Abstract
To achieve durable recognition as a promising animal experiment-abandoning tool in ophthalmology, in vitro engineered tissue equivalents of the human cornea should exhibit proper morphogenesis. Regarding this issue, we were seeking for the natural cell microenvironment fulfilling the minimum requirements to allow human corneal keratinocytes to develop a balanced epithelial morphology with regular spatial appearance of tissue homeostatic biomarkers. Hence, we established cocultures of 3D cell-based collagen scaffolds comprising immortalized corneal keratinocytes combined with a gradual cornea-derived in vivo-like cell microenvironment, together with immortalized stromal fibroblasts alone (nonholistic) or fibroblasts and immortalized endothelial cells (holistic). With matched non-holistic microenvironments revealing mostly flattened cells and putative apical cell ablation foci at day 6, and 9 in HE stains, holistic counterparts yielded proper epithelial stratification with cell flattening restricted to apical layers. Concordantly, RT2-PCR showed a tremendous increase in gene expression for progressive and terminal biomarkers of corneal keratinocyte differentiation, cytokeratin (CK) 12, and filaggrin (FIL), in response to nonholistic environments, while involucrin (INV) was moderately but significantly upregulated. Although visible, this increase was moderate in corneal keratinocytes with a holistic environment. On the protein level, indirect immunofluorescence revealed that only epithelia of holistic environments showed diminishment in CK19, counteracted by CK12 rising over time. This time-dependent progression in differentiation coincided with declined proliferation and tissue-regular focus of differentiation biomarkers inv and fil to suprabasal and apical cell layers. Our novel findings suggest the interplay of native tissue forming cell entities, important for balanced corneal epithelial morphogenesis. In addition, they provide evidence for a holistic cell microenvironment as a prerequisite for development of an in vitro engineered corneal epithelial tissue equivalent, exhibiting a regular appearance of tissue homeostatic biomarkers. Such equivalents will be promising tools in ophthalmology, for example, for mechanistic studies in basic research and/or testing of generics or preclinical validation of innovative cornea-tailored biomaterials, desired for regenerative strategies.
Introduction
In ophthalmology, experimental cornea-focusing issues of clinical relevance include, for instance, (1) extension of the knowledge of tissue homeostasis regulation, (2) testing of novel ocular surface generics, and (3) preclinical validation of innovative cornea-tailored biomaterials. For addressing this panel of issues, in vitro models comprising corneal tissue equivalents, would be the future means of choice, particularly in the context of minimizing or prospectively abandoning time-consuming and cost-intensive animal experiments. This aspect gains progressive importance, since animal studies have been shown among others, to fail in the safety evaluation of pharmaceutical drugs1 and have been discussed controversially as tools in medical research.2
For this reason, in vitro models should optimally comprise cells reflecting both the target species and the target tissue, to allow for the most possible explanatory power and thus result-obtained implications with respect to the in vivo situation. With focus on epithelia, which as surface or cavity-lining epithelia, for example, skin, oral mucosa and/or cornea, represent one of the main body tissue fractions in humans; adequate models in the first instance consist of monolayers of epithelial keratinocytes, derived from the native tissue of origin. Such keratinocyte primary cultures are suffering from two major limitations, regarding their suitability as powerful tools to address the aforementioned issues of clinical relevance. First, primary human keratinocytes underlie senescence-determined growth limitations3,4 and therefore, cannot be permanently mass propagated to high cell numbers, mandatory for reproducible in vitro testing. Second, when growing in single cell layers, keratinocytes fail to fulfill one of their essential tissue functions, namely, to develop a balanced morphogenesis, that is, stratified cell layers of distinct morphology.5 Such morphological distinction applies to the complex epithelia of skin epidermis, oral cavity buccal mucosa, as well as hard palate or gingival epithelium and cornea too. In this study, distinct cell morphologies correspond to respective layers, and in conjunction with the expression of biomarkers, for example, stem cell markers like cornea-attributed ABCG2, cytokeratins (CKs), involucrin, and filaggrin indicate certain stages of keratinocyte differentiation.6–11
Circumvention of these limitations is achieved by human keratinocyte immortalization,12–14 and generation of stratified epithelia in interactive 3D cell cocultures as described for skin,15 oral cavity,16,17 and cornea.18–22 Following immortalization, the resulting keratinocyte cell line should express most of the biomarkers, assigning to tissue homeostatic parameters such as differentiation, to reflect an authentic in vitro model system, suitable as an animal experiment substitute. Regarding this issue, we have recently shown that HPV 16 E6/E7-immortalized human corneal keratinocytes (IHCK) exposed to various biomechanical external cues are still capable to express the above-mentioned biomarkers, critical for corneal tissue homeostasis.11 In this context, spontaneous keratinocyte immortalization, a very seldom observed phenomenon, and the use of HPV-16 E6 and E7 genes as immortalizing agents are reported to maintain the cells' normal differentiation behavior.17,23 By contrast, employment of the SV40 T-antigen early region genes has been described, for instance, for mammary epithelial cells to be accompanied with aberrant differentiation, loss of DNA damage response, karyotypic instability and, in some cases, tumorigenicity with matched hTERT/catalytic telomerase subunit-immortalized counterparts.24 Thus, it appears likely that optimized tissue-authentic in vitro models should consist of target cells immortalized by a smooth modus operandi, that is, a biomarker-conservative immortalizing agent. Moreover, wherever possible, the model cell-type constituents, essential for developing the in vitro tissue equivalent should be immortalized uniformly, which means, by use of the same agent. This is important to obviate variations of cell/tissue-innate biomarker expression, hampering interpretation of results with respect to their transferability to the in vivo situation. In light of these critical issues, the aforementioned cornea models suffer from nonuniformity, since they comprise immortalized/primary cell combinations19–21 or in the case of uniformity,18 consist of only primary cells, which can hardly be used for reproducible in vitro testing, due to the previously described growth limitations.
Hence, to address the initially specified questions of clinical relevance by concomitantly fulfilling native tissue demands, in vitro tissue equivalents based on interacting species-specific target cells as in situ mimics are more and more needed. This becomes evident by the novelty of the European Union guidelines for preclinical testing of medical devices and drug compounds, also applying to ophthalmology, which will open the road for proof of concept studies, employing the described in vitro models as in vitro method-based animal experiment substitutes.25 In this context, they may become very useful in in vitro studies investigating the inflammatory and toxicity potential of drug ingredients in ophthalmology over initial time periods.26,27 The progressively maturated 3D corneal epithelial model following cultivation periods of 8 or 9 days presented in this study is suitable for short-term exposure testing, for example, acute toxicity or induction of cell apoptosis in a more physiological context. Investigations about substance effects on cell differentiation and/or proliferation, for example, can be applied to a premature 3D corneal epithelial model cultivated for 6 days.
Therefore, the present study aimed at seeking a cell microenvironment for immortalized corneal keratinocytes to develop balanced morphogenesis of a normal corneal epithelium, substantiated by regular histoarchitecture and expression of tissue-innate biomarkers, rendering cornerstone parameters of tissue homeostasis.
Materials and Methods
Cell culture
Primary cells were won as explant cultures after informed consent and approval by the ethics commission of the Albert-Ludwigs-University Freiburg (vote #: 307/09). Explant cultures of corneal epithelial cells, which were derived from epithelial sheets grown from limbal explants as described recently,11 corneal fibroblasts, and endothelial cells were immortalized as described before using the open reading frames of the E6/E7 oncogenes of the human papillomavirus type 16, according to the protocol established by Pear et al.28 Preliminary experiments showed successful and sustained immortalization and persistent expression of representative biomarkers of all cell types under study. Therefore, IHCK were used for experiments following passage 77, immortalized human corneal fibroblasts (IHCF) from passage 8, and immortalized human corneal endothelial cells (IHCE) from passage 20. Previous experiments with all immortalized cell types under study proved stable expression of tissue-relevant biomarkers, stable proliferation behavior, and tissue-innate cell morphology over the passage numbers applied in this work.
Cocultures comprising combinations of corneal keratinocytes with holistic and nonholistic cell microenvironments
Cocultures were fabricated as previously described.17 Briefly, 4.5×105 IHCF and 1×105 IHCE (holistic mode) or only 4.5×105 IHCF (nonholistic mode) were trypsinized, counted, and resuspended in 1 mL of fetal calf serum (Biochrome, Berlin, Germany). This suspension was mixed in a beaker chilled on ice with 8 mL of collagen solution (5 mg/mL; Gibco, Life Technologies, Grand Island, NY) and 1 mL HBSS 10×(Gibco, Life Technologies). By drop-wise addition of 1 M sodium hydroxide (∼30 μL/drop), the pH was adjusted to 7.2–7.6 (seen by the color change of the indicator phenol red in the HBSS to salmon colored to purple). Next, the gel was pipetted in a 24-well plate (1 mL per well) before gels were incubated without a culture medium at 37°C, 97% humidity, and 5% CO2. After 2 h, 1 mL of FAD medium (Ham's F12/Dulbecco's modified Eagle medium (DMEM): mixing ratio 1:3; Biochrom), 5% fetal calf serum (FCS), 100 μg/mL kanamycin (Sigma, Mannheim, Germany), 100 μg/mL neomycin (Sigma), and the supplements of KGM2 (Promocell, Heidelberg, Germany) were added. After an incubation period of 24 h, 1×106 IHCK were seeded on the gels containing either only IHCF (nonholistic mode) or IHCF and IHCE (holistic mode). After another 24 h, the gels were transferred from the 24-well plate to a 6-well plate containing 5 mL of fresh FAD medium. The viability and morphology of the IHCF and IHCE embedded in the collagen gel matrix were controlled daily by phase-contrast microscopy. After a cultivation period of 5, 6, and 9 days, the gels were fixed in Technovit 9100 (Heraeus Kulzer GmbH, Wehrheim, Germany) according to the manufacturer's instructions, respectively. Consecutively, slides of 10 μm thickness were cut for HE staining and for indirect immunofluorescence (IIF). Before staining, the samples were deplastinated in 2-methoxyethyl acetate (Sigma) three times for 20 min, respectively, following rehydration by a decreasing alcohol series (100%, 90%, 80%, 70%, 50%, 30%, and phosphate-buffered saline [PBS], each step for 5 min at room temperature). Rehydrated samples were instantly used for staining. Staining for HE was performed using a staining robot (Leica ST5010-CV5030 Integrated Workstation; Leica Microsystems GmbH, Wetzlar, Germany), IIF was performed as described below.
For quantitative reverse transcriptase polymerase chain reaction (RT-PCR), the cocultures were propagated in six-well plates. Two types of cocultures and one monoculture were performed: (mode 1, holistic) 4.5×105 IHCF and 1×105 IHCE were seeded in a six-well plate, while (mode 2, nonholistic) only 4.5×105 IHCF were seeded in a six-well plate. After cultivation in the FAD medium for 24 h, a transwell cell culture insert (Merck Millipore, Billerica, MA) was placed in the wells, respectively. Additionally, cell culture inserts were placed in six-well plates, devoid of IHCF and IHCE (mode 3). Then, 1×106 IHCK were seeded on the surface of the transwell inlays. By using this operation mode, 3D spatially separated growth of IHCK from holistic and nonholistic microenvironments was warranted. In analogy to the cocultures established on collagen gels, cells here can also only communicate through diffusible growth factors,17 and keratinocyte RNA contaminations with microenvironmental cell RNA can be excluded. These setups were cultivated for a time period of 5, 6, and 9 days. All cells were kept under standard cell culture conditions: 37°C, 97% humidity, and 5% CO2.
Indirect immunofluorescence
All antibodies were diluted in Dulbecco's phosphate-buffered saline (DPBS) (Life Technologies, Grand Island, NY) plus 0.05% Tween (Bio-Rad Laboratories GmbH, Munich, Germany). The amount of primary antibodies varied between 2–10 μg/mL (Table 1), whereas the amount of secondary antibodies was 10 μg/mL. In PBS, rehydrated slides were incubated with primary antibodies for 1 h and washed three times with PBS for 15 min, respectively. Thereafter, they were incubated with a secondary antibody (AlexaFluor® 488 goat anti-mouse or goat anti-rabbit; both Life Technologies) for 45 min in the dark. After washing three times with PBS for 15 min, cell nuclei were counterstained by a 300 nM 4′,6-diamino-2–phenylindole dihydrochloride solution (DAPI; Biomeda, Foster City, CA) for 10 min. The cells were washed two times with PBS followed by rinsing one time with water. Finally, samples were embedded in a mounting medium Fluoromount G (Southern Biotech, Birmingham, AL). For imaging and analysis, a BZ-9000 BIOREVO (Keyence, Neu-Isenburg, Germany) and associated software were used.
Table 1.
Primary Antibodies Used for Indirect Immunofluorescence
| Antibodies | Amount (antibody) per volume | Manufacturer |
|---|---|---|
| Anti-ABCG2 (rabbit monoclonal) | 10 μg/mL | Merck KG aA, Darmstadt, Germany |
| Anti-cytokeratin 12 (rabbit monoclonal) | 2 μg/mL | Santa Cruz Biotechnology, Heidelberg, Germany |
| Anti-cytokeratin 19 (mouse monoclonal) | 2 μg/mL | Santa Cruz Biotechnology, Heidelberg, Germany |
| Anti-filaggrin (rabbit monoclonal) | 4 μg/mL | Abcam, Cambridge, United Kingdom |
| Anti-involucrin (mouse monoclonal) | 4 μg/mL | Abcam, Cambridge, United Kingdom |
| Anti-Ki-67 (rabbit polyclonal) | 10 μg/mL | Abcam, Cambridge, United Kingdom |
RNA isolation and quantitative real-time PCR
After cultivation periods of 5, 6, and 9 days, IHCK from the cell culture inserts were lysed with RLT buffer (Qiagen, Hilden, Germany) and homogenized with QIAshredder columns according to the manufacturer's instructions (Qiagen), followed by total RNA isolation using the RNeasy mini kit (Qiagen) in accordance to the manufacturer's instructions. RNA concentrations and degree of degradation were measured using the Experion Automated Electrophoresis system (Bio-Rad Laboratories GmbH). First-strand cDNA synthesis was conducted using the RevertAid™ First-Strand cDNA Synthesis Kit (Thermo Scientific, Offenbach, Germany). For normalization, the cDNA concentration was adjusted to 5 ng/μL for each PCR. RT-PCR was performed using primers for ATP-binding cassette subfamily G member 2 (ABCG2, PPH01526B), filaggrin (FIL, PPH24283A), involucrin (INV, PPH01911A), CK12 (PPH09776A), and CK19 (PPH01004E), for Ki-67 (MKI67, PPH01024E), for glyceraldehyde 3-phosphate dehydrogenase (GAPDH, PPH00150E) and actin-β (ACTB, PPH00073E) (all primers: RT2-PCR Primer Set; Qiagen). The analysis was conducted in a CFX96 real-time PCR detection system (Bio-Rad Laboratories GmbH). The standard temperature profile included an initial denaturation step for 10 min at 95°C, followed by 40 cycles of denaturation at 95°C for 15 s, annealing, and extension at 60°C for 1 min.
The relative expression levels of each mRNA were analyzed using a modification of the ΔΔCT equation, which allows counting for differences in efficiencies (E=10−1/slope) between the PCRs.29 The data were normalized to the index CT of the GAPDH and β-actin and nonmodulated housekeeping genes and always referred to the relative gene expression values of the cell's RNA derived from samples of a cultivation period of 5 days. The data presented reflect the mean of three measurements of independent biological replicates (n=9,±SD). The statistical analysis was performed using the Student's t-test. p-Values ≤0.01 were considered significant.
Results
Balanced corneal epithelial morphogenesis as reflected by histoarchitecture reveals dependency from holistic natural cell microenvironment
Among the corneal constituents, the cornea epithelium as the outermost tissue is a squamous stratified epithelium. It comprises a single layer of cuboidal columnar basal cells, which acquire a flattened shape during their natural bi-directed spatial migration trajectory, that is, centripetally, starting from outer limbal borders, and horizontally, from Bowman's membrane adjacent sites to the apical surface layers.30 Based on this morphogenesis and its resulting cell morphologies, we were interested if IHCK were capable to resemble histoarchitecture of the normal corneal epithelial phenotype either cocultured with immortalized stromal fibroblasts only or with a combination, consisting of immortalized fibroblasts and immortalized endothelial cells. Regarding the human cornea, both fibroblasts and endothelial cells render cell types of the natural epithelial cell microenvironment. Hence, we have referred to the stromal fibroblasts only as nonholistic and the fibroblast–endothelial cell combination as the holistic corneal keratinocyte microenvironment in the established cocultures. In preliminary tests, we established the corneal epithelial cells in 3D cocultures, that is, the nonholistic and holistic microenvironment, up to 14 days. Similar cultivation times were used in previous studies for organotypic gingival cocultures.17,31,32 These preliminary tests on the aforementioned corneal cocultures revealed an only marginal maturation before day 5, and epithelial disarrangements starting from day 10 onward. Consequently, three cultivation times were chosen to investigate the characteristics of balanced epithelial morphogenesis: (1) 5 days for premature epithelial differentiation, (2) 6 days for progression of differentiation, and (3) 9 days for completion of differentiation, including terminal differentiation. These cultivation periods are in line with already published corneal epithelial in vitro models.19,22
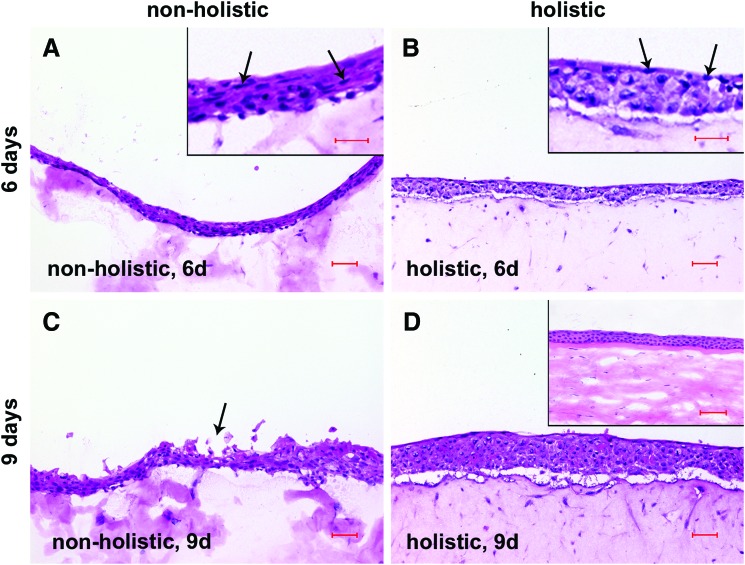
As shown in Figure 1, epithelial morphogenesis clearly discriminated nonholistic from holistic regimes, by revealing different outcomes. Whereas nonholistic microenvironments yielded epithelia, harboring mostly flattened keratinocytes already at day 6 (Fig. 1A), holistic counterparts here displayed columnar basal cells (Fig. 1B): cocultures with fibroblasts led to stratified epithelia integrating four up to six cell layers, with the occurrence of cell flattening frequently already visible in basal or second layer cells (Fig. 1A, arrows in inlay). By contrast, in the presence of fibroblasts and endothelial cells, corneal keratinocytes at this time point have formed a basal cell layer of nonflattened columnar cells, while cell flattening in the three to six layers consisting of the epithelial compartment appeared primarily restricted to the most apical layer (Fig. 1B, arrows in inlay). Aforementioned epithelial histoarchitecture by trend also applied to day 9 in response to a holistic cell environment, whereat the number of layers has increased to a maximum of 8 (Fig. 1D), indicating a balanced morphogenesis resembling the limbal central cornea transition zone comparable to the situation found in native cornea (Fig. 1D, inlay).11 Whereas holistic environments yielded proper epithelial entity, nonholistic counterparts displayed entity derangement by the presence of a high frequency of possible apical cell ablation foci (Fig. 1C, arrow), which may indicate the loss of epithelial integrity. The outcome of keratinocyte's development strongly suggests a dependency from the holistic natural microenvironment for balanced morphogenesis, leading to a corneal epithelial in vitro tissue equivalent.
FIG. 1.
HE stain of immortalized human corneal keratinocytes (IHCK) cocultured for 6 and 9 days with immortalized human corneal fibroblasts (IHCF) (A, C, nonholistic mode) and cocultured with IHCF and immortalized human corneal endothelial cells (IHCE) (B, D, holistic mode). Inlays in (A, B) show representative magnifications of the respective figure, arrows indicate flattened cells. Arrow in (C) indicates entity derangement. Inlay in (D) shows a native human cornea. Scale bars in (A–D) correspond to 50 μm. Scale bars in the inserts in (A) and (B) correspond to 20 μm and to 100 μm for the insert in D. Color images available online at www.liebertpub.com/tec
Gene expression revealed microenvironmental regulation of typical corneal biomarkers
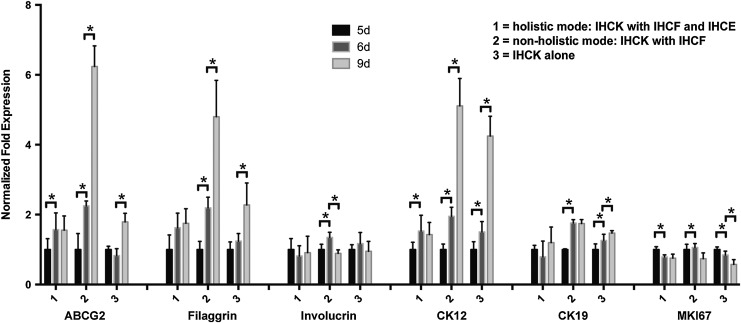
In stratified epithelia-like skin, oral mucosa, or cornea, all belonging to the self-renewing body tissues, homeostasis is bound to the fine-tuned balance of proliferation and differentiation,33 the latter subdivided in certain stages and characterized by expression of distinct biomarkers, including involucrin and filaggrin, also detectable in native corneal tissue sections.11,34 To analyze the expression response of such biomarkers in our immortalized corneal epithelial keratinocytes exposed to different microenvironments, we employed RT2-PCR (qPCR) for the stem cell marker ABCG2,35 for terminal differentiation markers filaggrin (FIL) and involucrin (INV),34 CK19 and CK12, discriminating less from progressively differentiated cell stages,36 as well as for the proliferation marker Ki-67 (MKI67).37
In addition to the holistic and nonholistic natural cell microenvironments, we detected the status of mRNA transcription levels also in solitary grown corneal keratinocytes at the respective time points, to obtain information about auto- and paracrine-mediated keratinocyte self-regulation of biomarker gene expression, that is, the autonomous cornea epithelial cell base situation. This strategy allows for evaluation of the decisive role of natural microenvironment cell entities on regulation of biomarker gene expression. Hence, Figure 2 depicts the 3 modes: holistic (1) and nonholistic (2) microenvironments, whereas mode (3) shows the corneal keratinocytes alone. Intriguingly, the most striking modulation of gene expression was observed for modus 2 and for ABCG2, FIL, and CK12, in particular (Fig. 2: ABCG2, filaggrin, CK12, mode 2). These modulations included drastic transcriptional increases by comparing mRNA levels at day 6 with day 9, which was already adumbrated with significance, when matching day 5 with day 6 (Fig. 2: ABCG2, filaggrin, CK12, mode 2). Such gene expression profiles may indicate obvious imbalance in the natural expression of the respective tissue homeostatic biomarkers regarding the normal corneal epithelial situation, by suggesting overshooting differentiation. Modulation for CK12 in mode 2 and 3 were extensively significant (Fig. 2: CK12, mode 3). While modulations were striking in mode 2, mode 3 normally revealed clear though less extensive significance, by matching day 6 with 9 (Fig. 2: ABCG2, filaggrin, mode 3). This gene expression situation, seen for base situation mode 3, may corroborate the aforementioned overshoot in differentiation, also when corneal keratinocytes were grown devoid of essential microenvironmental cell partners. In mode 1, that is, the holistic environment, corneal keratinocyte gene expression for all 3 markers showed a moderate, and only in parts, significant increase, when transcription at day 5 was matched with day 6, while alterations between day 6 and 9 remained below significance (Fig. 2: ABCG2, filaggrin, CK12, mode 1). Regarding inv, the second biomarker of terminal differentiation, significant changes could only be detected for mode 2. Changes were characterized by an increase between days 5 and 6, and a decrease between days 6 and 9 (Fig. 2: involucrin, mode 2), herewith again pointing to acceleration of corneal keratinocyte differentiation in response to a natural, but nonholistic cell microenvironment. Significant upregulation of CK19, the indicator of less differentiated stages in corneal epithelial cells applied to nonholistic mode 2 and base mode 3 too, particularly when transcription at day 5 and 6 was compared, but remained fairly constant in holistic mode 1 (Fig. 2: CK19, modes 1–3). While in modes 1 and 3, transcripts for the proliferation marker MKI67 significantly ceased, comparing day 5 with day 6, a converse significant increase was denoted for mode 2 (Fig. 2: MKI67, modes 1–3), thereby emphasizing the highest frequency of striking or significant transcriptional changes to accumulate on the nonholistic cell microenvironment. The results obtained from our gene expression studies may hypothesize that alternations in the corneal keratinocyte biomarker transcription appeared to be regulated by the provided setting conditions, that is, no microenvironmental cell partner, nonholistic configuration of cell partners, and holistic cell partner configuration, respectively.
FIG. 2.
Normalized gene expression by IHCK as coculture or monoculture: (mode 1) IHCK cocultured with IHCF and IHCE; (mode 2) IHCK cocultured with IHCF or (mode 3) IHCK only. RNA was isolated after 5, 6, and 9 days of cultivation. Data were obtained using the ΔΔCt-method29 and normalized to day 5 of the respective mode and to the housekeeping genes glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin. Asterisks indicate significant up-/downregulation (p≤0.01; n=9).
Progressive regular appearance of tissue homeostatic biomarkers is limited to holistic cell microenvironments
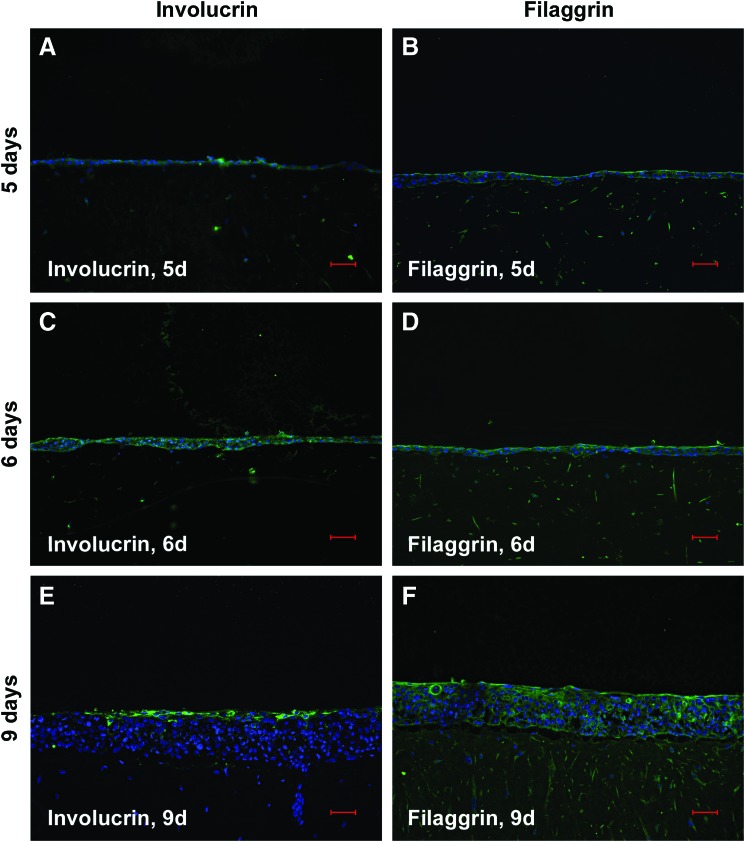
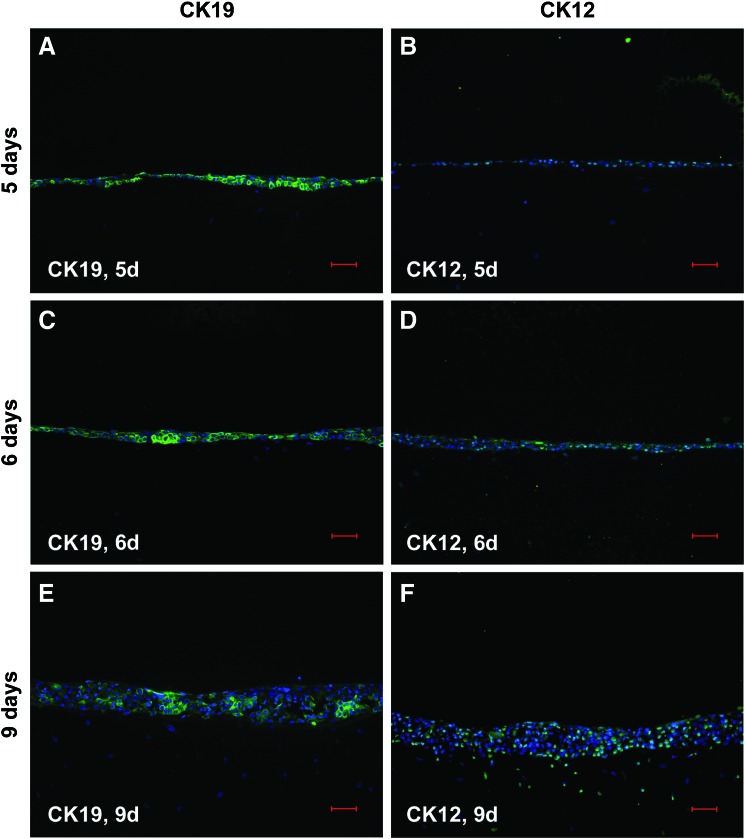
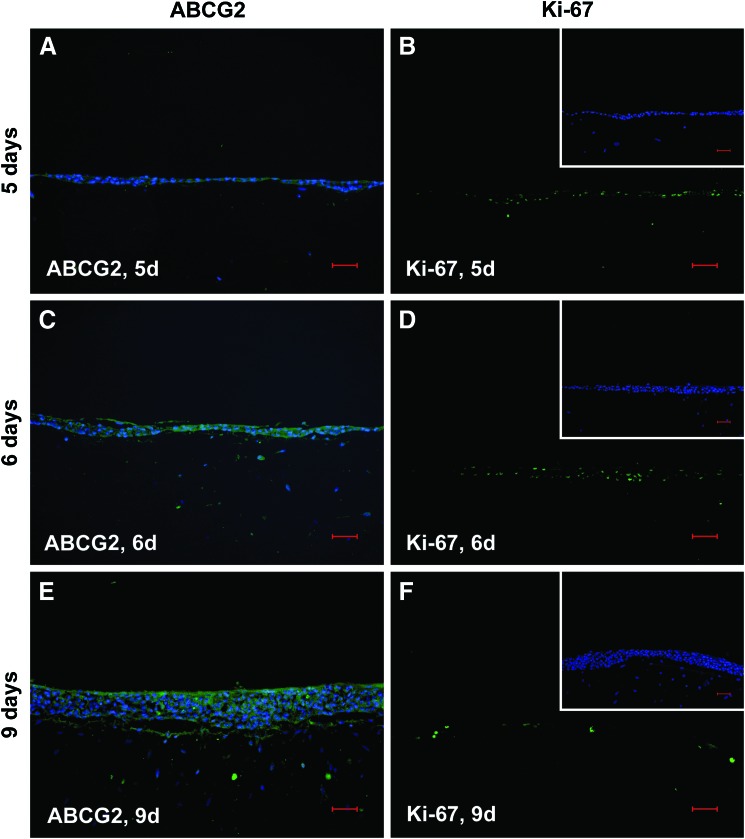
As particularly suggested by the in parts significant increase in gene expression of biomarkers characteristic for progressive and terminal corneal keratinocyte differentiation in case of mode 2, we hypothesized by analogy spatial distribution of such tissue homeostatic markers by trend reflecting an according immunolocalization. Since in this context, the most striking changes were concerning culture periods of 6 and 9 days, we focused on immunohistochemically detected IIF biomarker patterns at these time points. As exemplified in Figure 3 for inv, its fluorescence signal distribution was in fact capturing the entire epithelium, although varying in intensity at day 6, when in mode 2, immortalized corneal keratinocytes were cocultured in the presence of a natural but nonholistic cell microenvironment, as substantiated by the fibroblasts (Fig. 3A). In accordance with the above-mentioned gene expression profiles, this spatiotemporal inv distribution may indicate the regulative role of the cell microenvironment for biomarker topography, which in turn reflects premature corneal epithelial differentiation for mode 2. At day 9, inv fluorescence was only very faint, whereat the signal preferentially decorated apical layer cells and some of those putatively scraped off from the epithelium due to terminal differentiation (Fig. 3B). This faint inv fluorescence was unexpected but concomitant with the observed topography may support mode 2 determined premature in vitro epithelial differentiation as already implied by gene expression and inv topography at the earlier time point. Against this background, we were next interested whether a holistic cell microenvironment would fulfill the needs for corneal keratinocytes to exhibit regular epithelial appearance regarding the panel of tested biomarkers. With respect to terminal differentiation, inv and fil focused preferably on the epithelial apical zone at day 9 in the holistic microenvironment situation, mode 1 (Fig. 4E, F), while at day 5 (Fig. 4A, B) and 6 (Fig. 4C, D), they were also apparent at subapical sites, including suprabasal, parabasal, and, in part, also basal cells. This immunolocalization may indicate time-dependent corneal epithelial normalization of terminal differentiation marker topography in response to holistic mode 1. For CK19 and CK12, indicating less and progressive corneal keratinocyte differentiation stages, a vice versa situation was observable inside the mode 1 corresponding epithelial compartments (Fig. 5). The high incidence of CK19-positive cells, detectable at day 5 (Fig. 5A), progressively diminished at days 6 (Fig. 5C) and 9 (Fig. 5E), while a constant rising of CK12-positive cells could be denoted (Fig. 5B, D, and F: day 5, 6 and 9), and in both cases, markers expressing cells were not restricted to a distinct cell layer or layers at later time points (Fig. 5C, D: day 6; Fig. 5E, F: day 9). The stem cell marker ABCG2 was present at all three time points (Fig. 6A, C, E: day 5, 6, and 9), with the focus of positive cells to the uppermost cell layers at day 9 (Fig. 6E). Proliferation as detected by Ki-67 fairly coincided with ABCG2’ apical preference at day 9 (Fig. 6F), although indicating a decline of cycling cells at this culture period with matched earlier periods of 6 (Fig. 6D) and 5 days (Fig. 6B), respectively, showing no spatial restrictions. This is in accordance with the MKI67 expression results, when comparing decreasing mRNA levels in the holistic mode starting from day 9. The biomarkers of corneal epithelial homeostasis tested in response to mode 1, the holistic microenvironment, reveal time dependency for both expression and immunolocalization. Herewith, they suggest resemblance to normal native corneal epithelial sites, that is, the limbus and central cornea, by their fairly coinciding and thus regular spatial appearance, particularly at culture period day 6 and 9.
FIG. 3.
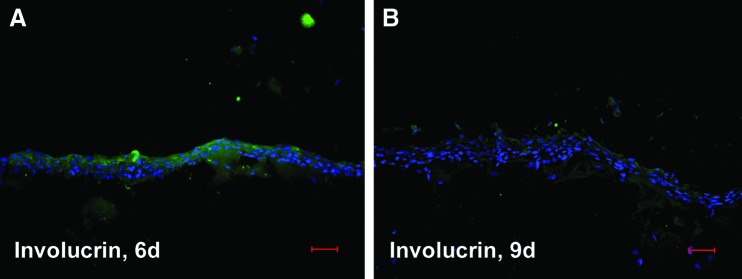
Indirect immunofluorescence (IIF) of IHCK for involucrin (green). IHCK are cocultured with IHCF (nonholistic mode) for 6 days (A) and 9 days (B). Cell nuclei are counterstained by DAPI (blue). Scale bars represent 50 μm. Color images available online at www.liebertpub.com/tec
FIG. 4.
IIF of IHCK for involucrin (A, C, E; green) and filaggrin (B, D, F; green). IHCK are cocultured with IHCF and IHCE embedded in collagen gel (holistic mode) for 5 days (A, B), 6 days (C, D), and for 9 days (E, F). Cell nuclei are counterstained by DAPI (blue). Scale bars correspond to 50 μm. Color images available online at www.liebertpub.com/tec
FIG. 5.
IHCK are cocultured in the holistic mode for 5 days (A, B), 6 days (C, D), and for 9 days (E, F). IHCK are stained by IIF for cytokeratin 19 (CK19; A, C, E; green) and cytokeratin 12 (CK12; B, D, F; green), while cell nuclei are counterstained by DAPI (blue). Scale bars represent 50 μm. Color images available online at www.liebertpub.com/tec
FIG. 6.
IIF of IHCK for ABCG2 (A, C, E; green) and Ki-67 (B, D, F; green). IHCK are cocultured in holistic mode for 5 days (A, B), 6 days (C, D), and for 9 days (E, F). Cell nuclei are counterstained by DAPI (blue). For better visibility of Ki-67-positive cells, cell counterstain is shifted to the respective inlays. Scale bars represent 50 μm. Color images available online at www.liebertpub.com/tec
Discussion
To face the demands of an in vitro method-based animal experiment substitute, in vitro generated tissue equivalents should fulfill some basic criteria for prospectively rendering them hopeful candidates. In the first place, they should comprise species-specific cells. Second, they should include cells of the target tissue, wherever possible, to be addressed by the aspired tests. And third, morphogenesis should yield a tissue equivalent fairly resembling the native counterpart regarding histoarchitecture and regular spatial appearance of tissue homeostatic biomarkers. To adequately take into account these criteria in the context of human ocular surface, we were scanning for the minimum natural cell environmental conditions, to allow human corneal keratinocytes for exhibition of proper morphogenesis.
As a further prerequisite for establishing human corneal epithelial tissue equivalents as precious tools, for example, preclinical validation of ocular surface-directed drugs and/or biomaterials, their constituting cell entities, namely keratinocytes, fibroblasts,38 as well as endothelial cells, should be permanently available in high cell numbers to assure reproducible testing. To achieve these goals, immortalization of corneal cells is mandatory, thereby bypassing natural growth limitations of primary cells.39 To exclude variations in corneal epithelial equivalent outcome, that is, morphogenesis and spatiotemporal expression of biomarkers from different cell transformation stages, we employed immortalized human corneal cells in this study, previously established by the own group, using the E6/E7 oncogenes of HPV-16 as a uniform immortalization platform. This is important, since it has been shown for a long time that the use of SV-40 large T-antigen as an epithelial keratinocyte immortalizing agent leads to serious damage in expressing tissue-innate biomarkers assigning to differentiation.40 Impact on epithelial cell differentiation has more recently been reported also for using SV40 T-antigen early region genes only.24 Moreover, corneal model systems existing so far often comprise cells of different transformation stages or primary cells only,18–21 thereby hampering elaboration of durable and/or consistent conclusions to be drawn from their results, and thus, implications to the in vivo situation.
As suggested by corneal keratinocyte morphogenesis, the yielding corneal epithelial tissue equivalents were able to exhibit morphological features also seen in the tissue of origin, exclusively in response to holistic microenvironments. Herein, the presence of both fibroblasts and endothelial cells seemed to be crucial for the accomplishment of balanced morphogenesis, as indicated at day 6, where cuboidal columnar basal cells were visible, but also at day 9, when stratified cell layers harbored flattened cells. Although not analyzed in detail, it cannot be excluded that mutual interplay of all three cell entities through diffusible growth factors enables the different cell types to fulfill their tasks similar to the in vivo situation. Thereby, they essentially contribute to the cause of the morphological outcome observed, which resembles sites of the limbus-central cornea transition zone, also found in the autochthonous normal corneal epithelium.11 This assumption appears possible, since for other epithelial entities like oral mucosa and skin, such growth factor dependencies and interplays have extensively been described in epithelial descending in vitro tissue equivalents.17,41–44
In the presence of natural, but nonholistic cell microenvironments (mode 2), as substantiated by fibroblasts only, the presence of fully spread and thus flattened epithelial cells, already visible at day 6, in conjunction with foci of obvious cell ablation strongly suggests premature corneal keratinocyte differentiation concomitant with deranged epithelial entity. This suggestion of differentiation prematurity was reinforced by the transcription profiles, particularly indicated by the overshooting gene expression for biomarkers FIL and CK12, and to a less though significant extent for inv too. All biomarkers are reflecting progressive and terminal keratinocyte differentiation stages in the context of tissue homeostasis of normal native stratified epithelia, including human cornea.11 The inverse relation between cuboidal, that is, rounded, less differentiated, and spread, that is, a flattened differentiated cell shape, the latter going along with induction of terminal differentiation marker inv, has been extensively studied in cultures of human new-born foreskin keratinocytes.45 This supports our aforementioned suggestion. Coinciding with the morphological features of cell flattening, observed in 5-day-old corneal epithelial equivalents in response to mode 2 and described by Watt and coworkers for cultured epithelial cells, pan-epithelial inv expression was detectable by IIF in these equivalents. This coincidence thereby reflects an analogy among the situation of inv induction in spread cells described for the previously mentioned in vitro monolayer cultures and our 3D coculture epithelial equivalent situation, which has developed under natural, but nonholistic cell microenvironmental conditions. An unexpected and thus intriguing finding was the drastic increase of ABCG2 mRNA synthesis in IHCK, particularly seen in response to mode 2. Although a significant transcriptional increase has also been detected for the other modes when cell microenvironments were holistic and/or missing, in case of mode 1, the apparent difference between the significances observed, was less extensive. Although normally indicating stem cell character in many cell entities, also including corneal keratinocytes, the significances in ABCG2 elevation in our study may be more associated with the in vitro coculture situation, which per se is comparable with a regenerative tissue situation, rather than with stem cell characteristics. The regenerative situation provided by the coculture setup can be hypothesized veritable, due to a limited number of corneal keratinocytes on the top of the collagen scaffold, initiating morphogenesis and thus development of an epithelial tissue equivalent, like in an epithelium after wounding. The authenticity of this hypothesis is substantiated by animal experiments focusing on skeletal muscle, where the ABCG2 increase has been denoted in cells upon injury. Moreover, muscle regeneration has been found impaired by consequently yielding fewer myofibrils in mice devoid of the ABCG2 gene.46 In the context of our tissue equivalents, ABCG2 as putative indicator of a regenerative situation may reveal a balance toward normal tissue formation for the holistic mode 1 microenvironment, as indicated by the potential steady-state transcription levels found at day 5 and 9. On the other hand, transcription profiles particularly detected for mode 2, and also mode 3, may point to imbalances in such tissue formation.
Conclusion
In summary, a balanced situation for the chronology of corneal keratinocyte morphogenesis appears to be clearly determined and thus regulated by the cellular microenvironment, as strongly evidenced by the comparison of nonholistic/mode 2 with holistic/mode 1 microenvironmental counterparts. Normal tissue typical apical cell flattening in uppermost layers, concomitant with a faint increase in differentiation biomarker gene expression, and finally, a regular apical protein appearance of inv and fil in conjunction with CK19 diminishment and CK12 elevation, has been only observed in corneal epithelial tissue equivalents responding to mode 1. The holistic microenvironment may therefore be considered a model system, allowing corneal keratinocytes for transiently exhibiting mimics of in vivo tissue homeostasis also under in vitro conditions. This conclusion is backed up by the described morphogenesis and spatiotemporal immunolocalization of corneal homeostatic biomarkers, suggesting chronological progression of differentiation accompanied by proliferation decline over time. By these features, mode 1 yielding engineered tissue equivalents resemble tissue sites of the normal native corneal limbal central cornea transition zone.11 By contrast, mode 2 led to pan-epithelial inv abundance already at day 6, and almost lack of this terminal marker at day 9 herewith proving imbalanced microenvironmental regulation of corneal keratinocyte development. The presence of transient tissue homeostasis mimics in vitro observed for mode 1 is coinciding with tissue equivalents of other 3D coculture models derived from oral mucosa and skin, and here also substantiated by morphogenesis as well as proliferation and differentiation.16,17,47 Based on our observations, the holistic mode 1 microenvironment has been identified as the critical prerequisite for proper corneal tissue equivalent development, rendering it a promising candidate for an in vitro method-based animal experiment substitute with emphasis on experimental clinically relevant cornea-focusing issues.
Acknowledgments
We are grateful to Yrgalem Abreha for excellent technical assistance. This work has been supported by a grant from the Deutsche Forschungsgemeinschaft/DFG to Philipp Eberwein: EB-478/2-1 and Pascal Tomakidi: TO-198/10-1.
Disclosure Statement
The authors confirm that there are no conflicts of interest.
References
- 1.Pearson R.M.In-vitro techniques: can they replace animal testing? Hum Reprod 1,559, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Matthews R.A.Medical progress depends on animal models—doesn't it? J R Soc Med 101,95, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickson M.A., Hahn W.C., Ino Y., Ronfard V., Wu J.Y., Weinberg R.A., et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol 20,1436, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivetti di Val Cervo P., Lena A.M., Nicoloso M., Rossi S., Mancini M., Zhou H., et al. p63-microRNA feedback in keratinocyte senescence. Proc Natl Acad Sci U S A 109,1133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fusenig N.E., Limat A., Stark H.J., and Breitkreutz D.Modulation of the differentiated phenotype of keratinocytes of the hair follicle and from epidermis. J Dermatol Sci 7 Suppl,S142, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Presland R.B., and Dale B.A.Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med 11,383, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Schlotzer-Schrehardt U., and Kruse F.E.Identification and characterization of limbal stem cells. Exp Eye Res 81,247, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Koster M.I., and Roop D.R.Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol 23,93, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bragulla H.H., and Homberger D.G.Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat 214,516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S.K., Teng Y., Wong H.K., Ng T.K., Huang L., Lei P., et al. MicroRNA-145 regulates human corneal epithelial differentiation. PLoS One 6,e21249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberwein P., Steinberg T., Schulz S., Zimmermann D., Accardi R., Beck D., et al. Expression of keratinocyte biomarkers is governed by environmental biomechanics. Eur J Cell Biol 90,1029, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Chapman S., Liu X., Meyers C., Schlegel R., and McBride A.A.Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest 120,2619, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Offord E.A., Sharif N.A., Mace K., Tromvoukis Y., Spillare E.A., Avanti O., et al. Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Invest Ophthal Vis Sci 40,1091, 1999 [PubMed] [Google Scholar]
- 14.Schmedt T., Chen Y., Nguyen T.T., Li S., Bonanno J.A., and Jurkunas U.V.Telomerase immortalization of human corneal endothelial cells yields functional hexagonal monolayers. PLoS One 7,e51427, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boehnke K., Mirancea N., Pavesio A., Fusenig N.E., Boukamp P., and Stark H.J.Effects of fibroblasts and microenvironment on epidermal regeneration and tissue function in long-term skin equivalents. Eur J Cell Biol 86,731, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Tomakidi P., Breitkreutz D., Fusenig N.E., Zoller J., Kohl A., and Komposch G.Establishment of oral mucosa phenotype in vitro in correlation to epithelial anchorage. Cell Tissue Res 292,355, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Roesch-Ely M., Steinberg T., Bosch F.X., Mussig E., Whitaker N., Wiest T., et al. Organotypic co-cultures allow for immortalized human gingival keratinocytes to reconstitute a gingival epithelial phenotype in vitro. Differentiation 74,622, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Orwin E.J., and Hubel A.In vitro culture characteristics of corneal epithelial, endothelial, and keratocyte cells in a native collagen matrix. Tissue Eng 6,307, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Reichl S., Bednarz J., and Muller-Goymann C.C.Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br J Ophthalmol 88,560, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer L., Bednarz J., Müller-Goymann C., and Reichl S.Esteraseaktivität eines organotypischen humanen Kornea-Konstrukts (HCC) als In-vitro-Modell für Permeationsuntersuchungen. Der Ophthalmol 102,971, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Papini S., Rosellini A., Nardi M., Giannarini C., and Revoltella R.P.Selective growth and expansion of human corneal epithelial basal stem cells in a three-dimensional-organ culture. Differentiation 73,61, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Proulx S., d'Arc Uwamaliya J., Carrier P., Deschambeault A., Audet C., Giasson C.J., et al. Reconstruction of a human cornea by the self-assembly approach of tissue engineering using the three native cell types. Mol Vis 16,2192, 2010 [PMC free article] [PubMed] [Google Scholar]
- 23.Fusenig N.E., and Boukamp P.Multiple stages and genetic alterations in immortalization, malignant transformation, and tumor progression of human skin keratinocytes. Mol Carcinog 23,144, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Toouli C.D., Huschtscha L.I., Neumann A.A., Noble J.R., Colgin L.M., Hukku B., et al. Comparison of human mammary epithelial cells immortalized by simian virus 40 T-Antigen or by the telomerase catalytic subunit. Oncogene 21,128, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Commission E.Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 and of the council of 22 September 2010 on the protection of animals used for scientific purposes. 2010. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF
- 26.Guenoun J.M., Baudouin C., Rat P., Pauly A., Warnet J.M., and Brignole-Baudouin F.In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci 46,2444, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Choy C.K., Cho P., Boost M.V., and Benzie I.F.Do multipurpose solutions damage porcine corneal epithelial cells? Optom Vis Sci 86,E447, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Pear W.S., Nolan G.P., Scott M.L., and Baltimore D.Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A 90,8392, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Ordonez P., and Di Girolamo N.Limbal epithelial stem cells: role of the niche microenvironment. Stem cells 30,100, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Mussig E., Steinberg T., Kohl A., Chamulitrat W., Komposch G., and Tomakidi P.Discrimination of epithelium-like and fibroblast-like phenotypes derived from ethanol-treated immortalised human gingival keratinocytes in epithelial equivalents. Cell Tissue Res 332,57, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Winter S., Kohl A., Huppertz A., Herold-Mende C., Wiest T., Komposch G., et al. Expression of mRNAs encoding for growth factors, ECM molecules, and MMP13 in mono-cultures and co-cultures of human periodontal ligament fibroblasts and alveolar bone cells. Cell Tissue Res 319,467, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Fuchs E., and Horsley V.More than one way to skin. Genes Dev 22,976, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong L., Corrales R.M., Chen Z., Villarreal A.L., De Paiva C.S., Beuerman R., et al. Expression and regulation of cornified envelope proteins in human corneal epithelium. Invest Ophthalmol Vis Sci 47,1938, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding X.W., Wu J.H., and Jiang C.P.ABCG2: a potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sci 86,631, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Nubile M., Lanzini M., Miri A., Pocobelli A., Calienno R., Curcio C., et al. In vivo confocal microscopy in diagnosis of limbal stem cell deficiency. Am J Ophthalmol 155,220, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Cresta F.B., and Alves M.R.[Evaluation of the corneal epithelium kinetics using cell proliferation markers]. Arq Bras Oftalmol 70,953, 2007 [DOI] [PubMed] [Google Scholar]
- 38.West-Mays J.A., and Dwivedi D.J.The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol 38,1625, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundberg A.S., Randell S.H., Stewart S.A., Elenbaas B., Hartwell K.A., Brooks M.W., et al. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene 21,4577, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Steinberg M.L., and Defendi V.Transformation and immortalization of human keratinocytes by SV40. J Invest Dermatol 81,131s, 1983 [DOI] [PubMed] [Google Scholar]
- 41.Smola H., Thiekotter G., and Fusenig N.E.Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J Cell Biol 122,417, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maas-Szabowski N., Stark H.J., and Fusenig N.E.Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J Invest Dermatol 114,1075, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Maas-Szabowski N., Szabowski A., Stark H.J., Andrecht S., Kolbus A., Schorpp-Kistner M., et al. Organotypic cocultures with genetically modified mouse fibroblasts as a tool to dissect molecular mechanisms regulating keratinocyte growth and differentiation. J Invest Dermatol 116,816, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Stark H.J., Szabowski A., Fusenig N.E., and Maas-Szabowski N.Organotypic cocultures as skin equivalents: a complex and sophisticated in vitro system. Biol Proc Online 6,55, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watt F.M., Jordan P.W., and O'Neill C.H.Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc Natl Acad Sci U S A 85,5576, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doyle M.J., Zhou S., Tanaka K.K., Pisconti A., Farina N.H., Sorrentino B.P., et al. Abcg2 labels multiple cell types in skeletal muscle and participates in muscle regeneration. J Cell Biol 195,147, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maas-Szabowski N., Starker A., and Fusenig N.E.Epidermal tissue regeneration and stromal interaction in HaCaT cells is initiated by TGF-alpha. J Cell Sci 116,2937, 2003 [DOI] [PubMed] [Google Scholar]