Abstract
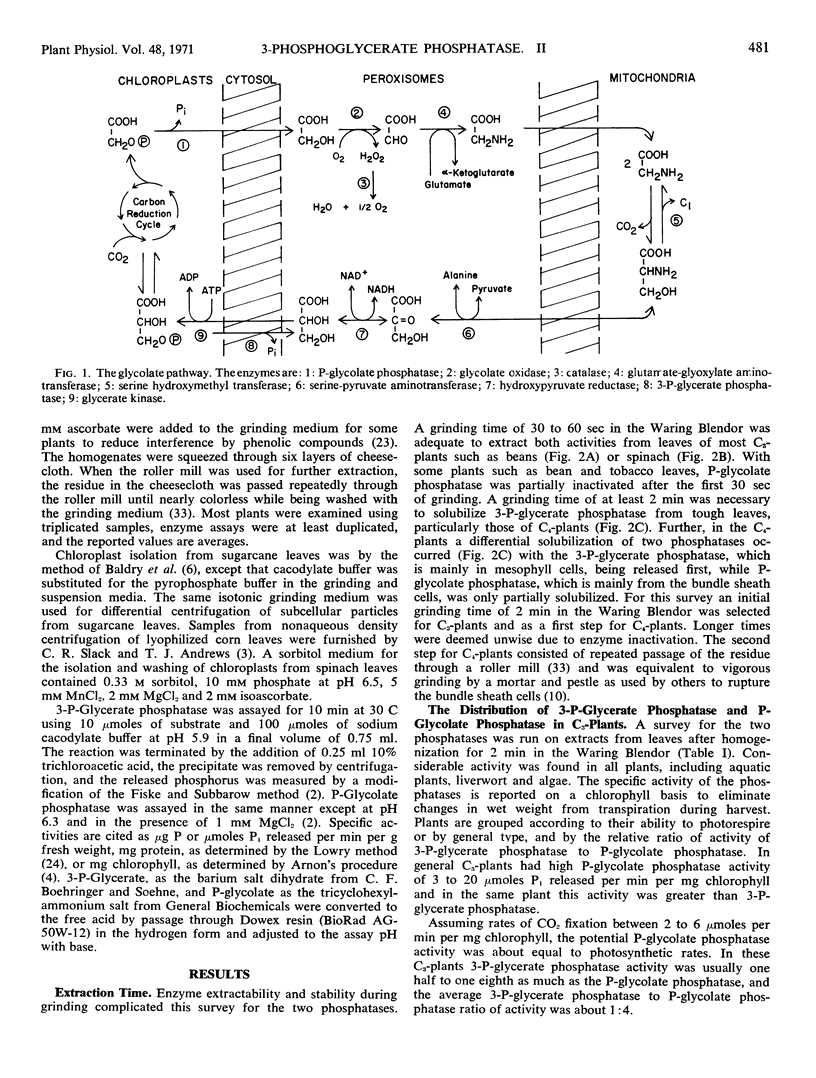
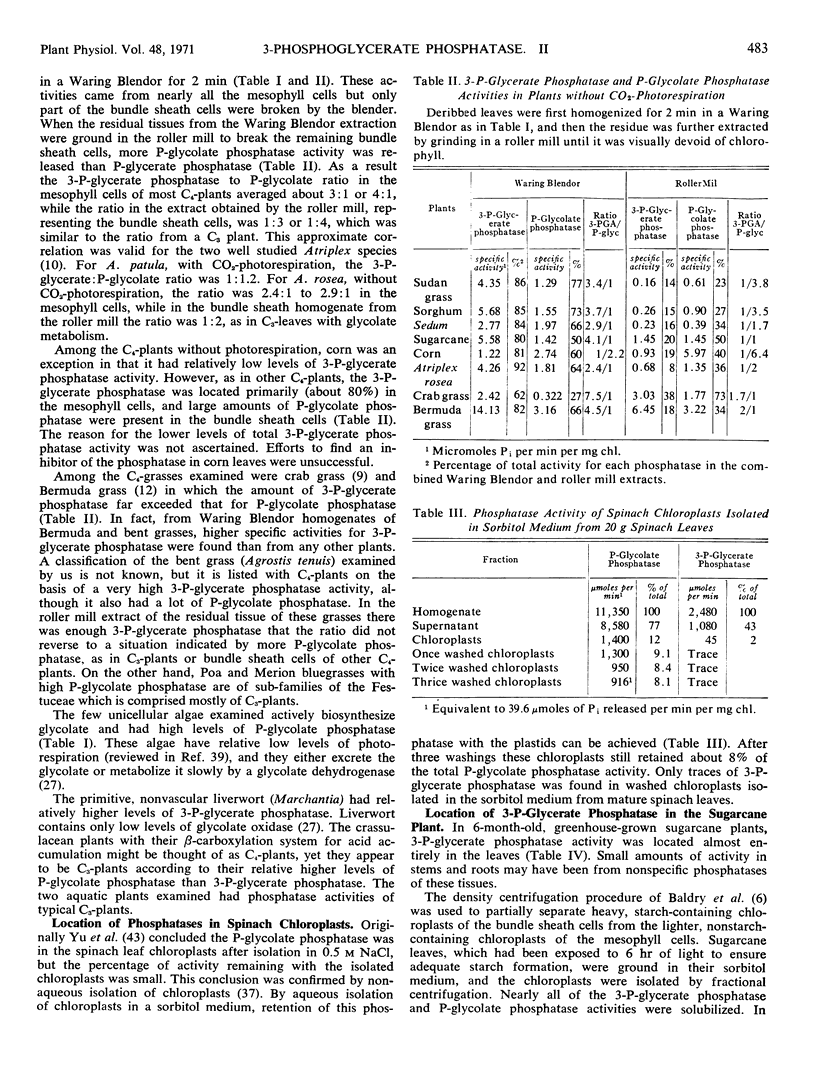
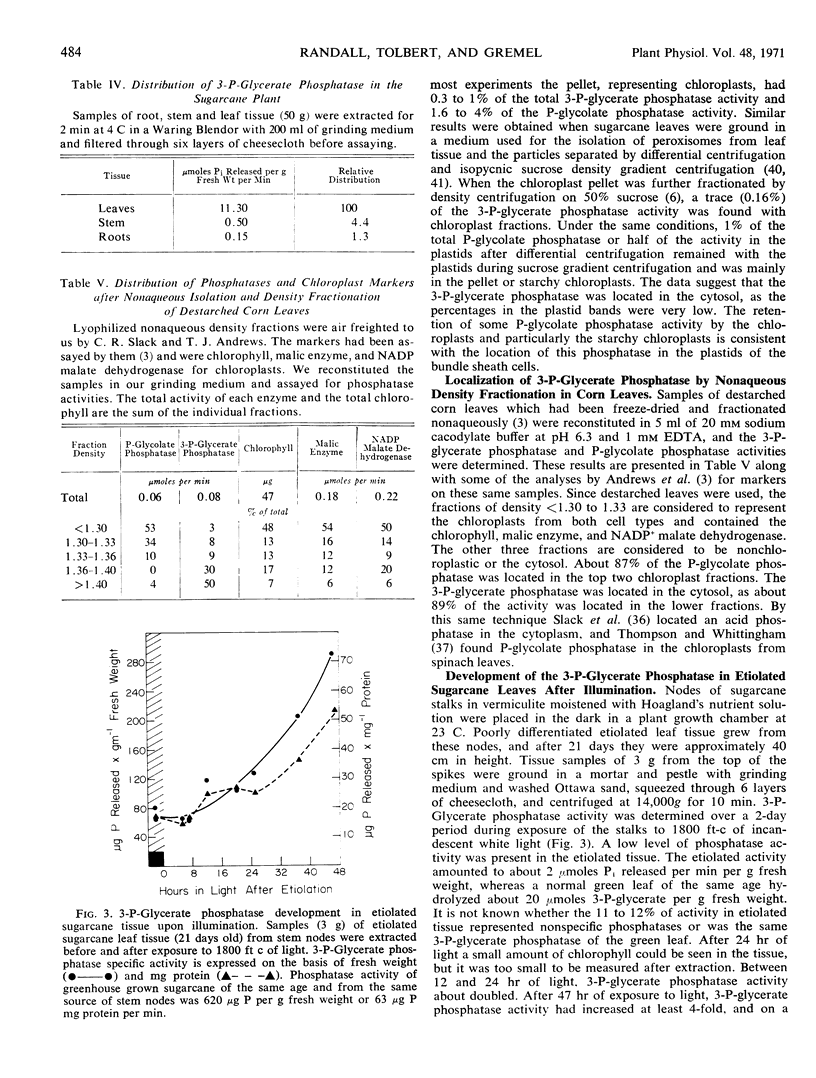
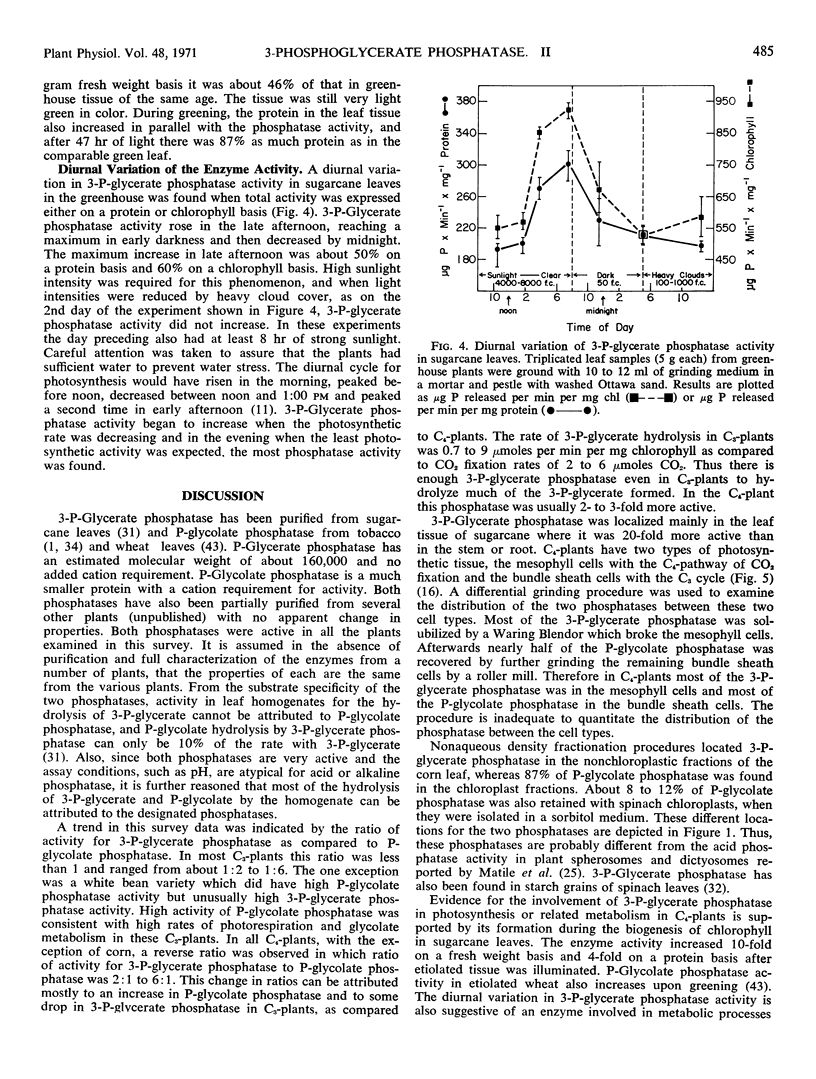
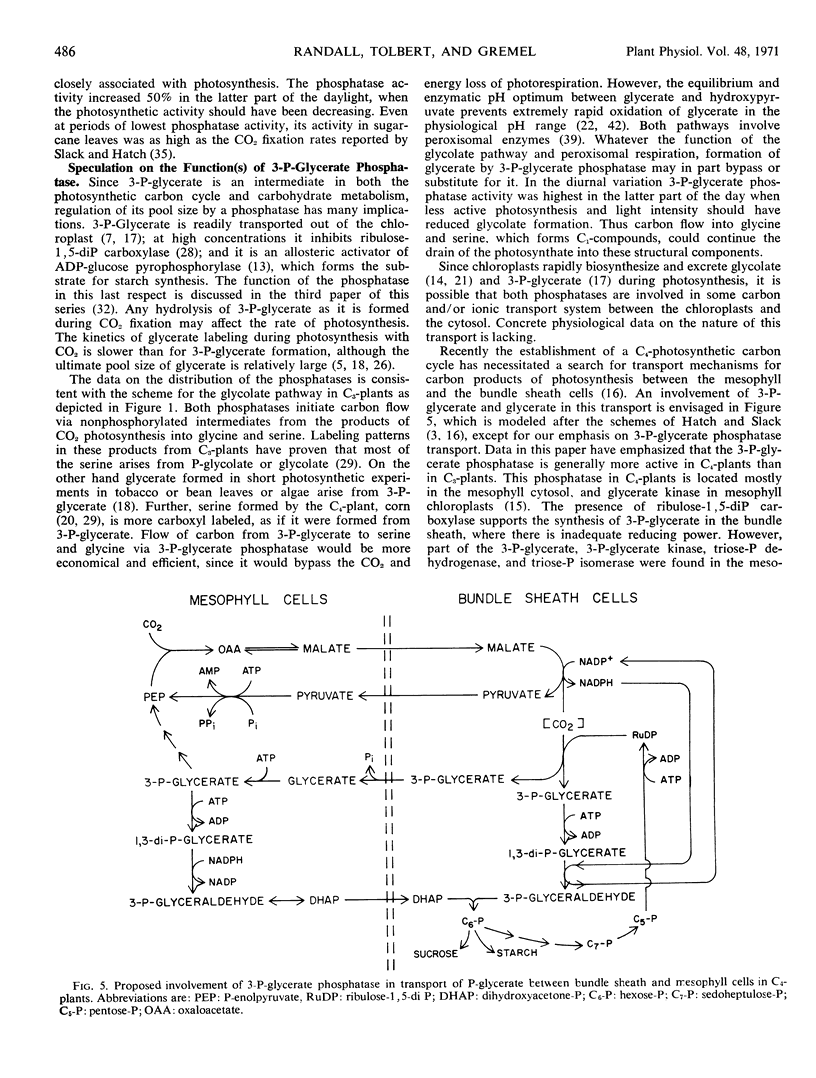
3-Phosphoglycerate phosphatase and phosphoglycolate phosphatase were found in leaves of all 52 plants examined. Activities of both phosphatases varied widely between 1 to 20 micromoles per minute per milligram chlorophyll. Plants were grouped into two categories based upon the relative ratio of activity of 3-phosphoglycerate phosphatase to phosphoglycolate phosphatase. This ratio varied between 2:1 to 4:1 in the C4-plants except corn leaves which had a low level of 3-phosphoglycerate phosphatase. This ratio was reversed and varied between 1:2 to 1:6 in all C3-plants except one bean variety which had large amounts of both phosphatases. By differential grinding procedures for C4 plants a major part of the 3-phosphoglycerate phosphatase was found in the mesophyll cells and P-glycolate phosphatase in the bundle sheath cells. Phosphoglycolate phosphatase, but not 3-phosphoglycerate phosphatase, was located in chloroplasts of C3- and C4- plants. Formation of 3-phosphoglycerate phosphatase increased 4- to 12-fold during greening of etiolated sugarcane leaves. This cytosol phosphatase displayed a diurnal variation in sugarcane leaves by increasing 50% during late daylight hours and early evening. It is proposed that the soluble form of 3-phosphoglycerate phosphatase is necessary for carbon transport between the bundle sheath and mesophyll cells during photosynthesis by C4-plants. In C3- and C4-plants this phosphatase initiates the conversion of 3-phosphoglycerate to serine which is an alternate metabolic pathway to glycolate metabolism and photorespiration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENSON A. A., BASSHAM J. A., CALVIN M., HALL A. G., HIRSCH H. E., KAWAGUCHI S., LYNCH V., TOLBERT N. E. The path of carbon in photosynthesis. XV. Ribulose and sedoheptulose. J Biol Chem. 1952 May;196(2):703–716. doi: 10.2172/915054. [DOI] [PubMed] [Google Scholar]
- Black C. C., Mollenhauer H. H. Structure and distribution of chloroplasts and other organelles in leaves with various rates of photosynthesis. Plant Physiol. 1971 Jan;47(1):15–23. doi: 10.1104/pp.47.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. NADP-specific malate dehydrogenase and glycerate kinase in leaves and evidence for their location in chloroplasts. Biochem Biophys Res Commun. 1969 Mar 10;34(5):589–593. doi: 10.1016/0006-291x(69)90778-5. [DOI] [PubMed] [Google Scholar]
- Hess J. L., Tolbert N. E. Glycolate pathway in algae. Plant Physiol. 1967 Mar;42(3):371–379. doi: 10.1104/pp.42.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. L., Tolbert N. E. Glycolate, glycine, serine, and glycerate formation during photosynthesis by tobacco leaves. J Biol Chem. 1966 Dec 10;241(23):5705–5711. [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. The C4-dicarboxylic acid pathway of photosynthesis. Identification of intermediates and products and quantitative evidence for the route of carbon flow. Biochem J. 1969 Aug;114(1):127–134. doi: 10.1042/bj1140127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEARNEY P. C., TOLBERT N. E. Appearance of glycolate and related products of photosynthesis outside of chloroplasts. Arch Biochem Biophys. 1962 Jul;98:164–171. doi: 10.1016/0003-9861(62)90162-5. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Warren W. A. The kinetic properties of spinach leaf glyoxylic acid reductase. J Biol Chem. 1970 Aug 10;245(15):3831–3839. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. Glycolate dehydrogenase in green algae. Arch Biochem Biophys. 1970 Nov;141(1):102–110. doi: 10.1016/0003-9861(70)90112-8. [DOI] [PubMed] [Google Scholar]
- RABSON R., TOLBERTNE, KEARNEY P. C. Formation of serine and glyceric acid by the glycolate pathway. Arch Biochem Biophys. 1962 Jul;98:154–163. doi: 10.1016/0003-9861(62)90161-3. [DOI] [PubMed] [Google Scholar]
- RICHARDSON K. E., TOLBERT N. E. Phosphoglycolic acid phosphatase. J Biol Chem. 1961 May;236:1285–1290. [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E. 3-Phosphoglycerate Phosphatase in Plants: III. Activity Associated with Starch Particles. Plant Physiol. 1971 Oct;48(4):488–492. doi: 10.1104/pp.48.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E. 3-Phosphoglycerate phosphatase in plants. I. Isolation and characterization from sugarcane leaves. J Biol Chem. 1971 Sep 10;246(17):5510–5517. [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J. 1967 Jun;103(3):660–665. doi: 10.1042/bj1030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D., Goodchild D. J. Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 Sep;114(3):489–498. doi: 10.1042/bj1140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. M., Whittingham C. P. Intracellular localisation of phosphoglycollate phosphatase and glyoxalate reductase. Biochim Biophys Acta. 1967;143(3):642–644. doi: 10.1016/0005-2728(67)90074-6. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Yu Y. L., Tolbert N. E., Orth G. M. Isolation and Distribution of Phosphoglycolate Phosphatase. Plant Physiol. 1964 Jul;39(4):643–647. doi: 10.1104/pp.39.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]


