Abstract
The general approach in heart valve tissue engineering is to mimic the shape of the native valve in the attempt to recreate the natural haemodynamics. In this article, we report the fabrication of the first tissue-engineered heart valve (TEHV) based on a tubular leaflet design, where the function of the leaflets of semilunar heart valves is performed by a simple tubular construct sutured along a circumferential line at the root and at three single points at the sinotubular junction. The tubular design is a recent development in pericardial (nonviable) bioprostheses, which has attracted interest because of the simplicity of the construction and the reliability of the implantation technique. Here we push the potential of the concept further from the fabrication and material point of view to realize the tube-in-tube valve: an autologous, living HV with remodelling and growing capability, physiological haemocompatibility, simple to construct and fast to implant. We developed two different fabrication/conditioning procedures and produced fibrin-based constructs embedding cells from the ovine umbilical cord artery according to the two different approaches. Tissue formation was confirmed by histology and immunohistology. The design of the tube-in-tube foresees the possibility of using a textile coscaffold (here demonstrated with a warp-knitted mesh) to achieve enhanced mechanical properties in vision of implantation in the aortic position. The tube-in-tube represents an attractive alternative to the conventional design of TEHVs aiming at reproducing the valvular geometry.
Introduction
Semilunar heart valves are complex structures, which ensure the unidirectional blood circulation. Valvular dysfunction can lead to cardiac insufficiency and finally to an increased mortality.1,2 The occurrence of acquired valvular heart diseases is rising drastically in industrialized countries as a consequence of the demographic aging and thus, an increased risk of degeneration of the valvular apparatus.3,4 The conventional therapy of severe defects is the replacement of the diseased valve with mechanical or biological prostheses.5 Although life-saving, these treatment options present several disadvantages, such as the need for life-long anticoagulation therapy or failure due to degeneration and calcification.6 The major drawback of the available valve prostheses is the missing capability for growth and remodelling. This results in the need for reoperations in paediatric patients to match the size of the valve to the somatic growth and in elderly patients for whom the average duration of a bioprosthesis is not sufficient anymore due to the increased life expectancy.7,8 Various approaches to create viable semilunar heart valves with remodelling capabilities have been developed by means of tissue engineering (TE).9,10 The general approach is to mimic the shape of the native valve to recreate the natural haemodynamics11 either by replicating the whole valvular apparatus, including the vascular part (wall)12–15 or by reproducing only the cusps to be sutured to the native wall.16–19 Both approaches have important limitations. The recreation of the complete valve is difficult due to its complex geometry, including the sinuses of Valsalva and the curved leaflets with the triangular coaptation.20 Furthermore, the native wall and cusps are different in tissue composition and organization and thus, crucially heterogeneous in biomechanical properties,20,21 a condition difficult to reproduce starting from a homogeneous material like in most TE approaches. Suturing single leaflets is a time-demanding task prone to misplacement and thus, to incorrect functioning of the valve.
A new design for a stentless heart valve bioprosthesis that focuses on the function and not on recreating the geometry of a native valve was developed by Mueller and von Segesser22 and brought to the market as 3F Aortic Bioprosthesis (3F Therapeutics, Inc.). The function of the semilunar cusps is performed by a simple tubular structure sutured along a circumferential line at the aortic root, as well as at three single points at the sinotubular junction (single point attached commissures [SPACs]).23 The back-flow of blood leads to the collapsing of the distal part of the tube and thus, to the closing of the vessel. This valve design permits the preservation of the sinuses of Valsalva with a reliable and safe implantation technique.24 Cox and colleagues25 compared the 3F tubular valve made with glutaraldehyde treated equine pericardium with a commercial stentless bioprosthetic valve on haemodynamic performance and stress distribution. Their study revealed equal or greater effective orifice area, lower transvalvular gradients and longer durability in accelerated tests. A glutaraldehyde-treated autologous ovine pericardial valve with a modified tubular design has been developed by Goetz and colleagues and tested in the arterial circulation of the adult sheep.26 Pericardial valves present the typical problems of biological valve substitutes, such as calcific degeneration, especially in younger patients.27 Furthermore, as a consequence of the chemical treatment, they are not viable anymore and therefore, they inherently lack the capability of continuous remodelling to adapt to the changing haemodynamic environment and to repair accumulating damage to the extracellular matrix (ECM) during routine function.
Here we exploit the tubular leaflet design following a TE approach to produce an autologous, living HV with remodelling and growing capability, physiological haemocompatibility, and simple to construct and fast to implant. We refer to it as tube-in-tube valve. The moulding technique applied in this work results in a complete tubular structure in one single piece that, differently from the reported pericardial bioprostheses, does not require any suture or gluing, and therefore, does not have any critical point prone to calcification.28
The main scaffold material for tissue-engineered heart valves (TEHVs) used by our group and others29–31 is fibrin. This natural polymer offers a broad range of advantageous properties, including its autologous origin, the rapid polymerization, the tuneable degradation via protease inhibitors and the manufacturability into 3D geometries.12,32
Two different approaches were developed to realize the tube-in-tube, mainly differing in the way the construct is conditioned. In the first approach, the tubular structure is moulded and before the dynamic conditioning it is sutured into a silicon tube representing the aortic wall, including the sinuses of Valsalva. In this way the valve is conditioned with functional opening and closing cycles in the same configuration it will have in vivo. In the second approach the valve is maintained in a tubular configuration via silicone connectors fixed at its ends and conditioned by cycling stretching/distension till the point of implantation.
Both approaches were realized with fibrin as the only scaffold material to achieve a truly autologous valve that could be eventually implanted in the pulmonary position and additionally with a textile coscaffold to improve the mechanical properties for implantation in the arterial circulation.
Viable tube-in-tube valves were produced with ovine umbilical artery cells and conditioned in bioreactors following both approaches. The tissue development was analyzed by histology and immunohistochemistry.
Methods
Cell isolation and culture
Vascular cells were isolated from ovine umbilical arteries, which were harvested during caesarean section of sheep. All animals received humane care in compliance with the European Convention on Animal Care. The isolation protocol was the same previously described for cell isolation from the ovine carotid artery.33 After removing the adventitia and following removal of endothelial cells by 1 mg/mL collagenase (Sigma) pretreatment, the arteries were minced into 1 to 2 mm rings. The tissue pieces were bathed in primary cell culture media (DMEM with 10% foetal bovine serum (FBS) and 1% antibiotic/antimycotic solution, all Gibco) for primary explant culture, and maintained in a humidified incubator at 37°C and 5% CO2. In this way, we obtained a mixed population of smooth muscle cells and (myo)fibroblasts as verified by the presence of alpha-smooth muscle actin (alpha-SMA)-positive cells and total absence of expression of von Willebrand factor. Upon confluency, the cells were serially passaged (1:3) using 0.25% trypsin/0.02% EDTA solution (Gibco) till a sufficient cell number for generation of a valve construct was obtained. Cells in passage six to eight were used.
Fibrin composition
Human fibrinogen (Calbiochem) was dissolved in purified water and dialysed with a cut-off membrane (Novodirect) of 6000–8000 MW overnight against Tris-buffered saline (TBS). The fibrinogen concentration after sterile filtration was estimated by measuring light absorbance at 280 nm with a spectrophotometer (Spectronic Genesys™ 6; Thermo Fisher Scientific GmbH). The final concentration of the fibrinogen solution was adjusted to 10 mg/mL with sterile TBS. The fibrin gel consisted of 3.5 mL fibrinogen solution (10 mg/mL), 1.4 mL TBS containing 70×106 umbilical artery cells (10×106 cells/mL) and 1.05 mL 50 mM CaCl2 (Sigma) in TBS. Fibrin polymerizsation was initialized by adding 1.05 mL of thrombin solution (40 U/mL; Sigma).
Concept of tubular TEHVs
The concept of the tubular design is illustrated in Figure 1. The valve function is obtained by suturing the tubular construct with the SPAC technique. Two different approaches were followed for the conditioning of the valve, both were implemented using fibrin as the only scaffold material and, alternatively, with a textile coscaffold. The textile was a polyethylene terephthalate (PET) warp-knitted tubular structure thermostabilized at 200°C for 8 min at the desired diameter.
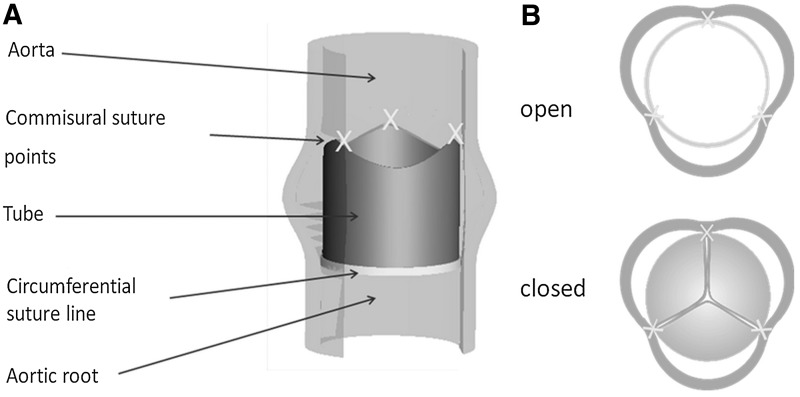
FIG. 1.
Principle of the tube-in-tube. (A) Sutured in the aortic or pulmonary position. (B) In systolic (open) and diastolic (closed) phase.
Approach 1: tubular construct to be conditioned with closing and opening cycles
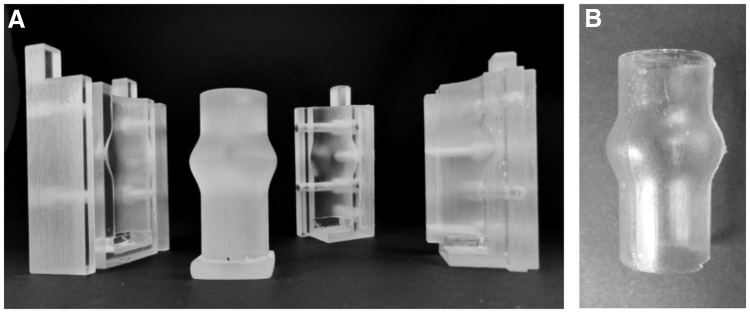
The mould consists of an inner cylinder and two outer shells (Fig. 2A) designed with the CAD program Pro/Engineering 5.0® (PTC; Needham) and produced by 3D Rapid Prototyping (Objet Eden350) using Fullcure 720 as material (Objetwith). After assembling the shells and the cylinder, the fibrinogen solution, and the other components (including the cells) were inserted with a mixing nozzle and left to polymerize for 45 min. The formed fibrin tube was then carefully removed from the inner cylinder. The tubular TEHV was subsequently sutured with 6-0 polybutester sutures (Vascufil®; Covidien's Syneture) in a silicone tube featuring the sinuses of Valsalva (Fig. 3B). The mould parts to produce the silicone tube (Fig. 3A) were designed with a CAD program according to the geometry reported by Reul et al.34 and rapid prototyped. Polydimethylsiloxane (SYLGARD® 184; Dow Corning) was utilized for the moulding of the tube.
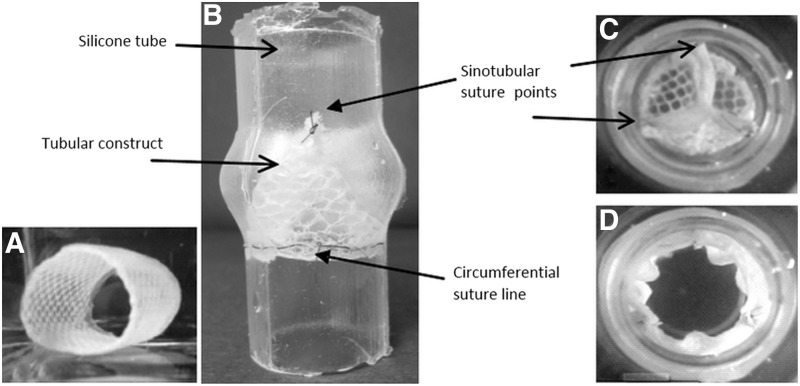
FIG. 2.
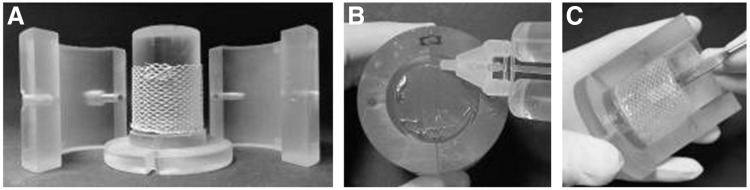
Moulding of the tubular construct. (A) inner cylinder with PET mesh and two shells. (B) Components for fibrin gel are inserted in mould. (C) After polymerization, the TEHV is carefully removed from the mould. PET, polyethylene terephthalate; TEHV, tissue-engineered heart valve.
FIG. 3.
(A) Rapid prototyped parts for the moulding of (B) a silicone tube featuring the sinuses of Valsalva.
Approach 2: tubular construct to be conditioned by cyclic stretching/distension
To mount the tubular construct in a bioreactor to be conditioned by cyclic stretching, the moulding technique was slightly changed to include two silicone rubber connectors (Elastosil M4641; R&G Faserverbundwerkstoffe) with a partially embedded PET warp-knitted mesh. The free standing part of the mesh was subsequently embedded in the fibrin gel during its polymerization creating continuity between the silicone holder and the tissue-engineered structure. The mesh could either be a complete structure in between the two holders in the case of a textile reinforced valve or a thin ring at each end of the tubular construct in case of a valve with only fibrin as scaffold. One of the two rings features three small squares to facilitate the suturing at the sinotubular junction. After the conditioning period, the connectors can be simply removed by cutting them off.
Conditioning of tubular TEHVs
All bioreactor components were sterilised using low-temperature hydrogen peroxide gas plasma (STERRAD® 100S Sterilisation System) 1 week before use. Tissue-engineered tubular constructs were cultured according to the two different approaches in the bioreactor system for 21 days at 37°C, 5% CO2, and 21% O2. The constructs were cultured in low glucose DMEM with 10% (FBS), antibiotic/antimycotic solution, 1.0 mM L-ascorbic acid 2-phosphate (Sigma) and 1.6 μL/mL tranexamic acid (1000 mg/mL; Pfizer). Culture medium within the bioreactor was replaced every 3 days. Culture conditions were monitored daily by measuring lactate, glucose, pO2, pCO2, and pH levels on a blood-gas analyser (Blood gas analyzer ABL 800 Flex; Radiometer).
The bioreactor consisted of two chambers communicating through the valve. The lower chamber functioned as ventricle pumping the medium through the valve into the upper chamber, which was partially filled with air and separated from the atmosphere by a sterile filter (0.2 μm; Millipore). The system was similar to the one previously described33 with the difference that in the present work it was actuated by means of a voice-coil motor (Type 810; Maschinenfabrik Mönninghoff GmbH & Co.KG) with a piston displacing the stroke volume through a silicon membrane, instead of using a respirator pump to displace the membrane. The movement of the piston of the voice-coil actuator was controlled through a LabVIEW 7.1 (National Instruments Germany GmbH) application.
For approach 1 the conditions chosen for this study were, an increase in pulse rate (and correspondingly valve opening/closing frequency) from 25 to 50 b.p.m. with the piston displacing the membrane upwards for 1/3 of the cycle and returning to the rest position in the remaining 2/3 of the cycle (375–750 mL/min) over 21 days with a pressure difference of 15 mm H2O over the valve leaflets. The frequency was increased in steps of 5 b.p.m. every 3 to 4 days.
For approach 2 (conditioning by cyclic stretching/distension), the pulse rate was also increased from 25 to 50 b.p.m. over 21 days. The duty cycle was 50% (the tubular construct being in the rest position for half of the cycle). The stroke volume was incrementally adjusted by increasing the actuator's amplitude to obtain increasing strain reaching a maximum of 15% per cycle. The strain was quantified by analysis of high speed videos (EX-ZR100; CASIO Europe GmbH) of the tubular construct during conditioning as the ratio between the diameter of the construct in the stretched and rest position by means of ImageJ (NIH).
Tissue-engineered valves were produced with and without textile coscaffold (approach 1, without textile, n=3; approach 1 with textile, n=3; approach 2 without textile, n=3) and cultivated dynamically for 21 days. Control tubular valves (n=3, without textile) were cultivated for 21 days under static conditions in a system equivalent to the bioreactor with respect to oxygen exchange.
Analysis of the influence of the tube diameter on opening and closing behavior
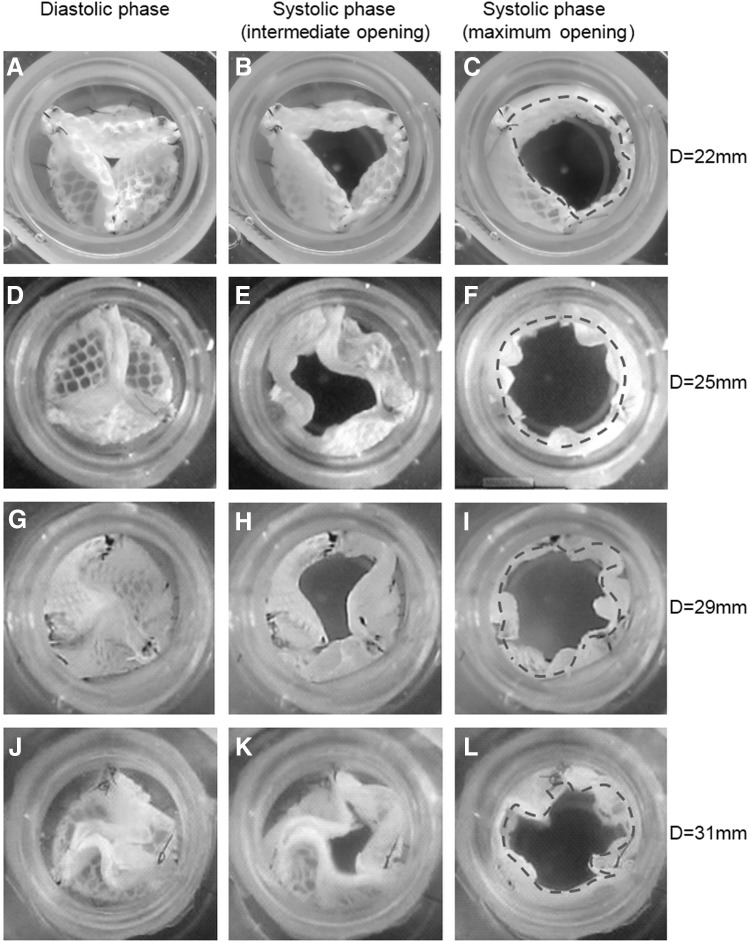
TEHV with textile reinforcement were moulded with different diameters (D=22, 25, 29, 31 mm). They were sutured into the silicone tube (inner diameter=22 mm) featuring the sinuses of Valsalva and the construct was mounted in a bioreactor system. Cyclic opening and closing of the valves was recorded with a high-speed camera (Casio Exilim EX-ZR100, 480 frames per second) and a comparison of their behavior was performed by analysing frames at the complete closure, partial opening, and complete opening for each valve.
Tissue analysis
Histology
Carnoy's-fixed, paraffin-embedded native ovine aortic and pulmonary valves and tissue-engineered valves were sectioned at 4 μm thickness longitudinally. Sections were subsequently stained using hematoxylin and eosin (H&E) for analysis of general tissue morphology and analysed using routine bright field light microscopy (AxioImager D1; Carl Zeiss GmbH), and images were acquired using a digital colour camera (AxioCam MRc; Carl Zeiss GmbH).
Immunohistochemistry
Nonspecific sites on Carnoy's-fixed, paraffin-embedded sections from native pulmonary valves and tissue-engineered valves were blocked, and cells were permeabilized with 5% normal goat serum (Sigma) in 0.1% Triton-phosphate-buffered saline (Sigma). Sections were incubated for 1 h at 37°C with the following primary antibodies: 1:1000 mouse monoclonal anti α-SMA (Sigma), 1:200 rabbit monoclonal anti-type I collagen, 1:25 rabbit anti-type III collagen (Acris Antibodies GmbH) and 1:200 rabbit monoclonal anti-elastin (Fitzgerald). The sections were then incubated for 1h at room temperature with either rhodamine- or fluorescein-conjugated goat-anti-mouse or goat-anti-rabbit secondary antibodies (1:400; Invitrogen), or biotinylated secondary antibodies (Dako). In the case of biotinylated staining, the procedure continued with a 1h incubation of streptavidin/tetramethylrhodamine isothiocyanate (TRITC; Acris Antibodies GmbH) at 37°C. Cells were counterstained with DAPI nucleic acid stain (Molecular Probes). Native ovine pulmonary valve tissue samples served as positive controls. As negative controls, samples were incubated with the secondary antibody only. Samples were viewed using a microscope equipped for epi-illumination (AxioObserver Z1; Carl Zeiss GmbH). Images were acquired using a digital camera (AxioCam MRm; Carl Zeiss GmbH).
Results
Proof of principle
The results of the moulding procedure are shown in Figure 4 for approach 1 with textile reinforcement and in Figure 5 for approach 2 with only fibrin as scaffold material. Figure 4C and D and Figure 5B–D show the way the tubular constructs were conditioned for both approaches. The constructs were obtained as one single piece without the need for gluing of suturing of different components.
FIG. 4.
Approach 1: (A) Tubular valve with textile reinforcement, (B) sutured in a silicone tube featuring the sinuses of Valsalva seen from the lateral side and from the vascular side in (C) closed and (D) open position.
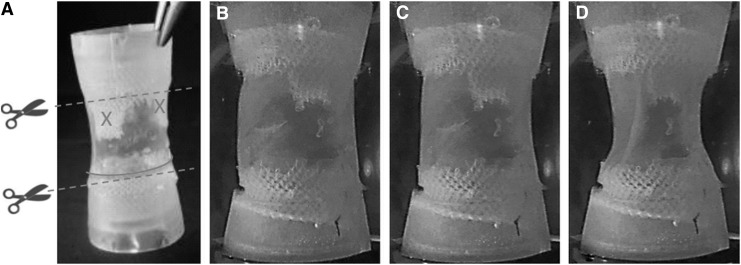
FIG. 5.
Approach 2: (A) Tubular valve after demoulding and (B–D) during cyclic stretching in the bioreactor. The dotted lines in A indicate the cutting directions to release the valve from the connectors at the time of implantation, the solid line and the crosses indicate the points of suture of the valve to the root.
Influence of the tube diameter on closing and opening behavior
When sutured into a silicone tube with inner diameter of 22 mm, a tubular construct with outer diameter of 22 mm resulted in insufficient closure with missing central coaptation of the leaflets (Fig. 6A) and an incomplete opening because of restrained leaflets (Fig. 6C). In case of a diameter of 25 mm, the valve's orifice area was adequate and the closure complete (Fig. 6D–F). The free edges exhibited a slight twisting in the closed position. This twist became more pronounced with a further increase of the tubular diameter resulting in S-shaped coaptation lines in the 29 mm structure (Fig. 6G) and in impaired coaptation in the 31 mm structure (Fig. 6J) due to excessive material. Correspondingly, the maximum opening area became smaller (Fig. 6K, L).
FIG. 6.
Still images of closing and opening cycles of tube-in-tube valves with different diameters sutured in the same silicone tube (D=22 mm). Behavior of the tubular construct with a diameter of 22 mm (A–C), 25 mm (D–F), 29 mm (G–I), and 31 mm (J–L). The dashed lines indicate the valvular orifices.
Tissue analysis
Histology
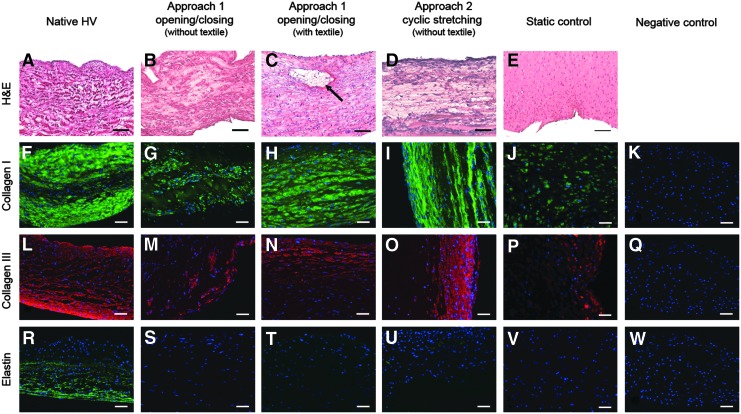
The H&E staining demonstrates living cells surrounded by connective tissue in all the tubular structures (Fig. 7B–E). In the case of constructs without textile reinforcement, in the central portion the cells appear to be less densely distributed, while when a textile mesh was present, the cells are homogeneously distributed throughout the whole thickness. Figure 7C shows that the textile was well enclosed in the developed tissue (black arrow).
FIG. 7.
Histological and fluorescence immunohistochemical micrographs of native ovine pulmonary valve (wall), dynamically and statically conditioned tissue-engineered tubular valves. Negative controls demonstrated undetectable levels of staining. Scale bars=100 μm (A–E), 50 μm (F–W). The black arrow indicates the textile structure.
Immunohistochemistry
Type I (Fig. 7G–J) and III (Fig. 7M–P) collagen, the most abundant protein components of the native valve tissue, were markedly expressed and had mostly a longitudinal orientation in the dynamically conditioned constructs, while they were deposited in a lower amount and did not show any orientation in the statically conditioned ones (Fig. 7J, P).
Elastin, an essential component of the native ECM, was hardly detectable in the engineered valves and only traces could be seen (Fig. 7S–V). Negative controls for all markers studied (reacted in the absence of primary antibody) exhibited undetectable levels of staining (Fig. 7K, Q, W).
Discussion
In this paper, we reported the realization of the first tissue-engineered heart valve based on a tubular leaflet design. The concept of the tubular design consists in replacing the leaflets of semilunar heart valves with a tubular structure sutured at three SPACs at the sinotubular junction and along a circumferential line at the annulus.35 Biological (nonviable) stentless heart valves based on the tubular leaflet design have been shown to be attractive alternatives to commercially available biological valves in terms of haemodynamic performance and durability. The tubular construct can be reliably implanted in standard operation times and it is functional even if implanted asymmetrically.26,35
We exploited the potential of this concept further by adding to the simplicity of construction and the reliability of the implantation the key advantages of tissue engineering to produce a living fibrin-based valve potentially able to self-repair, remodel, and grow.
In vitro and in vivo studies with tubular valves made of chemically treated pericardium have shown promising results. Cox and colleague showed superior haemodynamic performance and a higher durability of an equine pericardial tubular valve (3F Aortic Bioprosthesis; 3F Therapeutics, Inc.) with respect to commercial biological valves.25 Clinical studies confirmed the haemodynamic performance of the 3F valve;36–38 however, they also showed higher incidence of neurological postoperative thromboembolic complications.24,37 It can be speculated that the complications are related to the material of the valve. Equine pericardium was chosen due to its flexibility and tensile strength; however, it has been shown that this type of pericardial tissue exhibits a drastically higher calcification and inflammatory response when implanted subcutaneously in rats compared to bovine or porcine tissue.39 No thromboembolic complications occurred when tubular leaflets made of autologous pericardium were implanted in the subcoronary aortic position of the sheep. Although the study was shorter in time (up till 11 months), the authors argue that the autologous pericardium briefly treated with glutaraldehyde is a superior material. Still, endocarditis as a consequence of the material cannot be excluded as Al Halees and colleagues40 reported an increased incidence in humans with autologous pericardial valves briefly treated with glutaraldehyde. The authors speculate that the short glutaraldehyde treatment reduces the toxicity of the pericardium but not in the measure to prevent endocarditis as effectively as fully tanned pericardium. In any case, a chemically treated tissue is not viable, consequently the valve will not be able to remodel and self-repair and will be prone to calcific degeneration as all biological heart valve prostheses. An autologous tissue-engineered equivalent would be free of such shortcomings and could fully exploit the tremendous potential of the tubular design.
Animal studies on fibrin-based tissue-engineered heart valves29,41 revealed an absence of thrombus formation, calcification, stenosis, or aneurysm development. This together with the excellent tissue remodelling shows the potential of TEHVs based on an autologous fibrin scaffold. A confluent monolayer of endothelial cells was found on the whole valve surface 3 months after implantation29 and extensive endothelialization was observed on the root lumen but not on the leaflet 8 weeks after implantation.41 Flanagan and colleagues, differently from Syedain and coworkers, performed a short endothelialization process in vitro (30 min), but no efficiency of the process was reported. While it can be argued that sheep are able to endothelialize a variety of prostheses that are implanted in the circulation,42 pericardial tissue valves were only partially covered with endothelial cells after 11 months in vivo in the sheep model.26 We have recently shown that a complete endothelial layer can be easily achieved in vitro with autologous cells on fibrin-based constructs to provide physiological haemocompatibility already at the moment of implantation.43
Also from the fabrication point of view, the methods we developed have the clear advantage of resulting in one piece-constructs, without the need to use glue, which could result in undesired stiffening or suture material which could influence negatively the flexibility of the leaflets and be the cause of calcification.26 The 3F valve has three longitudinal suture lines running for the whole height of the tubular construct, and the valve presented by Goetz and colleagues has one.
We have shown that the choice of the tubular diameter with respect to that of the root is crucial for the correct functioning of the tubular valve. Marom et al.44 demonstrated in a numeric model the decrease of coaptation of the aortic cusps with the increase of the annulus' dimensions. In agreement with this we showed that a diameter of the tubular construct equal to that of the sinotubular junction resulted in insufficient closure, reduced effective orifice area and incomplete coaptation. The leaflets' performance improved by increasing the tubular construct's diameter. However, a further increase of the diameter resulted in a hindered coaptation, diminished effective orifice area and folding of the construct was needed to be able to fit it to a root, which had a smaller inner diameter. The crimped textile/tissue will likely be cause of in vivo thrombi formation. The problem can be straightforwardly solved with a conical design (Fig. 8A) so that the bigger diameter of the construct, which is fixed at three points, can be chosen optimally with respect to the closure and opening dynamics, while the smaller diameter can be chosen according to the root's dimension. However, on average, the aortic annulus' diameter is 15 to 20% larger compared with the sinotubular junction in humans,45 so that a cylindrical TEHV could be adequate. Natural variations, as well as aortic dilation have to be considered and ideally the correct shape and dimensions will be determined for each patient after ultrasound investigation of the native valve. The fabrication technique can be readily adapted using a tailor-made mould produced by 3D Rapid Prototyping. The same holds for the design variant proposed by Goetz and coworkers26 consisting in a tubular valve with 3D embossed geometry closer to the natural leaflets and therefore, a more physiological stress distribution. Xiong and coworkers46 proved the better mechanical performance of such design with respect to the plain tubular construct in a finite element study.
FIG. 8.
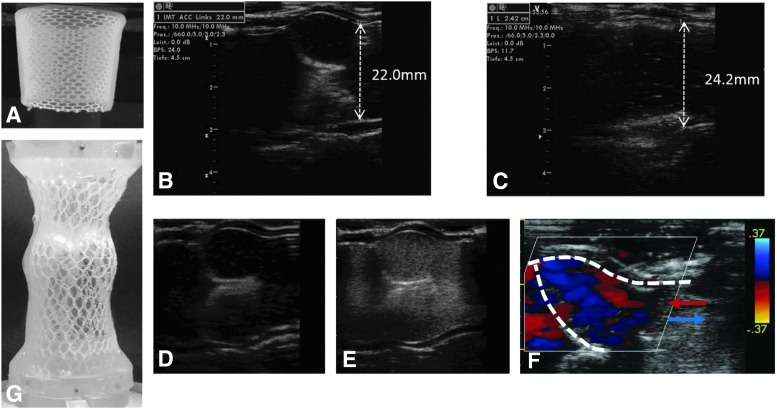
(A) Conical shaped textile reinforced tubular construct; (B, C) ultrasound images of the tube-in-tube in closed and open position under a 10% increase of the silicone tube's diameter at the level of the commissures during systole; (D, E) ultrasound images of the valve before and after injection of air bubbles as contrast agent; (F) Doppler ultrasound of the closing valve showing copresence of flows with opposite direction. The red and blue arrows indicate flow towards and away from the leaflet; (G) textile reinforced aortic root featuring the sinuses of Valsalva.
The right proportions are crucial for the forces applied at the commissures during functioning of the valve. If the free edges are longer than the radius of the sinotubular junction, the SPACs are exposed to only minor forces in the radial direction.35 Since a frequent cause for failure of commercially available bioprosthetic valves is the tearing at the commissural posts47 shown to be the points of greatest stress,48 this is a clear advantage of a properly dimensioned tubular heart valve. When mounted in a home-made flow-loop bioreactor, the tube-in-tube efficiently opened and closed under flow conditions resulting in a 10% dilatation at the level of the commissures during the systolic phase (Fig. 8B, C) as demonstrate by ultrasound images (Vivid-i ultrasound machine (GE Healthcare) equipped with a 13-MHz linear ultrasound probe). A 7%–12% dilatation has been quantified as physiological in different studies.49–51 With the same set-up we could also show that there is an efficient replacement of the volume in correspondence of the sinuses of Valsalva. The injection in the flow-loop of air bubbles as a ultrasound contrast agent, resulted in a valve with the outflow tract completely filled with bubbles within one stroke (Fig. 8D, E). This is only possible if there is flow recirculation at the level of the sinuses, which has also been shown by Doppler ultrasound (Fig. 8F) where the red and blue colours represent flow towards and away from the closing leaflet, respectively. These results suggest that the tube-in-tube concept has no significant dead volume that could lead to thrombus formation.
The two different fabrication approaches proposed in this paper differ mainly for the conditioning of the construct. The mechanical stimulation is of crucial importance for the development and the organization of the extracellular matrix.52 In the first approach the tubular construct is mounted in a silicone tube featuring the sinuses of Valsalva with the SPAC technique and it is stimulated with opening and closing cycles via a pulsatile flow of medium through the silicon tube. The frequency of the pulse is incremented in time as it happens during the fetal heart valve development.53 In the second approach, the tubular construct is cyclically stretched and distended with incremental strain amplitude, frequency and duty cycle. Syedain and colleagues have clearly show that cyclic distension/stretching can be used to improve mechanical and biochemical properties and structural organization of tissue-engineered tubular constructs,54 especially with a protocol of increasing frequency and duty cycle in time. Both approaches were realized, viable constructs were conditioned in bioreactors for 21 days and clear ECM proteins deposition was observed for all of them, mostly with a longitudinal orientation as a result of the dynamic conditioning. The static control exhibited lower ECM production and no orientation in agreement with our previous comparative study on the influence of dynamic conditioning on the in vitro development of fibrin-based heart valves33 and results presented by other groups as well.55–57 With the preliminary results shown here, it is not possible to conclude if there is a clear difference between the two approaches. The optimization of both protocols with respect to tissue formation is subject of ongoing research. Ultimately, which approach should be preferred will be determined in an in vivo study with a thorough analysis of the tube-in-tube performance.
The fabrication methods we developed allow for the introduction of a textile coscaffold with the aim of enhancing the mechanical properties to produce a heart valve able to withstand the arterial circulation. We have recently shown that TE vascular grafts based on a fibrin-textile scaffold could be produced in a short bioreactor cultivation time (2 weeks) and successfully implanted in the arterial circulation of the sheep for 3 months.58 Also in that case, the textile coscaffold was a plain warp-knitted mesh. In the aortic position the root-sparing procedure is the preferred one and therefore, the tube-in-tube concept has high potential. In cases in which the root needs also to be substituted, it is possible to tissue engineer this part as well, as a separate element. Figure 8G shows the proof of principle of a textile-reinforced fibrin construct featuring the sinuses of Valsalva. The advantage would be that the two components (leaflets and root) can be produced and conditioned independently to reproduce the heterogeneity of the native heart valves.
Conclusion
A fibrin-based tubular TEHV is an attractive alternative to the conventional design of semilunar valve prostheses. It combines the simplicity of construction and the reliability of implantation with the advantages of a living structure, that is, self-repair, remodelling and physiological haemodynamics. The design can be easily tailored on the anatomical details of the patient's valve through the production of a rapid prototyped mould. The developed methods foresee the use of textile reinforcement to improve the mechanical properties in vision of an implantation in the aortic position. We believe that with a more sophisticated textile composite scaffold (e.g., warp-knitted mesh in combination with electrospun elastic fibres) and a proper cell source, the tube-in-tube can be prepared in a limited preoperative time.
Acknowledgments
The authors thank the Fördergemeinschaft Deutsche Kinderherzzentren e.V. for financial support.
Disclosure Statement
No competing financial interests exist.
References
- 1.Otto C.M., Lind B.K., Kitzman D.W., Gersh B.J., and Siscovick D.S.Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 341,142, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Dujardin K.S., Enriquez-Sarano M., Schaff H.V., Bailey K.R., Seward J.B., and Tajik A.J.Mortality and morbidity of aortic regurgitation in clinical practice. A long-term follow-up study. Circulation 99,1851, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., and Enriquez-Sarano M.Burden of valvular heart diseases: a population-based study. Lancet 368,1005, 2006 [DOI] [PubMed] [Google Scholar]
- 4.D'arcy J.L., Prendergast B.D., Chambers J.B., Ray S.G., and Bridgewater B.Valvular heart disease: the next cardiac epidemic. Heart 97,91, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Vahanian A., Alfieri O., Andreotti F., Antunes M.J., Baron-Esquivias G., Baumgartner H., Borger M.A., Carrel T.P., De Bonis M., Evangelista A., Falk V., Iung B., Lancellotti P., Pierard L., Price S., Schafers H.J., Schuler G., Stepinska J., Swedberg K., Takkenberg J., Von Oppell U.O., Windecker S., Zamorano J.L., Zembala M., Bax J.J., Ceconi C., Dean V., Deaton C., Fagard R., Funck-Brentano C., Hasdai D., Hoes A., Kirchhof P., Knuuti J., Kolh P., Mcdonagh T., Moulin C., Popescu B.A., Reiner Z., Sechtem U., Sirnes P.A., Tendera M., Torbicki A., Von Segesser L., Badano L.P., Bunc M., Claeys M.J., Drinkovic N., Filippatos G., Habib G., Kappetein A.P., Kassab R., Lip G.Y., Moat N., Nickenig G., Otto C.M., Pepper J., Piazza N., Pieper P.G., Rosenhek R., Shuka N., Schwammenthal E., Schwitter J., Mas P.T., Trindade P.T., and Walther T.Guidelines on the management of valvular heart disease (version 2012): the joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 33,2451, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Schoen F.J., and Levy R.J.Pathology of substitute heart valves: new concepts and developments. J Card Surg 9,222, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Eitz T., Fritzsche D., Kleikamp G., Zittermann A., Horstkotte D., and Korfer R.Reoperation of the aortic valve in octogenarians. Ann Thorac Surg 82,1385, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Hanania G., Michel P.L., Montely J.M., Warembourg H., Nardi O., Leguerrier A., Agnino A., Despins P., Legault B., Petit H., and Bouraindeloup M.[The long term (15 years) evolution after valvular replacement with mechanical prosthesis or bioprosthesis between the age of 60 and 70 years]. Arch Mal Coeur Vaiss 97,7, 2004 [PubMed] [Google Scholar]
- 9.Mol A., Smits A.I., Bouten C.V., and Baaijens F.P.Tissue engineering of heart valves: advances and current challenges. Expert Rev Med Devices 6,259, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Schoen F.J.Heart valve tissue engineering: quo vadis? Curr Opin Biotechnol 22,698, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Butcher J.T., Mahler G.J., and Hockaday L.A.Aortic valve disease and treatment: the need for naturally engineered solutions. Adv Drug Deliv Rev 63,242, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Jockenhoevel S., Chalabi K., Sachweh J.S., Groesdonk H.V., Demircan L., Grossmann M., Zund G., and Messmer B.J.Tissue engineering: complete autologous valve conduit—a new moulding technique. Thorac Cardiovasc Surg 49,287, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Sodian R., Sperling J.S., Martin D.P., Egozy A., Stock U., Mayer J.E., Jr. and Vacanti J.P.Fabrication of a trileaflet heart valve scaffold from a polyhydroxyalkanoate biopolyester for use in tissue engineering. Tissue Eng 6,183, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Cox M.A., Kortsmit J., Driessen N., Bouten C.V., and Baaijens F.P.Tissue-engineered heart valves develop native-like collagen fiber architecture. Tissue Eng Part A 16,1527, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Neidert M.R., and Tranquillo R.T.Tissue-engineered valves with commissural alignment. Tissue Eng 12,891, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Shinoka T., Breuer C.K., Tanel R.E., Zund G., Miura T., Ma P.X., Langer R., Vacanti J.P., and Mayer J.E., Jr.Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann Thorac Surg 60,S513, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Klopsch C., Gabel R., Kaminski A., Mark P., Wang W., Toelk A., Delyagina E., Kleiner G., Koch L., Chichkov B., Mela P., Jockenhoevel S., Ma N., and Steinhoff G.Spray- and laser-assisted biomaterial processing for fast and efficient autologous cell-plus-matrix tissue engineering. J Tissue Eng Regen Med 2012. [Epub ahead of print]; DOI: 10.1002/term.1657 [DOI] [PubMed] [Google Scholar]
- 18.Courtney T., Sacks M.S., Stankus J., Guan J., and Wagner W.R.Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials 27,3631, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hong H., Dong N., Shi J., Chen S., Guo C., Hu P., and Qi H.Fabrication of a novel hybrid heart valve leaflet for tissue engineering: an in vitro study. Artif Organs 33,554, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Sauren A.A., Van Hout M.C., Van Steenhoven A.A., Veldpaus F.E., and Janssen J.D.The mechanical properties of porcine aortic valve tissues. J Biomech 16,327, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Sacks M.S., and Yoganathan A.P.Heart valve function: a biomechanical perspective. Philos Trans R Soc Lond B Biol Sci 362,1369, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller X.M., and Von Segesser L.K.A new equine pericardial stentless valve. J Thorac Cardiovasc Surg 125,1405, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Goetz W.A., Lim H.S., Lansac E., Weber P.A., and Duran C.M.A temporarily stented, autologous pericardial aortic valve prosthesis. J Heart Valve Dis 11,696, 2002 [PubMed] [Google Scholar]
- 24.Linneweber J., Heinbokel T., Christ T., Claus B., Kossagk C., and Konertz W.Clinical experience with the ATS 3F stentless aortic bioprosthesis: five years' follow up. J Heart Valve Dis 19,772, 2010 [PubMed] [Google Scholar]
- 25.Cox J.L., Ad N., Myers K., Gharib M., and Quijano R.C.Tubular heart valves: a new tissue prosthesis design—preclinical evaluation of the 3F aortic bioprosthesis. J Thorac Cardiovasc Surg 130,520, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Goetz W.A., Tan T.E., Lim K.H., Salgues Sle H., Grousson N., Xiong F., Chua Y.L., and Yeo J.H.Truly stentless molded autologous pericardial aortic valve prosthesis with single point attached commissures in a sheep model. Eur J Cardiothorac Surg 33,548, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Schoen F.J., and Levy R.J.Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg 79,1072, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Myers J.L.Invited letter concerning: calcification after end-to-end arterial anastomoses. J Thorac Cardiovasc Surg 99,380, 1990 [PubMed] [Google Scholar]
- 29.Flanagan T.C., Sachweh J.S., Frese J., Schnoring H., Gronloh N., Koch S., Tolba R.H., Schmitz-Rode T., and Jockenhoevel S.In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model. Tissue Eng Part A 15,2965, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Robinson P.S., Johnson S.L., Evans M.C., Barocas V.H., and Tranquillo R.T.Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Eng Part A 14,83, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Mol A., Rutten M.C., Driessen N.J., Bouten C.V., Zund G., Baaijens F.P., and Hoerstrup S.P.Autologous human tissue-engineered heart valves: prospects for systemic application. Circulation 114,I152, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Cholewinski E., Dietrich M., Flanagan T.C., Schmitz-Rode T., and Jockenhoevel S.Tranexamic acid—an alternative to aprotinin in fibrin-based cardiovascular tissue engineering. Tissue Eng Part A 15,3645, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Flanagan T.C., Cornelissen C., Koch S., Tschoeke B., Sachweh J.S., Schmitz-Rode T., and Jockenhoevel S.The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials 28,3388, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Reul H., Vahlbruch A., Giersiepen M., Schmitz-Rode T., Hirtz V., and Effert S.The geometry of the aortic root in health, at valve disease and after valve replacement. J Biomech 23,181, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Goetz W.A., Lim K.H., Ibled R., Grousson N., Satgues S.L.H., and Yeo J.H.Forces at single point attached commissures (SPAC) in pericardial aortic valve prosthesis. Eur J Cardio-Thorac 29,150, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Doss M., Martens S., Wood J.P., Miskovic A., Christodoulou T., Wimmer-Greinecker G., and Moritz A.Aortic leaflet replacement with the new 3F stentless aortic bioprosthesis. Ann Thorac Surg 79,682, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Risteski P., Adami C., Papadopoulos N., Sirat A.S., Moritz A., and Doss M.Leaflet replacement for aortic stenosis using the 3f stentless aortic bioprosthesis: midterm results. Ann Thorac Surg 93,1134, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Eckstein F.S., Tevaearai H., Keller D., Schmidli J., Immer F.F., Seiler C., Saner H., and Carrel T.P.Early clinical experience with a new tubular equine pericardial stentless aortic valve. Heart Surg Forum 7,E498, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Masumoto H., Watanabe T., Sakai Y., and Ueda Y.[Experimental study of calcification of the xenopericardia]. Kyobu Geka 53,468, 2000 [PubMed] [Google Scholar]
- 40.Al Halees Z., Al Shahid M., Al Sanei A., Sallehuddin A., and Duran C.Up to 16 years follow-up of aortic valve reconstruction with pericardium: a stentless readily available cheap valve? Eur J Cardiothorac Surg 28,200, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Syedain Z., Lahti M., Johnson S., Robinson P., Ruth G., Bianco R., and Tranquillo R.Implantation of a tissue-engineered heart valve from human fibroblasts exhibiting short term function in the sheep pulmonary artery. Cardiovasc Eng Tech 2,101, 2011 [Google Scholar]
- 42.Hoffman D., Gong G., Liao K., Macaluso F., Nikolic S.D., and Frater R.W.Spontaneous host endothelial growth on bioprostheses. Influence of fixation. Circulation 86,II75, 1992 [PubMed] [Google Scholar]
- 43.Weinandy S., Rongen L., Schreiber F., Cornelissen C., Flanagan T.C., Mahnken A., Gries T., Schmitz-Rode T., and Jockenhoevel S.The BioStent: novel concept for a viable stent structure. Tissue Eng Part A 18,1818, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Marom G., Haj-Ali R., Rosenfeld M., Schafers H.J., and Raanani E.Aortic root numeric model: annulus diameter prediction of effective height and coaptation in post-aortic valve repair. J Thorac Cardiovasc Surg 145,406, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Kunzelman K.S., Grande K.J., David T.E., Cochran R.P., and Verrier E.D.Aortic root and valve relationships. Impact on surgical repair. J Thorac Cardiovasc Surg 107,162, 1994 [PubMed] [Google Scholar]
- 46.Xiong F.L., Goetz W.A., Chong C.K., Chua Y.L., Pfeifer S., Wintermantel E., and Yeo J.H.Finite element investigation of stentless pericardial aortic valves: relevance of leaflet geometry. Ann Biomed Eng 38,1908, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Spray T.L., and Roberts W.C.Structural changes in porcine xenografts used as substitute cardiac valves. Gross and histologic observations in 51 glutaraldehyde-preserved Hancock valves in 41 patients. Am J Cardiol 40,319, 1977 [DOI] [PubMed] [Google Scholar]
- 48.Christie G.W.Computer modelling of bioprosthetic heart valves. Eur J Cardiothorac Surg 6Suppl 1,S95, 1992 [PubMed] [Google Scholar]
- 49.Thubrikar M., Bosher L.P., and Nolan S.P.The mechanism of opening of the aortic valve. J Thorac Cardiovasc Surg 77,863, 1979 [PubMed] [Google Scholar]
- 50.Robicsek F., Thubrikar M.J., and Fokin A.A.Cause of degenerative disease of the trileaflet aortic valve: review of subject and presentation of a new theory. Ann Thorac Surg 73,1346, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Pang D.C., Choo S.J., Luo H.H., Shomura Y.U., Daniel S., Nikolic S., Cheung D.T., Oury J.H., and Duran C.M.Significant increase of aortic root volume and commissural area occurs prior to aortic valve opening. J Heart Valve Dis 9,9, 2000 [PubMed] [Google Scholar]
- 52.Freed L.E., Guilak F., Guo X.E., Gray M.L., Tranquillo R., Holmes J.W., Radisic M., Sefton M.V., Kaplan D., and Vunjak-Novakovic G.Advanced tools for tissue engineering: scaffolds, bioreactors, and signaling. Tissue Eng 12,3285, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Stock U.A., and Vacanti J.P.Cardiovascular physiology during fetal development and implications for tissue engineering. Tissue Eng 7,1, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Syedain Z.H., and Tranquillo R.T.Controlled cyclic stretch bioreactor for tissue-engineered heart valves. Biomaterials 30,4078, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Syedain Z.H., Weinberg J.S., and Tranquillo R.T.Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci U S A 105,6537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubbens M.P., Mol A., Boerboom R.A., Bank R.A., Baaijens F.P., and Bouten C.V.Intermittent straining accelerates the development of tissue properties in engineered heart valve tissue. Tissue Eng Part A 15,999, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Gandaglia A., Bagno A., Naso F., Spina M., and Gerosa G.Cells, scaffolds and bioreactors for tissue-engineered heart valves: a journey from basic concepts to contemporary developmental innovations. Eur J Cardiothorac Surg 39,523, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Koch S., Flanagan T.C., Sachweh J.S., Tanios F., Schnoering H., Deichmann T., Ella V., Kellomaki M., Gronloh N., Gries T., Tolba R., Schmitz-Rode T., and Jockenhoevel S.Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials 31,4731, 2010 [DOI] [PubMed] [Google Scholar]