Abstract
The amniotic membrane (AM) is a naturally derived biomaterial that possesses biological and mechanical properties of great importance for tissue engineering. The aim of our study was to determine whether the AM enables the formation of a normal urinary bladder epithelium—urothelium— and to reveal any differences in the urothelial cell (UC) growth and differentiation when using different AM scaffolds. Cryopreserved human AM was used as a scaffold in three different ways. Normal porcine UCs were seeded on the AM epithelium (eAM), denuded AM (dAM), and stromal AM (sAM) and were cultured for 3 weeks. UC growth on AM scaffolds was monitored daily. By using electron microscopy, histochemical and immunofluorescence techniques, we here provide evidence that all three AM scaffolds enable the development of the urothelium. The fastest growth and the highest differentiation of UCs were demonstrated on the sAM scaffold, which enables the development of tissue-engineered urothelium with molecular and ultrastructural properties comparable to that of the native urothelium. Most importantly, the highly differentiated urothelia on the sAM scaffolds provide important experimental models for future drug delivery studies and developing tissue engineering strategies considering that subtle differences are identified before translation to the clinical settings.
Introduction
The amniotic membrane (AM) is the innermost layer of the placenta. It consists of a monolayer of amniotic epithelial cells (AECs), a thick basal lamina, and an avascular stroma, which is further subdivided into compact, fibroblast, and spongy layers.1 The AM has unique biological and mechanical qualities that render it a desirable biomaterial for tissue engineering.
Studies on the cryopreserved AM have revealed the presence of various growth factors such as epidermal growth factor (EGF), transforming growth factor (TGF)-α, -β1, -β2, and -β3, keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor, and also platelet-derived growth factor (PDGF).2 These growth factors might contribute to AM ability to reduce scarring and inflammation, to enhance wound healing, and promote epithelialization.3 The AM also possesses antimicrobial properties, and shows low or no immunogenicity since it has not been associated with graft rejection after transplantation.4,5 Since the AM must bear the load of pressure from the amniotic fluid during gestation, it has also the mechanical strength important to withstand the stress induced during the growth of tissue.6 Besides the attractive biological properties, the AM is easy to obtain and is readily available.
The AM has been successfully applied in a variety of clinical studies, such as management of burns7 and ulcers,8 tissue reconstruction of the vagina,9 and prevention of postoperative adhesions,10 but is still most widely used in ophthalmology for ocular surface reconstruction.11–13
The AM can be used as a scaffold either with the amniotic epithelium (epithelial AM [eAM]) or without it (denuded AM [dAM]).14 In addition, the AM stromal side (AM stroma [sAM]) also has proven to be a promising scaffold.15,16
The urothelium is a three- to five-layered epithelium, covering the mucosal surface of the renal pelvis, ureter, urinary bladder, and proximal urethra.17 The primary function of the urothelium is to provide the tightest and most impermeable barrier in the body, that is, the blood–urine barrier.18 Since the urinary tract is exposed to a variety of possible injures that may lead to organ damage or loss, the appropriate scaffold, which would enable proliferation and differentiation of urothelial cells (UCs) in vitro as well as their implantation into organisms, would have a number of applications. So far, two published studies investigated the suitability of the AM scaffold for UC cultivation, but showed contradictory results. Sharifiaghdas et al.19 confirmed urothelium-like tissue formation using dAM as a scaffold for mouse UCs, whereas Sartoneva et al.20 described the dAM scaffold as inappropriate for human UC cultivation. Due to the current controversy of AM suitability for UCs, the detailed molecular and ultrastructural status of the UCs on AM scaffolds should be provided.
The present study investigates the suitability of eAM, dAM, and sAM as scaffolds for porcine UCs. Our aim was to determine whether the AM enables the formation of a normal urothelium and to reveal any differences in UC growth and differentiation when using different AM scaffolds. For this aim, we defined the histochemical composition of the AM scaffolds, monitored the UC growth on different AM scaffolds, determined the UC phenotype with immunofluorescence analysis of differentiation-related markers, and studied the UC ultrastructure with electron microscopy.
Materials and Methods
Preparation of tissue constructs of human AM scaffolds and porcine UCs
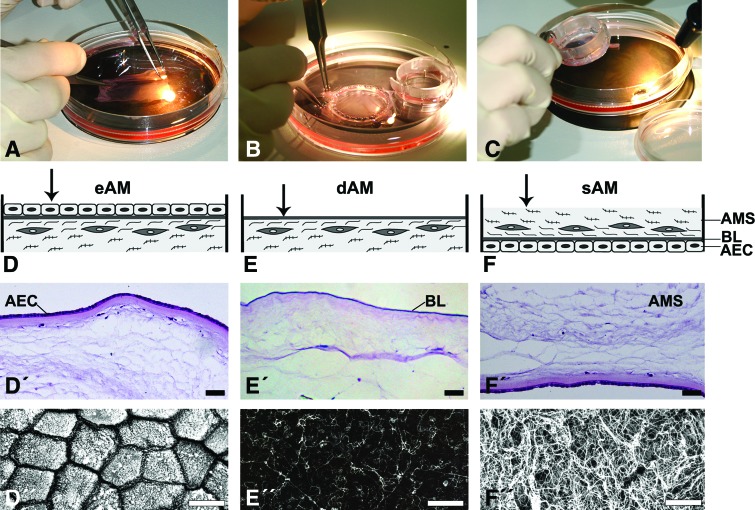
The use of human AM was approved by the National Medical Ethics Committee and prepared following the standard procedure.21 Briefly, human AMs were obtained with informed consent at the time of elective caesarean sections from volunteer mothers. Human immunodeficiency virus, syphilis, and hepatitis B and C had been excluded by serological tests. The placenta was washed aseptically with phosphate-buffered saline (PBS) containing 50 μg/mL penicillin, 50 μg/mL streptomycin, 100 μg/mL neomycin, and 2.5 μg/mL amphotericin B. The AM was then manually separated from the horion, cut into pieces of 4×4 cm2, and cryopreserved at −80°C in the Eagle's medium and glycerol in a volume ratio 1:1. Before use, frozen AMs were thawed, washed with sterile PBS and culture medium, and fastened with membrane holders of 14 or 25 mm in diameter (CellCrown; Scafdex) (Fig. 1A–C) with the epithelial side facing upward (eAM; dAM) or downward (sAM) (Fig. 1D-F). To obtain the dAM, half of the AMs fastened with the epithelial side upward were then deprived of AECs by a 22-min incubation in the proteolytic enzyme thermolysin (1:50; Sigma), at 37°C (Fig. 1E). After the incubation with thermolysin, AMs were immediately rinsed and washed two times for 15 min in PBS, with shaking to remove cellular debris.
FIG. 1.
Preparation of the amniotic membrane (AM) scaffolds. (A–C) AMs were thawed, washed with sterile phosphate-buffered saline and culture medium, and then fastened with the membrane holders of 14 or 25 mm in diameter. (D–F) Schematic representation of three different AM scaffolds. Urothelial cells (UCs) were seeded on (D, D′, D′′) epithelial AM (eAM), which is directly on amniotic epithelial cells (AEC), on (E, E′, E′′) denuded AM (dAM), which is on amniotic basal lamina (BL), and on (F, F′, F′′) stromal AM (sAM), which is on the spongy layer (SL) of AM stroma (AMS). Before UC seeding, the histology and topography of each AM scaffold was evaluated. Histological sections showed uniform lining of AEC on eAM and sAM scaffolds (D′, F′) and completely removed AECs after thermolysin treatment (E′). Scanning electron micrographs showed (D′′) the apical surface of AECs, (E′′) amniotic basal lamina after thermolysin treatment, and (F′′) spongy layer of sAM. Scale bars: (D′–F′) 100 μm, (D′′–F′′) 10 μm. Color images available online at www.liebertpub.com/tec
Porcine urinary bladders were obtained from a local abattoir. Primary and secondary UC cultures were established and subcultured as described previously.22 UCs were seeded on differently prepared AM scaffolds (Fig. 1D'–F'') at a seeding density of 2×105/cm2.
The culture medium, consisting of a 1:1 mixture of the MCDB 153 medium (Sigma) and Advanced-DMEM medium (Invitrogen, Gibco), supplemented with 2.5% fetal bovine serum (Invitrogen), adenine (15 μg/mL), insulin (5 μg/mL), hydrocortisone (0.5 μg/mL), phosphoetanolamine (0.1M) (all Sigma), and glutamax (4 mM) (Invitrogen), was changed three times a week. Tissue constructs were maintained at 37°C in a humidified atmosphere of 5% CO2 (v/v) in air for 3 weeks.
Analysis of UC growth on different AM scaffolds
To evaluate the growth of UCs on different AM scaffolds, UCs were observed with a transmitted light inverted microscope Eclipse TE300 (Nikon). The first 3 days and at the end of each week, eight random images of each UC culture were taken. The area of AM scaffold covered with UCs was evaluated with ImageJ software, measuring this area as a percentage of the total field view area.
Histological, histochemical, and immunofluorescence analysis
The AM itself, the 3-week UC cultures grown on different AM scaffolds, that is, tissue constructs and porcine urinary bladder in vivo, were fixed in 4% paraformaldehyde for histological and histochemical staining or in 1% paraformaldehyde for immunofluorescence. Samples were dehydrated through a graded series of ethanol into xylene and embedded in paraffin wax. Once dewaxed, paraffin sections were stained with hematoxylin–eosin for histology, Periodic Acid Schiff stain (PAS) for neutral proteoglycans, Alcian Blue for acid proteoglycans, or Van Gieson for collagen fibers (all Sigma) or incubated in a rabbit polyclonal anti-collagen IV antibody (1:200; Abcam) or a rabbit polyclonal anti-collagen VII antibody (1:200; Calbiochem, Merck) for immunolabeling of basal lamina. Goat anti-rabbit IgG conjugated with AlexaFluor 555 (1:200; Invitrogen) were used as secondary antibodies. After washing in PBS, the samples were mounted in the mounting medium Vectashield with DAPI (Vector Laboratories) for DNA labeling. Sections were observed with a transmitted light or inverted fluorescence microscope Eclipse TE300 (Nikon).
After 3 weeks in culture, tissue constructs were fixed in ice-cold absolute ethanol for 25 min at room temperature. Following the preincubation in the 1% bovine serum albumin (Sigma), uroplakins were immunolocalized by a rabbit polyclonal anti-uroplakin antibody (1:1000, Prof. T.-T. Sun, New York University, School of Medicine), and tight junctional protein occludin by a rabbit polyclonal anti-occludin antibody (1:20; Zymed). Goat anti-rabbit IgG conjugated with AlexaFluor 488 (1:200; Invitrogen) were used as secondary antibodies. After washing, the samples were mounted in the mounting medium Vectashield with DAPI (Vector Laboratories) for DNA labeling. Tissue constructs were examined with a fluorescence microscope AxioImager.Z1 equipped with ApoTome (Zeiss). To quantitatively evaluate the fluorescence intensity of uroplakins, 20 random images of superficial UCs for each tissue construct were made. Using AxioVision 4.8 software (Zeiss) the fluorescence intensity of uroplakins for each image was measured.
Transmission and scanning electron microscopy
AM scaffolds, 3-week tissue constructs of urothelium on AM scaffolds, and porcine urinary bladder in vivo were prepared as described previously.23,24 Briefly, they were fixed with 4% (w/v) paraformaldehyde and 2.5% (v/v) glutaraldehyde in a 0.1 M cacodylate buffer, pH 7.4 for 2 h and 45 min. The fixation was followed by overnight rising in the 0.1 M cacodylate buffer and a postfixation in 1% (w/v) osmium tetroxide for 1 h at 4°C. For transmission electron microscopy (TEM), the samples were then dehydrated in a graded series of ethanol and embedded in Epon (Serva Electrophoresis). Ultrathin sections were contrasted with uranil acetate and lead citrate and observed in a transmission electron microscope (Philips CM100). After dehydration through a graded series of acetone, the samples for scanning electron microscopy (SEM) were dried at the critical point, spattered with gold, and observed in a scanning electron microscope (Jeol 840A).
Analysis of superficial UC size and differentiation on different AM scaffolds
To evaluate the size and differentiation of 3-week superficial UCs on different AM scaffolds, nine random images of each 3-week tissue construct were taken using SEM and further analyzed with ImageJ software. For UC size evaluation, a maximum calliper diameter of 20 superficial UCs randomly selected from images of each tissue construct was measured. For UC differentiation assessment, areas with highly and poorly differentiated superficial UCs were measured from each image taken. Highly differentiated UCs were categorized as cells with their apical plasma membrane mostly shaped into rounded ridges or microridges, and poorly differentiated UCs were categorized as the cells, which have their apical plasma membrane shaped into microvilli or ropy ridges.
Statistical analysis
All data are presented as mean±standard error. Statistical analysis was performed using a two-tailed Student's t-test. p-Values of<0.05 were considered statistically significant.
Results
AM scaffold characterization
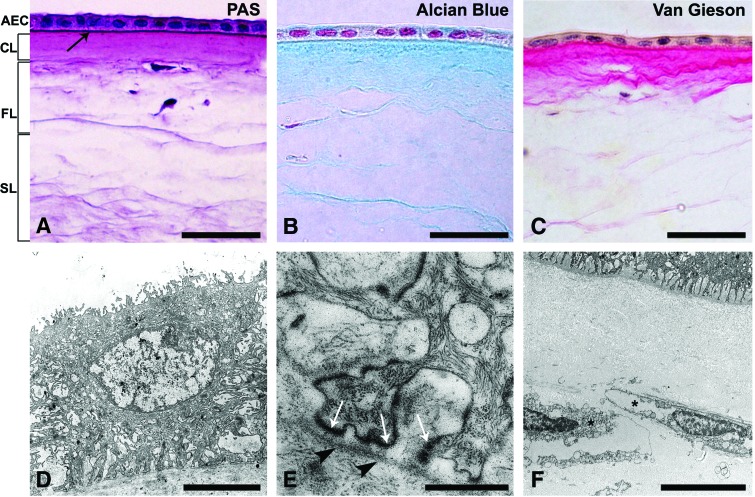
All AMs were composed of an epithelial monolayer, basal lamina, and stromal matrix consisting of compact, fibroblast, and spongy layers (Fig. 2). Staining with PAS, Alcian Blue, and Van Gieson showed the presence of neutral proteoglycans, acid proteoglycans, and collagens in AM stroma, respectively (Fig. 2A–C). TEM confirmed the characteristic AM structure, but has furthermore showed damaged and devitalized AECs and fibroblasts (Fig. 2D–F). During 3 weeks in culture, the gradual exfoliation of AECs was observed on the eAM and sAM scaffolds.
FIG. 2.
AM scaffold characterization. (A) The AM consists of a monolayer of AEC, basal lamina (arrow), and stromal matrix, further composed of compact (CL), fibroblast (FL), and spongy layers (SL). Using Periodic Acid Schiff reaction, thick amniotic basal lamina (arrow) and neutral proteoglycans (purple-red) in AM stroma were demonstrated. Staining with Alcian Blue (B) and Van Gieson (C) confirmed the presence of acid proteoglycans (blue) and collagen fibers (light red) in the AM stromal matrix, respectively. Collagens were also abundant in the basal lamina. (D) Transmission electron microscopy revealed damaged AECs with a vacuolar cytoplasm. (E) Their cell base formed podocyte-like processes attached to the thick basal lamina (arrowheads) by hemidesmosomes (white arrows). (F) Fibroblasts (*) in the fibroblast layer of AM stroma were damaged and devitalized. Scale bars: (A–C) 50 μm, (D) 5 μm, (E) 500 nm, and (F) 10 μm. Color images available online at www.liebertpub.com/tec
UC growth on different AM scaffolds
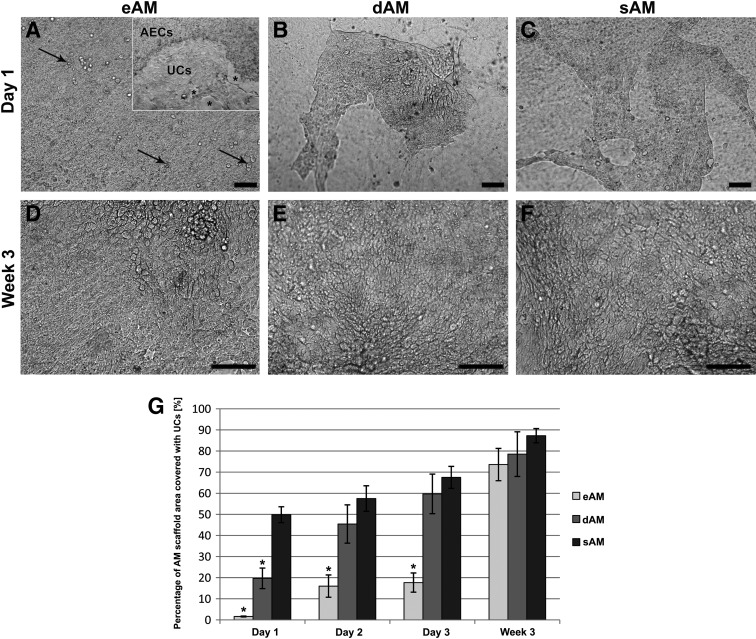
UCs proliferated most rapidly on the sAM scaffold where large islands of UCs, covering 50% of the scaffold surface, were formed already the first day after seeding (Fig. 3A–C). After 1 week, proliferation decreased and a significant difference between different AM scaffolds was no longer evident. A similar trend of UC growth was seen on the dAM. On the eAM, the proliferation of UCs was the slowest. In all AM scaffolds, the UCs overgrew almost the entire scaffold surface in 3 weeks (Fig. 3D–G), although the three-to-five-layered urothelium was formed only on the sAM (Fig. 5A–I).
FIG. 3.
UCs on different AM scaffolds observed with transmitted light inverted microscope. (A) The first day after seeding, single UCs attached on eAM (black arrows). (A) On the third day, the UCs on eAM already formed small islands. Gradually with the UC expansion, displacement of AECs was noted. Asterisks indicate the area of exfoliated AECs. (D, G) In the following days, the UC proliferation on eAM remained slow, but continuous. (B) On dAM, the first day after seeding, UCs formed large islands and (E) continued to proliferate until just confluence. (C) The fastest UC proliferation was noted on sAM, where already on the first day after seeding, UCs covered half of the scaffold surface. (F) After the first days, the UC proliferation on sAM decelerated and in the third week, the difference in UC growth between AM scaffolds was no longer significant. (G) Graph displays mean percentage of AM scaffold area covered with UCs and standard error, *p<0.05. Scale bars: 100 μm.
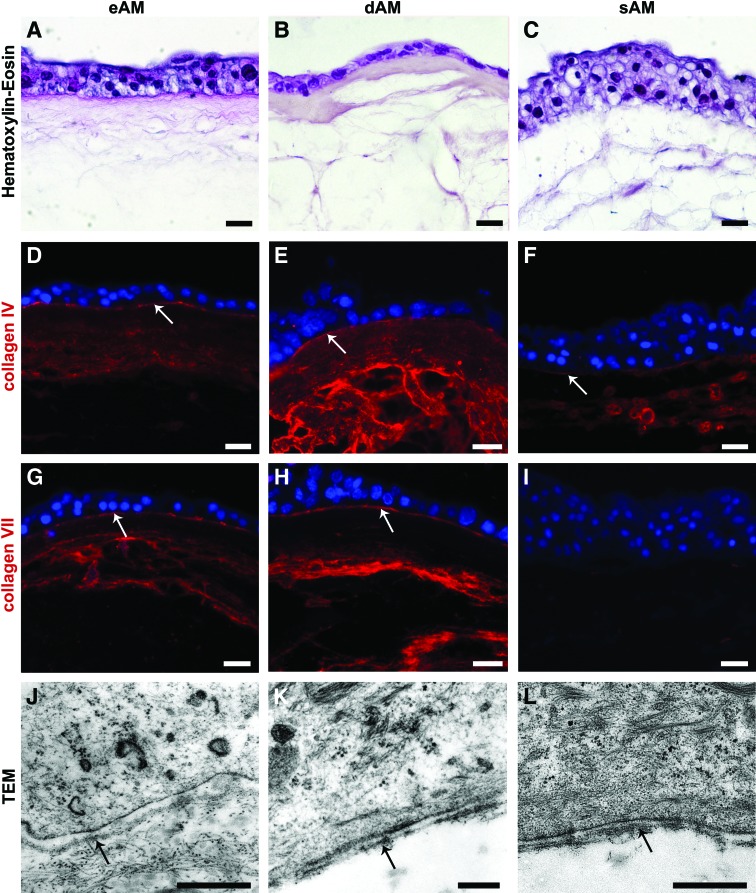
FIG. 5.
Histological, histochemical, and ultrastructural analyses of established tissue constructs. (A–C) Hematoxylin–eosin. The urothelium was established on all three different scaffolds. On eAM and dAM, the urothelium was two-to-four layered, on sAM three-to-five layered. (D–I) Immunolabeling with anti-collagen IV and anti-collagen VII and (J–L) TEM, showed that on the eAM and dAM scaffolds, UCs attached on to the AM basal lamina (white and black arrows on D, E, G, H, J, and K). On the sAM scaffold, the UCs formed the basal lamina (F), expressing collagen IV (white arrow on F), but not collagen VII (I). DNA (nuclei) is blue. Scale bars: (A–I) 20 μm, (J, L) 500 nm, (K) 200 nm. Color images available online at www.liebertpub.com/tec
The molecular characterization of UCs grown on different AM scaffolds
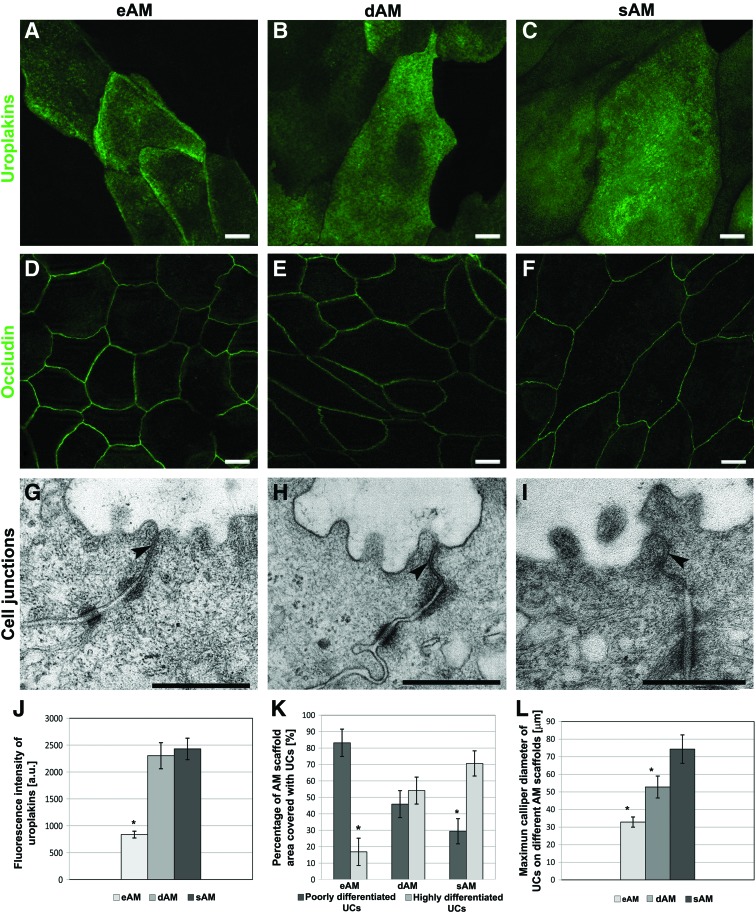
Expression of uroplakins was demonstrated in all 3-week-old tissue constructs (Fig. 4A–C). However, the fluorescence intensity of uroplakins varied, depending on which AM scaffold the UCs were cultured (Fig. 4J). The highest fluorescence intensity of uroplakins was measured in UCs cultured on sAM (2429±178 a.u.). A slightly lower fluorescence intensity was measured in UCs on dAM (2303±242 a.u.), while the fluorescence intensity of uroplakins was significantly lower (837±63 a.u.) in UCs on eAM compared to the other two.
FIG. 4.
The distribution of urothelial differentiation-related markers and ultrastructural analysis of UCs grown on AM scaffolds. (A–F) After 3 weeks of UC cultivation, the expression of (A–C) uroplakins and (D–F) occludin was demonstrated in UCs on different AM scaffolds. (G–I) The presence of well-developed cell junctions was confirmed with transmission electron microscopy (TEM), further indicating that the most developed tight junctions (arrowheads) and membranes overlap, which fasten two superficial UCs better together, were established on the sAM scaffold. (J–L) After 3 weeks, the superficial UCs on the eAM scaffold had the lowest fluorescence intensity of uroplakins (J), they were poorly differentiated (K) and small (L). The superficial UCs on the dAM were larger (L) and reached a higher stage of differentiation (K). Additionally, the uroplakin fluorescence intensity was higher (J). The largest (L) and most differentiated superficial UCs (K) with the highest fluorescence intensity of uroplakins were found on the sAM scaffold (J). Graphs display mean and standard error, *p<0.05. Scale bars: (A–F) 10 μm, (G–I) 500 nm. Color images available online at www.liebertpub.com/tec
The expression of occludin revealed well-developed tight junctions between the adjacent UCs on all three AM scaffolds (Fig. 4D–F). However, the tight junctions between superficial UCs on eAM and dAM were more diffusely labeled with anti-occludin antibodies, while the most developed tight junctions were established on the sAM scaffold. This was shown by thin and continuous lines of occludin immunolabeling and was further confirmed by TEM. Additionally, on sAM, the overlapping plasma membranes of adjacent superficial UCs were found (Fig. 4G–I).
Immunofluorescence of collagen IV and TEM revealed that the UCs formed the basal lamina de novo when cultured on the sAM scaffold (Fig. 5F, L). However, de novo formed basal lamina did not express collagen VII (Fig. 5I). On the eAM and dAM scaffold, the immunolabeling of collagen IV and collagen VII (Fig.5D, E, G, H) confirmed that UCs attached on AM basal lamina (Supplementary Fig. S1A–D; Supplementary Data are available online at www.liebertpub.com/tec).
The ultrastructural characterization of UCs grown on different AM scaffolds
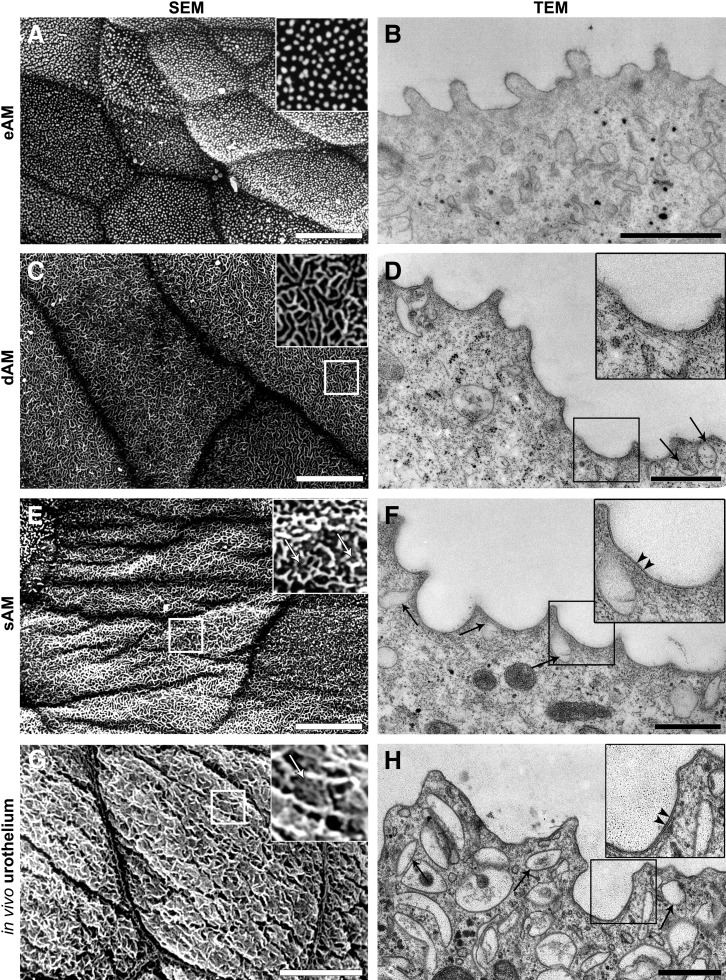
To determine the differentiation of UCs grown on different AM scaffolds, the ultrastructure of UCs was examined by TEM and SEM. After 3 weeks, most of the superficial UCs on eAM were poorly differentiated (Fig. 4K). Superficial UCs were small (32.9±2.9 μm in diameter) (Fig. 4L) with their apical plasma membrane covered with microvilli (83.2%±8.3%) (Fig. 6A, B). On dAM, larger (52.8±6.2 μm) and more differentiated UCs were found (Fig. 4K, L). More than half of the superficial UCs exhibited an apical plasma membrane shaped into ropy and rounded ridges (54.1%±8.12%); in addition, UCs with microridges were also found (Fig. 6C, D). UCs seeded on sAM reached the highest differentiation stage (Fig. 4K). Superficial UCs were the largest of all (74.4±8.1 μm) (Fig. 4L), with their apical plasma membrane shaped mostly into rounded ridges and microridges (70.62%±7.65%), and with the highest density of discoidal or fusiform-shaped vesicles in the apical cytoplasm (Fig. 6E, F). Urothelia on sAM reached the differentiation stage comparable to that of urothelia in vivo (Fig. 6G, H).
FIG. 6.
The apical plasma membrane ultrastructure of UCs grown on different AM scaffolds 3 weeks after seeding and of the UCs in vivo. (A, B) After 3 weeks, most of the superficial UCs on eAM were undifferentiated and the apical plasma membrane was shaped into microvilli. (C, D) The majority of the superficial UCs grown on dAM were partially differentiated. Their apical plasma membrane was shaped into rounded ridges with regions of scalloped appearance as shown on the transmission electron micrographs. Additionally, a few discoidal or fusiform-shaped vesicles (DFVs) in the apical cytoplasm of superficial UCs were found (black arrows). (E, F) The superficial UCs reached the highest differentiation stage when cultured on the sAM scaffold. After 3 weeks, their apical plasma membrane was shaped into rounded ridges, including microridges (white arrows on enlarged image on E) and was asymmetrically thickened in the region of the urothelial plaques (arrowheads on enlarged image on F). Furthermore, TEM confirmed the presence of DFVs in the apical cytoplasm (arrows on F) of superficial UCs. (G, H) Superficial UCs of urothelium in vivo exhibited an apical plasma membrane shaped into microridges (white arrow on enlarged image on G) with asymmetrically thickened regions of the urothelial plaques (arrowheads on enlarged image on H) and expressed a high density of DFVs in the apical cytoplasm (black arrows). Details in large white frames on (A), (C), (E), and (G) are three times enlarged images of corresponding smaller frames; similarly, the images in large black frames on (D), (F), and (H) present two times enlarged corresponding smaller frames. Scale bars: (A, C, E, G) 10 μm, (B, D, F, H) 500 nm.
Discussion
Choosing an appropriate biomaterial for scaffolding is an important step in tissue engineering.25 Scaffolds must provide an appropriate environment for cell growth and differentiation, enable formation of a functional tissue substitute, and be able to efficiently integrate into the host tissue when transplanted in vivo. The biological scaffolding materials are an appealing choice, as naturally derived scaffolds are usually embedded with bioactive molecules and thus possess signaling cues that enhance cell growth and differentiation.26
This study is the first to compare the suitability of eAM, dAM, and sAM as scaffolds for urothelia. The suitability of different AM scaffolds was assessed considering the growth and differentiation of UCs. As extracellular matrix components of the AM basal lamina create an almost native scaffold for cell seeding,1 the optimal growth of UCs was expected on dAM. However, the UC growth was the fastest and the differentiation the highest when UCs were seeded on the sAM scaffold. The possible reasons why UCs on sAM have the quickest adhesion and proliferation and the highest differentiation are the following. First, the AM stromal matrix is rich with collagens I, III, IV, VII, laminin, fibronectin, and proteoglycans,1,27 which are presented also in urinary bladder lamina propria,28 thus partially explaining the usefulness of sAM scaffolds as an appropriate substrate for UC proliferation and differentiation. Laminin and fibronectin are also potent chemoattractants for epithelial cells29 and as such, probably serve as promoters of UC migration. Second, cells in vivo exist in a three-dimensional environment generated and maintained by multiple cell–cell and cell–matrix interactions.30 Proteoglycans, which are especially reached in the spongy layer of AM stroma could affect these complex interactions and induce UC adhesion and growth. Third, cryopreserved AM contains various growth factors, many of which are of the epithelial origin, however, in less amount, the same growth factors are found in the AM stromal matrix.2 Both AM stroma and urinary bladder lamina propria contain EGF, TGFα, KGF, HGF, bFGF, and TGFβ1 and -β2,1,28 suggesting that the AM stromal matrix is a source of those growth factors, which can primary induce UC adhesion, proliferation, and differentiation. Furthermore, the growth factors released from damaged AECs could also stimulate UC growth on sAM during the first days of cultivation. According to our results, AECs of the sAM scaffold gradually exfoliate, further explaining why the growth of UCs on sAM decelerates with time.
Growth of UCs was the slowest on eAM, that is, when seeded directly on AECs. AECs, act as a physical barrier, making it difficult for cells to attach.14 At first, UCs on eAM formed small islands. Later on, the AECs were gradually displaced by UCs, allowing the UCs to attach on to the AM basal lamina and expand more rapidly. Similar was shown by Kruse et al.,31 who assumed that devitalized AECs probably serve as a feeder layer for migrating cells. The slowest growth of UCs on the eAM scaffold could be the reason for their poor differentiation at the time of fixation. Sartoneva et al.20 reported that the dAM scaffold is nonsuitable for human UC cultivation. Our findings using the dAM scaffold demonstrate the establishment of the urothelium with partially differentiated superficial UCs. The discrepancy may result from the difference in AM scaffold preparation; in contrast to their study where viable dAMs were used, we used cryopreserved AMs.
The urothelial blood–urine barrier is the tightest and most impermeable barrier in the body. The exceptionally high transepithelial resistance of urothelium results from a high apical plasma membrane transcellular resistance combined with paracellular resistance of tight junctions.32,33 In the present study, we found that the superficial UCs on sAM were the largest and displayed molecular (uroplakins, occludin) and ultrastructural properties (urothelial plaques, microridges, well-developed tight junctions, and membrane overlaps) of superficial UCs in highly differentiated urothelia. Furthermore, we found that the size of UCs correlated with their differentiation stage, that is, small UCs were less differentiated than larger UCs, which showed properties of highly differentiated UCs. It is known that large cells have less cell-to-cell contacts and therefore could have a higher transepithelial resistance and lower solute flux than smaller cells.34 Since in urothelia with small superficial UCs, cell-to-cell junctions were more extensive, meaning that potential tight junctions imperfections and leakages are more likely to occur, we believe that the size of UCs is beside the molecular and ultrastructural markers an important marker of the urothelial cytodifferentiation.
Basal lamina formation is a complex process requiring dynamic interactions between epithelial cells and their environment. Type IV collagen is an important structural component of basal lamina35 and is presented ubiquitously in all basal laminas.36 Together with the laminins, the network of collagen IV provides the basis for further basal lamina formation.37 The collagen VII is the major structural component of the anchoring fibrils, which anchor the basal lamina to the underlying stroma.38 The formation of anchoring fibrils is one of the final steps in basal lamina assembly and indicates the mature basal lamina.39 The AM basal lamina contains collagen IV and VII, which further confirms that when seeded on eAM and dAM scaffolds, UCs attached on AM basal lamina. When cultured on sAM, UCs formed basal lamina de novo. De novo formed basal lamina expressed collagen IV, but not collagen VII, suggesting that, it was not fully developed. Nevertheless, the UCs on the sAM reached a high stage of differentiation, indicating on the importance of the self-constructed basal lamina for the formation of a highly differentiated urothelium.
An increasing number of studies suggest that in addition to the paracrine activity of growth factors, the scaffold topography is the key factor directing cell proliferation and differentiation.26,40 Due to the high collagen and proteoglycan content, the growth factors bound on heparan residues, and its topography, the AM stromal matrix meets the criteria for a good scaffolding biomaterial.
The AM has antimicrobial properties that decrease the risk of postoperative infection.41 When used as a patch in ocular surface reconstructions, AM was shown to reduce inflammation by entrapping the inflammatory cells and inducing their apoptosis.42 Furthermore, Resch et al.43 demonstrated that the stroma of the transplanted AM can integrate into the host corneal tissue. Recently, the AM was also used as a scaffold for the engineering of the capillary structure by printing technology,44 suggesting that the AM, which is primary avascular tissue, could also be vascularized. All these promising findings illustrate the behavior of AM scaffolds in vivo and thereby set the promising background for the potential use of our highly differentiated tissue constructs in reconstructive urology.
When considering in vivo transplantation, it is important that tissue-engineered urothelium already forms a functional phenotype to provide immediate protection of the underlying stroma.24,45 To our knowledge, the human AM is currently the only scaffold which enables the in vitro development of tissue-engineered porcine urothelium with molecular and ultrastructural properties comparable to that of native urothelium, making such an urothelium a useful research tool for difficult-to-source human urothelium. Despite these encouraging results, further preclinical studies concerning the integration of the established constructs in vivo should be addressed before considering their use in clinical applications.
Conclusions
The present study demonstrates cultivation of UCs on three different AM scaffolds. Our findings show that the AM is a suitable scaffold for UCs as it supports UC proliferation and promotes their differentiation and formation into normal urothelium. Most importantly, we demonstrate that the sAM scaffold enables the development of tissue-engineered urothelium with molecular and ultrastructural properties comparable to that of native urothelium. Apart from the fact that such differentiated tissue-engineered urothelia could be in the future potentially applicable for in vivo transplantation procedures, it currently presents an excellent in vitro research tool for understanding the molecular and functional relationships in urothelial differentiation and could also be used as a model for drug delivery studies.
Supplementary Material
Acknowledgments
This work was supported by the Slovenian Research Agency (Grant No. P3-0108). The authors would like to express their appreciation to Professor Dr. Kristijan Jezernik and Professor Dr. Rok Romih for their continuous support. We thank Professor Dr. Tung-Tien Sun (New York University, School of Medicine), for uroplakin antibodies, Dr. Tina Cirman (Blood Transfusion Centre of Slovenia, Ljubljana) for preparing the AM, Eva Lasič for proofreading the manuscript, and Linda Štrus and Sanja Čabraja for technical assistance.
Disclosure Statement
No competing financial interests exist.
References
- 1.Niknejad H., Habibollah P., Jorjani M., Ahmadiani A., Ghanavi J., and Seifalian A.M.Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 15,88, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Koizumi N.J., Inatomi T.J., Sotozono C.J., Fullwood N.J., Quantock A.J., and Kinoshita S.Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 20,173, 2000 [PubMed] [Google Scholar]
- 3.Mamede A.C., Carvalho M.J., Abrantes A.M., Laranjo M., Maia C.J., and Botelho M.F.Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res 349,447, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Adinolfi M., Akle C.A., McColl I., Fensom A.H., Tansley L., Connolly P., et al. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature 295,325, 1982 [DOI] [PubMed] [Google Scholar]
- 5.Houlihan J.M., Biro P.A., Harper H.M., Jenkinson H.J., and Holmes C.H.The human amnion is a site of MHC class Ib expression: evidence for the expression of HLA-E and HLA-G. J Immunol 154,5665, 1995 [PubMed] [Google Scholar]
- 6.Riau A.K., Beuerman R.W., Lim L.S., and Mehta J.S.Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 3,216, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Mohammadi A.A., Seyed Jafari S.M., Kiasat M., Tavakkolian A.R., Imani M.T., Ayaz M., et al. Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns 39,349, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Mermet I., Pottier N., Sainthillier J.M., Malugani C., Cairey-Remonnay S., Maddens S., and Riethmuller D.Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen 15,459, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Sarwar I., Sultana R., Nisa R.U., and Qayyum I.Vaginoplasty by using amnion graft in patients of vaginal agenesis associated with Mayor-Rokitansky-Kuster-Hauser syndrome. J Ayub Med Coll Abbottabad 22,7, 2010 [PubMed] [Google Scholar]
- 10.Young R.L., Cota J., Zund G., Mason B.A., and Wheeler J.M.The use of an amniotic membrane graft to prevent postoperative adhesions. Fertil Steril 55,624, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Meller D., Pauklin M., Thomasen H., Westekemper H., and Steuhl K.P.Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int 108,243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura T., Sekiyama E., Takaoka M., Bentley A.J., Yokoi N., Fullwood N.J., et al. The use of trehalose-treated freeze-dried amniotic membrane for ocular surface reconstruction. Biomaterials 29,3729, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Kreft M.E., and Dragin U.Amniotic membrane in tissue engineering and regenerative medicine. Zdrav Vestn 79,707, 2010 [Google Scholar]
- 14.Koizumi N., Rigby H., Fullwood N.J., Kawasaki S., Tanioka H., Koizumi K., et al. Comparison of intact and denuded amniotic membrane as a substrate for cell-suspension culture of human limbal epithelial cells. Graefes Arch Clin Exp Ophthalmol 24,123, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Chen Y.J., Chung M.C., Jane Yao C.C., Huang C.H., Chang H.H., Jeng J.H., et al. The effects of acellular amniotic membrane matrix on osteogenic differentiation and ERK1/2 signaling in human dental apical papilla cells. Biomaterials 33,455, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Espana E.M., He H., Kawakita T., Di Pascuale M.A., Raju V.K., Liu C.Y., et al. Human keratocytes cultured on amniotic membrane stroma preserve morphology and express keratocan. Invest Ophthalmol Vis Sci 12,5136, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Romih R., Korosec P., de Mello W., Jr., and Jezernik K.Differentiation of epithelial cells in the urinary tract. Cell Tissue Res 320,259, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kreft M.E., Jezernik K., Kreft M., and Romih R.Apical plasma membrane traffic in superficial cells of bladder urothelium. Ann N Y Acad Sci 1152,18, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Sharifiaghdas F., Hamzehiesfahani N., Moghadasali R., Ghaemimanesh F., and Baharvand H.Human amniotic membrane as a suitable matrix for growth of mouse urothelial cells in comparison with human peritoneal and omentum membranes. Urol J 4,71, 2007 [PubMed] [Google Scholar]
- 20.Sartoneva R., Haimi S., Miettinen S., Mannerström B., Haaparanta A.M., Sándor G.K., et al. Comparison of a poly-L-lactide-co-ɛ-caprolactone and human amniotic membrane for urothelium tissue engineering applications. J R Soc interface 58,671, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikek K., Pfeifer V., and Drnovsek-Olup B.Amniotic membrane transplantation in the ocular surgery. Zdrav Vestn 73,419, 2004 [Google Scholar]
- 22.Kreft M.E., Hudoklin S., and Sterle M.Establishment and characterization of primary and subsequent subcultures of normal mouse urothelial cells. Folia Biol (Praha) 51,126, 2005 [PubMed] [Google Scholar]
- 23.Višnjar T., Kocbek P., and Kreft M.E.Hyperplasia as a mechanism for rapid resealing urothelial injuries and maintaining high transepithelial resistance. Histochem Cell Biol 137,177, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Kreft M.E., and Robenek H.Freeze-fracture replica immunolabelling reveals urothelial plaques in cultured urothelial cells. PLoS One 7,e38509, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mano J.F., Silva G.A., Azevedo H.S., Malafaya P.B., Sousa R.A., Silva S.S., et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface 4,999, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsiao Y.C., Lee H.W., Chen Y.T., Young T.H., and Yang T.L.The impact of compositional topography of amniotic membrane scaffold on tissue morphogenesis of salvary gland. Biomaterials 32,442, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Meinert M., Eriksen G.V., Petersen A.C., et al. Proteoglycans and hyaluronan in human fetal membranes. Am J Obstet Gynecol 184,679, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Andersson K.E., and McCloskey K.D.Lamina propria: the functional center of the bladder? Neurourol Urodyn 2013. [Epub ahead of print]; DOI: 10.1002/nau.22465 [DOI] [PubMed] [Google Scholar]
- 29.Ohshima M., Tokunaga K., Sato S., Maeno M., and Otsuka K.Laminin- and fibronectin-like molecules produced by periodontal ligament fibroblasts under serum-free culture are potent chemoattractants for gingival epithelial cells. J Periodontal Res 38,175, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Seidler D.G., Schaefer L., Robenek H., Iozzo R.V., Kresse H., and Schönherr E.A physiologic three-dimensional cell culture system to investigate the role of decorin in matrix organisation and cell survival. Biochem Biophys Res Commun 332,1162, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Kruse F.E., Joussen A.M., Rohrscnieder K., You L., Sinn B., Baumann J., et al. Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefes Arch Clin Exp Ophthalmol 238,68, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Negrete H.O., Lavelle J.P., Berg J., Lewis S.A., and Zeidel M.L.Permeability properties of the intact mammalian bladder epithelium. Am J Physiol 271,F886, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Kreft M.E., Hudoklin S., Jezernik K., and Romih R.Formation and maintenance of blood-urine barrier in urothelium. Protoplasma 246,3, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Anderson J.M., and Van Itallie C.M.Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1,a002584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelse K., Pöschl E., and Aigner T.Collagens-structure, function, and biosynthesis. Adv Drug Deliv Rev 55,1531, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Tanjore H., and Kalluri R.The role of type IV collagen and basement membranes in cancer progression and metastasis. Am J Pathol 168,715, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalluri R.Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3,422, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Sakai L.Y., Keene D.R., Morris N.P., and Burgeson R.E.Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol 103,1577, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yurchenco P.D., and Schittny J.C.Molecular architecture of basement membranes. FASEB J 4,1577, 1990 [DOI] [PubMed] [Google Scholar]
- 40.Bettinger C.J., Langer R., and Borenstein J.T.Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem Int Ed Engl 48,5406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talmi Y.P., Sigler L., Inge E., Finkelstein Y., and Zohar Y.Antibacterial properties of human amniotic membranes. Placenta 12,285, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Liu T., Zhai H., Xu Y., Dong Y., Sun Y., Zang X., et al. Amniotic membrane traps and induces apoptosis of inflammatory cells in ocular surface chemical burn. Mol Vis 18,2137, 2012 [PMC free article] [PubMed] [Google Scholar]
- 43.Resch M.D., Schlötzer-Schrehardt U., Hofmann-Rummelt C., Sauer R., Cursiefen C., Kruse F.E., et al. Adhesion structures of amniotic membranes integrated into human corneas. Invest Ophthalmol Vis Sci 47,1853, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Yoshida T., Komaki M., Hattori H., Negishi J., Kishida A., Morita I., et al. Therapeutic angiogenesis by implantation of a capillary structure constituted of human adipose tissue microvascular endothelial cells. Arterioscler Thromb Vasc Biol 30,1300, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Turner A.M., Subramaniam R., Thomas D.F., and Southgate J.Generation of a functional, differentiated porcine urothelial tissue in vitro. Eur Urol 54,1423, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.