Abstract
Purpose.
We have recently provided evidence showing that luminal lymphatic valves are formed right after the onset of corneal inflammatory lymphangiogenesis (LG). The purpose of this study was to further characterize the long-term time course, spatial distribution, directional orientation, and functional implications of the valve formation in relation to corneal LG.
Methods.
Corneal LG was induced in normal adult BALB/c mice by a modified suture placement model with equal distribution in the nasal and temporal side. Whole-mount corneas were harvested every 2 weeks for up to 8 weeks post suturing for immunofluorescent microscopic assays. Quantitative analysis on both lymphatic vessels and valves was performed by using National Institutes of Health ImageJ software. Corneal lymphatic live imaging was performed to show functional drainage of the valves.
Results.
Lymphatic vessel invasion areas at 4, 6, and 8 weeks were significantly less than the peak at 2 weeks post corneal suturing. In contrast, the ratio of lymphatic valves to vessel invasion area was at its lowest at 2 weeks with a peak approximately at 6 weeks post suturing. Lymphatic valves were more localized in the nasal quadrant at all time points studied, and most of the well-formed valves were directionally oriented toward the limbus. The lymphatic valves function to guide lymphatic drainage outside the cornea.
Conclusions.
This study presents new insights into corneal lymphatic valve formation and function in inflammatory LG. Further investigation on lymphatic valves may provide novel strategies to interfere with lymphatic maturation and function and to treat lymphatic-related disorders.
Keywords: lymphangiogenesis, lymphatic valve, corneal inflammation
This study provides the first evidence on the time course, spatial distribution, and directional orientation with functional relevance of corneal lymphatic valves in inflammatory lymphangiogenesis. Further investigation may offer new therapeutic strategies for lymphatic disorders.
Introduction
The lymphatic vasculature system includes an extensive network of vessels that penetrates through most tissues of the body and carries out important functions complementing the blood circulatory system. A large portion of the fluids leaked out of the blood capillaries in the peripheral tissues are returned back to the blood circulation via the lymphatic channel. Hence, the lymphatic system is critically important in maintaining body fluid homeostasis, dietary fat absorption, and immune surveillance. Until recently, this system has been overlooked by the more readily visible blood vascular system. The recent identification of several lymphatic specific markers, such as lymphatic vessel endothelial hyaluronic acid receptor (LYVE)-1, vascular endothelial growth factor receptor (VEGFR)-3, and prospero homeobox protein (Prox)-1, have allowed for vigorous exploration into the active growth of lymphatic vessels during both developmental and pathologic processes.1–3 This has greatly advanced our knowledge of the functional roles of the lymphatic vasculatures in a wide array of systemic conditions, such as autoimmune diseases, hypertension, obesity, and cancer.4,5
The transparent and alymphatic cornea serves as an ideal tissue to study the development of new lymphatic vessels, commonly referred to as lymphangiogenesis (LG).6,7 This is largely due to the fact that LG can be readily induced in the cornea after a traumatic, infectious, or chemical insult,8–10 and its evaluation is clearly straightforward since there are no pre-existing or background vessels to consider. Using corneal models of LG, researchers have been able to show that the lymphatic pathway is associated with prevalent ocular pathologies including dry eye and herpes simplex keratitis.8,11 Moreover, it has been defined as a primary mediator of corneal transplant rejection.12,13
We have recently provided the first evidence on the presence of luminal valves inside newly formed lymphatic vessels in inflamed cornea.14 We have shown that corneal lymphatic valves express a high level of integrin α-9 (Itga-9), which belongs to a family of cell surface receptors that bind to extracellular matrix proteins and mediate cellular functions such as migration, survival, and differentiation.15 Luminal valve formation, or valvulogenesis, offers a new concept in our understanding of corneal LG and its progression, since lymphatic valves are considered important structures for directing lymph flow as well as the overall proper function of the lymphatic system. Unlike the blood circulatory system, the lymphatic channel is a unidirectional network with blind-ended lymphatic capillaries that are connected to increasingly larger collecting vessels. Interstitial fluid reabsorbed in the peripheral tissues first enters the lymphatic capillaries before travelling through the collecting vessels and returning to the blood circulation via the thoracic or right lymphatic duct.4 Luminal valves are therefore structurally positioned to facilitate one-way trafficking and to prevent backflow toward terminal capillaries in peripheral tissues. The importance of lymphatic valves is highlighted by the fact that Itga-9 gene knockout mice die shortly after birth owing to fatal bilateral chylothorax, a disease characterized by lymphatic fluid accumulation in the pleural cavity.16
Our previous study reveals that luminal lymphatic valves are formed shortly after the onset of corneal inflammatory LG; however, several important questions still remain unanswered up to this stage: (1) Does lymphatic valve formation peak around the same time as corneal LG? (2) Are lymphatic valves evenly distributed in the cornea, or do they also follow a nasal dominant pattern similarly as lymphatic vessels, as we have recently reported?17 (3) How are lymphatic valves oriented in the cornea? Are they formed in a unidirectional alignment allowing for proper lymph flow away from the central cornea? and (4) What are functional relevance of these valves in inflamed cornea? This current study was designed to address these questions. Results from this study should provide an important frame of reference for investigating lymphatic valvulogenesis in relation to LG initiation, progression, maturation, and function. This study should also help to define the critical time window and route for therapeutic intervention of lymphatic-related disorders, which occur both inside and outside the eye.
Methods
Mice and Anesthesia
Eight- to 12-week-old normal adult male BALB/c mice (Taconic Farms, Germantown, NY) were used in the experiments. All mice were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and all protocols were approved by the Animal Care and Use Committee, University of California, Berkeley. Mice were anesthetized by using a mixture of ketamine, xylazine, and acepromazine (50 mg/kg, 10 mg/kg, and 1 mg/kg body weight, respectively) for each surgical procedure.
Corneal Suture Placement Model of Lymphangiogenesis
A modified model of suture-induced corneal inflammation was used to stimulate growth of new lymphatic vessels into the cornea, similarly as described previously.14,18,19 Briefly, three 11-0 nylon sutures (AROSurgical, Newport Beach, CA) were placed intrastromally without penetrating into the anterior chamber and were circumferentially spaced 120° from one another. To appreciate the nasal versus temporal distribution of lymphatic vessels and valves, the sutures were placed with one at 12 o'clock and two others evenly distributed in the nasal and temporal side, as illustrated in Figure 1A. Sutures were left in place and whole-mount corneas were harvested at 2, 4, 6, and 8 weeks post suturing. Repeated experiments (at least twice) resulted in a total study sample size of 58 mice consisting of study groups varying between 10 to 17 mice for each time point.
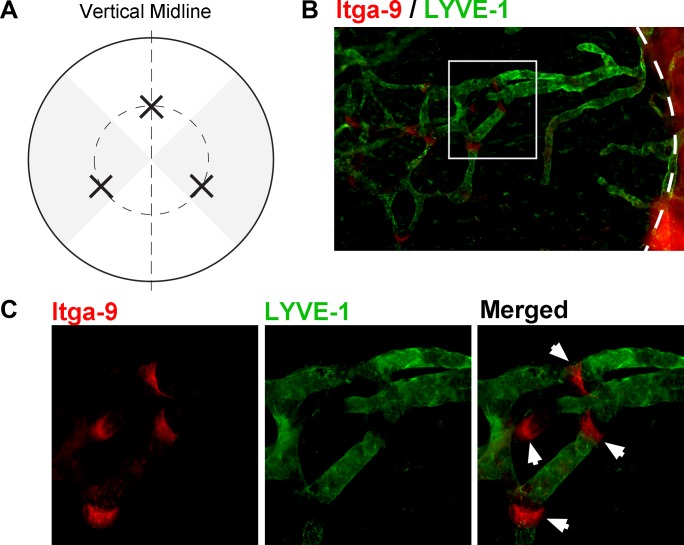
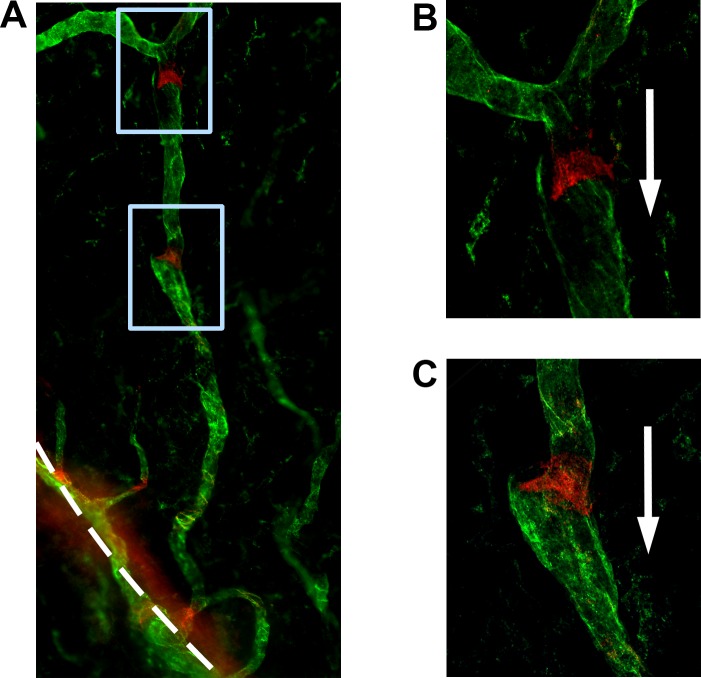
Figure 1.
Modified suture placement model to evaluate corneal lymphatic valves with Itga-9 expression. (A) Schematic diagram illustrating the three sutures (indicated by X) placed at the 4-, 8-, and 12-o'clock positions of the cornea. Outer circle: Limbus. Inner circle: 1.5-mm demarcation of suture placement. Vertical line: Demarcation between the nasal and temporal sides. Grey shaded regions: Areas evaluated for nasal and temporal quadrant data. (B, C) Representative micrographs showing Itga-9 expression on luminal valves of LYVE-1+ vessels, as indicated by white arrows (dashed line delineates the limbus). Itga-9: Red; LYVE-1: Green. (C) Higher magnification of boxed area in (B). Original magnifications: ×100 (B) and ×200 (C).
Immunofluorescent Microscopic Assays
The experiments were performed according to our standard protocol.14,19 Briefly, excised whole-mount full-thickness corneas were fixed in acetone and sequentially incubated with purified rabbit anti-mouse LYVE-1 (Abcam, Cambridge, MA) antibodies alone or together with goat anti-mouse Itga-9 antibodies (R&D Systems, Minneapolis, MN). Samples were visualized by aminomethylcoumarin acetate or FITC-conjugated donkey anti-rabbit and Cy3-conjugated donkey anti-goat antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Double-stained samples were covered with Vector Shield mounting medium (Vector Laboratories, Burlingame, CA) and examined by an AxioImager M1 epifluorescence deconvolution microscope with AxioVision 4.8 software (Carl Zeiss AG, Göttingen, Germany). In addition, the samples were evaluated with a LSM 780 NLO AxioExaminer confocal microscope (Carl Zeiss AG). Confocal z-stack images were compiled with National Institutes of Health (NIH; Bethesda, MD) ImageJ processing software to generate three-dimensional images of lymphatic valve leaflet morphology.20
Lymphatic Vessel and Valve Quantification
As shown in Figure 1, the cornea was divided into four equal quadrants in reference to the vertical midline passing through the 6- and 12-o'clock positions. The nasal and temporal quadrants were used for analysis of lymphatic vessel and luminal valve distribution for each sample. For LG evaluation, LYVE-1+ vascular structures were analyzed by using NIH ImageJ software, as described previously.17,20,21 Briefly, the lymphatic invasion area was normalized to the total corneal area to obtain a percentage coverage score for each sample. The total corneal area was measured by outlining the innermost lymphatic vessels of the limbal arcade, and lymphatic invasion area was determined by tracing out the contours of the LYVE-1+ lymphatic network inside the cornea. For luminal valve evaluation, focal Itga-9+/LYVE-1− areas running along the length of the LYVE-1+ vessels were identified as valves (Figs. 1B, 1C) and quantified for each sample. Additionally, a subset of 8-week corneas post suturing was evaluated for valvular directional orientation either toward terminal vessels inside the cornea or the limbus.
Live Imaging of Corneal Lymphatic Vessels
In vivo imaging of corneal lymphatic vessels was performed by using high-molecular-weight FITC-labeled dextran (2000 kDa; Sigma-Aldrich Corp., St. Louis, MO), as reported recently.22,23 The dye drainage was imaged with a customized imaging system including an adjustable eye and head stage holder and the Axio Zoom.V16 microscope (Carl Zeiss AG).
Statistical Analysis
Analysis of corneal LG and lymphatic valves was performed by using one-way ANOVA to compare the means of all time points. Post hoc pairwise comparisons between the study time points were done with Tukey's honestly significant difference test. Wilcoxon signed rank test was used for nasal versus temporal polarity analysis. All statistical analysis was done with Prism software (GraphPad, La Jolla, CA) with P < 0.05 considered significant. Data are expressed as mean ± SEM.
Results
Time Course of Lymphatic Vessel Formation in Inflamed Cornea
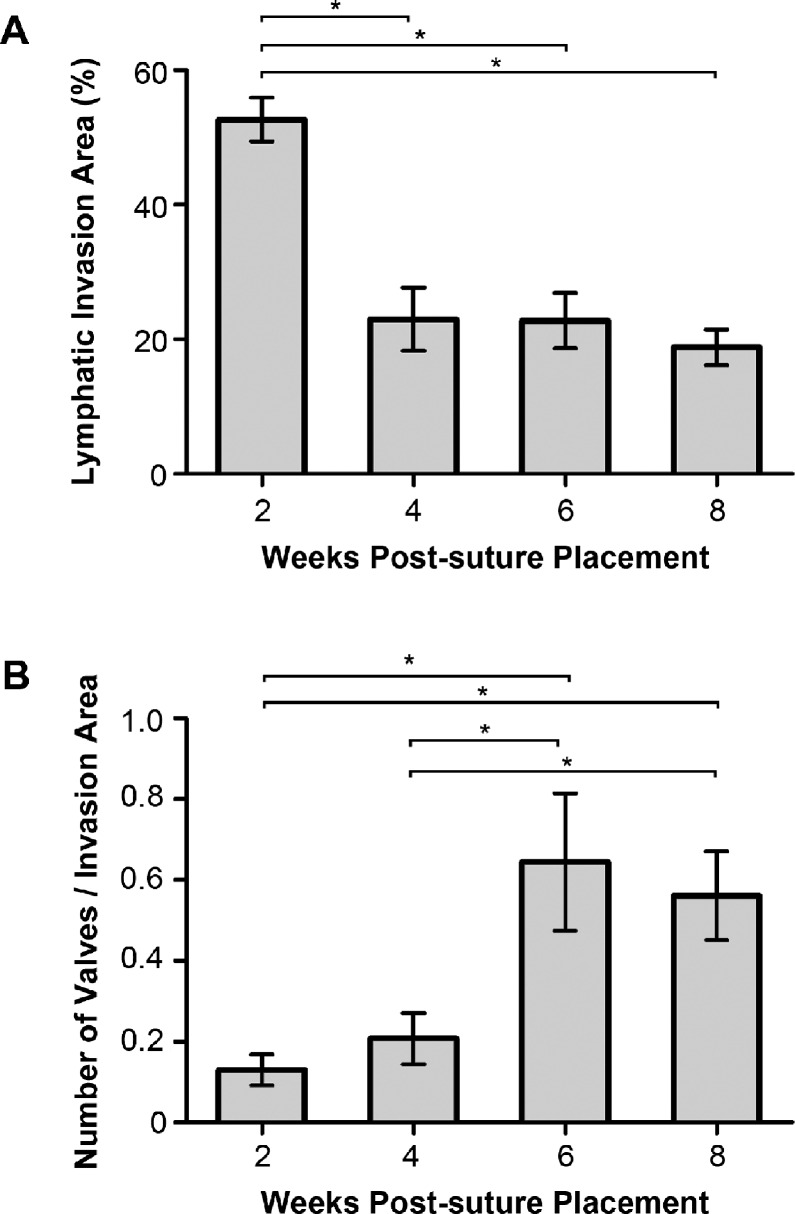
We first set out to define the time course of lymphatic vessel formation during the 8-week time period with the suture placement model we used. One-way ANOVA analysis revealed a significant difference in the mean lymphatic invasion area between the four study time points (P < 0.01). As shown in Figure 2A, corneal invasion area peaked at 2 weeks and gradually declined after that. The invasion area at 4 weeks post suturing was significantly lower than that at the 2-week time point (P < 0.05), and it remained low at 6- and 8-week time points as well, compared to the 2-week data (P < 0.05).
Figure 2.
Time course of lymphatic vessel and valve formation during corneal inflammation. (A) Summarized data from repetitive experiments showing greater lymphatic vessel invasion area at 2 weeks than at 4, 6, and 8 weeks after suture placement. (B) Summarized data showing significantly greater ratio of valves to invasion area at 6 and 8 weeks than at 2 and 4 weeks after suture placement. *P < 0.05.
Time Course of Lymphatic Valve Formation in Inflamed Cornea
After determining the time course of lymphatic vessel formation during the 8-week period, we next evaluated the change in the ratio of valve quantity to lymphatic invasion area over time by the immunofluorescent microscopic analysis. A significant difference in this parameter was revealed between the four study time points (ANOVA, P < 0.01), as presented in Figure 2B. The value of the ratio increased with time and was significantly greater at the 6-week compared to the earlier 2- and 4-week time points (P < 0.05). It remained greater at 8 weeks than at 2 and 4 weeks post suturing as well (P < 0.05).
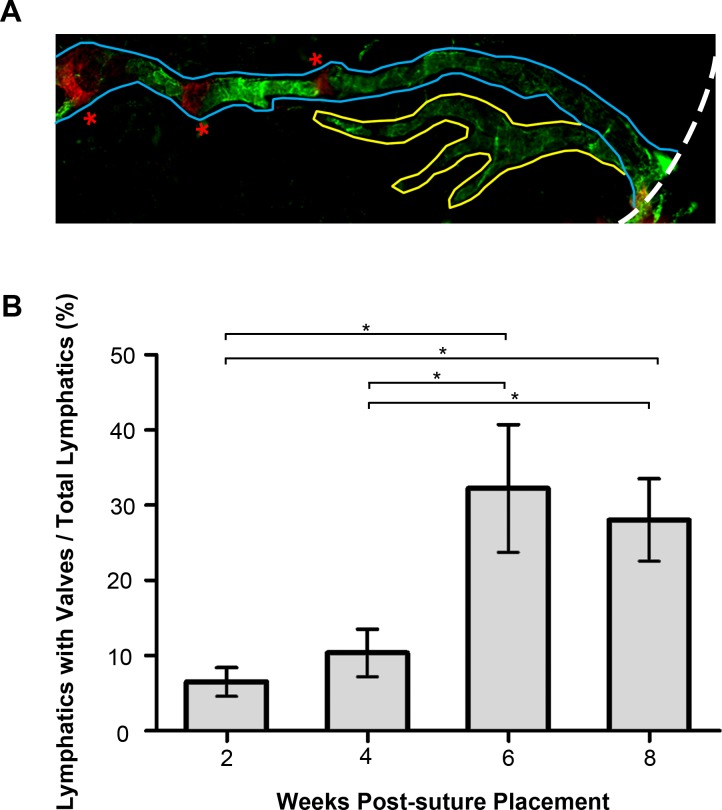
To more specifically assess the percentage of lymphatic vessels equipped with luminal valves over all lymphatic vessels formed (with or without valves) at a given time point, we manually traced out individual lymphatic vessels equipped with valves starting from the limbal area up to the innermost portion encroaching the central cornea for each sample (as demonstrated in Fig. 3A), and this additional analysis showed a significant difference in the percentage of lymphatic vessels possessing valves over total vessels between the four study time points (ANOVA, P < 0.01). As summarized in Figure 3B from repetitive experiments, the percentage of vessels possessing valves at the 2- and 4-week time points was significantly less than that at the 6- and 8-week time points (P < 0.05).
Figure 3.
The percentage of lymphatic vessels equipped with valves increased over time postsuture placement. (A) Schematic micrograph illustrating lymphatic vessels possessing valves (demarcated in blue) and lymphatic vessels without valves (demarcated in yellow). Dashed white line delineates the limbus; red asterisks indicate valves with Itga-9 red staining. LYVE-1: Green. Total lymphatic quantification includes both vessel types. Original magnification: ×100. (B) Summarized data from repetitive experiments showing greater percentage of vessels equipped with valves at 6 and 8 weeks than at 2 and 4 weeks after suture placement. *P < 0.05.
Differential Distribution of Lymphatic Vessel and Valves in the Nasal Versus Temporal Cornea During Inflammation
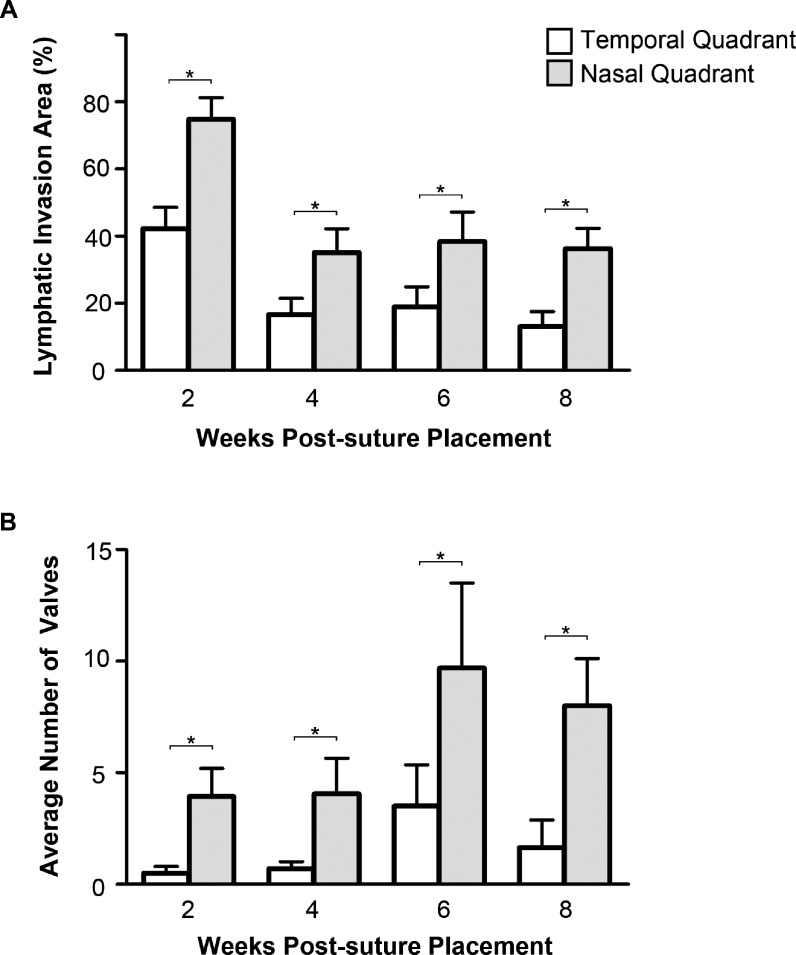
We next examined whether lymphatic vessel and valve formation were differentially distributed between the nasal and temporal quadrants in the inflamed cornea. As summarized in Figure 4A, lymphatic vessels were more observed in the nasal than the temporal quadrant at all time points studied (P ≤ 0.01). Likewise, there were significantly more lymphatic valves located in the nasal than the temporal cornea at all time points studied (Fig. 4B, P < 0.05).
Figure 4.
Comparison of nasal and temporal lymphatic vessel and valve distribution in inflamed cornea. (A) Summarized data showing the lymphatic invasion area was significantly greater in the nasal quadrant at all time points (2, 4, 6, and 8 weeks) post suturing. (B) Summarized data showing the distribution of lymphatic valves was also significantly greater in the nasal quadrant at all time points post suturing. *P < 0.05.
Directional Orientation of Corneal Lymphatic Valves and Functional Relevance for Lymphatic Drainage
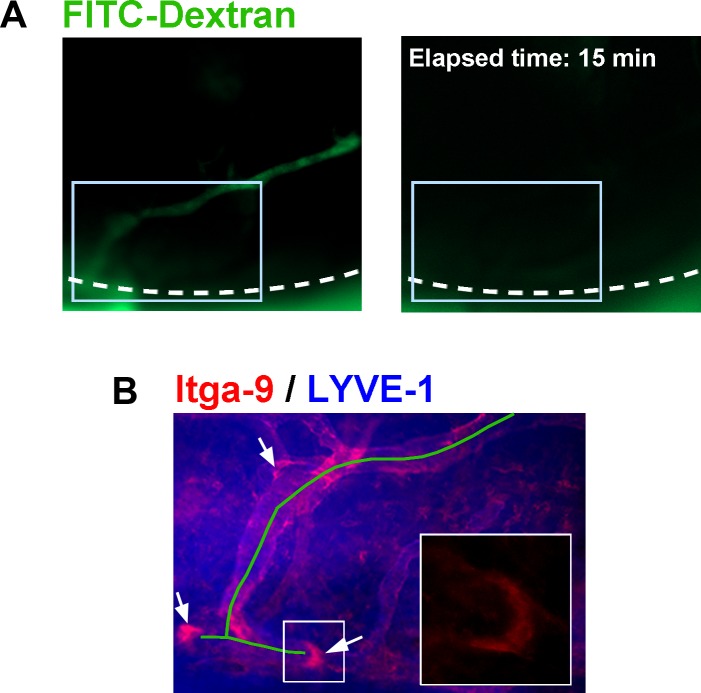
Previous embryonic studies in other tissues have shown that lymphatic valve orientation is related to the direction of fluid flow.24 Upon further examination on the morphologic structures of lymphatic valves induced by inflammation in this corneal study, it was found that corneal lymphatic valves tended to be oriented in a manner that promotes fluid transport toward the limbal vessels. Among all 103 well-formed valves detected in the 8-week corneas post suturing, predominantly 98% (101/103) were oriented toward the limbal direction, as shown in Figures 5A through 5C. To further assess the functional relevance of the valves in lymph drainage, we next performed the in vivo lymphatic drainage experiment with a large molecule FITC-dextran, a specific dye for lymph flow, as previously reported and confirmed in this study (Supplementary Fig. S1).22,23 Additionally, the presence and position of lymphatic valves in the cornea was assessed by ex vivo immunofluorescent analysis. As shown in Figures 6A (left panel) and 6B, our data demonstrated that along the lymphatic branches, which were highlighted by FITC-dextran flow, several lymphatic valves were positioned to direct lymphatic drainage toward the limbus. As a result, the lymphatic dye inside these vessels cleared out within a period of 15 minutes (Fig. 6A, right panel).
Figure 5.
Directional orientation of valves toward the limbus in inflamed cornea. (A–C) Representative micrographs showing directional orientation (indicated by white arrows) of lymphatic valves toward the limbus (indicated by dashed white line). (B, C) Higher magnification views of boxed area in (A). Itga-9: Red; LYVE-1: Green. Original magnifications: ×200 (A) and ×400 (B, C).
Figure 6.
Lymphatic valves direct lymphatic drainage in inflamed cornea. (A) Representative time-lapse micrographs of corneal live imaging showing the clearance of lymphatic-specific dye of FITC-dextran within a period of 15 minutes. Dashed lines delineate the limbus. (B) Immunostaining micrograph of the boxed area (A) showing that the lymphatic branches that were filled with FITC-dextran (indicated by green line) were equipped with valves (indicated by arrows) that were oriented to facilitate proper lymphatic drainage outside the cornea to the limbus. Inset: Higher magnification view showing valve orientation in boxed area of (B). Itga-9: Red; LYVE-1: Blue. Original magnifications: ×40 (A), ×100 (B), and ×630 (inset of B).
Discussion
This study established for the first time the long-term time course of lymphatic luminal valve formation in relation to corneal inflammatory LG. We demonstrated that the proportion of lymphatic vessels possessing luminal valves peaks at approximately 1 month after the peak of corneal vessel invasion, and the progression of valve formation occurs more dominantly in the nasal versus temporal side. More importantly, corneal lymphatic valves were directionally oriented toward the limbal area to guide lymphatic drainage out of the central cornea.
Our finding that lymphatic vessel formation peaked at 2 weeks post suturing is consistent with a previous study using similar suture placement model, as reported by another research group.25 The finding on the polarized distribution of lymphatic vessels and valves is also consistent with our previous 2-week report that newly formed lymphatic vessels are more situated at the nasal side.17 However, the current study was more comprehensive since it covered a much longer time period (up to 8 weeks after suturing) and presented the first evidence on polarized lymphatic valve distribution as well. Taken together, we have now a well-defined model to study corneal lymphatic vessel and luminal valve formation from early to late stages.
The increase of luminal lymphatic valves on vessels with time strongly suggests that the valves can serve as an indicator of vessel maturation. The presence of valves as corneal LG proceeds may drive the selection of vessels that can persist and become functional with the maturation process. As a result of this selection, those vessels without developing valves may undergo regression after remodeling. The total lymphatic vessels indeed decreased with time (Fig. 1A). Future investigation on this phenomenon may therefore reveal new therapeutic targets to interfere with lymphatic valve formation and maturation. It is also possible that various types of lymphatic vessels are formed inside the cornea and they function differently whether equipped with valves or not, which also warrants further investigation. The concept that luminal lymphatic valves can be formed and maintained inside corneal lymphatic vessels challenges the commonly accepted belief, based on developmental studies in other tissues, that capillary-type lymphatics are not considered to be equipped with luminal valvular structures.14 These luminal valves are different from lymphatic microvalves, which is a term used to describe a valve system at the level of the endothelium, formed by specialized interendothelial junctions between lymphatic endothelial cells.26 This terminology of microvalves has been used recently to describe LYVE-1–negative areas in corneal lymphatics.27 It is yet to be fully determined whether luminal and interendothelial junctions co-exist along and inside corneal lymphatic vessels.
Our finding on the luminal valves may define a category of terminal lymphatics that exist in certain tissues (such as the cornea) under certain circumstances (such as inflammation). It will be important to determine whether this new category of lymphatics exists in other tissues during other pathologic conditions, such as cancers or infections, which is beyond the scope of this study. Because normal cornea is devoid of any lymphatic structures, this study using the cornea allowed us a unique opportunity to particularly investigate pathologic LG with valvulogenesis, since all lymphatic vessels and luminal valves detected in the cornea are newly formed in response to the pathologic stimulation of inflammation.
The fact that corneal luminal lymphatic valves are oriented toward limbal vessels and function to guide lymphatic flow away from central cornea highly suggests that these valves are formed to enable lymphatic drainage outside the diseased cornea. This makes sense since it is known that the cornea becomes edematous and thickens after a pathologic stimulation,22,28,29 and lymphatic trafficking from the cornea to outside draining lymph nodes is needed to elicit an immune response.29,30 However, as we discussed earlier, it still remains to be determined whether all lymphatic vessels in the cornea need to develop valves before they can function, or whether the lymphatic vessels equipped with these valves are specifically formed to carry out this drainage function in the diseased cornea. Further investigation on the mechanisms by which the luminal valves are formed and properly oriented, whether through signal transduction of mechanical forces, concentration gradient of molecular factors, or a combination of both, will be of great value in furthering our fundamental understanding of lymphatic valvulogenesis in more detail. Interestingly and complementary to this study, during the review process of our current manuscript, another article, published by Katsuta et al.,23 has shown that EphrinB2-EphB4 signals are involved in corneal lymphatic valve formation induced by alkali burns, and EphB4 is expressed on luminal lymphatic valves in the cornea, as well as Itga-9.
Compared to blood vessels that have been studied extensively in the past, lymphatic research is relatively new and we are just beginning to better understand the wide array of diseases associated with lymphatic dysfunction in the cornea as well as systemically in the body. Because the cornea has proven to be a powerful tool for blood vessel research in broad fields (such as tumor growth and metastasis) during the past few decades,7,31,32 we hope that results from this study and future investigation using the cornea will help to elucidate the mechanisms of the pathologic processes LG and lymphatic valvulogenesis and to provide new avenues of therapy for relevant lymphatic disorders that occur both inside and outside the eye.
Acknowledgments
The authors thank Samuel Coleman and Wenjuan Feng for assistance with confocal microscopy and statistical analysis.
Supported in part by research grants from the National Institutes of Health, University of California at Berkeley (LC), and National Institutes of Health K-12 training grant (TT).
Disclosure: T. Truong, None; E. Huang, None; D. Yuen, None; L. Chen, None
References
- 1. Kaipainen A, Korhonen J, Mustonen T, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995; 92: 3566–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999; 144: 789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999; 98: 769–778 [DOI] [PubMed] [Google Scholar]
- 4. Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010; 140: 460–476 [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010; 24: 2115–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003; 22: 273–281 [DOI] [PubMed] [Google Scholar]
- 7. Chen L, Hann B, Wu L. Experimental models to study lymphatic and blood vascular metastasis. J Surg Oncol. 2011; 103: 475–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010; 207: 101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ling S, Lin H, Liang L, et al. Development of new lymphatic vessels in alkali-burned corneas. Acta Ophthalmol. 2009; 87: 315–322 [DOI] [PubMed] [Google Scholar]
- 10. Chen L. Ocular lymphatics: state-of-the-art review. Lymphology. 2009; 42: 66–76 [PMC free article] [PubMed] [Google Scholar]
- 11. Goyal S, Chauhan SK, El Annan J, Nallasamy N, Zhang Q, Dana R. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol. 2010; 128: 819–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010; 184: 535–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang H, Grimaldo S, Yuen D, Chen L. Combined blockade of VEGFR-3 and VLA-1 markedly promotes high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2011; 52: 6529–6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Truong T, Altiok E, Yuen D, Ecoiffier T, Chen L. Novel characterization of lymphatic valve formation during corneal inflammation. PLoS One. 2011; 6: e21918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008; 8: 604–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang XZ, Wu JF, Ferrando R, et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000; 20: 5208–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ecoiffier T, Yuen D, Chen L. Differential distribution of blood and lymphatic vessels in the murine cornea. Invest Ophthalmol Vis Sci. 2010; 51: 2436–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen L, Cursiefen C, Barabino S, Zhang Q, Dana MR. Novel expression and characterization of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1) by conjunctival cells. Invest Ophthalmol Vis Sci. 2005; 46: 4536–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grimaldo S, Yuen D, Ecoiffier T, Chen L. Very late antigen-1 mediates corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2011; 52: 4808–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneider CA, Rasband WS, Eliceiri KW. NIH. Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004; 113: 1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuen D, Wu X, Kwan AC, et al. Live imaging of newly formed lymphatic vessels in the cornea. Cell Res. 2011; 21: 1745–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katsuta H, Fukushima Y, Maruyama K, et al. EphrinB2-EphB4 signals regulate formation and maintenance of funnel-shaped valves in corneal lymphatic capillaries. Invest Ophthalmol Vis Sci. 2013; 54: 4102–4108 [DOI] [PubMed] [Google Scholar]
- 24. Bazigou E, Xie S, Chen C, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009; 17: 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006; 25: 443–447 [DOI] [PubMed] [Google Scholar]
- 26. Trzewik J, Mallipattu SK, Artmann GM, Delano FA, Schmid-Schonbein GW. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J. 2001; 15: 1711–1717 [DOI] [PubMed] [Google Scholar]
- 27. Nakao S, Zandi S, Faez S, Kohno R, Hafezi-Moghadam A. Discontinuous LYVE-1 expression in corneal limbal lymphatics: dual function as microvalves and immunological hot spots. FASEB J. 2011; 26: 808–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J, Xin L, Shan B, et al. PEAKS DB: de novo sequencing assisted database search for sensitive and accurate peptide identification. Mol Cell Proteomics. 2012; 11: M111.010587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004; 10: 813–815 [DOI] [PubMed] [Google Scholar]
- 30. Rodin G, Yuen D, Mischitelle A, et al. Traumatic stress in acute leukemia. Psychooncology. 2013; 22: 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rogers MS, Birsner AE, D'Amato RJ. The mouse cornea micropocket angiogenesis assay. Nat Protoc. 2007; 2: 2545–2550 [DOI] [PubMed] [Google Scholar]
- 32. Gimbrone MA Jr, Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974; 52: 413–427 [DOI] [PubMed] [Google Scholar]