Abstract
Recently, several studies have strongly suggested that age-related decline in episodic memory is associated with deficits in hippocampal pattern separation (orthogonalizing overlapping experiences using distinct neural codes). The same studies also link these deficits to neurobiological features such as dentate/CA3 representational rigidity and perforant path loss. This decline in pattern separation is thought to underlie behavioral deficits in discriminating similar stimuli on pictorial tasks. Similar pictorial stimuli invoke interference both in the perceptual and conceptual domains, and do not allow one to be disentangled from another. For example, it is very difficult to design a set of pictorial stimuli that are perceptually similar yet conceptually unrelated. Verbal stimuli, on the other hand, allow experimenters to independently manipulate conceptual and perceptual interference. We tested discrimination on conceptually similar (semantically related) and perceptually similar (phonologically related) verbal stimuli in young (mean age 20) and older adults (mean age 69), and find that older adults are selectively impaired in perceptual pattern separation. This deficit was not secondary to failure in working memory, attention, or visual processing. Based on past studies, we suggest that perceptual discrimination relies on recollection while conceptual discrimination relies more on gist. Our results fit well within the notion that recollection but not familiarity (i.e. gist) is impaired in older adults, and suggests that the impairment observed in pictorial tasks may be driven mostly by failure in perceptual and not conceptual pattern separation.
Keywords: hippocampus, pattern separation, perceptual interference, conceptual interference, phonological, semantic
Introduction
Long-term memory function is frequently reported to decline in the elderly. Specific age-related declines have been reliably documented in episodic memory (Craik FIM, 1980), spatial memory (Newman and Kaszniak, 2000), recollection (Jennings and Jacoby, 1997), contextual memory (Spencer and Raz, 1995), associative memory (Silver et al., 2012), and source memory (Schacter et al., 1991; Schacter et al., 1994). All of these mnemonic abilities require detailed contextual representations to be formed, which relies in part on intact pattern separation. Pattern separation is the neural computational process by which similar experiences are stored using distinct neural representations and is thought to rely heavily on the hippocampal dentate gyrus (DG) and CA3 subregions [c.f. (Yassa and Stark, 2011) for review]. Without intact pattern separation, memories can easily interfere with one another and source confusion arises.
Recently, several studies have strongly suggested that pattern separation abilities decline with age. On a behavioral level, these studies have shown that aging is associated with worse performance on tasks that require fine-level discrimination among similar “lures” using both objects (Toner et al., 2009; Yassa et al., 2011a; Yassa et al., 2011b; Yassa et al., 2010b) and spatial layouts (Holden and Gilbert, 2012; Holden et al., 2012; Stark et al., 2010). These tasks were designed to tax neural pattern separation abilities, and some have been shown in fMRI studies to rely on intact processing specifically in the DG/CA3 region (Yassa et al., 2011a; Yassa et al., 2011b; Yassa et al., 2010b), as well as the integrity of the perforant path input to these subfields (Yassa et al., 2011b; Yassa et al., 2010a).
In this work, it is unclear whether the behavioral deficit reported in older adults is due to an increase in conceptual or perceptual interference. Both types of interference occur in any task that uses pictorial stimuli, and they are often confounded with one another. That is, items that are perceptually similar will most often also be conceptually similar. This is a limitation of using pictorial stimuli and can be averted by using verbal stimuli instead. In the verbal domain, words can be manipulated such that they are either perceptually (i.e., phonologically) similar or conceptually (i.e., semantically) similar. For example, “curtain” and “certain” are perceptually similar yet conceptually unrelated, whereas “closet” and “drawer” are conceptually related yet perceptually distinct.
Using a design that shares some features with the Deese-Roediger and McDermott (DRM) paradigm (Roediger and Mcdermott, 1995), we developed two conditions, one testing recognition in the presence of perceptual similarity and the other in the presence of conceptual similarity. In the original DRM task, participants learned lists of closely related lures (e.g., “chair”, “desk”, “recliner”) and later during recognition falsely recognized a related word that was not on the list (e.g., “couch”). The design here differed in two respects: (1) each lure item during recognition was related to only one item in the study list, and (2) we included a perceptual similarity condition as an independent manipulation. The word lists for both perceptually and conceptually similar items were based on published work (Mechelli et al., 2007). We compared two samples of young and older adults on these tasks. This design allowed us to disentangle the contributions of perceptual and conceptual interference and address an issue that prior work using pictorial tasks could not address.
Participants
18 young adults (mean age = 19.67 years SD 1.24) and 21 healthy older adults (mean age = 69.48 years SD 7.33) participated in the perceptual discrimination task while 18 young adults (mean age = 19.56 years SD 1.04) and 21 healthy older adults (mean age = 69.19 years SD 8.18) participated in the conceptual discrimination task. All participants were recruited by flyer and online advertising around the Johns Hopkins campus and surrounding local community. All participants were cognitively intact, did not suffer from major neurological, psychiatric or health conditions, spoke English as their native language, and were right-handed. Before testing, all participants provided written informed consent, and were debriefed on the experiment at the conclusion of testing.
Behavioral tasks and analyses
All experiments were programmed in Psychtoolbox 3.0 running under MATLAB (Natick, MA) release R2010a. The words presented in all experiments were based on Mechelli et al.’s (2007) previous work. All words were concrete nouns, with an average frequency of 45 occurrences per million (range 10–600). Words were considered conceptually similar if they were related in semantic or categorical meaning, while phonologically similar words shared at least the first phoneme with most sharing the first two or three phonemes. Experiment 1 used phonologically similar lures while Experiment 2 used conceptually similar lures. Otherwise, all parameters were held constant across the two experiments. Each experiment involved two distinct phases. During the first phase (encoding), participants were shown a list of words (n = 134 items) on the screen, each for 2000 ms (500 ms inter-stimulus interval [ISI]) and asked to indicate whether the word represented an indoor or outdoor object or action. Since some of the words were more abstract than others, participants were told that there was no right or wrong answer, but to follow their instinct as to how the word made them feel. During the second phase (recognition), participants were again shown a list of words, each for 2000 ms (500 ms ISI) and asked if the word was previously shown during the first phase (i.e., “old”) or not shown during the first phase (i.e., “new”). They provided responses using two keyboard buttons. The words shown during recognition were divided evenly between targets (repeated items), lures (similar items) and foils (novel items) (n = 67 items each). See Fig 1 for example stimuli and experiment logic.
Figure 1. Task stimuli and parameters.
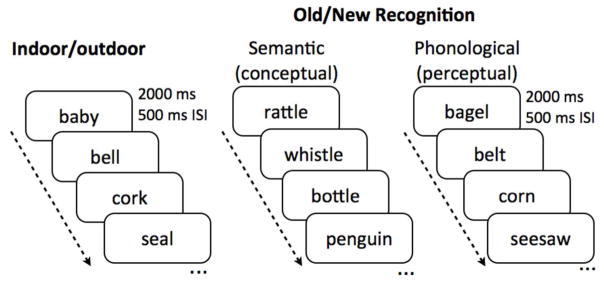
During the first phase, participants were given an indoor/outdoor judgment task with stimuli presented for 2000 ms with a 500 ms inter-stimulus interval (ISI). During the second phase, participants were given an old/new recognition test. Participants were randomized to either the semantic condition (testing conceptual interference) or the phonological condition (testing perceptual interference).
We calculated two critical measures of performance in each experiment. The first was d’ calculated as z(Hits)-z(False alarms) based on the target and foil items. This is a standard measure of recognition performance. The second was a normalized lure discrimination index (zLDI) calculated as z [p(“New”|Lure)-p(“New”|Target)] which estimates discrimination ability (i.e., the behavioral assay of pattern separation) and corrects for response bias. Raw response rates are presented in Table 1.
Table 1.
Group response distributions across tasks
| Young | Aged | |||
|---|---|---|---|---|
| Conceptual | Old | New | Old | New |
| Targets | 0.790 (0.102) | 0.210 (0.102) | 0.784 (0.155) | 0.216 (0.155) |
| Lures | 0.126 (0.083) | 0.874 (0.083) | 0.148 (0.116) | 0.852 (0.116) |
| Foils | 0.085 (0.066) | 0.915 (0.066) | 0.088 (0.088) | 0.912 (0.088) |
| Perceptual | Old | New | Old | New |
| Targets | 0.826 (0.118) | 0.174 (0.118) | 0.753 (0.131) | 0.247 (0.131) |
| Lures | 0.152 (0.090) | 0.848 (0.090) | 0.205 (0.152) | 0.795 (0.152) |
| Foils | 0.099 (0.079) | 0.901 (0.079) | 0.165 (0.157) | 0.835 (0.157) |
Results presented as mean (standard deviation)
Analyses of performance across groups and tasks were conducted using two-way Analysis of Variance (ANOVA) with age group (aged vs. young) and task (perceptual vs. conceptual) as fixed factors. The dependent variables were summary performance statistics (i.e. d’ and zLDI). All statistics were conducted using IBM SPSS v. 20 (Armonk, NY). Final alpha for all significance tests was set at .05 two-tailed.
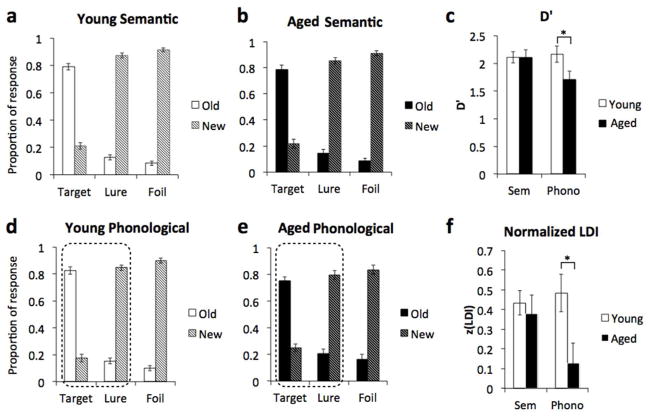
Impaired perceptual but not conceptual discrimination in older adults
Young adults and older adults performed very well on the conceptual task with no significant differences between groups on any of the conditions (Fig 2ab). Both recognition (d’) and discrimination (zLDI) were not significantly different in young and older adults (all p’s > .05) (Fig 2c). On the perceptual task, however, there was a difference between age groups approaching significance in d’ [t(37)=1.82, p=.076)], and a significant group difference in zLDI (t(37) = 2.552, p=.015) (Fig 2f). We further assessed this difference in a two-way ANOVA with age and task as fixed factors. For d’ we found no main effect of age or task, but a significant age x task interaction (F(1,74)=4.08, p=.047). For zLDI we found a significant main effect of age [F(1,75)=5.12, p=.027] and a marginally significant interaction [F(1,74) =2.79, p=.1].
Figure 2. Behavioral performance on semantic and phonological interference tests.
Performance of young adults on the semantic interference task (a) is comparable to performance of the older adults (b). Both D’ (c) and normalized lure discrimination index (zLDI) (f) show comparable performance on the semantic (Sem) version. Performance of young adults on the phonological interference task (d) was significantly better than performance of older adults (e). Both D’ (c) and zLDI (f) show decreased performance in the older adults compared to young adults on the Phonological (Phono) version.
Controlling perceptual/attentional influences: match-to-sample task
In order to control for declines in perceptual abilities in older adults that may confound the results of the age comparison in the perceptual condition, we ran a control match-to-sample task in a separate, stimulus naïve sample of eight (mean age 20.13 SD 1.13) young adults and six (mean age 76.17 SD 7.41) older adults. In this task, participants were shown the same word pairs shown in the phonological conditions, except that each word was yoked to its similar lure, separated by a noise mask to remove the sensory trace (see Fig 3a). The first word appeared for 2000 ms, a noise mask followed for 500 ms, then the second word appeared for another 2000 ms. Once the second word appeared, participants made a judgment as to whether the second word was the same or different from the first word in the pair. Comparison of young vs. aged in the control task was done using the nonparametric Mann-Whitney U test as group size was too small to approximate a normal distribution.
Figure 3. Control match-to-sample task.
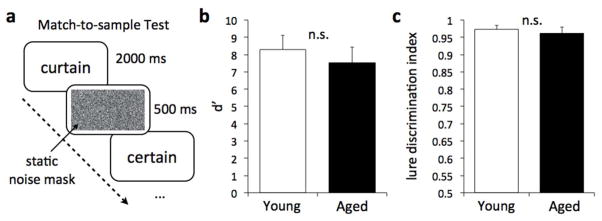
To control for non-mnemonic perceptual influences such as attention, vigilance, working memory, visual deficits, etc. we used a match-to-sample task with yoked pairs of interference-evoking words separated by a 500 ms static noise mask to eliminate the iconic trace. We see no performance differences between young and older adults on this task.
Performance on the match-to-sample task in both young and older adults was very similar and both at ceiling (all p’s>.05; Fig 3bc), suggesting that age-related perceptual/attentional or working memory deficits could not explain the deficit in phonological discrimination and that this deficit is primarily the result of proactive interference (i.e. a mnemonic process). It is worth noting that at-ceiling performance does not allow us to investigate more fine-level differences among groups. For example, it is possible that by making the task more difficult (i.e., briefer presentation time, increased number of words, etc.) we would be able to detect a perceptual/attentional or working memory deficit in older adults that may contribute to the mnemonic deficit. However, we chose to hold constant all of our parameters so that the memory effect can be isolated. While we cannot say for certain whether in other situations perceptual/attentional or working memory deficits would contribute to age-related failures in mnemonic discrimination, we can be reasonably assured that it cannot explain the mnemonic findings reported in this study.
Perceptual vs. conceptual discrimination in aging
Taken together, our data suggest that age-related mnemonic discrimination deficits are principally due to perceptual and not conceptual interference. This is consistent with the notion that conceptual knowledge is preserved with age and it is possible that having a larger conceptual framework could aid with performance of this task. For example, recent studies have shown that semantic relatedness could improve associative memory task performance in elderly but not young adults (Badham et al., 2012; Crespo-Garcia et al., 2012; Patterson et al., 2009).
Accurate recognition in lure discrimination tasks such as the DRM or the task discussed herein is thought to depend on both gist information and item-specific recollection (recalling exact item features and contextual details), whereas false recognition is thought to rely solely on gist information (Schacter et al., 1997b). It is possible that correctly discriminating perceptual lures disproportionately relies on item-specific recollection compared to correctly discriminating conceptual lures. It is precisely this item-specific recollection that is also sensitive to pattern separation deficits (Norman, 2010; Yonelinas et al., 2010), thus the perceptual condition may be more sensitive to such deficits than the conceptual condition. This may explain why older adults may struggle with perceptual but not conceptual interference in our task.
Consistent with this account, Schacter and colleagues (Schacter et al., 1997b) have also previously showed that conceptual false recognition was associated with more “Remember” responses and perceptual false recognition was associated with more “Know” responses. This suggests that conceptual false recognition may be mediated by illusory recollection while perceptual false recognition may be mediated by gist memory or familiarity. A large body of work has consistently shown that recollection declines in older adults and familiarity is generally spared (Bastin and Van der Linden, 2003; Duverne et al., 2008; Healy et al., 2005; Jennings and Jacoby, 1997; Prull et al., 2006; Toth and Parks, 2006). This bias towards gist processing with age may explain the increased perceptual false alarms in our task, which are likely more dependent on false familiarity or gist rather than illusory recollection.
Several past studies using DRM-type tasks where word lists were created with single critical lures missing and tested during recognition have shown that older adults, under some circumstances, show increased conceptual false alarms compared to young adults (Norman and Schacter, 1997; Tun et al., 1998; Watson et al., 2001). While this seems inconsistent with our data, it is possible that when older adults are allowed to create a more generalized semantic representation during study using multiple exemplars from the same category, gist information becomes harder to overcome during test and thus false recognition arises. Also, without comparison with phonologically similar lures, it is difficult to determine whether the effect is general to all forms of discrimination or specific to conceptual discrimination. To our knowledge, only one study (Budson et al., 2003) using a similar DRM-design also investigated phonological similarity and found that older adults were impaired on both semantic and phonological discrimination.
It is difficult to compare our results directly to the study by Budson and colleagues, since the word lists the authors used included multiple lures during study (adapted from the DRM procedure) and not single related items as in our experiment. These two types of designs may differ substantially in terms of underlying semantic representations but can offer complementary information, and an important future direction for this research would be to systematically vary the number of related lures in study lists to investigate this difference among paradigms.
Mechanistic basis for pattern separation impairment
Although we could only assess behavioral discrimination in this study and not neural pattern separation per se, the data is consistent with a failure in pattern separation of perceptually similar inputs. The mechanism for this is thought to be dependent on an age-related shift in computational bias from pattern separation to pattern completion, as a result of the disinhibition in the CA3 region, which leads to hyperexcitability of place cells in older rats (Wilson et al., 2006) and BOLD fMRI hyperactivity in older humans (Yassa et al., 2010a). As activity increases in the CA3 region, which is rich with recurrent collaterals that are capable of auto-association, the dynamic balance between pattern separation and pattern completion shifts in favor of completion. We have also shown in the past that aging is associated with CA3/dentate representational rigidity (inability to elicit a novelty response) while viewing similar but not identical lures, the extent of which predicted behavioral discrimination deficits (Yassa et al. 2011b). In the same study, we also demonstrate that this rigidity is related to perforant path integrity, as assessed with high-resolution diffusion tensor imaging. Taken together, these data suggest that several features in the hippocampal network including structural and functional changes may underlie the observed age-related impairment in pattern separation. The data in this particular paper support the notion that not all types of memory are susceptible to this impairment and that conceptual discrimination (which may utilize other non-hippocampal mechanisms) is relatively immune from this deficit.
Limitations and future directions
Our study also has some notable limitations. The task designs did not allow us to parametrically investigate the level of interference (i.e., how phonologically or semantically similar items were). This type of manipulation based on amounts of interference could be informative as far as understanding pattern separation dynamics and would be essential for determining the neural signatures that may underlie phonological and semantic interference. Both tasks were also relatively easy with lure correct rejections around 80%. This is likely because we only used a single item during study to elicit each false alarm during test, unlike the DRM design, which uses an entire list of words to elicit each false alarm. While our design further demonstrates the power of the DRM concept (false recognition even with a single interfering item), future experiments systematically varying the number of related items during study are also valuable. Our age groups were also selected at the extremes of the aging continuum (20 year olds vs. 70 year olds), thus we cannot determine the age when this deficit starts to manifest. A lifespan approach is necessary to address this question.
In conclusion, our results suggest that aging is associated with the impairment of distinct processing of phonologically related lures and not semantically related lures. This contributes to a small but growing literature on age-related memory impairments in pattern separation and further suggests that age-related impairments may be disproportionately due to failures in perceptual rather than conceptual processing.
Acknowledgments
M.A.Y. is supported by grants from the NIA P50 AG05146 and R01 AG034613. M.L. is supported by a Provost Undergraduate Research Award.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest in relation to this manuscript.
References
- Badham SP, Estes Z, Maylor EA. Integrative and semantic relations equally alleviate age-related associative memory deficits. Psychol Aging. 2012;27(1):141–52. doi: 10.1037/a0023924. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: a study of the effects of test format and aging. Neuropsychology. 2003;17(1):14–24. [PubMed] [Google Scholar]
- Budson AE, Sullivan AL, Daffner KR, Schacter DL. Semantic versus phonological false recognition in aging and Alzheimer’s disease. Brain Cogn. 2003;51(3):251–61. doi: 10.1016/s0278-2626(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Craik FIMSE. In: Poon LWFJ, Cermak L, Arenberg D, Thompson LW, editors. Age differences in memory: the roles of attention and depth of processing; New directions in memory and aging: Proceedings of the George Talland memorial conference; Hillsdale, NJ: Lawrence Erlbaum; 1980. pp. 95–112. [Google Scholar]
- Crespo-Garcia M, Cantero JL, Atienza M. Effects of semantic relatedness on age-related associative memory deficits: the role of theta oscillations. Neuroimage. 2012;61(4):1235–48. doi: 10.1016/j.neuroimage.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Dodson CS, Bawa S, Slotnick SD. Aging, source memory, and misrecollections. J Exp Psychol Learn Mem Cogn. 2007;33(1):169–81. doi: 10.1037/0278-7393.33.1.169. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiol Aging. 2008;29(12):1902–16. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Healy MR, Light LL, Chung C. Dual-process models of associative recognition in young and older adults: evidence from receiver operating characteristics. J Exp Psychol Learn Mem Cogn. 2005;31(4):768–88. doi: 10.1037/0278-7393.31.4.768. [DOI] [PubMed] [Google Scholar]
- Holden HM, Gilbert PE. Less efficient pattern separation may contribute to age-related spatial memory deficits. Front Aging Neurosci. 2012;4:9. doi: 10.3389/fnagi.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden HM, Hoebel C, Loftis K, Gilbert PE. Spatial pattern separation in cognitively normal young and older adults. Hippocampus. 2012;22(9):1826–32. doi: 10.1002/hipo.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. An opposition procedure for detecting age-related deficits in recollection: telling effects of repetition. Psychol Aging. 1997;12(2):352–61. doi: 10.1037//0882-7974.12.2.352. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Josephs O, Lambon Ralph MA, McClelland JL, Price CJ. Dissociating stimulus-driven semantic and phonological effect during reading and naming. Hum Brain Mapp. 2007;28(3):205–17. doi: 10.1002/hbm.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MC, Kaszniak AW. Spatial memory and aging: Performance on a human analog of the Morris water maze. Aging Neuropsychology and Cognition. 2000;7(2):86–93. [Google Scholar]
- Norman KA. How hippocampus and cortex contribute to recognition memory: revisiting the complementary learning systems model. Hippocampus. 2010;20(11):1217–27. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, Schacter DL. False recognition in younger and older adults: exploring the characteristics of illusory memories. Mem Cognit. 1997;25(6):838–48. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- Patterson MM, Light LL, Van Ocker JC, Olfman D. Discriminating semantic from episodic relatedness in young and older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2009;16(5):535–62. doi: 10.1080/13825580902866638. [DOI] [PubMed] [Google Scholar]
- Prull MW, Dawes LL, Martin AM, 3rd, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: adult age differences and neuropsychological test correlates. Psychol Aging. 2006;21(1):107–18. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Roediger HL, Mcdermott KB. Creating False Memories - Remembering Words Not Presented in Lists. Journal of Experimental Psychology-Learning Memory and Cognition. 1995;21(4):803–814. [Google Scholar]
- Schacter DL, Kaszniak AW, Kihlstrom JF, Valdiserri M. The Relation between Source Memory and Aging. Psychology and Aging. 1991;6(4):559–568. doi: 10.1037//0882-7974.6.4.559. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Koutstaal W, Norman KA. False memories and aging. Trends Cogn Sci. 1997a;1(6):229–36. doi: 10.1016/S1364-6613(97)01068-1. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Osowiecki D, Kaszniak AW, Kihlstrom JF, Valdiserri M. Source Memory - Extending the Boundaries of Age-Related Deficits. Psychology and Aging. 1994;9(1):81–89. doi: 10.1037//0882-7974.9.1.81. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Verfaellie M, Anes MD. Illusory memories in amnesic patients: conceptual and perceptual false recognition. Neuropsychology. 1997b;11(3):331–42. doi: 10.1037//0894-4105.11.3.331. [DOI] [PubMed] [Google Scholar]
- Silver H, Goodman C, Bilker WB. Impairment in associative memory in healthy aging is distinct from that in other types of episodic memory. Psychiatry Res. 2012;197(1–2):135–9. doi: 10.1016/j.psychres.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging. 1995;10(4):527–39. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17(6):284–8. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16(5):338–42. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Toth JP, Parks CM. Effects of age on estimated familiarity in the process dissociation procedure: the role of noncriterial recollection. Mem Cognit. 2006;34(3):527–37. doi: 10.3758/bf03193576. [DOI] [PubMed] [Google Scholar]
- Tun PA, Wingfield A, Rosen MJ, Blanchard L. Response latencies for false memories: gist-based processes in normal aging. Psychol Aging. 1998;13(2):230–41. doi: 10.1037//0882-7974.13.2.230. [DOI] [PubMed] [Google Scholar]
- Watson JM, Balota DA, Sergent-Marshall SD. Semantic, phonological, and hybrid veridical and false memories in healthy older adults and in individuals with dementia of the Alzheimer type. Neuropsychology. 2001;15(2):254–67. [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29(12):662–70. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011a;21(9):968–79. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011b;108(21):8873–8. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci U S A. 2010a;107(28):12687–91. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34(10):515–25. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010b;51(3):1242–52. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Aly M, Wang WC, Koen JD. Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus. 2010;20(11):1178–94. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]