Abstract
Key features of bone tissue structure and composition are capable of directing cellular behavior towards the generation of new bone tissue. Bone tissue, as well as materials derived from bone, have a long and successful history of use as bone grafting materials. Recent developments in design and processing of synthetic scaffolding systems has allowed the replication of the bone’s desirable biological activity in easy to fabricate polymeric materials with nano-scale features exposed on the surface. The biological response to these new tissue-engineering scaffold materials oftentimes exceeds that seen on scaffolds produced using biological materials.
Key Worlds: Bone tissue engineering, Biomimetic, Nanofiber, Scaffold, Thermally induced phase separation
Bone Structure and Function
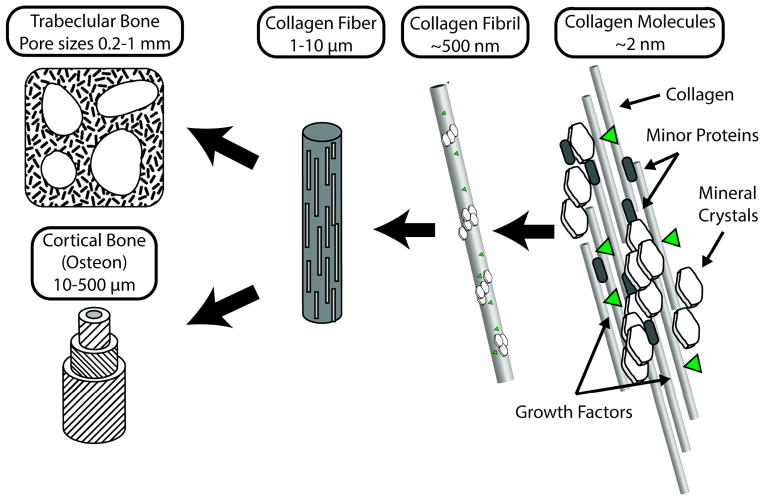
As the primary structural material of the body, bone tissue must withstand a variety of static and dynamic loading conditions. Bone tissue is a composite of tough, though flexible, collagen fibers reinforced with calcium phosphate nanocrystals. The composite structure of bone gives it much greater stiffness than typical tissues while at the same time providing surprisingly high fracture toughness and resistance to damage, especially compared to other biological tissues 1. As a living tissue bone reacts to physical stimuli, growing in response to imposed loads, and capable of detecting damaged (but not yet completely fractured) areas of tissue and replacing them though a process called remodeling. Bone tissue can be described as a polymer composite of collagen reinforced with calcium phosphate nanocrystals which is arranged in a highly organized structure over multiple size scales (Fig 1). Collagen proteins ~200 nm long and 2–3 nm in diameter self-assemble into fibrillar structures, which are then further aggregated into fibers ~200 nm in diameter. The regular stacking pattern of collagen molecules in a fiber leaves small gaps which in bone are occupied by plate-shaped nanocrystals 10–20 nm in length and 2–3 nm wide and made of a mineral that closely resembles the synthetic mineral hydroxyapatite (Ca10(PO4)6(OH)2) 2.
Figure 1.
Organization of bone tissue from its smallest components (right) to whole tissues (left). Bone is a composite of collagen fibers reinforced with calcium phosphate nanocrystals arranged in a semi-regular pattern. Other components include minor proteins and growth factors. Mineralized collagen is aggregated into small fibrils, which further combine to form fibers a few microns in diameter and several mm long. In trabecular (spongy) bone the mineralized fibers are semi-randomly laid out in struts forming an open cell foam. Cortical bone is composed of circular osteons which feature aligned sheets of mineralized fibers wrapped around a central hollow core.
Depending on the type of bone, these mineralized fibers are arranged into larger tissue-level structures. Woven bone has a random organization of mineralized fibers, and is a transient bone structure produced during skeletal development and initial response to injury. Trabecular, or spongy bone, fills the interior of many bones and is porous open cell foam with pore sizes on the order of 0.5–1 mm. Mineralized fibrils run parallel to the surface of the trabecular bone struts (Fig. 1). In the long bones of the arms and legs, cortical bone, highly adapted to resisting on-axis compressive loading, is composed of cylindrical structures termed osteons which are 1–2 cm long, 1–2 mm wide, and made up of concentric sheets of mineralized fibers oriented at ~90 degrees from previous layers, a structure strikingly similar to plywood or carbon-fiber composite materials (Fig. 1) 2.
Though the structure of bone tissue varies at the micro- and macro- size scales, the basic building block – the mineralized collagen fiber, is common to all bony tissues. The intrinsic properties of these mineralized collagen fibers, combined with other minor protein components (<5% by weight), provide biological signals that direct bone producing cells – osteoblasts – to form bone tissue. Type I collagen has over 40 cellular binding sites and other minor components of bone tissue display binding locations as well 3. These minor components include growth factors such as bone morphogenic proteins (BMPs) and proteins such as bone sialoprotein, osteocalcin 4, osteonectin 5, and matrix GLA protein 6, most of which are thought to help direct mineral formation and integrate deposited crystals into the collagen matrix as well as possessing additional biological activity. The combination of these major and minor components of bone tissue provides a strong osteogenic, or bone-generating, signal to adjacent cells, stimulating adult stem cells to differentiate into bone-producing cells (osteoblasts), and increasing the rate of new tissue formation by mature osteoblasts.
While bone has limited regenerative capability, some injuries heal poorly due to anatomical location, patient age, or may be simply too large for the body to repair the defect by itself. Any space larger than ~5 mm will not heal naturally, and requires clinical intervention to ensure healing 7,8. While a simple fracture rarely results in a gap larger then this critical size, traumatic injuries, cancers and infections of the bone, or simply age-related degeneration and fracture, can result in voids where bone regrowth is desired, but would not happen naturally. The number of these conditions makes bone the most commonly transplanted tissue after blood, with over 500,000 grafting procedures performed in the US annually 9. To meet the needs of these patients, surgeons have developed a variety of methods and have used a variety of materials to restore bone tissue, or at a minimum restore mechanical integrity of the wound site.
Early Approaches to Bone Regeneration
Since bone is primarily a structural tissue simply restoring mechanical integrity is often sufficient, and various types of metallic hardware have been long utilized for this purpose. While these can effectively hold pieces of bone together, the stress concentrations induced at the implant attachment sites can cause loosening and implant failure over time. Mechanical fixation is usually a temporary measure (which may last decades, in cases such as artificial joints), providing stability and holding bone in place long enough for re-integration of the bone fragments to occur. However this strategy is only effective if there is sufficient healthy bone fragments available to reassemble. Oftentimes even after reassembly of the available fragments, gaps still exist that must be filled with bone or materials that lead to bone regeneration. Hence in recent years the focus has shifted to materials not just capable of restoring mechanical integrity to the tissue, but to regenerating bone as it originally existed, leaving only healthy natural tissue.
To replace bone tissue with a material that will eventually become bone, the first choice of surgeons is to use pieces of the patient’s own bone. Bone autografts – from a patient’s own body - are the gold standard of treatment, with success rates well over 90% in most cases 9,10. Their use is limited by amount of bone that be safely transplanted, and some diseases can preclude their use altogether. Extraction of an autograft is essentially a second surgery, with all of the attendant risks and complications, and can include long-term pain and numbness at the extraction site 11.
To overcome the expense and trauma associated with autograft harvesting, allografts – donor tissue – have been proposed as a solution not limited by graft size or patient condition. While showing similar efficacy to autografts, donor tissue is in short supply, and issues of potential disease transmission and tissue rejection make their use a clear second choice to autografts. Nonetheless for specific conditions where very large portions of bone are being replaced, allografts are often an excellent choice 12.
As obtaining autografts is often not practical, and allografts have significant drawbacks, an alternative is to utilize highly processed, biologically sourced, components of bone tissue. Bone tissue can be fired to burn off all organic material leaving only the mineral fraction, producing mineral chips that the body still recognizes as bone to some degree. Alternately, collagen purified from bovine hides can be fabricated into highly porous foams, which also allow for cellular colonization and eventual tissue regrowth. While economical and easy to use, these products work best in constrained environments where fragments are not likely to shift or migrate out of the wound site. Their bioactivity is somewhat less then that of autograft or allograft as they lack key components of bone tissue, especially the osteogenic growth factors embedded in living bone. These mineral and collagen products are often the first choice for applications where immediate load bearing is not required, such as dental applications and spinal fusion procedures 10,13.
In recent years these purified bone components have been supplemented with bone morphogenic proteins (BMPs), which strongly encourage bone formation, even in ectopic locations (away from other bony tissues), a process normally possible by the typical osteogenic cellular signaling and control mechanism. Their efficacy is such that collagen scaffolds containing BMP have become the most common grafting material for spinal fusion procedures 14. Controlling the release of BMP, and providing physiologically useful quantities at the wound site, but not elsewhere in the body, has proven a challenge, and severe complications including deaths have been reported due to excessive BMP diffusing out from the graft site 15,16. Due to their highly effective biological properties, extensive research has been conducted into immobilizing or controlling BMP release at the wound site, allowing for much smaller amounts to be used, providing the same efficacy without the risks 17. Recent studies have investigated utilizing multiple growth factors, such as combinations of BMP and vascular endothelial growth factor (VEGF) to accelerate healing by encouraging blood vessel formation in the wound site18,19.
All of the currently available materials for bone replacement share a basic similarity – they replicate some component of natural bone tissue in order to induce a similar biological response. While effective at ensuring bioactivity, limiting material selection to biological sources puts constraints on the ultimate properties of the material. Synthetic materials offer significant advantages in cost, consistency, and ease of regulatory approval. Through careful design it may even be possible to enhance the biologically signaling a material provides, healing a wound site faster than any bone-based material would allow.
Biomimetic Design of Synthetic Bone Replacement Materials
Structure and Composition
Recently it has become clear that aside from chemical signaling, the shape a cell takes can powerfully affect its differentiation and behavior 20. Experiments with micro-patterned surfaces show that pits and groves can control cell shape, with subsequent effects on proliferation rate, motility, differentiation, and extra-cellular matrix production 21,22. Bone tissue at its smallest scale is composed of mineralized collagen fibers on the scale of 1 μm in diameter. These fibers are exposed on any bone surface, and the resulting surface morphology likely provides osteogenic signaling to attached cells.
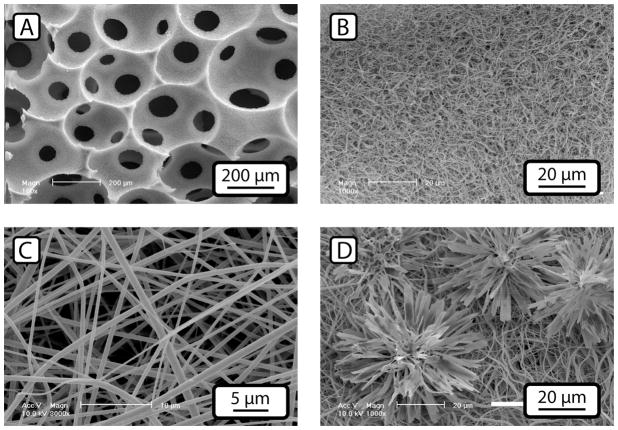
Three techniques have recently been utilized to create three-dimensional scaffolding materials with nano-scale features similar in shape to collagen fibers; thermally induced phase separation (TIPS), electrospinning, and self-assembly (SA). TIPS utilizes phase separation to induce the formation of polymeric nanofibers 50–500 nm in diameter (Fig. 2a and b), and is compatible with both synthetic polymers such as poly(L-lactic acid) (PLLA) and naturally derived polymers such as gelatin 23,24. Electrospinning uses electric charge to draw out a spun filament to a micron or nanometer diameter, which collects on a collection plate as a porous mat of interlocking fibers (Fig. 2c) 25. This technique can be used with nearly any polymeric material, and polymer-polymer blends and polymer-ceramic composite fibers can be easily produced. Self-assembly takes cues from self-organizing structures in nature, either replicating the fiber formation capability of collagen-like proteins, or using amphiphilic molecules to create nano-scale tubular micelles that can be further consolidated into a gel 26. All three techniques have their advantages and disadvantages as tissue engineering scaffold materials, and several recent reviews cover them in depth 27–29. All have achieved some measure of success in bone tissue engineering. Other techniques for producing porous bone tissue scaffolds include freeze-drying, gas-foaming, porogen leaching, and solid free-form fabrication, among others 30. In general, these techniques, by themselves, are incapable of producing the nano-fibrous structure easily obtained though self-assembly, electrospinning, or TIPS, but can be utilized to provide controlled macroporosity to a nanofibrous material.
Figure 2.
Different types of nano-fibrous scaffolding materials. Phase separated poly-lactic acid scaffolds (A and B) can be produced as open-cell foams whose surface is a mat of fibers approximately 100 nm in diameter. Electrospun materials (C) typically form loose meshes of fibers ~1 micron in diameter. Both types of nano-fiber materials can be enhanced with the addition of calcium phosphate minerals. (D) shows a phase-separated scaffold surface treated with an electrodeposition process, resulting in flower-shaped mineral deposits while still leaving the nano-fibers exposed. Figures 2a and 2b adapted from Wei et al., 2009, permission for re-use obtained from Elsevier Publishing. Figs. 2c and 2d adapted from He at al. 2010, permission for re-use obtained from John Wiley and Sons.
While the mechanism is not fully understood, nano-fibrous materials in general seem to increase osteogenic differentiation of precursor cells, and ultimately lead to increased bone growth, compared to flat materials of the same composition26,31–36. The most striking aspect of this osteoinductive behavior is that it occurs with numerous material types not typically considered bioactive, fabricated in a variety of ways, and verified both in vitro and in vivo using both human and animal cells. The common factor appears to be a surface comprised of numerous small fibers less than ~1 μm in width, and it can be inferred that this nano-fiber architecture may be directly affecting cellular behavior. Although the mechanisms remain not fully understood, a few important findings have been made. Cells appeared to be less spread on nanofiber surfaces compared to flat films, the down regulation of the RhoA-ROCK signaling pathway on a TIPS nanofiber surface led to increased expression of bone sialoprotein by osteoblasts33. Modulation of cellular attachment and adhesion size and spacing has been shown to affect osteogenic differentiation20,37. It is likely that nanofiber surfaces modulate cellular adhesion, possibly though selective protein adsorption onto the scaffold surface38, in such a way as to promote osteogenic activity.
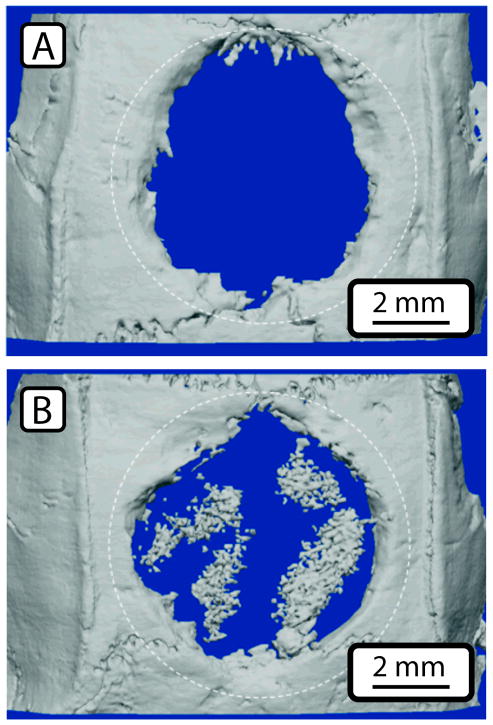
In animal studies, a TIPS-based polymer scaffold produced isolated islands of bone formation in the interior of the scaffold in a mouse calvarial defect model, and regenerated bone volume was greatly enhanced compared to a smooth-walled scaffold with a similar microstructure (Fig. 3)39. Electrospun and self-assembled scaffolds have also demonstrated bone tissue formation in subcutaneous animal models26,40, but no bone void healing studies have been conducted utilizing these materials.
Figure 3.
Repair of calvarial (skull) defect in mice using a PLLA scaffold with a smooth pore surface (A) or a phase-separated scaffold with nanofibrous structure (B). Dotted lines show the extent of the original defect. By 6 weeks bone has grown into both scaffolds, but the nano-fiber scaffold (B) shows extensive bone growth in the center of the scaffold. Figure adapted from Woo et al., 2009, permission obtained from Mary-Ann Liebert Inc.
To further replicate the biological properties of bone ECM, calcium phosphate (CaP), typically in the form of hydroxyapatite (HA), can be incorporated into scaffolding materials. CaP minerals have a long history of being utilized as biomaterials, and when used alone or combined with other materials such as collagen have shown to immediately up-regulate osteogenic markers as well as increase extra-cellular matrix deposition 41,42, partially explaining the osteogenic properties of bone tissue and bone-mineral grafting products. Aside from biological effects, CaP addition to polymer scaffolds can greatly improve their mechanical properties, to the point of nearing parity with native bone tissue. Recent studies have focused on replicating the anisotropic mechanical properties of bone through the use of plate or needle shaped CaP particles43, and the use of nanoparticles to improve reinforcement particle distribution and enhance biological activity44.
CaP particles can be directly incorporated into the scaffold, but the particles are often unevenly distributed, and can only be added at low concentrations, limiting the range of possible designs. Alternately, CaP minerals can be deposited in situ in an already fabricated scaffold. Soaking a scaffold in a supersaturated solution of calcium phosphate similar to the body’s interstitial fluid will deposit a film of partially carbonated hydroxyapatite, a near exact chemical match for native bone mineral 31,45. This coating takes weeks to form, often resulting in scaffold degradation during fabrication. However they do appear to enhance bioactivity and rate of bone regeneration in some simple animal models, demonstrating the benefits of incorporating CaP into a nanofibrous scaffold.
Recent efforts have focused on speeding up mineral deposition, and changing the morphology of the deposited mineral from a brittle coating to micro- or nano-size particles with high surface area. Peptide amphiphile nano-fibers have been fabricated with a calcium chelating region that acts as a CaP nucleation site, leading to the formation of CaP nano-crystals similar in shape to those occurring naturally in bone tissue 26, although even with this enhancement CaP takes days to deposit. This technique presumably could be utilized to enhance calcium phosphate deposition in other polymers functionalized with calcium chelating regions, but little work has been done in this area. An electrodeposition process has been shown to rapidly deposit carbonated hydroxyapatite on both electrospun and TIPS-based scaffolds. CaP morphologies ranging from films of bladelike crystals to star-shaped rosettes can be produced (Fig. 2d), allowing for precise tailoring of the CaP reinforcing particles. A scaffold mineralized with this electrodeposition technique caused increased cell proliferation and osteogenic activity in MC3T3-E1 mouse pre-osteoblasts in a preliminary experiment 46.
Lastly, a scaffold should ideally degrade at the rate new tissue forms, eventually leaving only healthy tissue. This can occur in one of two ways – biological (enzymatic) degradation using the body’s natural remodeling mechanisms, or chemical degradation, typically though hydrolysis of polymer chains. Natural materials such as collagen and calcium phosphate can be degraded by the body using the same mechanisms responsible for bone remodeling, but this option is not available to artificial materials, unless biologically degradable domains are added to polymer chains directly or as crosslinkers. Biologically degradable molecules can be used as cross-linkers for otherwise non-degradable materials, allowing the material to be remodeled in the same way as natural tissue. This technique can turn a cell-impermeable gel into a matrix easily traversed by encapsulated cells 47,48. Alternately chemically degrading materials, such as PLLA, can be tailored to have a specific degradation rate, and issues of cell infiltration can be overcome through an open-cell porous structure.
Bioactive Molecule Addition
In the early 1990s, the binding location of the primary cell-matrix adhesion proteins, the integrins, was determined, and a number of short peptide sequences were identified which allow a cell to attach to a substrate containing them. Numerous binding motifs have been identified, but the RGD (Arginine-Glycine-Aspartic Acid) sequence is the best studied 49, and has been attached to a variety of artificial surfaces in order to increase bioactivity. See Hersel et al. for an excellent review of the various permutations of polymer formulations and binding strategies that have been tried. While this technique can effectively increase bioactivity of otherwise inert surfaces, materials with nano-fibrous features or bioactive mineral components already interact strongly with cells, and additional benefit from RGD or similar functionalization would likely only be minor.
These same peptide-binding techniques can be utilized to bind growth factor peptides to the same scaffold surfaces, providing additional tissue-specific signaling mechanisms. Covalently linking growth factors to a scaffold carries numerous advantages. The local concentration of growth factor can be maintained at a high level within the scaffold without requiring excessive growth factor molecules, or worry about diffusion of factors out of the wound site causing undesirable side effects or tumor generation 50. Techniques can also be used to pattern different growth factors though a scaffold, facilitating the development of multi-phasic scaffolds, for example to repair an osteochondral defect.
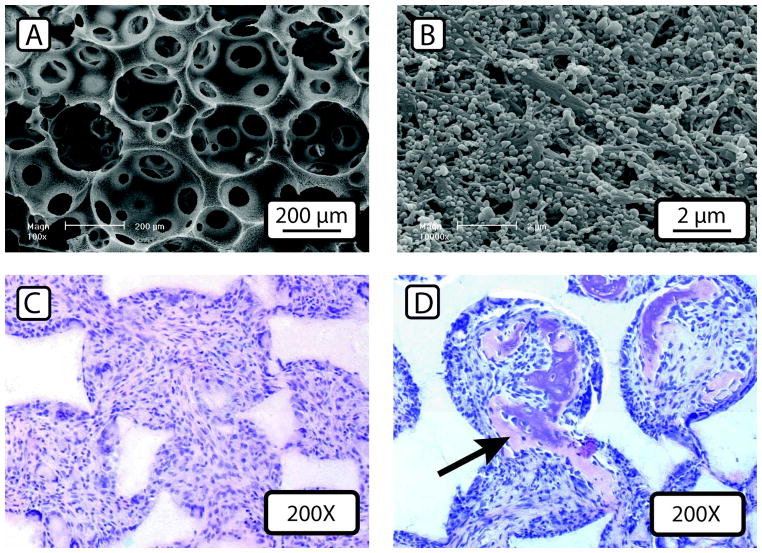
Aside from permanent fixation of growth factors, various methods of controlling growth factor release have been developed. Embedding BMP deeply in scaffold materials can slow their release, but then BMP release is inextricably tied to scaffold degradation rate, complicating scaffold design. Degradable microspheres or other particles can be utilized to deliver the drug of interest51. By controlling sphere size, thickness, and polymer composition, low-level release over weeks is possible 50, and sphere properties can be tailored independently of scaffold design. Multiple varieties of microsphere can be used to deliver multiple growth factors52. Immobilization of multiple microspheres on a scaffold pore surface can maintain their individual release profiles53. In vivo experiments show increased bone tissue formation when using BMP-releasing microspheres immobilized on the pore surface of a nanofibrous scaffold (Fig. 4)54. The greatest differences were seen at later time points, when the traditionally delivered BMP had long since diffused out of the wound site, suggesting that low-level BMP delivery over the entire course of healing is more effective than a single pulse of growth factor upon implantation. More studies are required to determine the optimum long-term dosing rates and timeframes of factor delivery to maximize bone regeneration while minimizing potential side effects caused by high BMP or other growth factors doses.
Figure 4.
Nanofibrous scaffold loaded with BMP-containing microspheres at low (A) and (B) high magnification. Microsphere addition does not change scaffold morphology, even at very high loading levels. (C) and (D) show histological sections of scaffolds subcutaneously implanted in the backs of mice for 3 weeks. BMP-soaked scaffolds (C) show minimal bone tissue formation, but using delayed-release microspheres results substantially more bone tissue formation, as indicated by a black arrow (D). Figures adapted from Wei et al., 2007, permission obtained from Elsevier Publishing.
The Future of Bone Tissue Engineering
Techniques for producing synthetic bioactive bone scaffolds have the potential to significantly improve the performance of bone graft replacement materials. In some cases, the biological response to these materials seemingly exceeds that of bone or scaffolds produced using biological materials. Instead of the long-standing goal of merely replicating natural tissue regeneration, artificial materials and structures could be utilized in the future to exceed the body’s natural healing response, and for the first time, offer a bone graft replacement material with a clear advantage over traditional autografts. All the more remarkable is that these gains are made with non-optimized scaffold systems, and with little understanding of how these systems achieve their superior results compared to traditional bone replacement materials. A more complete understanding of the interrelated mechanisms involved in bone tissue regeneration, when combined with scaffolds precisely tailored to guide that behavior to maximum tissue regeneration rate, could potentially lead to treatments far more effective then those today.
Acknowledgments
We gratefully acknowledge the financial support from the NIH (NIDCR DE022327 & DE015384), DOD (W81XWH-12-2-0008), and NSF (DMR-1206575). RK was partially supported by an NIH/NIDCR Training Grant (T32 DE007057) at the University of Michigan School of Dentistry.
References
- 1.Weiner S, Wagner HD. Ann Rev of Mat Sci. 1998;28:271–98. [Google Scholar]
- 2.Rho JY, Kuhn-Spearing L, Zioupos P. Med Eng & Phys. 1998;20:92–102. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 3.Di Lullo GA, et al. J Bio Chem. 2002;277:4223–31. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 4.Hauschka PV, Wians FH. Anatomical Record. 1989;224:180–8. doi: 10.1002/ar.1092240208. [DOI] [PubMed] [Google Scholar]
- 5.Bolander ME, et al. Proc Nat Acad Sci. 1988;85:2919–23. doi: 10.1073/pnas.85.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price PA, et al. Proc Nat Acad Sci. 1976;73:1447–51. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petite H, et al. Tissue-engineered bone regeneration. Nature biotechnology. 2000;18:959–63. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz JP, Hollinger JO. Clin Ortho Rel Res. 1986;205:299–308. [PubMed] [Google Scholar]
- 9.Greenwald AS, et al. J Bone Joint Surg. 2001;83-A(Suppl):98–103. doi: 10.2106/00004623-200100022-00007. [DOI] [PubMed] [Google Scholar]
- 10.Knesser U, et al. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seiler JG, Johnson J. J South Ortho Assoc. 2000;9:91–7. [PubMed] [Google Scholar]
- 12.De Long WG, et al. J Bone Joint Surg. 2007;89:649–58. doi: 10.2106/JBJS.F.00465. [DOI] [PubMed] [Google Scholar]
- 13.Janicki P, Schmidmaier G. Injury. 2011;42(Suppl 2):S77–81. doi: 10.1016/j.injury.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Geiger M, Li R, Friess W. Adv Drug Delivery Rev. 2003;55:1613–29. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Chrastil J, et al. Spine. 2013 Preprint. [Google Scholar]
- 16.Lubelski D, et al. J Spinal Dis Tech. 2013 doi: 10.1097/BSD.0b013e31823f3139. Preprint. [DOI] [PubMed] [Google Scholar]
- 17.Bessa PC, Casal M, Reis RL. J Tiss Eng Regen Med. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 18.Peng H, et al. J Clin Inv. 2002;110:751–9. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang YC, et al. J Bone Min Res. 2005;20:848–57. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 20.McBeath R, et al. Dev Cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 21.Kolind K, et al. Biomaterials. 2012;33:6626–33. doi: 10.1016/j.biomaterials.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 22.Curtis S, et al. Biophys Chem. 2001;94:275–83. doi: 10.1016/s0301-4622(01)00247-2. [DOI] [PubMed] [Google Scholar]
- 23.Ma PX, Zhang R. J Biomed Mat Res. 1999;46:60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Ma PX. Biomaterials. 2009;30:4094–103. doi: 10.1016/j.biomaterials.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doshi J, Reneker D. J Electostat. 1995;35:151–60. [Google Scholar]
- 26.Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684–8. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 27.Ma PX. Adv Drug Deliv Rev. 2008;60:184–98. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holzwarth JM, Ma PX. Biomaterials. 2011;32:9622–9. doi: 10.1016/j.biomaterials.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teo WE, Ramakrishna S. Nanotechnology. 2006;17:R89–R106. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 30.Hutmacher DW. Biomaterials. 2000;21:2529–43. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 31.Whited BM, et al. Biomaterials. 2011;32:2294–304. doi: 10.1016/j.biomaterials.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Badami AS, et al. Biomaterials. 2006;27:596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Liu X, Ma PX. Biomaterials. 2008;29:3815–21. doi: 10.1016/j.biomaterials.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith LA, et al. Biomaterials. 2010;31:5526–35. doi: 10.1016/j.biomaterials.2010.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo KM, et al. Biomaterials. 2007;28:335–43. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 36.McCullen SD, et al. Biomed Mat. 2009;4:035002. doi: 10.1088/1748-6041/4/3/035002. [DOI] [PubMed] [Google Scholar]
- 37.Cavalcanti-Adam EA, et al. Biophys J. 2007;92:2964–74. doi: 10.1529/biophysj.106.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo K, et al. J Biomed Mat Res A. 2003;67:531–7. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 39.Woo KM, et al. Tiss Eng A. 2009;15:2155–62. doi: 10.1089/ten.tea.2008.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seyedjafari E, et al. Biomacromolecules. 2010;11:3118–25. doi: 10.1021/bm1009238. [DOI] [PubMed] [Google Scholar]
- 41.Shu R, et al. J Biomed Mat Res A. 2003;67:1196–204. doi: 10.1002/jbm.a.20021. [DOI] [PubMed] [Google Scholar]
- 42.Beck GR. J Cell Biochem. 2003;90:234–43. doi: 10.1002/jcb.10622. [DOI] [PubMed] [Google Scholar]
- 43.Converse G, et al. Biomaterials. 2007;28:927–35. doi: 10.1016/j.biomaterials.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 44.Shi Z, et al. Acta Biomat. 2009;5:338–45. doi: 10.1016/j.actbio.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 45.Al-Munajjed AA, et al. J Biomed Mat Res B. 2009;90:584–91. doi: 10.1002/jbm.b.31320. [DOI] [PubMed] [Google Scholar]
- 46.He C, et al. Adv Fun Mat. 2010;20:3568–76. doi: 10.1002/adfm.201000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almany L, Seliktar D. Biomaterials. 2005;26:2467–77. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 48.Mann BK, et al. Biomaterials. 2001;22:3045–51. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 49.Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385–415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 50.Tessmar JK, Göpferich AM. Adv Drug Deliv Rev. 2007;59:274–91. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 51.Mehta M, et al. Adv Drug Deliv Rev. 2012;64:1257–76. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen FM, et al. Biomaterials. 2010;31:6279–308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 53.Wei G. Growth Factor-Delivering Nano-Fibrous Scaffolds for Bone Tissue Regeneration. University of Michigan; 2006. [Google Scholar]
- 54.Wei G, et al. Biomaterials. 2007;28:2087–96. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]