Abstract
Purpose.
To investigate the impact of misclassification of age-related macular degeneration (AMD) on the baseline intensity and estimated effects of age, sex, and the Y402H variant in the complement factor H (CFH) gene on incidence, progression, and regression of AMD.
Methods.
The Beaver Dam Eye Study, a longitudinal population-based study of age-related eye diseases conducted in the city and township of Beaver Dam, Wisconsin, performed examinations every 5 years during a 20-year period (1988–1990 through 2008–2010). Study participants (N = 4379) aged 43 to 86 years at the baseline examination had retinal photographs taken at baseline and up to four subsequent examinations. Multistate models with misclassification in continuous time were used to model the effects of age, sex, and CFH genotype on incidence, progression, and regression of AMD and mortality.
Results.
After accounting for AMD misclassification, the occurrence of any AMD regression was rare (1%–4%), while it was relatively common (14%–21%) in models that do not account for misclassification. Failure to account for misclassification attenuated estimated age effects on incidence and progression to moderately severe early AMD and attenuated estimated CFH effects on incidence and progressions to moderately severe and severe early AMD.
Conclusions.
Apparent regression of AMD can largely, if not completely, be explained by misclassification. Estimated age effects on incidence and progression to moderately severe early AMD and estimated CFH effects on incidence and progressions to moderately severe and severe early AMD were attenuated in multistate models that did not account for misclassification.
Keywords: age-related macular degeneration, CFH, epidemiology
We investigate the impact of misclassification of AMD on the baseline intensity and covariate effects on incidence, progression, and regression of AMD. Regression of AMD can largely be explained by misclassification, and effects of age and CFH were attenuated in models without misclassification.
Introduction
Information regarding the development and progression of age-related macular degeneration (AMD) has emerged from clinical and epidemiologic studies.1–11 This has resulted in the development of severity scales with categories ranging from no or minimal AMD lesions not severe enough to categorize the earliest level of early AMD through the most severe lesions (neovascular AMD and/or geographic atrophy) that categorize the presence of late or advanced AMD.6,12–14 Most scales use size, type and area of drusen, and pigmentary abnormalities of the retinal pigment epithelium (RPE) to define the intermediate severity levels of early AMD. Assessments of AMD lesions that characterize its severity from photographs are subject to misclassification owing to media opacity, photographic artifacts, and grader errors.
While most epidemiologic studies have investigated the incidence and progression of AMD, few studies have examined regression.2,5,9,11 In the Chesapeake Bay Waterman Study, large soft drusen disappeared in 34% of eyes during a 5-year follow-up.2 In the Melton Mowbray study, 20% of soft drusen regressed during a 7-year period.9 The Beaver Dam Eye Study (BDES), using side-by-side longitudinal review to minimize false-positive progression and regression of AMD severity, previously reported a cumulative disappearance of soft indistinct drusen, during a 15-year period, of 18% in the absence of the appearance of more severe lesions such as RPE depigmentation, geographic atrophy, or exudative macular degeneration.11 It is unclear to what extent these high rates of apparent AMD regression reflect misclassification of AMD state rather than true regression of the disease.
Most studies that have examined relationships of the Y402H polymorphism in the complement factor H (CFH) gene on chromosome 1q have shown a relationship to incidence of early or progression to late AMD.12–15 Few have considered progression through various stages of early AMD or regression of AMD, and none have accounted for potential misclassification of AMD state.16–19
Multistate models (MSMs) with misclassification can be used to gain greater insight into the true course of incidence, progression, and regression of AMD. We used MSMs with misclassification to model transitions from the observed state at a study visit to the observed state at a subsequent visit.20–24 In practice, disease states are observed with error at intermittent study visits, and exact transition times are not observed; even when the exact date of death is known, the AMD state at death is unknown.25–28 In this article, we used MSMs with misclassification to examine the impact of misclassification on baseline intensity and the estimated effects of age, sex, and the Y402H variant in the CFH gene on incidence, progression, and regression of AMD as well as mortality in the population-based BDES.
Methods
Population
Methods used to identify the population have been described previously.29–34 Characteristics of the population at each examination and reasons for nonparticipation appear elsewhere.30–34
Procedures and Definitions
Similar procedures were used at all examinations.3,5,35–39 Data were collected with institutional review board approval from the University of Wisconsin-Madison, informed consent was obtained from each participant at each examination, and the study adhered to the tenets of the Declaration of Helsinki. Pertinent parts of the examination consisted of taking stereoscopic 30° color fundus photographs centered on the disc (Diabetic Retinopathy Study standard field 1) and macula (Diabetic Retinopathy Study standard field 2) and a nonstereoscopic color fundus photograph temporal to but including the fovea of each eye.
Grading procedures have been described previously.37–39 Information on gradability has been published elsewhere.3,5,11,39,40 In this study, AMD status in each eye was classified by using a 5-step severity scale (Table 1).41 Subjects were classified as to severity level on the basis of the worse eye.
Table 1.
The 5-Level Beaver Dam Eye Study AMD Severity Scale
|
Level |
Label |
Description |
| 1 | No AMD | Hard drusen or small soft drusen (<125 μm in diameter) only, regardless of area of involvement, and no pigmentary abnormality (defined as presence of increased retinal pigment or RPE depigmentation) present. |
| 2 | Minimally severe early AMD | Hard drusen or small soft drusen (<125 μm in diameter), regardless of area of involvement, with any pigmentary abnormality OR soft drusen (≥125 μm in diameter) with drusen area < 196,350 μm2 (equivalent to a circle with a diameter of 500 μm) and no pigmentary abnormalities. |
| 3 | Moderately severe early AMD | Soft drusen (≥125 μm in diameter) with drusen area < 196,350 μm2 (equivalent to a circle with a diameter of 500 μm) and with any pigmentary abnormality OR soft drusen (≥125 μm in diameter) with drusen area ≥ 196,350 μm2 (equivalent to a circle with a diameter of 500 μm) with or without increased retinal pigment but no RPE depigmentation. |
| 4 | Severe early AMD | Soft drusen (≥125 μm in diameter) with drusen area ≥ 196,350 μm2 (equivalent to a circle with a diameter of 500 μm) and RPE depigmentation present, with or without increased retinal pigment. |
| 5 | Late AMD | Pure geographic atrophy in the absence of exudative macular degeneration OR exudative macular degeneration with or without geographic atrophy present. |
Reprinted with permission from Gangnon RE, Lee KE, Klein BE, Iyengar SK, Sivakumaran TA, Klein R. Effect of the Y402H variant in the complement factor H gene on the incidence and progression of age-related macular degeneration: results from multistate models applied to the Beaver Dam Eye Study. Arch Ophthalmol. 2012;130:1169–1176. Copyright 2012 American Medical Association. All rights reserved.
Information on alleles for the Y402H polymorphism for CFH was available for 4479 participants. Distributions of other characteristics for these subjects did not differ from the rest of the population (data not shown).
Vital status was monitored by reading the obituaries in local newspapers and by making annual telephone contact. Persons not known to have died but who could not be contacted had their survival time entered as their last contact date.
Statistical Analysis
Incidence, progression, and regression of AMD and mortality were modeled by using MSMs in continuous time with misclassification, where the observed data are states assumed to be misclassifications of the true, underlying states. We identified mutually exclusive and exhaustive states representing the current status (current AMD severity level or death) of each subject at a given age. The observed AMD level is subject to measurement error. Replicate single gradings of AMD from fundus photographs were used to estimate the matrix of misclassification probabilities, using a latent class model for diagnostic accuracy in the absence of a known gold standard42,43; the extension of Lo's estimator was used to ensure stochastic ordering of the misclassification probabilities.44,45 To be specific, the likelihood of a set of k gradings of a single photograph, g1, g2,…, gk, is given by Σs π(s) p(g1|s) p(g2|s)…p(gk|s), where s is the unknown (latent) true grade for the photograph, π(s) is the prevalence of grade s in the reproducibility sample, and p(g|s) is the probability of observed grade g for a photograph with true grade s. This model assumes that (1) the gradings (g1, g2,…, gk) are conditionally independent given the true AMD state (s) and (2) the (mis)classification probabilities are constant within and across subjects. With these assumptions, the unknown parameters (π(s), p(g|s)) can be estimated via maximum likelihood.
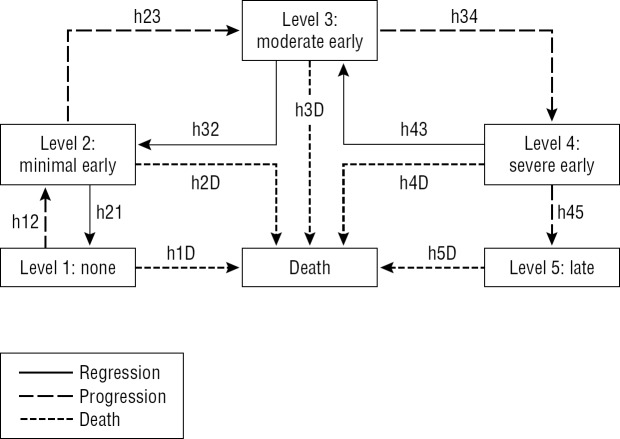
The Figure illustrates the underlying MSM; arrows indicate possible instantaneous transitions. Instantaneous transitions (the next state to which the individual moves and the time of the change) were allowed between adjacent AMD states with one exception; based on clinical observation, regression from late AMD (level 5) to severe early AMD (level 4) was not allowed. Model selection based on the Akaike information criterion (AIC)46 was used to evaluate the need for covariate effects on other instantaneous transitions (incidence/progression, regression, or mortality).
Figure.
Transition diagram for 5-level AMD scale. Arrows indicate possible instantaneous transitions between states. hXY indicates hazard for instantaneous transition from state X (1, 2, 3, 4, or 5) to state Y (1, 2, 3, 4, 5, or D [death]). Reprinted with permission from Gangnon RE, Lee KE, Klein BE, Iyengar SK, Sivakumaran TA, Klein R. Effect of the Y402H variant in the complement factor H gene on the incidence and progression of age-related macular degeneration: results from multistate models applied to the Beaver Dam Eye Study. Arch Ophthalmol. 2012;130:1169–1176. Copyright 2012 American Medical Association. All rights reserved.
Transitions are governed by up to 12 transition intensities, one for each possible instantaneous transition between states (represented by arrows in the Fig.), which represent the hazard of moving between states. Dependence of transition intensities on age, sex, and CFH Y402H genotype was specified by using log-linear regression models. Age was entered as a linear term and updated annually. Sex and CFH Y402H genotype were entered by using indicator variables. Covariate effects on transitions within the AMD scale were unconstrained. Covariate effects on transitions to death were constrained to be equal, but intercepts were allowed to vary.
Analyses were conducted in R47 by using the msm package.48 Covariate effects on transition intensities are summarized as hazard ratios. We calculated estimated transition probabilities to each AMD state (and death) after 5 years and estimated cumulative incidence of each AMD state (and death) for specified subgroups. Cumulative incidence calculations used annual assessments of AMD status; subjects were assigned to the most severe AMD state observed at or before the current age.
Results
Of the 4973 subjects seen at any study visit, 134 were excluded for ungradable AMD status at all visits, and 460 were excluded for missing CFH Y402H genotype; 4379 subjects contributed data from 12,640 BDES follow-up intervals (up to four per subject). Table 2 displays characteristics of subjects at the start of each interval by observed AMD level. Subjects with more severe AMD were older and more likely to be female, to have CFH Y402H genotype CT or CC, and to be seen at later visits. Similar relationships were seen among subjects with partial AMD severity information (data not shown).
Table 2.
Characteristics of the Cohort by Current AMD Status (Worse Eye) at the Beginning of the Beaver Dam Eye Study Follow-up Intervals
| No. (%)* |
|||||
|
Characteristic |
No AMD, L1, n
= 8474 |
Minimal Early AMD, L2, n
= 1524 |
Moderate Early AMD, L3, n
= 833 |
Severe Early AMD, L4, n
= 250 |
Late AMD, L5, n
= 294 |
| Age, mean (SD), y | 63 (10) | 67 (10) | 73 (10) | 76 (9) | 80 (8) |
| Male sex | 3644 (43) | 736 (48) | 357 (43) | 82 (33) | 106 (36) |
| Participated in examination | |||||
| BDES 1 | 3151 (37) | 530 (35) | 247 (30) | 47 (19) | 75 (26) |
| BDES 2 | 2185 (26) | 444 (29) | 239 (29) | 64 (26) | 67 (23) |
| BDES 3 | 1724 (20) | 316 (21) | 197 (24) | 70 (28) | 70 (24) |
| BDES 4 | 1414 (17) | 234 (15) | 150 (18) | 69 (28) | 82 (28) |
| CFH Y402H genotype | |||||
| TT | 3482 (41) | 594 (39) | 287 (34) | 66 (26) | 43 (15) |
| CT | 3903 (46) | 743 (49) | 404 (48) | 138 (55) | 180 (61) |
| CC | 1089 (13) | 187 (12) | 142 (17) | 46 (18) | 71 (24) |
L, level. Reprinted with permission from Gangnon RE, Lee KE, Klein BE, Iyengar SK, Sivakumaran TA, Klein R. Effect of the Y402H variant in the complement factor H gene on the incidence and progression of age-related macular degeneration: results from multistate models applied to the Beaver Dam Eye Study. Arch Ophthalmol. 2012;130:1169–1176. Copyright 2012 American Medical Association. All rights reserved.
No. (%) of subject follow-up intervals.
Misclassification of AMD State
Replicate AMD grades were available for 89 different retinal photograph sets. The number of replicate grades for a given photograph ranged from 17 to 74 (median, 20; mean, 36). Table 3 displays the observed agreement of replicate grades for the same photographs. Observed agreement of the replicate grade for no AMD was 96% exact agreement, for level 2 it was 62%, for level 3 it was 39%, for level 4 it was 59%, and for late AMD it was 87%.
Table 3.
Observed Agreement of Replicate Gradings of Same Photographs
|
AMD Level (Second Grading) |
AMD Level (First Grading) |
|||||||||
|
No AMD (Level 1)
N
= 101,159 |
Minimal Early AMD (Level 2)
N
= 11,614 |
Moderate Early AMD (Level 3)
N
= 11,333 |
Severe Early AMD (Level 4)
N
= 14,591 |
Late AMD (Level 5)
N
= 31,847 |
||||||
|
No.* |
% |
No.* |
% |
No.* |
% |
No.* |
% |
No.* |
% |
|
| 1 | 97,158 | 96 | 1,808 | 16 | 1,379 | 12 | 768 | <1 | 46 | <1 |
| 2 | 1,808 | 2 | 7,202 | 62 | 2,505 | 22 | 99 | <1 | 0 | 0 |
| 3 | 1,379 | 1 | 2,505 | 22 | 4,434 | 39 | 2,032 | 18 | 983 | 3 |
| 4 | 768 | <1 | 99 | <1 | 2,032 | 18 | 8,590 | 59 | 3,102 | 10 |
| 5 | 46 | <1 | 0 | 0 | 983 | 9 | 3,102 | 10 | 27,716 | 87 |
* No., N, number of paired gradings.
Table 4 displays estimated misclassification probabilities for the underlying, unobserved true AMD states. There is an estimated probability of <1% that a participant with no AMD will be falsely graded as having AMD, but the probabilities that subjects with level 2 or level 3 will be falsely graded as having no AMD are relatively higher (16.2% and 6.5%, respectively). The probability of overgrading level 2 as level 3 was 20.9%, level 3 as level 4 was 28.4%, and level 4 as late AMD was 23.2%. The probability of undergrading level 4 as level 3 was 10.8%.
Table 4.
Estimated Misclassification Probabilities (%, 95% Confidence Interval)
|
True State |
Observed State |
||||
|
Level 1 |
Level 2 |
Level 3 |
Level 4 |
Level 5 |
|
| Level 1 | 99.31 | 0.23 | 0.39 | 0.03 | 0.04 |
| (97.74–99.79) | (0.01–0.61) | (0.01–1.69) | (0.00–0.15) | (0.00–0.06) | |
| Level 2 | 16.22 | 62.37 | 20.87 | 0.47 | 0.06 |
| (2.32–29.78) | (47.66–84.57) | (5.49–27.38) | (0.01–1.55) | (0.02–0.15) | |
| Level 3 | 6.47 | 2.29 | 61.98 | 28.35 | 0.90 |
| (0.06–17.70) | (0.03–30.20) | (37.10–90.97) | (1.97–41.10) | (0.05–1.57) | |
| Level 4 | 0.07 | 1.75 | 10.79 | 64.16 | 23.24 |
| (0.03–3.51) | (0.04–11.03) | (0.06–52.74) | (43.36–94.10) | (0.23–39.85) | |
| Level 5 | 0.03 | 0.03 | 3.31 | 1.82 | 94.81 |
| (0.01–0.06) | (0.01–0.06) | (0.02–8.73) | (0.10–17.95) | (79.11–99.71) | |
Level 1, no AMD; level 2, minimally severe early AMD; level 3, moderately severe early AMD; level 4, severe early AMD; level 5, late AMD.
Covariate Effects on Transition Intensities
Parameter estimates are presented in Table 5 for the MSM that does not account for misclassification and in Table 6 for the MSM that accounts for misclassification. When accounting for misclassification, a reduced model with no covariate effects (age, sex, or CFH genotype) on regression (level 2 to level 1, level 3 to level 2, or level 4 to level 3) was selected by AIC.
Table 5.
Estimated Covariate Effects on Transition Intensities or Hazards Without Accounting for Misclassification
|
Incidence or Progression (95% CI) |
Regression (95% CI) |
Mortality (95% CI) |
||||||
|
Level 1 to Level 2 |
Level 2 to Level 3 |
Level 3 to Level 4 |
Level 4 to Level 5 |
Level 2 to Level 1 |
Level 3 to Level 2 |
Level 4 to Level 3 |
Any AMD to Death |
|
| Baseline intensity, | ||||||||
| relative to mortality | 1.17 | 5.18 | 3.98 | 3.52 | 2.52 | 4.17 | 5.17 | |
| (0.99–1.41) | (3.79–7.08) | (2.60–6.07) | (1.87–6.60) | (1.74–3.66) | (2.56–6.78) | (1.89–14.1) | ||
| Covariate HR | ||||||||
| Age, per 5 years | 1.43 | 1.31 | 1.24 | 1.27 | 1.04 | 0.91 | 0.91 | 1.66 |
| (1.37–1.49) | (1.24–1.39) | (1.14–1.34) | (1.14–1.41) | (0.95–1.13) | (0.81–1.03) | (0.68–1.21) | (1.61–1.70) | |
| Sex, male | 0.96 | 0.88 | 0.69 | 1.17 | 0.83 | 1.30 | 0.46 | 1.55 |
| (0.82–1.13) | (0.72–1.08) | (0.52–0.91) | (0.82–1.68) | (0.60–1.14) | (0.83–2.04) | (0.14–1.50) | (1.42–1.69) | |
| CFH Y402H genotype | ||||||||
| TT | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| CT | 1.35 | 1.09 | 1.44 | 1.60 | 0.87 | 0.49 | 0.49 | 0.94 |
| (1.13–1.60) | (0.87–1.36) | (1.04–1.98) | (1.03–2.48) | (0.62–1.22) | (0.23–1.04) | (0.16–1.48) | (0.85–1.01) | |
| CC | 1.98 | 1.73 | 1.30 | 1.72 | 0.92 | 0.60 | 0.41 | 0.98 |
| (1.57–2.49) | (1.29–2.33) | (0.86–1.94) | (1.01–2.91) | (0.54–1.56) | (0.37–0.97) | (0.09–1.83) | (0.86–1.12) | |
| AMD severity | ||||||||
| Level 1 | 1.00 | |||||||
| Level 2 | 1.04 | |||||||
| (0.82–1.32) | ||||||||
| Level 3 | 1.01 | |||||||
| (0.79–1.30) | ||||||||
| Level 4 | 1.05 | |||||||
| (0.71–1.55) | ||||||||
| Level 5 | 1.37 | |||||||
| (1.15–1.62) | ||||||||
CI, confidence interval; HR, hazard ratio. Level 1, no AMD; level 2, minimally severe early AMD; level 3, moderately severe early AMD; level 4, severe early AMD; level 5, late AMD. Estimates are mutually adjusted for all other covariates listed in the table. Bold text indicates results that were significant at the 5% level. Reprinted with permission from Gangnon RE, Lee KE, Klein BE, Iyengar SK, Sivakumaran TA, Klein R. Effect of the Y402H variant in the complement factor H gene on the incidence and progression of age-related macular degeneration: results from multistate models applied to the Beaver Dam Eye Study. Arch Ophthalmol. 2012;130:1169–1176. Copyright 2012 American Medical Association. All rights reserved. Modified to add the Baseline Intensity row.
Table 6.
Estimated Covariate Effects on Transition Intensities or Hazards Accounting for Misclassification
|
Incidence or Progression (95% CI) |
Regression (95% CI) |
Mortality (95% CI) |
||||||
|
Level 1 to Level 2 |
Level 2 to Level 3 |
Level 3 to Level 4 |
Level 4 to Level 5 |
Level 2 to Level 1 |
Level 3 to Level 2 |
Level 4 to Level 3 |
Any AMD to Death |
|
| Baseline intensity, | ||||||||
| relative to mortality | 0.88 | 1.54 | 4.73 | 2.49 | 0.44 | 0.10 | 0.20 | |
| (0.70–1.41) | (1.07–2.21) | (1.89–11.83) | (0.80–7.70) | (0.29–0.68) | (0.01–1.70) | (0.02–2.20) | ||
| Covariate HR | ||||||||
| Age, per 5 years | 1.58 | 1.51 | 1.29 | 1.25 | 1.67 | |||
| (1.50–1.67) | (1.39–1.64) | (1.14–1.46) | (0.99–1.57) | (1.62–1.71) | ||||
| Sex, male | 0.90 | 0.63 | 0.85 | 1.25 | 1.55 | |||
| (0.74–1.10) | (0.47–0.85) | (0.56–1.29) | (0.99–1.57) | (1.42–1.70) | ||||
| CFH Y402H genotype | ||||||||
| TT | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||
| CT | 1.46 | 1.61 | 1.72 | 1.58 | 0.94 | |||
| (1.17–1.82) | (1.18–2.20) | (1.07–2.76) | (0.63–3.99) | (0.86–1.03) | ||||
| CC | 2.27 | 2.75 | 1.62 | 1.39 | 1.00 | |||
| (1.71–3.01) | (1.81–4.18) | (0.92–2.83) | (0.45–4.32) | (0.87–1.14) | ||||
| AMD severity | ||||||||
| Level 1 | 1.00 | |||||||
| Level 2 | 1.13 | |||||||
| (0.93–1.36) | ||||||||
| Level 3 | 0.68 | |||||||
| (0.35–1.30) | ||||||||
| Level 4 | 1.60 | |||||||
| (0.94–2.75) | ||||||||
| Level 5 | 1.23 | |||||||
| (1.01–1.50) | ||||||||
Level 1, no AMD; level 2, minimally severe early AMD; level 3, moderately severe early AMD; level 4, severe early AMD; level 5, late AMD. Estimates are mutually adjusted for all other covariates listed in the table. Bold text indicates results that were significant at the 5% level.
Older age was associated with increased AMD incidence and progression and mortality (Tables 5, 6). Being male was associated with increased mortality, but not with AMD incidence or progression. CFH Y402H genotypes CC and CT were associated, relative to genotype TT, with increased AMD incidence and progression, but not with mortality. Late AMD was associated with increased mortality relative to no AMD, but earlier stages of AMD were not.
While misclassification did not change the qualitative findings for covariate effects, it had substantial impacts on the quantitative results. Estimated age effects on incidence and progression to moderately severe early AMD and estimated CFH effects on incidence and progressions to moderately severe and severe early AMD were attenuated in MSMs that did not account for misclassification. Estimated baseline intensities for incidence/progression and regression of AMD were exaggerated in MSMs that did not account for misclassification. The estimated effect of late AMD on mortality was similarly exaggerated in MSMs that did not account for misclassification (Tables 5, 6).
Five-Year Transition Probabilities by Age, Sex, and CFH Y402H Genotype
For individuals free of AMD at age 50 years, rates of progression to level 2 (incidence of early AMD) at age 55 years was lower in MSMs that accounted for misclassification (1.2%–2.9%) than in MSMs that did not account for misclassification (2.1%–4.2%). For those free of AMD at age 70 years, rates of progression to level 2 at age 75 years were similar between MSMs that accounted for misclassification (6.1%–12.8%) and MSMs that did not (6.7%–11.3%). At age 90 years, progression to level 2 or above at age 95 years was somewhat higher in MSMs that accounted for misclassification (10.8%–32.0%) than in MSMs that did not (10.1%–26.7%), while rates of progression to level 3 or above were similar between the two models (3.0%–22.9% with misclassification; 4.4%–18.3% without).
For individuals with AMD at level 2 at age 50 years, regression to no AMD by age 55 years was common (11.7%–16.0%) in MSMs without misclassification, but relatively infrequent (3.8%–3.9%) in MSMs with misclassification; progression to level 3 was much less common in MSMs with misclassification (1.9%–7.7% vs. 9.1%–18.9%). At age 70 years, regression remained common (8.6%–13.9%) in MSMs without misclassification and infrequent (2.8%–3.2%) in MSMs with misclassification, while progression to level 3 or above was less frequent in MSMs with misclassification (8.7%–33.4% vs. 23.5%–45.7%). At age 90 years, regression was infrequent (1.3%–4.4%) in MSMs without misclassification and rare (0.3%–0.9%) in MSMs with misclassification; progression to level 3 or above was less frequent in MSMs with misclassification (12.8%–40.4% vs. 17.7%–38.5%).
Cumulative Incidence of AMD for Individuals With No AMD at Age 45 Years
Estimated cumulative incidence of level 2 or higher was lower in MSMs with misclassification than in MSMs without misclassification. For example, cumulative incidence of level 2 by age 65 years was 16.1% (vs. 23.0%) for women with the CC genotype and 6.6% (vs. 11.7%) for men with the TT genotype; by age 80 years, it was 47.5% (vs. 61.2%) and 21.1% (vs. 34.1%), respectively; by age 100 years, it was 64.8% (vs. 67.7%) and 31.6% (vs. 38.6%), respectively. Cumulative incidence of late AMD was similarly lower in MSMs without misclassification.
Simulated Realizations of AMD History for Individuals With No AMD at Age 45 Years
We simulated realizations of AMD history for 100 subjects (for each sex and CFH genotype combination) free of AMD at age 45 years, from the MSM with misclassification and the MSM without misclassification. The occurrence of any AMD regression was rare (1%–4%) using the MSM that accounted for misclassification, while it was relatively common (14%–21%) using the MSM that did not account for misclassification.
Discussion
In a cohort followed up for 20 years, we showed that, after accounting for misclassification of observed AMD states, true regression of AMD is rare (lifetime occurrence of 1%–4%). Relatively high rates of apparent regression in AMD levels between consecutive BDES visits (10% of eyes at level 2; 9% at level 3; and 7% at level 4) likely reflect misclassification due to media opacity, photographic artifacts, and grader error. While multiple gradings for AMD, including masked side-by-side comparisons of photographs, can minimize the impact of grader error, it is difficult, if not impossible, to remove other sources of misclassification directly. The MSMs with misclassification used here provide a valuable tool for accounting for misclassification analytically.
Misclassification rates used in the MSMs were estimated from replicate single gradings of photographs by two graders rather than the full grading system, which uses a preliminary grading, a detailed grading, edits, adjudication, and longitudinal review of apparent progression. As such, the misclassification estimates used in these analyses are overestimates of the errors in the grading system. For example, after longitudinal review, rates of apparent progression by one or more steps on a 5-step scale between consecutive BDES visits decrease by up to 4% (from 17%–13%), while rates of apparent regression decrease by up to 2.4% (from 7.5%–5.1%). However, our assessment of misclassification from replicate gradings of a single photograph fails to account for other sources of misclassification, including photographic quality and day-to-day biological variation in the appearance of the eye, which are likely greater in importance than the grading error. As such, if anything, the misclassification estimates used in the MSMs may be understating the true magnitude of misclassification.
In conclusion, we provide an approach using MSMs to adjust for the effect of misclassification of the progression and regression of AMD assessed by grading of fundus photographs and categorizing AMD by using a 5-step AMD severity scale. These data strongly suggest the need for multiple gradings by multiple graders, including longitudinal review, to attempt to reduce misclassification as much as possible.
Acknowledgments
An abstract of this study was presented at the 2013 Spring Meeting of the Eastern North American Region (ENAR) International Biometric Society, Orlando, Florida, March 10–13, 2013.
Supported by The National Institutes of Health Grant EY06594 (RK, BEKK), which provided funding for the entire study including collection and analyses of data; further support for data analyses was provided by an unrestricted grant from Research to Prevent Blindness, New York, New York. These funding organizations had no role in the design and conduct of the study, in the collection, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript. REG and RK had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the National Institutes of Health.
Disclosure: R.E. Gangnon, None; K.E. Lee, None; B.E.K. Klein, None; S.K. Iyengar, None; T.A. Sivakumaran, None; R. Klein, None
References
- 1. Gass JD. Drusen and disciform macular detachment and degeneration. Arch Ophthalmol. 1973; 90: 206–217 [DOI] [PubMed] [Google Scholar]
- 2. Bressler NM, Munoz B, Maguire MG, et al. Five-year incidence and disappearance of drusen and retinal pigment epithelial abnormalities: Waterman study. Arch Ophthalmol. 1995; 113: 301–308 [DOI] [PubMed] [Google Scholar]
- 3. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997; 104: 7–21 [DOI] [PubMed] [Google Scholar]
- 4. Klaver CC, Assink JJ, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2001; 42: 2237–2241 [PubMed] [Google Scholar]
- 5. Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2002; 109: 1767–1779 [DOI] [PubMed] [Google Scholar]
- 6. van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam Study. Arch Ophthalmol. 2003; 121: 519–526 [DOI] [PubMed] [Google Scholar]
- 7. Mitchell P, Wang JJ, Foran S, Smith W. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002; 109: 1092–1097 [DOI] [PubMed] [Google Scholar]
- 8. Wang JJ, Foran S, Smith W, Mitchell P. Risk of age-related macular degeneration in eyes with macular drusen or hyperpigmentation: the Blue Mountains Eye Study cohort. Arch Ophthalmol. 2003; 121: 658–663 [DOI] [PubMed] [Google Scholar]
- 9. Sparrow JM, Dickinson AJ, Duke AM, Thompson JR, Gibson JM, Rosenthal AR. Seven year follow-up of age-related maculopathy in an elderly British population. Eye (Lond). 1997; 11: 315–324 [DOI] [PubMed] [Google Scholar]
- 10. Buch H, Nielsen NV, Vinding T, Jensen GB, Prause JU, la Cour M. 14-year incidence, progression, and visual morbidity of age-related maculopathy: the Copenhagen City Eye Study. Ophthalmology. 2005; 112: 787–798 [DOI] [PubMed] [Google Scholar]
- 11. Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007; 114: 253–262 [DOI] [PubMed] [Google Scholar]
- 12. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308: 385–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005; 308: 419–421 [DOI] [PubMed] [Google Scholar]
- 14. Edwards AO, Ritter R III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005; 308: 421–424 [DOI] [PubMed] [Google Scholar]
- 15. Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005; 14: 3227–3236 [DOI] [PubMed] [Google Scholar]
- 16. Seddon JM, Francis PJ, George S, Schultz DW, Rosner B, Klein ML. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007; 297: 1793–1800 [DOI] [PubMed] [Google Scholar]
- 17. Seddon JM, Reynolds R, Maller J, Fagerness JA, Daly MJ, Rosner B. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009; 50: 2044–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klein ML, Francis PJ, Ferris FL III, Hamon SC, Clemons TE. Risk assessment model for development of advanced age-related macular degeneration. Arch Ophthalmol. 2011; 129: 1543–1550 [DOI] [PubMed] [Google Scholar]
- 19. Seddon JM, Reynolds R, Yu Y, Daly MJ, Rosner B. Risk models for progression to advanced age-related macular degeneration using demographic, environmental, genetic, and ocular factors. Ophthalmology. 2011; 118: 2203–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods Med Res. 2002; 11: 91–115 [DOI] [PubMed] [Google Scholar]
- 21. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 22. Lawless JF. Statistical Models and Methods for Lifetime Data. 2nd ed. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 23. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [Google Scholar]
- 24. Andersen PK, Borgan O, Gill RD, Kieding N. Statistical Models Based on Counting Processes. 1st ed. New York, NY: Springer; 1993. [Google Scholar]
- 25. Commenges D. Inference for multi-state models from interval-censored data. Stat Methods Med Res. 2002; 11: 167–182 [DOI] [PubMed] [Google Scholar]
- 26. Gentleman RC, Lawless JF, Lindsey JC, Yan P. Multi-state Markov models for analysing incomplete disease history data with illustrations for HIV disease. Stat Med. 1994; 13: 805–821 [DOI] [PubMed] [Google Scholar]
- 27. Marshall G, Jones RH. Multi-state models and diabetic retinopathy. Stat Med. 1995; 14: 1975–1983 [DOI] [PubMed] [Google Scholar]
- 28. Gruger J, Kay R, Schumacher M. The validity of inferences based on incomplete observations in disease state models. Biometrics. 1991; 47: 595–605 [PubMed] [Google Scholar]
- 29. Linton KL, Klein BE, Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. Am J Epidemiol. 1991; 134: 1438–1446 [DOI] [PubMed] [Google Scholar]
- 30. Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991; 98: 1310–1315 [DOI] [PubMed] [Google Scholar]
- 31. Klein R, Klein BE, Lee KE. Changes in visual acuity in a population: the Beaver Dam Eye Study. Ophthalmology. 1996; 103: 1169–1178 [DOI] [PubMed] [Google Scholar]
- 32. Klein R, Klein BE, Lee KE, Cruickshanks KJ, Chappell RJ. Changes in visual acuity in a population over a 10-year period: the Beaver Dam Eye Study. Ophthalmology. 2001; 108: 1757–1766 [DOI] [PubMed] [Google Scholar]
- 33. Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006; 142: 539–549 [DOI] [PubMed] [Google Scholar]
- 34. Klein R, Lee KE, Gangnon RE, Klein BE. Incidence of visual impairment over a 20-year period: the Beaver Dam Eye Study. Ophthalmology. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klein R, Klein BE. The Beaver Dam Eye Study: Manual of Operations (Revised). Springfield, VA: US Department of Commerce; 1991; 74–112 NTIS Accession No. PB91-149823 [Google Scholar]
- 36. Klein R, Klein BE. The Beaver Dam Eye Study II: Manual of Operations. Springfield, VA: US Department of Commerce; 1995; 61–90 NTIS Accession No. PB95-273827 [Google Scholar]
- 37. Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991; 98: 1128–1134 [DOI] [PubMed] [Google Scholar]
- 38. Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. Springfield, VA: US Department of Commerce; 1991. NTIS Accession No. PB91-184267 [DOI] [PubMed] [Google Scholar]
- 39. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992; 99: 933–943 [DOI] [PubMed] [Google Scholar]
- 40. Klein R, Myers CE, Meuer SM, et al. Risk alleles in CFH and ARMS2 and the long term natural history of age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2013. In press doi:10.1001/jamaophthalmol.2013.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gangnon RE, Lee KE, Klein BE, Iyengar SK, Sivakumaran TA, Klein R. Effect of the Y402H variant in the complement factor H gene on the incidence and progression of age-related macular degeneration: results from multistate models applied to the Beaver Dam Eye Study. Arch Ophthalmol. 2012; 130: 1169–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hui SL, Walter SD. Estimating the error rates of diagnostic tests. Biometrics. 1980; 36: 167–171 [PubMed] [Google Scholar]
- 43. Jackson CH, Sharples LD, Thompson SG, Duffy SW, Couto E. Multistate Markov models for disease progression with classification error. J R Stat Soc Ser D Stat. 2003; 52: 193–209 [Google Scholar]
- 44. Lo SH. Estimation of distribution functions under order restrictions. Stat Decis. 1987; 5: 251–262 [Google Scholar]
- 45. Gangnon RE, King WN. Minimum distance estimation of the distribution functions of stochastically ordered random variables. J R Stat Soc Ser C Appl Stat. 2013; 51: 485–492 [Google Scholar]
- 46. Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control. 1974; 19: 716–723 [Google Scholar]
- 47. R Development Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 48. Jackson CH. Multi-state models for panel data: the msm package for R. J Stat Softw. 2011; 38: 1–28 [Google Scholar]