scaffolds generated with the following chondrocyte to MSC ratios: (a–c) 1:0, (d–f) 1:1, (g–i) 1:3, (j–l) 1:5, and (m–o) 0:1. ECM was deposited for (a, d, g, j, m) 7, (b, e, h, k, n) 14, and (c, f, i, l, o) 21 days before freeze–thaw devitalization. 10× magnification. Scale bar in (a) indicates 100 μm and applies to all images.
DNA and ECM content of devitalized constructs
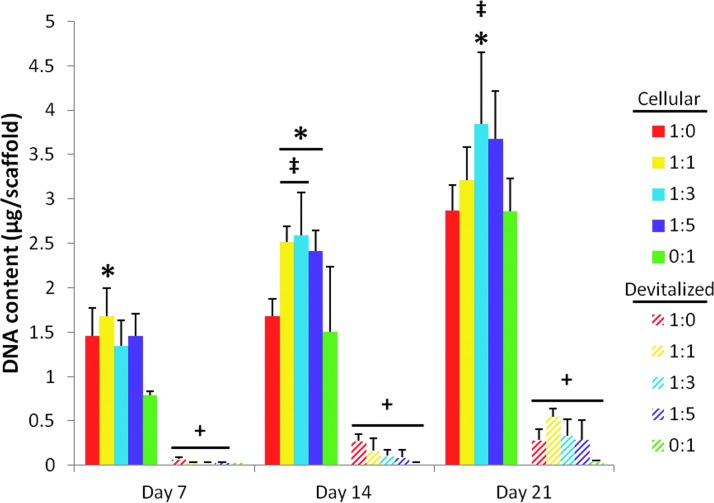
Figure 5 depicts the DNA content of the various PCL/ECM composite scaffolds before and following devitalization. Upon freeze–thaw, there was a significant decrease in the DNA content within the scaffolds for all cell ratios at each time point except 0:1 day 7 (Fig. 5, “+”). Large decreases in the scaffold DNA content were observed regardless of the initial DNA content before freeze–thaw. This indicated that the freeze–thaw devitalization process was effective at decellularizing the scaffolds, resulting in a primarily acellular product. None of the devitalized scaffolds differed significantly in terms of DNA content for any of the three ECM maturities investigated.
FIG. 5.
DNA content of PCL/ECM constructs both before (cellular) and after (devitalized) devitalization. PCL/ECM scaffolds were generated for 7, 14, or 21 days of culture with five different chondrocyte to MSC ratios. The DNA content for cellular PCL/ECM constructs, which is included here for comparison to determine the effect of devitalization, originally appeared as data represented by the 35,000-cells/scaffold seeding density in Figure 6 of Levorson et al.21 Data are presented as mean±standard deviation for (n=4). Statistical significance is indicated by the symbols defined in Table 2 (p<0.05).
Table 2.
Symbols Indicating Statistical Significance for Biochemical Data in Figures 5–7
| Symbol | Meaning |
|---|---|
| ‡ | Statistically significant difference (p<0.05) from culture ratio 1:0 at this particular time point. |
| * | Statistically significant difference (p<0.05) from culture ratio 0:1 at this time point. |
| + | Statistically significant difference (p<0.05) between cellular and acellular samples at this culture ratio and time point. |
| # | Statistically significant difference (p<0.05) between different coculture ratios within a cellularity state and time point. |
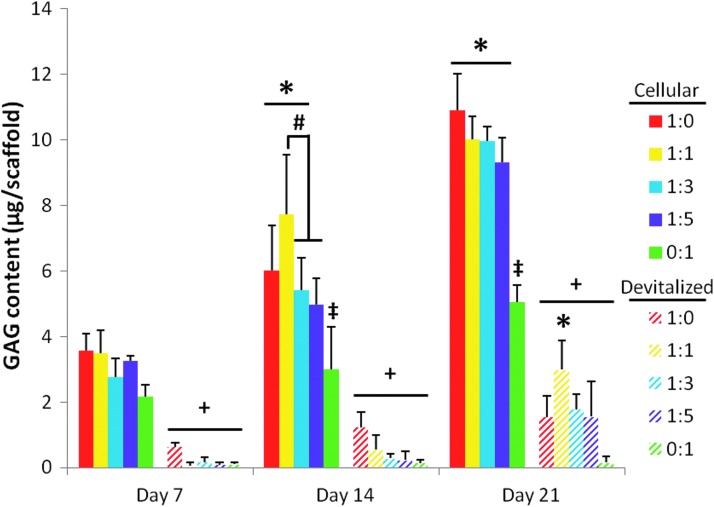
As was seen with DNA content within the scaffolds, upon freeze–thaw devitalization, the GAG content of the dPCL/ECM scaffolds decreased significantly for scaffolds generated by each coculture ratio as compared to the cellular PCL/ECM constructs (Fig. 6, “+”). This significant decrease in the GAG content following freeze–thaw processing was observed for all three of the ECM maturities investigated. Among the dPCL/ECM scaffolds, significant differences in GAG content were only observed for the most mature (day 21) ECM. Specifically, 1:1 d21 dPCL/ECM scaffolds contained significantly more GAG than MSC-only PCL/ECM controls (Fig. 6, “*”).
FIG. 6.
GAG content of PCL/ECM constructs both before (cellular) and after (devitalized) devitalization. PCL/ECM scaffolds were generated for 7, 14, or 21 days of culture with five different chondrocyte to MSC ratios. The GAG content for cellular PCL/ECM constructs, included here for comparison to determine the effect of devitalization, originally appeared as data represented by the 35,000-cells/scaffold seeding density in Figure 7 of Levorson et al.21 Data are presented as mean±standard deviation for (n=4). Statistical significance is indicated by the symbols defined in Table 2 (p<0.05).
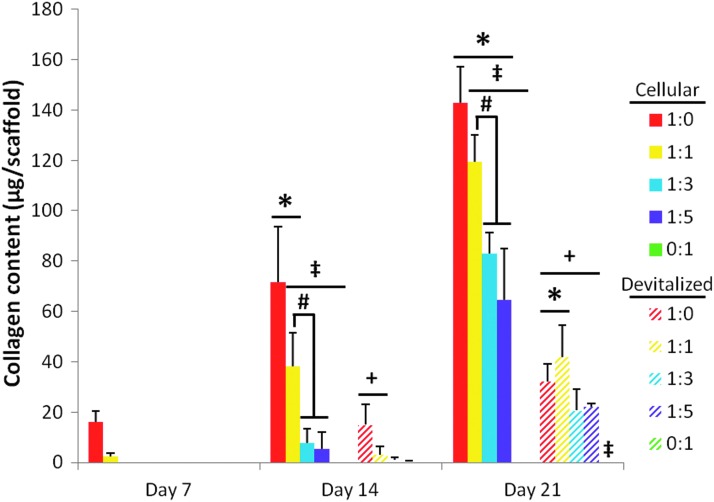
Figure 7 depicts the collagen content of PCL/ECM constructs both before and after devitalization. For the most mature ECM, developed for 21 days of culture, there was a significant decrease in collagen content observed upon devitalization of all four chondrocyte-containing groups (Fig. 7, “+”). Significant decreases in the collagen content after freeze–thaw were also observed for the day 14 1:0 and 1:1 ratio groups (Fig. 7, “+”). For the later ECM maturity level (day 21), dPCL/ECM scaffolds originally formed by cell ratios 1:0 and 1:1 resulted in higher collagen concentrations than scaffolds produced by MSCs alone (Fig. 7, “*”). The collagen contents of day 7 dPCL/ECM scaffolds and all 0:1 constructs were below the threshold of the assay. However, slight staining for Picrosirius red on samples from these groups (Fig. 3) indicated the presence of some collagen within these scaffolds, although minimal amounts.
FIG. 7.
Collagen content of PCL/ECM constructs both before (cellular) and after (devitalized) devitalization. PCL/ECM scaffolds were generated for 7, 14, or 21 days of culture with five different chondrocyte to MSC ratios. The DNA content for cellular PCL/ECM constructs, included here for comparison to determine the effect of devitalization, originally appeared as data represented by the 35,000-cells/scaffold seeding density in Figure 8 of Levorson et al.21 Data are presented as mean±standard deviation for (n=4). Statistical significance is indicated by the symbols defined in Table 2 (p<0.05).
Chondrogenic induction potential of PCL/ECM
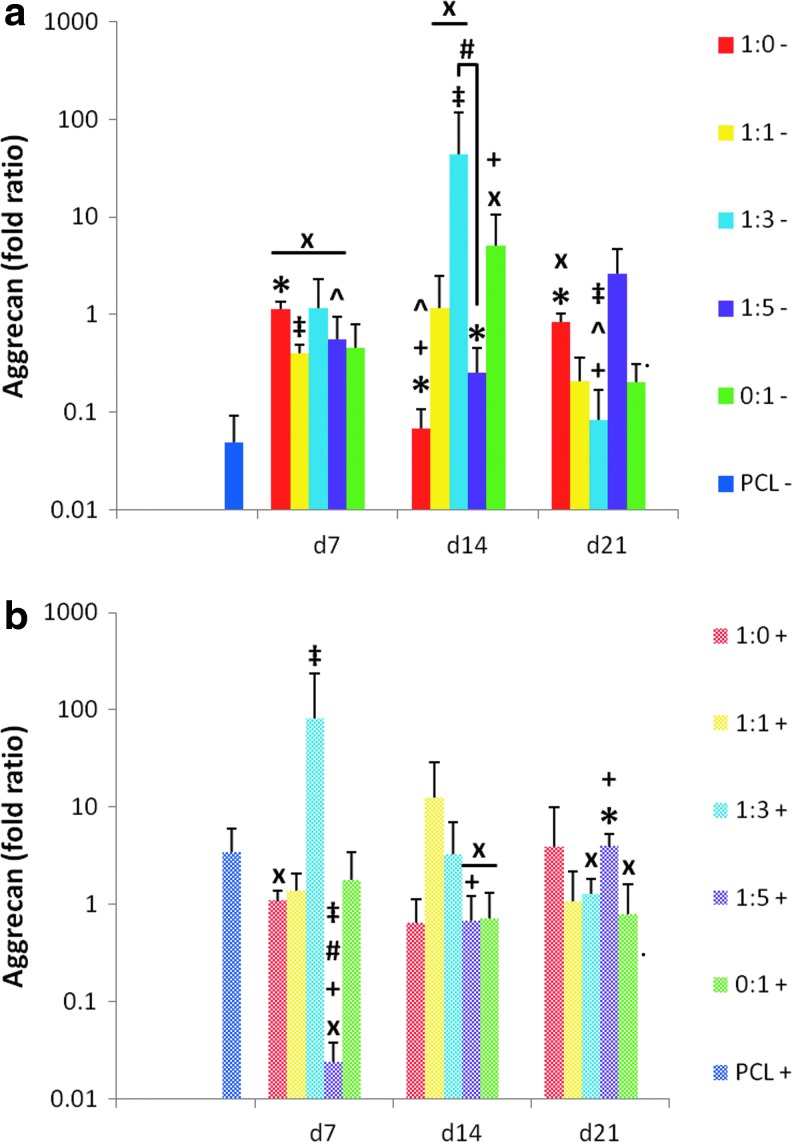
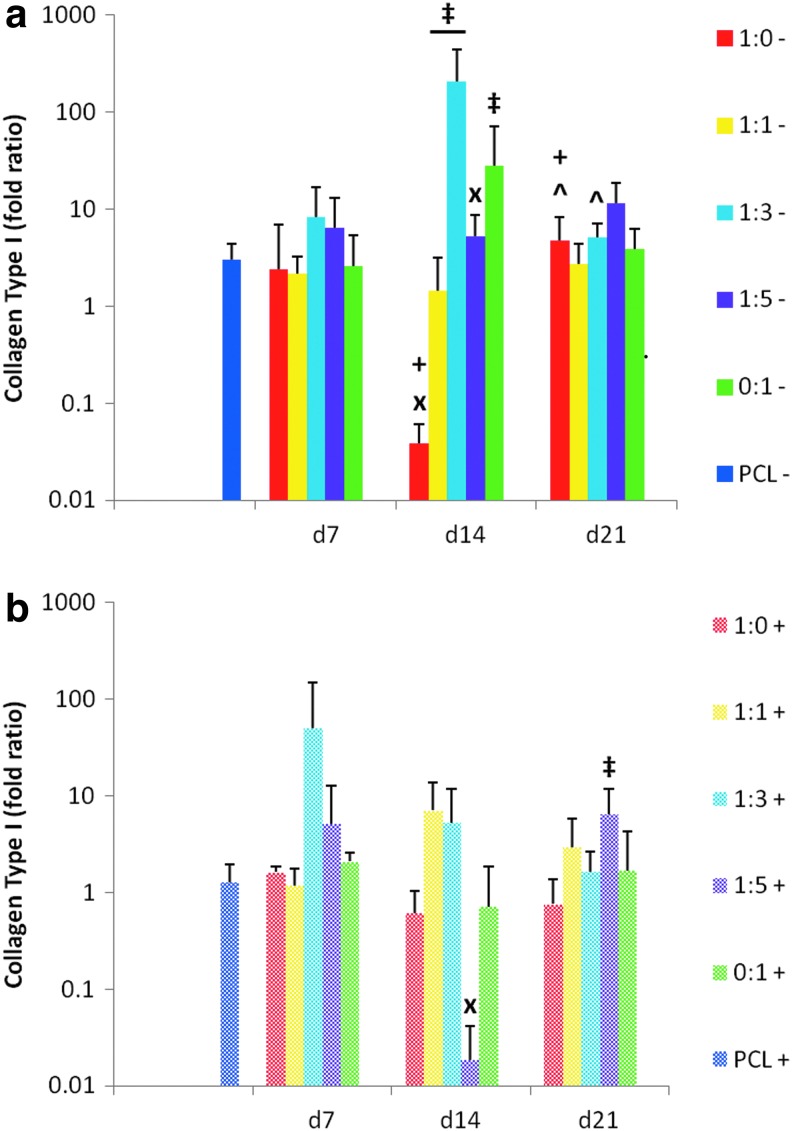
In addition to collagen type II, the expression of the proteoglycan aggrecan is one of the most commonly used markers of chondrogenesis and the articular chondrocyte phenotype (Fig. 8). A decrease in aggrecan expression was observed in MSCs cultured on plain PCL control scaffolds without TGF-β3 (“PCL−”) over the 21-day period. In the absence of TGF-β3, cell generated ECM within PCL scaffolds led to improved expression of aggrecan over the PCL controls (“PCL−”) for all five dPCL/ECM scaffold formulations when the ECM was relatively immature (Fig. 8a, “x”). In contrast, when reseeded MSCs on dPCL/ECM scaffolds were treated with TGF-β3, the only significant differences from the PCL control (“PCL+”) were the result of a lower aggrecan signal from the dPCL/ECM scaffold groups. Taking into consideration all ECM maturities investigated, in the presence of TGF-β3, the 1:1 coculture group was the only scaffold formulation that never differed significantly from the PCL+ control.
FIG. 8.
Relative gene expression of aggrecan expressed by MSCs seeded on dPCL/ECM scaffolds. dPCL/ECM scaffolds cultured in serum-free conditions (a) without growth factor supplements (−) and (b) with TGF-β3 (+). Culture duration was 21 days for all groups. Numeric ratios represent the ratio of chondrocytes to MSCs used to generate the ECM coating the scaffold, while d7, d14, and d21 distinguish the three ECM maturities evaluated. Data were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression and presented as fold change in aggrecan expression. Fold ratios are presented as mean±standard deviation (n=4). Symbols describing statistical significance are defined in Table 3 (p<0.05).
Table 3.
Symbols Indicating Statistical Significance for rt-PCR Data in Figures 8–11
| Symbol | Meaning |
|---|---|
| ‡ | Statistically significant difference (p<0.05) from 1:0 PCL/ECM for this particular ECM maturity and either absence or presence of TGF-β3 treatment. |
| * | Statistically significant difference (p<0.05) from culture ratio 0:1 PCL/ECM for this particular ECM maturity and TGF-β3 treatment. |
| ^ | Statistically significant difference (p<0.05) due to TGF-β3 treatment. |
| + | Statistically significant difference (p<0.05) due to ECM maturity. |
| # | Statistically significant difference (p<0.05) between different coculture ratios for this particular ECM maturity and TGF-β3 treatment. |
| x | Significant difference (p<0.05) from plain PCL control. |
PCL, poly(ɛ-caprolactone); ECM, extracellular matrix.
We hypothesized that dPCL/ECM scaffolds produced by culturing chondrocytes alone, which contained more cartilage-like ECM before and after devitalization (Figs. 6 and 7), would be the most successful at inducing chondrogenesis. For this reason, the aggrecan gene expression of MSCs reseeded on the four other dPCL/ECM scaffolds were compared to that of the MSCs reseeded on the 1:0 scaffold group. Only the MSCs on the 1:3+ d7 and 1:3− d14 scaffolds produced greater aggrecan signals than the corresponding 1:0 scaffolds (Fig. 8, “‡”). MSCs cultured on the other dPCL/ECM scaffolds had similar aggrecan expression levels as the corresponding 1:0 scaffolds except for groups 1:1− d7, 1:5+ d7, and 1:3− d21, which resulted in significantly lower aggrecan signals.
Comparing the dPCL/ECM scaffolds produced with chondrocytes and chondrocyte containing cocultures to the MSC generated (0:1) dPCL/ECM scaffolds, more differences in aggrecan expression were observed in groups without growth factor treatment. For both the least and most mature ECM (d7 and d21), chondrocyte-only derived (1:0−) scaffolds resulted in significantly higher aggrecan expression compared to the MSC-only derived (0:1−) samples (Fig. 8a, “*”). In contrast, the 1:0− and 1:5− constructs had lower aggrecan signals than 0:1− scaffolds with d14 ECM. ECM maturity had some effect on aggrecan expression. For the 1:0− and 1:3− groups, aggrecan expression was significantly different among the various ECM maturity levels (Fig. 8a, “+”). Within the 1:5+ scaffolds, aggrecan expression was found to increase steadily with increasing ECM maturity. Conversely, the 1:1 PCL/ECM scaffolds did not display a significant maturity dependent change in aggrecan expression for either TGF-β3 treatment group.
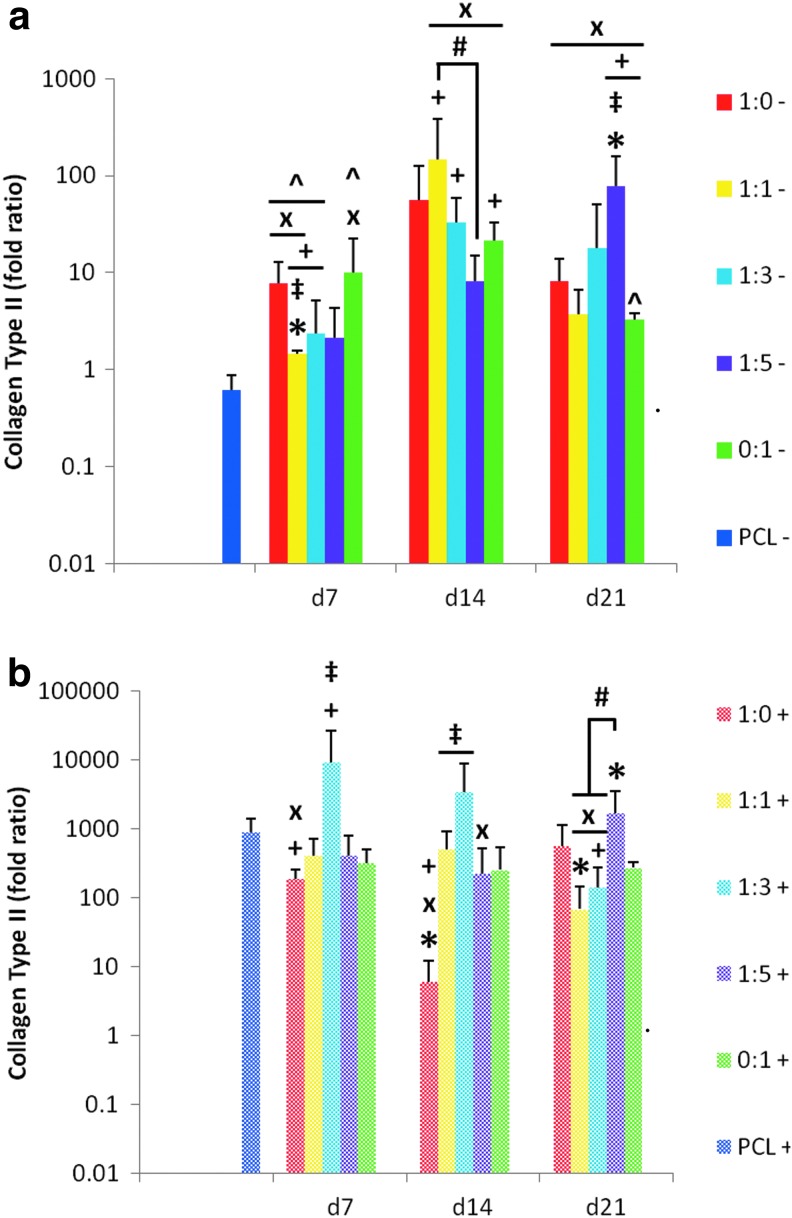
As the most abundant ECM component present within articular cartilage, increased collagen type II expression was used as a major indicator of chondrogenesis and maintenance of the chondrocytic phenotype (Fig. 9). In general, MSCs cultured on each of the dPCL/ECM scaffold formulations displayed increases in collagen II expression during the 21 days of culture both with and without growth factor administration. The only instance in which a decrease in collagen II expression occurred was in MSCs seeded on PCL controls and cultured without TGF-β3 (PCL−). Although not always significantly different, in the absence of TGF-β3, MSCs cultured on dPCL/ECM scaffolds expressed higher collagen II levels than PCL controls regardless of the cell types used to generate the ECM. Compared to the PCL− control, most dPCL/ECM scaffold types with the intermediate (d14) and most mature (d21) ECM exhibited significant increases in collagen II expression when cultured without TGF-β3, with the only exception being the 1:0− d14 scaffolds (Fig. 9a, “x”). For the least mature (d7) dPCL/ECM, only groups 1:0−, 1:1−, and 0:1− had significantly higher collagen II expression levels than the PCL− control. In contrast, when cultured with TGF-β3 supplemented medium none of the dPCL/ECM groups had significantly higher collagen II signals than MSCs on the corresponding plain PCL+ controls.
FIG. 9.
Relative gene expression of collagen type II expressed by MSCs seeded on dPCL/ECM scaffolds. PCL/ECM scaffolds cultured in serum-free conditions (a) without growth factor supplements (−) and (b) with TGF-β3 (+). Culture duration was 21 days for all groups. Numeric ratios represent the ratio of chondrocytes to MSCs used to generate the ECM coating the scaffold, while d7, d14, and d21 distinguish the three ECM maturities evaluated. Data were normalized to GAPDH expression and presented as fold change in collagen type II expression. Fold ratios are presented as mean±standard deviation (n=4). Symbols describing statistical significance are defined in Table 3 (p<0.05).
In the absence of supplemental growth factor, collagen II expression among the various groups was generally similar to that of the dPCL/ECM scaffolds produced by chondrocytes alone (1:0−). The exceptions being groups 1:5− d21 and 1:1− d7 (Fig. 9a, “‡”). With the addition of TGF-β3, more of the d7 and d14 dPCL/ECM scaffolds generated by means of coculture resulted in higher collagen II levels than the corresponding 1:0+ scaffolds (Fig. 9b, “‡”). For d21 ECM, no differences in collagen II expression levels were noted compared to MSCs cultured on 1:0+ dPCL/ECM. Similarly, most scaffolds led to similar collagen II levels as cells grown on MSC generated dPCL/ECM scaffolds with and without TGF-β3 supplementation. However, the 1:5 d21 dPCL/ECM scaffolds had higher collagen II expression levels than 0:1 dPCL/ECM regardless of growth factor treatment (Fig. 9, “*”).
Differences due to ECM maturity were noted within the 1:0+, 1:1−, 1:3−, and 1:5− dPCL/ECM scaffolds. Specifically within these scaffold types, the scaffolds cultured without growth factors showed an increase in collagen II signal with increasing ECM maturity from d7 to d14 or d21. In contrast, in the presence of TGF-β3, the collagen II expression of MSCs cultured on 1:0+ dPCL/ECM decreased as ECM maturity increased from d7 to d14 (Fig. 9, “+”).
Collagen type I expression within the constructs was quantified (Fig. 10) with the goal of minimizing expression levels. The MSCs cultured on dPCL/ECM scaffolds with and without additional growth factors generally exhibited a moderate increase in collagen I expression, shown by fold increases greater than 1, similar to the level seen for MSCs cultured on PCL controls. Only two scaffold formulations resulted in a decrease in collagen I expression compared to the corresponding PCL control, the 1:0− d14 group and the 1:5+ d14 group (Fig. 10, “x”). Within the unsupplemented day 14 dPCL/ECM scaffolds, all of the groups except the 1:5− constructs resulted in significantly higher collagen I expression than the 1:0− scaffolds of the same maturity (Fig. 10a, “‡”). TGF-β3 supplements during MSC culture on the dPCL/ECM scaffolds showed a minimal effect on collagen I expression. Specifically, only for the 1:5+ group, at the later ECM maturity state, there was a significant increase from the corresponding 1:0+ group. Additionally, of the growth factor supplemented cultures on dPCL/ECM scaffolds, only the d14 1:5+ group exhibited a significant difference in collagen type I expression compared to the PCL controls.
FIG. 10.
Relative gene expression of collagen type I expressed by MSCs seeded on dPCL/ECM scaffolds. PCL/ECM scaffolds cultured in serum-free conditions (a) without growth factor supplements (−) and (b) with TGF-β3 (+). Numeric ratios represent the ratio of chondrocytes to MSCs used to generate the ECM coating the scaffold, while d7, d14, and d21 distinguish the three ECM maturities evaluated. Data were normalized to GAPDH expression and presented as fold change in collagen type I expression. Fold ratios are presented as mean±standard deviation (n=4). Symbols describing statistical significance are defined in Table 3 (p<0.05).
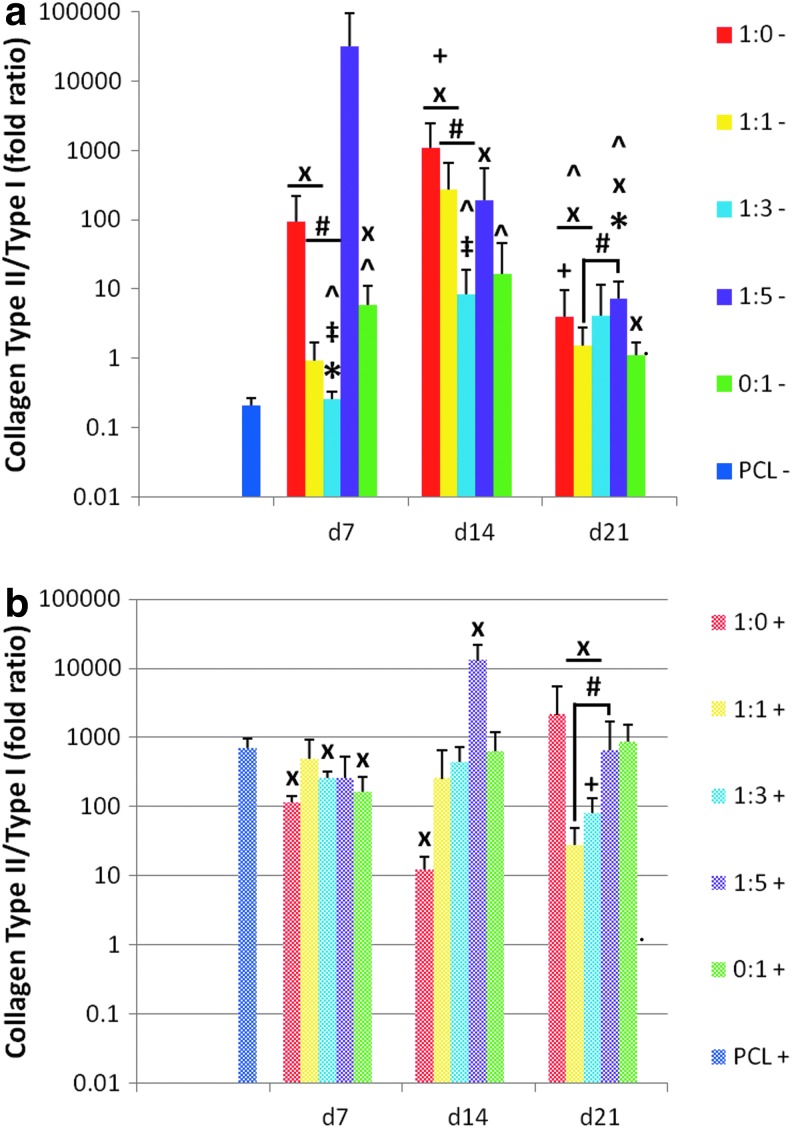
Whereas native articular cartilage is primarily composed of collagen type II and GAGs, some collagen type I is also present although in a relatively lower concentration than collagen type II. For this reason, we hypothesized that the successful induction of MSCs toward the chondrogenic lineage for articular cartilage regeneration would result in a higher ratio of collagen II to collagen I expression (Fig. 11). When cultured in the absence of additional growth factors, the dPCL/ECM scaffolds generally showed higher collagen II/I ratios than the corresponding PCL− control except for the 1:3− group at all ECM maturity levels, as well as the 1:5− d7 and 0:1− d14 groups (Fig. 11a, “x”). In contrast, the inclusion of growth factors led to lower collagen II/I expression ratios than the PCL+ control for some dPCL/ECM groups.
FIG. 11.
Ratio of relative gene expression of collagen type II to collagen type I as expressed by MSCs seeded on dPCL/ECM scaffolds. PCL/ECM scaffolds cultured in serum-free conditions (a) without growth factor supplements (−) and (b) with TGF-β3 (+). Culture duration was 21 days for all groups. Numeric ratios represent the ratio of chondrocytes to MSCs used to generate the ECM coating the scaffold, while d7, d14, and d21 distinguish the three ECM maturities evaluated. The proportion of collagen type II to type I fold ratio was calculated per individual sample. Ratios are presented as mean±standard deviation (n=4). Symbols describing statistical significance are defined in Table 3 (p<0.05).
The chondrocyte generated 1:0− and 1:0+ dPCL/ECM scaffolds approximated the collagen II/I ratios of the other groups except for scaffolds 1:3− d7 and d14, which had significantly lower expression ratios (Fig. 11, “‡”). Comparing the coculture generated dPCL/ECM groups that did not receive TGF-β3, there were significant differences in collagen II/I expression between 1:1− and 1:3− groups for d7 and d14 ECM maturities, with the 1:1− groups displaying the higher collagen II/I ratios (Fig. 11, “#”). For the most mature ECM state studied, the 1:1 and 1:5 groups differed significantly, with the 1:5 cultured scaffolds having higher collagen II/I expression levels than the 1:1 group both with and without TGF-β3 treatment.
Significant effects due to ECM maturity were seen in the 1:3+, 1:1−, and 1:0− scaffolds. For the dPCL/ECM scaffolds 1:1− and 1:0−, intermediate ECM maturities delivered more desirable collagen II/I ratios than the more mature ECM stage (Fig. 11, “+”). For 1:3+, the d21 ECM resulted in collagen II/I expression ratios that were significantly lower than for the two corresponding groups cultured on the less mature ECM.
Discussion
This study demonstrated the chondroinductive capacity of electrospun PCL scaffolds coated with devitalized cell generated cartilage-like ECM. Synthetic polymers offer the benefit of mechanical strength and tunable degradation rates. However, these synthetic polymers often require additional treatments to enhance cell attachment and proliferation as they are not innately bioactive and do little to direct cell differentiation and tissue regeneration.28 An alternative to synthetic polymers is naturally derived materials, such as hyaluronic acid and collagen, which provide cell signals necessary for tissue development. In addition to providing differentiation signals, ECM components have been shown to successfully act as motogenic factors useful for recruiting MSCs.29 As such, an ECM composite material could act to influence host cell migration upon implantation as well as provide necessary morphogenic factors to direct tissue regeneration.
Previous efforts toward utilizing ECM for cartilage tissue engineering scaffolds have centered around either native cartilage tissue isolated by various means and used in a minced or decellularized fashion or on individually isolated ECM components, such as collagen and GAGs, as lyophilized scaffolds or as coatings on synthetic polymer scaffolds.5,8,10,30 Whereas these approaches do benefit from the bioactivity of ECM, it is difficult to obtain autogeneic cartilage tissue, while avoiding donor-site morbidity in a patient. Additionally, allogeneic or xenogeneic sources carry with them concerns about potential immunogenicity issues. The other option of creating bioactive ECM scaffolds using isolated and purified proteins carries the benefit of a well-defined and reproducible scaffold. The benefit of a cell-derived ECM/polymer hybrid scaffold is knowledge that the proteins are deposited in their physiologically active conformations and that the ECM deposited contains other components, such as growth factors and regulatory proteins, which may be beneficial to cell recruitment and direction of tissue regeneration.16,17
Previous studies in our group have shown that cell generated devitalized ECM-coated scaffolds can direct differentiation of MSCs toward the osteogenic lineage.31–33 A study of chondrocyte produced devitalized dPCL/ECM scaffolds generated in a perfusion bioreactor for cartilage regeneration purposes demonstrated that cartilage-like ECM led to a reduction in collagen I expression and also acted to improve the GAG synthetic activity of MSCs when coupled with TGF-β1.18 Although several studies have shown that chondrocytes successfully produce cartilage-like ECM, it is difficult to obtain sufficient quantities of this cell type for tissue engineering efforts, especially due to dedifferentiation upon in vitro expansion.20 For this reason, the use of cocultures of chondrocytes together with MSCs has been investigated in an effort to utilize MSCs to stimulate ECM production thereby reducing the number of chondrocytes necessary to generate cell-derived polymer/ECM hybrid scaffolds.21,22
This study was designed to further examine and characterize the PCL/ECM constructs produced by cocultures of MSCs and chondrocytes that we described previously.21 The ultimate goal was to closely examine the chondrogenic potential of a cell generated ECM coating on a synthetic polymer scaffold. To accomplish this, it was first necessary to devitalize the PCL/ECM constructs to remove the influence of the cells employed to generate the ECM thereby isolating the effect of the ECM coating on the differentiation state of cells reseeded on the dPCL/ECM scaffolds. Previous work studying cell generated osteogenic PCL/ECM constructs showed that a simple freeze–thaw method was the least destructive to the ECM coating, while successfully removing cellular material when compared to a detergent method.34 For this reason, the freeze–thaw method was employed to devitalize the constructs characterized here.
Before examining the chondroinductive potential of the ECM on the scaffolds after devitalizing, it was necessary to characterize the postprocessing state of the scaffolds to determine the effect of freeze–thaw as well as to establish the baseline condition of the scaffolds before reseeding with MSCs. The freeze–thaw method successfully devitalized the scaffolds as was shown by the DNA assay and the nuclear fast red stained histological sections. Any remaining cellular matter appeared to primarily be located near the surfaces of the scaffolds where the ECM was the densest. The dense surface layer of the ECM could be responsible for limiting the complete removal of the cell debris.
We hypothesized that since the constructs cultured with the various cell ratios produced more ECM over time,21 the more mature dPCL/ECM constructs would contain greater amounts of ECM after devitalization. However, biochemical assays as well as histological staining showed that there were significant decreases in ECM contents of the scaffolds regardless of the initial ECM quantities before devitalization. This is likely to be the reason that there were no significant differences in the ECM content of dPCL/ECM scaffolds of differing ECM maturities. Compared to histological sections before devitalization,21 the dPCL/ECM scaffolds displayed decreases in the ECM content throughout the scaffold as well as at the surface. Additionally, all dPCL/ECM scaffolds investigated at all three ECM maturities showed a decrease in the GAG content due to freeze–thaw devitalization with only the 1:1 d21 scaffolds showing a significantly greater GAG content compared to another devitalized scaffold type. Whereas a decrease in GAG content due to processing was anticipated based on previous results,18,33 the lack of variation in the final GAG content after freeze–thaw was unexpected. However, it is understandable that devitalization by aqueous means led to attrition since GAGs are highly water soluble and such loss has been observed even with processing of ex vivo cartilage tissue.30,35,36
The observed effect of devitalization on collagen content of the dPCL/ECM scaffolds was similar to that observed for GAG content. In general, all of the scaffolds with appreciable collagen content before freeze–thaw exhibited significant losses in collagen content due to devitalization processing. In this case, the two d21 scaffolds generated with the greatest amount of chondrocytes, 1:0 and 1:1, exhibited significantly greater collagen contents than the MSC-only generated 0:1 d21 dPCL/ECM scaffolds. As with the loss of GAGs observed, histological sections stained with Picrosirius red showed that collagen was washed away from throughout the scaffold as a whole not just the outer surface. This suggests that some of the collagen was solubilized or disrupted and rinsed out of the hydrated scaffold during processing. These results suggest that it will be important to further optimize ECM adhesion on these scaffolds to reduce the loss of ECM following processing.
Following characterization of the dPCL/ECM scaffolds, the chondroinductive capacity of these scaffolds was assessed. Specifically, dPCL/ECM scaffolds were reseeded with MSCs and cultured in serum-free conditions for 21 days to elucidate any potential chondrogenic effects of the cell generated ECM. It was hypothesized that dPCL/ECM scaffolds found to contain greater amounts of ECM with an even distribution throughout the scaffold would result in the greatest levels of chondrogenic gene expression. In this case, positive chondrogenic induction was determined to be an increase in aggrecan, collagen type II, and collagen II/I expression with the maintenance or decrease in collagen type I expression particularly when compared to PCL control samples. This is because collagen I is the principal ECM component of fibrocartilage, which due to its reduced compressive strength is less desirable than regenerated articular cartilage, primarily composed of collagen II and proteoglycans.37,38 As the biochemical results showed that the devitalized chondrocyte and coculture generated scaffolds were not significantly different in ECM content, it was anticipated that MSCs cultured on the scaffolds generated with greater numbers of chondrocytes would deliver superior chondrogenic gene expression patterns. This hypothesis was based on the fact that chondrocytes are the primary cell type of cartilage and are naturally responsible for generating its ECM. We hypothesized that this ECM would serve as a signal to direct cellular differentiation within these scaffolds.
Supplementing the medium with TGF-β3 appeared to cause an induction of chondrogenesis in each scaffold type. As such, it seems that TGF-β3 treatments somewhat masked the subtle differences of the different ECM coatings especially when compared to the plain PCL controls. For this reason, our discussion of the optimal culture ratio and maturity resulting in the most chondrogenic dPCL/ECM scaffold will focus primarily on the gene expression of MSCs cultured without TGF-β3 supplements.
For samples cultured in serum-free medium without growth factor supplements, it was assumed that any change in gene expression that occurred was due to the scaffold, specifically the ECM coatings deposited by the different cell ratios. The ultimate goal was to determine which cell ratio generated the most chondrogenic ECM coating on a PCL scaffold, and this chondrogenic potential was determined by quantifying chondrogenic gene expression when compared to PCL controls. Day 7 and 21 chondrocyte-only (1:0) generated dPCL/ECM as well as day 7 and 14 1:1 produced dPCL/ECM scaffolds displayed the most favorable chondrogenic gene expression patterns over PCL controls. All four of these scaffold types exhibited significant increases in aggrecan, collagen type II, and collagen II/I expression without any change in collagen type I expression compared to uncoated PCL controls. In contrast to the promising results of the 1:0− d7 constructs, the corresponding group cultured with TGF-β3 supplementation (1:0+ d7) showed a reduction in aggrecan, collagen II, and collagen II/I expression as compared to PCL controls. However, it is important to note that for these genetic markers 1:0+ d7 displayed fold increases of ∼100 for collagen II and II/I expression and 1 for aggrecan expression over the expression of day 0 MSCs.
In contrast, day 7 1:3− and 1:5− scaffolds displayed the least favorable gene expression patterns with a significant improvement over PCL controls only for aggrecan expression. Similarly, day 21 1:3− scaffolds only exhibited a significant increase in collagen II expression over PCL controls. For these three scaffold types with the lowest chondrogenic capacity, the maturity of the cell generated ECM did not have a significant effect on gene expression. However, in the absence of TGF-β3, it also did not result in reduced chondrogenic expression compared to the PCL control. The gene expression results support the hypothesis that dPCL/ECM scaffolds generated with greater concentrations of chondrocytes would have a higher capacity for chondroinduction. Furthermore, dPCL/ECM scaffolds produced by cocultures containing as few as 50% chondrocytes with 14 days maturity approximated the chondrogenic gene expression patterns of MSCs cultured on scaffolds generated wholly by chondrocytes.
We hypothesized that the observed differences in gene expression following MSC culture on the dPCL/ECM scaffolds would be attributable to the maturity of the ECM that was established on the scaffold because the amount of ECM present on the various scaffolds following devitalization showed minimal differences. ECM maturity had a moderate influence on scaffold chondroinductive capacity in the absence of TGF-β3. In general, the intermediate (day 14) ECM maturity showed the greatest degree of chondrogenic potential within a scaffold type (Figs. 8–11, “+”). Furthermore, PCL/ECM scaffolds generated by the coculture of chondrocytes and MSCs in equal amounts (1:1) for 14 days displayed the best performance overall. This mirrors previous findings that suggested a 50% reduction of chondrocytes replaced by MSCs could result in the approximation of ECM production by chondrocytes alone.21 There is a need to further investigate the mechanism by which ECM maturity influences the bioactivity of the scaffolds in an effort to enhance the chondrogenic potential of these constructs.
Conclusions
This work examined the ability of ECM generated by the two cell types of the chondrogenic niche, chondrocytes and MSCs, to direct chondrogenesis of MSCs. Specifically, different ratios of chondrocytes and MSCs were used to coat fibrous synthetic polymer scaffolds with cartilage-like ECM. Those polymer/ECM hybrid constructs were then devitalized and characterized. It was determined that the freeze–thaw process resulted in significant losses in DNA and ECM content throughout all of the scaffold types. The presence of cartilage-like ECM produced by chondrocytes as well as a 1:1 ratio of chondrocytes to MSCs was determined to have a positive effect on aggrecan, collagen type II, and collagen II/I expression when compared to PCL controls. In this way, this study demonstrates that it is possible to develop a hybrid polymer/ECM scaffold to direct cartilage regeneration. Furthermore, this may be accomplished utilizing cocultures of chondrocytes and MSCs thereby reducing the number of chondrocytes necessary to produce chondrogenic hybrid scaffolds.
Acknowledgment
This work was supported from funding by the National Institutes of Health (R01AR57083).
Disclosure Statement
No competing financial interests exist.
References
- 1.Levorson E.J., Kasper F.K., and Mikos A.G.Scaffolds-flow perfusion bioreactor design. In: Ducheyne P., Healy K.E., Hutmacher D.W., and Kirkpatrick C.J., eds. Comprehensive Biomaterials, vol. 5 Oxford: Elsevier, 2011, pp. 1–11 [Google Scholar]
- 2.Chen W.-C., Yao C.-L., Chu I.M., and Wei Y.-H.Compare the effects of chondrogenesis by culture of human mesenchymal stem cells with various type of the chondroitin sulfate C. J Biosci Bioeng 111,226, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Lu H., Hoshiba T., Kawazoe N., Koda I., Song M., and Chen G.Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials 32,9658, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Murphy C.M., Matsiko A., Haugh M.G., Gleeson J.P., and O'Brien F.J.Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J Mech Behav Biomed Mater 11,53, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Barnes C.P., Pemble C.W., Brand D.D., Simpson D.G., and Bowlin G.L.Cross-Linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng 13,1593, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Chen W.-C., Yao C.-L., Wei Y.-H., and Chu I.M.Evaluating osteochondral defect repair potential of autologous rabbit bone marrow cells on type II collagen scaffold. Cytotechnology 63,13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan Y., Wang Z., Yan W., Wang S., Zhang S., and Jia J.Preparation of collagen-coated electrospun nanofibers by remote plasma treatment and their biological properties. J Biomater Sci Polym Ed 18,1153, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Farrell E., O'Brien F.J., Doyle P., Fischer J., Yannas I., Harley B.A., O'Connell B., Prendergast P.J., and Campbell V.A.A Collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng 12,459, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Jakobsen R., Shahdadfar A., Reinholt F., and Brinchmann J.Chondrogenesis in a hyaluronic acid scaffold: comparison between chondrocytes and MSC from bone marrow and adipose tissue. Knee Surg Sports Traumatol Arthrosc 18,1407, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Yang Z., Shi Y., Wei X., He J., Yang S., Dickson G., Tang J., Xiang J., Song C., and Li G.Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold. Tissue Eng Part C Methods 16,865, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Needham C.J., Williams A.K., Chew S.A., Kasper F.K., and Mikos A.G.Engineering a polymeric gene delivery vector based on poly(ethylenimine) and hyaluronic acid. Biomacromolecules 13,1429, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X., Park H., Liu G., Liu W., Cao Y., Tabata Y., Kasper F.K., and Mikos A.G.In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials 30,2741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holland T.A., Bodde E.W., Cuijpers V.M., Baggett L.S., Tabata Y., Mikos A.G., and Jansen J.A.Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthritis Cartilage 15,187, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Park H., Temenoff J.S., Tabata Y., Caplan A.I., Raphael R.M., Jansen J.A., and Mikos A.G.Effect of dual growth factor delivery on chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in injectable hydrogel composites. J Biomed Mater Res A 88A,889, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Cheng N.-C., Estes B.T., Awad H.A., and Guilak F.Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A 15,231, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thibault R.A., Mikos A.G., and Kasper F.K.Protein and mineral composition of osteogenic extracellular matrix constructs generated with a flow perfusion bioreactor. Biomacromolecules 12,4204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes M.E., Bossano C.M., Johnston C.M., Reis R.L., and Mikos A.G.In vitro localization of bone growth factors in constructs of biodegradable scaffolds seeded with marrow stromal cells and cultured in a flow perfusion bioreactor. Tissue Eng 12,177, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Liao J., Guo X., Grande-Allen K.J., Kasper F.K., and Mikos A.G.Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials 31,8911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darling E.M., Pritchett P.E., Evans B.A., Superfine R., Zauscher S., and Guilak F.Mechanical properties and gene expression of chondrocytes on micropatterned substrates following dedifferentiation in monolayer. Cell Mol Bioeng 2,395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cournil-Henrionnet C., Huselstein C., Wang Y., Galois L., Mainard D., Decot V., Netter P., Stoltz J.-F., Muller S., Gillet P., and Watrin-Pinzano A.Phenotypic analysis of cell surface markers and gene expression of human mesenchymal stem cells and chondrocytes during monolayer expansion. Biorheology 45,513, 2008 [PubMed] [Google Scholar]
- 21.Levorson E.J., Mountziaris P.M., Hu O., Kasper F.K., and Mikos A.G.Cell derived polymer/extracellular matrix composite scaffolds for cartilage regeneration, part 1: investigation of co-cultures and seeding densities for improved extracellular matrix deposition. Tissue Eng Part C Methods 2013. [Epub ahead of print]; DOI: 10.1089/ten.TEC.2013.0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meretoja V.V., Dahlin R.L., Kasper F.K., and Mikos A.G.Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials 33,6362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo X.-T., Guo S.-C., Xie H.-Q., Deng L., Zhi W., Xiang Z., Li X.-Q., and Yang Z.-M.Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone 45,42, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Meretoja V.V., Dahlin R.L., Wright S., Kasper F.K., and Mikos A.G.The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials 34,4266, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levorson E.J., Sreerekha P.R., Chennazhi K.P., Kasper F.K., Nair S.V., and Mikos A.G.Fabrication and characterization of multiscale electrospun scaffolds for cartilage regeneration. Biomed Mater 8,014103, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pham Q.P., Kurtis Kasper F., Scott Baggett L., Raphael R.M., Jansen J.A., and Mikos A.G.The influence of an in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials 29,2729, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan S., Jayakumar R., Chennazhi K.P., Levorson E.J., Mikos A.G., and Nair S.V.Multiscale fibrous scaffolds in regenerative medicine. Adv Polym Sci 246,2012 [Google Scholar]
- 29.Thibault M.M., Hoemann C.D., and Buschmann M.D.Fibronectin, vitronectin, and collagen I induce chemotaxis and haptotaxis of human and rabbit mesenchymal stem cells in a standardized transmembrane assay. Stem Cells Dev 16,489, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Benders K.E.M., Weeren P.R., Badylak S.F., Saris D.B.F., Dhert W.J.A., and Malda J.Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol 31,169, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Datta N., Holtorf H.L., Sikavitsas V.I., Jansen J.A., and Mikos A.G.Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials 26,971, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Datta N., Pham Q.P., Sharma U., Sikavitsas V.I., Jansen J.A., and Mikos A.G.In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci USA 103,2488, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thibault R.A., Scott Baggett L, Mikos A.G., and Kasper F.K.Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng Part A 16,431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thibault R.A., Mikos A.G., and Kasper F.K.Osteogenic differentiation of mesenchymal stem cells on demineralized and devitalized biodegradable polymer and extracellular matrix hybrid constructs. J Biomed Mater Res A 101A,1225, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolis S., Handley C.J., and Comper W.D.Passive loss of proteoglycan from articular cartilage explants. Biochim Biophys Acta 993,157, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Mahmood T.A., Shastri V.P., Van Blitterswijk C.A., Langer R., and Riesle J.Tissue engineering of bovine articular cartilage within porous poly(ether ester) copolymer scaffolds with different structures. Tissue Eng 11,1244, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Ortiguera C.J.Surgical treatment options for articular cartilage injuries of the knee. Northeast Florida Med 57,13, 2006 [Google Scholar]
- 38.McNickle A.G., Provencher M.T., and Cole B.J.Overview of existing cartilage repair technology. Sports Med Arthrosc Rev 16,196, 2008 [DOI] [PubMed] [Google Scholar]