Abstract
Graves' disease (GD) is a common autoimmune condition. At its core, stimulatory autoantibodies are directed at the thyroid-stimulating hormone receptor (TSHR), resulting in dysregulated thyroid gland activity and growth. Closely associated with GD is the ocular condition known as thyroid-associated ophthalmopathy (TAO). The pathogenesis of TAO remains enigmatic as do the connections between the thyroid and orbit. This review highlights the putative molecular mechanisms involved in TAO and suggests how these insights provide future directions for identifying therapeutic targets. Genetic, epigenetic, and environmental factors have been suggested as contributory to the development of GD and TAO. Thyroid-stimulating hormone receptor and insulin-like growth factor receptor (IGF-1R) are expressed at higher levels in the orbital connective tissue from individuals with TAO than in healthy tissues. Together, they form a functional complex and appear to promote signaling relevant to GD and TAO. Orbital fibroblasts display an array of cell surface receptors and generate a host of inflammatory molecules that may participate in T and B cell infiltration. Recently, a population of orbital fibroblasts has been putatively traced to bone marrow–derived progenitor cells, known as fibrocytes, as they express CD45, CD34, CXCR4, collagen I, functional TSHR, and thyroglobulin (Tg). Fibrocytes become more numerous in GD and we believe traffic to the orbit in TAO. Numerous attempts at developing complete animal models of GD have been largely unsuccessful, because they lack fidelity with the ocular manifestations seen in TAO. Better understanding of the pathogenesis of TAO and development of improved animal models should greatly accelerate the identification of medical therapy for this vexing medical problem.
Keywords: autoimmune, Graves' disease, inflammation
Introduction
Graves' disease (GD) is a common autoimmune condition associated with dysregulated thyroid gland activity and growth.1 Underlying glandular overactivity are stimulatory autoantibodies directed against the thyrotropin receptor (TSHR), known as thyroid-stimulating immunoglobulins (TSI).1 Their actions result in the overproduction of thyroid hormones, which in turn causes numerous metabolic abnormalities in peripheral tissues.1 Closely associated with GD is the manifestation localized in orbital connective tissues, known as thyroid-associated ophthalmopathy (TAO, also known as Graves' orbitopathy) that occurs in 25% to 50% of those with GD.2,3 The clinical features of TAO include periorbital edema, eyelid retraction, proptosis, strabismus, exposure keratopathy, and compressive neuropathy (Figs. 1, 2). They are disfiguring and also may threaten sight.4 The clinical course of TAO usually involves a self-limited active phase characterized by inflammation and tissue remodeling.4 This process typically lasts between 18 and 36 months, and is followed by the stable phase. The exact pathogenesis of TAO appears to involve complex molecular and cellular processes that have yet to be understood fully.
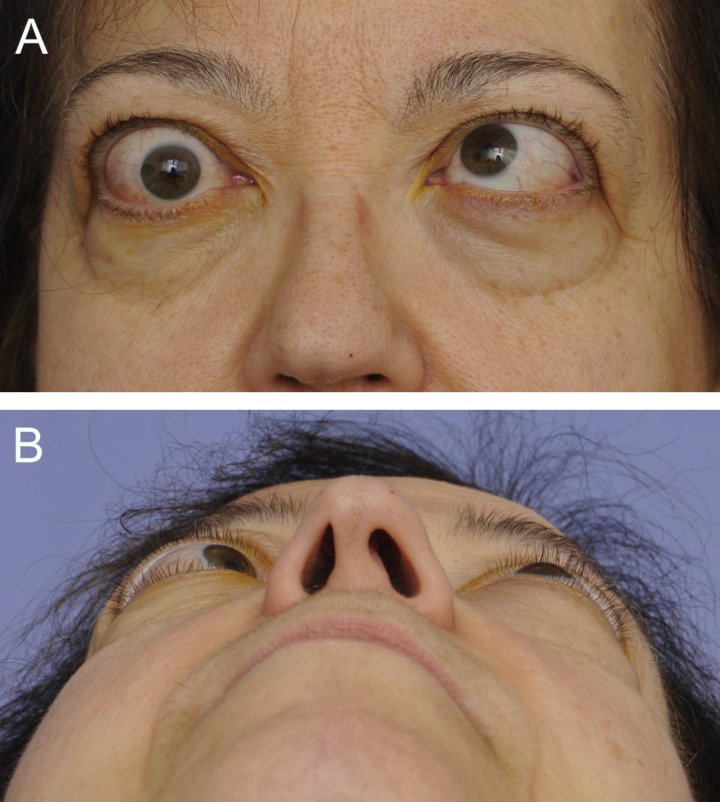
Figure 1.
A 61-year-old woman with TAO. (A) Frontal view demonstrating bilateral upper lid retraction (right greater than left) and bilateral lower lid retraction with inferior scleral show. Bilateral proptosis, lateral flare, chemosis, injection, caruncular edema, and significant left esotropia also are evident. (B) Worms-eye view highlighting proptosis (right greater than left).
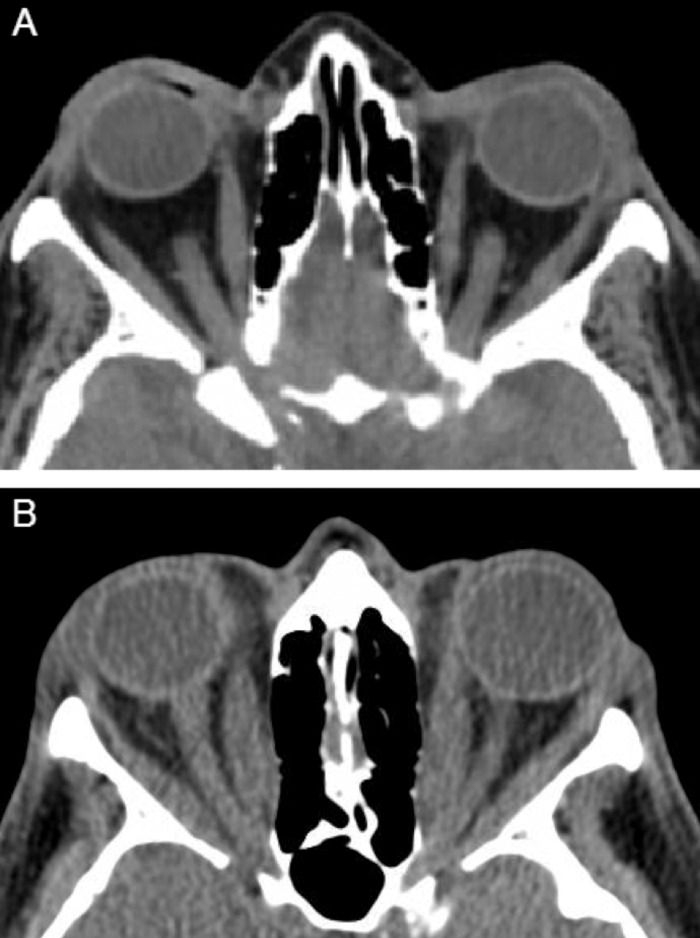
Figure 2.
Orbital CT without contrast. (A) Uninvolved orbits. (B) Patient with TAO demonstrating bilateral exophthalmos. Bilateral marked enlargement of the extraocular muscles, especially the medial, superior, and inferior rectus muscles, causing apical crowding of the optic nerve. Stranding can be observed around both optic nerves.
The connection between the orbit and the pathologic events occurring within the thyroid in GD remains enigmatic, as does any relationship existing between thyroid hormone excess and TAO. The first signs of ocular involvement can precede, accompany, or follow development of thyroid dysfunction. The concept that orbital disease is not provoked directly by abnormal thyroid hormone levels associated with GD is now widely accepted. Rather, most experts believe that TAO occurs as the consequence of underlying autoimmune processes. Its complexity and diversity of presentation, combined with the absence of complete animal models, have delayed solving TAO. However, recent progress in identifying at least some of the pathologic elements involved in GD has begun to accelerate our understanding of the disease. This review attempts to crystallize these advances, and in so doing, identify weaknesses in the current concepts, and provide a roadmap for future studies that should facilitate basic discovery and translate to the clinic.
Genetic, Epigenetic, and Environmental Risk Factors for TAO
Genetic Predisposition
Genetic and environmental factors contribute to the pathogenesis of GD.5 However, clear-cut differences between genetic variations associated with GD and those peculiar to the subset of individuals developing TAO have not yet been identified.6 Similar to other autoimmune conditions, GD and TAO are more prevalent among females.7 However, men with GD appear to be at greater risk of developing severe TAO.8,9 Prevalence of TAO also diverges with respect to ethnicity. For instance, Asians are less likely to suffer TAO than are their European counterparts.10 Increased incidence of GD among family members also indicates that genetic factors have a major role in susceptibility.5,11 A recent study investigated the prevalence of ocular and eyelid signs in first and second-degree relatives from a single family harboring multiples cases of GD, TAO, and Hashimoto's thyroiditis.12 The investigators reported that 33% of the euthyroid relatives had signs of TAO, such as upper lid retraction. These findings favor a genetic contribution to the development of TAO.12
Studies examining twins with GD were conducted by interrogating the Danish twin registry.5,13 These demonstrated concordance rates as high as 30% for GD in monozygotic compared to 3% in dizygotic twins.5,13 They indicated that approximately 79% of the risk for developing GD is attributable to genetics, while the remaining 21% derives from environmental factors.13 In addition, several reports have appeared identifying multiple susceptibility genes associated with GD. Among these polymorphisms are variations in genes regulating immune function, such as HLA-DR3,14,15 CTLA4,16 PTPN22,17 CD40,18 IL-2RA,19 FCRL3,20 and IL-23R.21 Others encode thyroid-specific proteins, such as TSHR22 and thyroglobulin (Tg).23
Identification of novel single-nucleotide polymorphisms (SNPs) in disease susceptibility genes further contributes to our understanding of the genetic basis underlying GD. The Interleukin-21 and IL-21R polymorphisms have been associated with autoimmune conditions, such as type 1 diabetes mellitus,24 juvenile idiopathic arthritis,25 psoriasis,26 celiac disease,27,28 ulcerative colitis,29 and multiple sclerosis.30 The SNPs within the IL-21 gene and those located within intron 1 of TSHR, such as rs2284720, also have been associated with GD and TAO.31–34 The SNP rs6479778, identified within the ARID5B gene at 10q locus,32,35 and SNP rs12147587, located within the NRXN3 gene at 14q locus,32 represent variations within genes that regulate adiposity and might predispose to GD.36,37
Because the vast majority of individuals with TAO have underlying GD, it would not be surprising that the two processes share disease susceptibility genes. One recent study examined polymorphisms of HLA, CTLA4, IL23R, and TSHR in a cohort with TAO and found no genetic differences compared to patients with GD without ocular involvement.38 Most studies have concluded that the gene polymorphisms thus far identified contribute little to overall disease susceptibility. None identified appears to convey sufficient risk for developing TAO to warrant prophylactic treatment in individuals with GD. The relative contributions of specific genetic and environmental factors for developing TAO remain to be quantified. Moreover, the susceptibility conferred appears complex and varies with ethnicity.
Epigenetics
Besides genetic factors, epigenetic determinants, such as heritable alternations in gene function, also may have a role in GD. These could contribute through alterations in DNA methylation, histone modifications, genomic imprinting, RNA interference, and X chromosome inactivation.39 As with genetic factors, those that emanate from the epigenenome and provide unequivocal causality have yet to be identified. Yin et al.39 found upward skewing of X chromosome inactivation (≥80% inactivation of one X chromosome in the same tissue) in GD when compared to healthy individuals. Yet, the mechanisms through which this inactivation leads to increased risk for GD are not yet known.39 Nonetheless, this phenomenon could ultimately explain the higher incidence of GD and TAO in women.40
A recent study has identified a Tg promoter nucleotide substitution (−1623 A/G SNP, rs180195) that may predispose to autoimmune thyroid disease.41 This G allele and G/G haplotype are more frequent in affected individuals, and interact epigenetically with IFNα following viral infections.41 Subsequently, interferon regulatory factor-1 (IRF-1) binds the Tg promoter at rs180195, resulting in enhanced mono-methylation of the Lys-4 residue of H3.41 Treatment with IFNα of thyroid cells transfected with a fragment of the Tg gene promoter fused to a reporter increases its activity only in the construct harboring the variant. Thus, it is possible that IFNα promotes IRF-1 binding to the variant Tg promoter, thereby directly modulating expression of gene(s) underlying thyroid autoimmunity.
Environmental Factors
Environmental factors, such as infectious agents, have been implicated in the initiation of immune responses to self-antigens.7 These might underlie the development of GD and TAO. Bacteria can induce inflammatory responses leading to aberrant expression of co-stimulatory molecules, including MHC class II. This process often results in presentation of self-antigens and the activation of antigen-specific T cells.7 Alternatively, infections can alter the expression of host proteins so that they become misrecognized as foreign.42 Molecular mimicry, resulting from primary sequence identity or conformational similarities to antigens, also could have a pathogenic role in the development of GD, as has been proposed in other autoimmune conditions.43–45
An early study reported that DNA from human foamy viruses (HFV), otherwise known as spuma viruses, had been detected in peripheral DNA from a majority of those with GD, but was undetectable in healthy controls.46 Subsequent studies have failed to confirm these findings.47,48 However, another report detected HFV proteins in diseased thyroid tissue.49 It remains unclear whether HFV infection might be associated with GD. A follow-up study utilizing more modern techniques could resolve this open question.
Yersinia enterocolitica was investigated initially for its participation in GD more than 40 years ago.50,51 The large proportion of individuals with GD in whom antibodies against Y. enterocolitica can be detected suggests that these bacteria might express proteins resembling those of the host.52,53 This concept is based in part on identification of high affinity TSH and TSI binding sites on Y. enterocolitica.54–56 Furthermore, mice immunized with Y. enterocolitica envelope proteins have been shown to develop anti-TSHR antibodies.50 A recent study demonstrated the outer membrane porin F protein of Y. enterocolitica cross-reacts immunologically with the leucine-rich domain of TSHR.57 Furthermore, early precursor B cells can expand when exposed to Y. enterocolitica porin proteins and undergo somatic hypermutation to acquire cross-immunogeneticity with TSHR.58 Although development of autoimmunity following certain infections has been suspected for many years, further study will be necessary before this mechanism can be linked causally to GD and TAO.
Cigarette smoking has been associated consistently with development and worsening of GD and TAO,59–61 as well as other forms of human autoimmunity.62,63 This connection was first described by Hagg and Asplund.64 Subsequent studies have confirmed their findings, and smoking has emerged as an important risk factor for GD and TAO with odds ratios of 1.9 (95% confidence intervals [CI], 1.1–3.2) and 7.7 (95% CI, 4.3–13.7), respectively.60 In individuals with GD who smoke more than 20 cigarettes per day, the relative risk for developing proptosis is 3.37 (1.50–7.58, P = 0.003) and as high as 7.04 (3.00–16.5, P < 0.0001) for developing diplopia.65 Risk for developing TAO relates more to the number of cigarettes smoked following development of GD than the life-cumulative smoking burden.65 In a matched case-control twin study, Brix et al.66 found that the discordant monozygotic twin with GD was more likely to have smoked when compared to the healthy sibling. A meta-analysis of studies investigating the association between smoking and thyroid diseases confirmed the increased risk for developing or worsening of TAO beyond that associated with GD.67 A retrospective analysis demonstrated that nonsmokers had a decreased risk of TAO progression, and better therapeutic response to orbital radiation and corticosteroids than did smokers.68 While the mechanism underlying the deleterious effects of smoking on TAO remains uncertain, its cessation appears to improve treatment response and to lower the risk of developing TAO de novo.
The Putative Role of TSHR in TAO
Thyroid-stimulating hormone receptor, a glycoprotein hormone receptor, is a member of the G protein coupled receptor family.69 It contains a ligand-binding extracellular domain (ectodomain), a transmembrane domain, and an intracellular domain (endodomain).69 Posttranslational intramolecular proteolytic cleavage of the extracellular domain results in the generation of the A-subunit, which exhibits immunoreactivity and is processed by antigen presenting cells.70 Thyroid-stimulating immunoglobulins and TSH binding to TSHR results in receptor activation and unregulated thyroid hormone production. This appears to be the basis for hyperthyroidism and the development of goiter in GD.1
The frequently encountered close temporal relationship between the onset of thyroid dysfunction and development of TAO suggests that GD and TAO might share a common etiology, and perhaps share a common autoantigen.71 In addition to thyroid epithelium, TSHR can be detected in several connective tissue/adipose depots, including those within the orbit.72,73 Levels of TSHR mRNA are considerably lower in orbital fat than those found in thyroid.74 They appear to be higher in orbital fibroblasts from patients with TAO compared to those from healthy donors.74 While the role of TSI in TAO has not been established, these antibodies can activate TSHR displayed on orbital fibroblasts and lead to downstream signaling and production of IL-6.75 While evidence suggesting that low-level TSHR expression on orbital fibroblasts is capable of transducing signals from TSI has been introduced, whether the receptor protein serves as an intraorbital antigen remains uncertain. To our knowledge, no compelling studies have demonstrated antigen-specific T cell infiltration of the orbit in TAO.
T and B Cells
In TAO, T and B cells infiltrate orbital fat (Fig. 3) and extraocular muscles.76 This pattern of lymphocyte recruitment shares similarities with that occurring in the thyroid.77,78 Both CD4+ and CD8+ T cells can be identified among the infiltrate, a process that apparently occurs early in TAO.79 Th1 predominates early in the disease, whereas a bias toward the Th2 phenotype can be found later.80 CD4+ Th17 T cells, which have been implicated in other autoimmune diseases, have yet to be identified in orbital infiltrates.81 Despite the variants of IL-23R that have been associated with TAO,21 and the increased frequency of circulating Th17 and Th22 cells in GD,82,83 the possible involvement of the Th17 pathway in TAO has yet to be examined carefully.
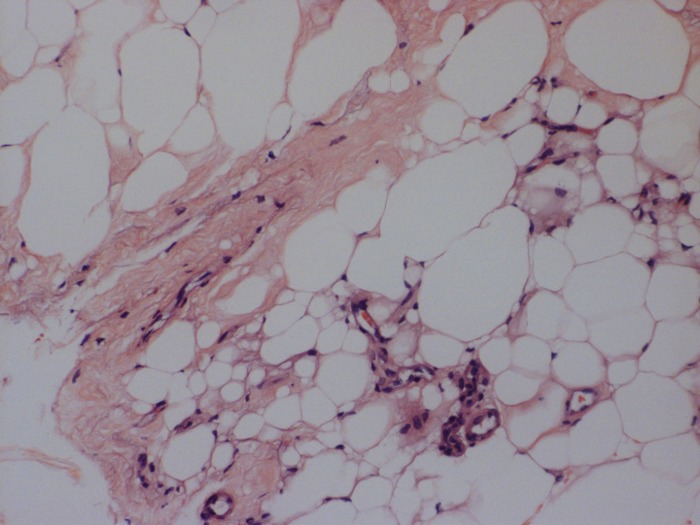
Figure 3.
Histologic examination of orbital tissue of a patient with GD (hematoxylin and eosin, ×20). Mononuclear cell infiltrate is seen within the orbital fat compartment.
Cytokines
Orbital tissue activation and remodeling associated with TAO appear to result from cytokine-dependent fibroblast activation.84 This might be attributed, at least in part, to the unusual susceptibility of orbital fibroblasts to the actions of pro-inflammatory cytokines.84 Evidence for involvement of specific cytokines derives from their detection in involved orbital fat. One study demonstrated immunoreactivity against IFNγ, TNFα, and IL-1α.85 Messenger RNA encoding cytokines, including TNFα, IL-1β, IFNγ, IL-4, IL-6, and IL-10, was detected in extraocular muscle and fat from patients with TAO.86 Prummel et al.87 found elevated serum soluble IL-2R (sIL-2R) levels in approximately 45% of individuals with TAO. Although these levels failed to correlate with disease activity or severity, those individuals with the highest levels exhibited enlarged extraocular muscles.87 Serum concentrations of IL-6 and soluble IL-6R were found to be elevated in GD compared to healthy individuals.88 These were even higher in the subset with active TAO.88 Serum IL-6 levels were higher in long-standing TAO.89 Serum soluble IL-2R, IL-6, IL-6R, TNFαR I, II, and sCD30 were elevated in patients with moderately severe untreated TAO compared to healthy individuals matched for sex, age, and smoking habits.90 Increased serum IL-17 levels were detected in TAO and were particularly elevated in those with active disease.91
Orbital Fibroblasts and the Putative Role of Bone Marrow–Derived Fibrocytes
A remaining central question concerns the identity of the primary autoimmune target in TAO. Extraocular muscle has been proposed by a few investigators,92–97 but most have focused on orbital fibroblasts.98 Supporting the latter point of view, infiltrating CD8+ T cells recognize orbital fibroblasts, and become activated through MHC class II and CD40-dependent signaling,7 suggesting that these cells represent autoimmune targets.
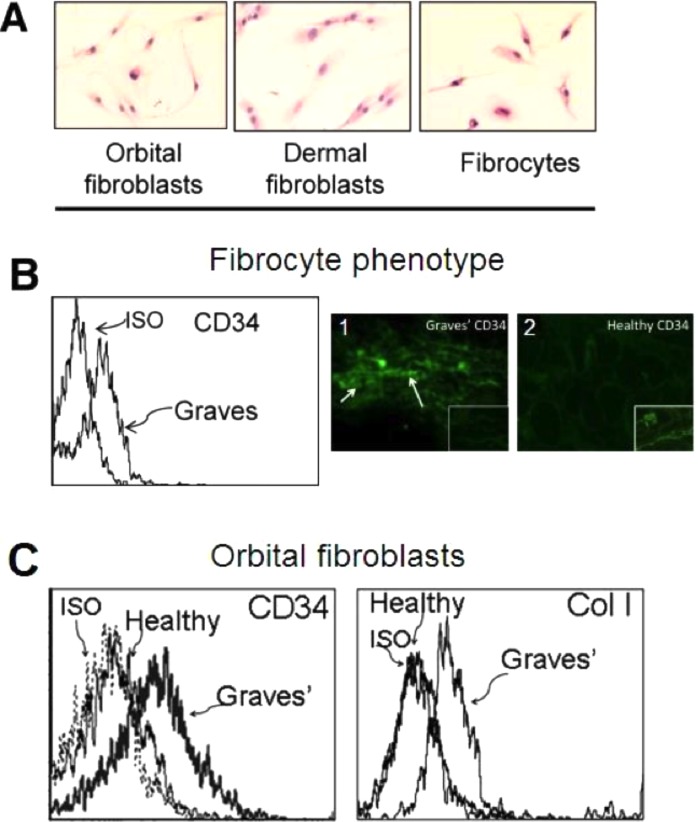
Orbital fibroblasts are a heterogeneous population of cells with complex structural and immunoregulatory functions.99,100 They comprise spindle- and fusiform-shaped cells, projecting two or three dendritic processes.101 Others are angular, with three or more dendritic processes. Thus, their shapes differ slightly from those of dermal fibroblasts. Their rate of cell division is predicated, at least in part, on whether they display the cell surface glycoprotein CD90, known as thymocyte antigen 1 (Thy-1).102 For the first time, Koumas et al.103,104 demonstrated that human orbital fibroblasts exhibited heterogeneous expression of Thy-1, and when separated into Thy-1+ and Thy-1− subsets, responded differently to extracellular stimuli, and showed distinct functionalities. When exposed to IL-1β or following CD40 ligation, Thy-1+ orbital fibroblasts produced considerably higher levels of PGE2 via upregulation of prostaglandin endoperoxide H synthase-2 (PGHS-2, also known as COX-2).104 Further, Thy-1+ orbital fibroblasts differentiated into myofibroblasts when treated with TGF-β, as was evidenced by strong immunofluorescence activity to α-SMA,105 whereas the Thy-1−-subset underwent adipogenesis when treated with a PPARγ agonist.103,104
The cellular attributes of orbital fibroblasts currently are thought to predispose to the pathologic processes associated with TAO.84 They display unique arrays of costimulatory molecules and cell surface receptors for various cytokines and growth factors.84 It is the particular profile of inflammatory cues to which they respond that appears to set them apart from other fibroblasts. For instance, leukoregulin, IL-1β, and CD40 ligand (also known as CD40L or CD154) vigorously induce PGHS-2 in orbital fibroblasts when compared to dermal fibroblasts.106–108 A major aspect of phenotypic divergence of orbital fibroblasts appears to relate to the disparities with which the IL-1 receptor antagonists (IL-1RA) isoforms are expressed.109,110 Unlike those from the skin, orbital fibroblasts express vanishingly low levels of secreted IL-1RA (sIL-1RA), the antagonist molecule that has the dominant role in blocking IL-1–derived signaling. Instead, intracellular IL-1RA is far more highly expressed and inducible in these cells. The exaggerated induction of PGHS-2 resulting from cytokines, such as IL-1β, is mediated through enhanced PGHS-2 gene promoter activity and mRNA stability.106,108 The upregulation of PGHS-2 was found to be accompanied by dramatically increased PGE2 production.107 Orbital fibroblasts express PGE2 receptors and respond to this prostanoid by developing multiple long cytoplasmic processes111 and generating cyclic adenosine monophosphate.112 In addition, PGE2 influences B cell class-switching,113 T cell differentiation,114 and mast cell degranulation,115 all of which might have roles in TAO. Hwang et al.116 recognized that orbital fibroblasts from patients with TAO display higher levels of CD40 than do cells derived from healthy donors. These levels are further upregulated by IFNγ. When ligated with CD40L, they produce hyaluronan117 as well as IL-6, IL-8, and MCP-1.116 Interleukin-6 drives immunoglobulin production, development of plasma cells,118 IL-4 synthesis, and biases T cells toward Th2 development.119 Monocyte chemotactic factor-1, a powerful chemoattractant, may be involved in promoting mononuclear cell infiltration in TAO.120 Interleukin-16 and RANTES121 also are produced by orbital fibroblasts, once they are activated by cytokines, such as IL-1β122 and IgGs,123 from patients with GD through the IGF-1 receptor pathway.124 Thus, fibroblasts may have important roles in T cell infiltration of the orbit and B cell differentiation.
The embryonic origins of orbital fibroblasts have been debated for many years. Recently, a potential explanation for the cellular heterogeneity found in TAO orbital connective tissue has been provided by the recognition that a subset of these cells apparently derives from the bone marrow.74 Progenitor cells, known as fibrocytes, have been found in these orbital tissues from individuals with TAO, but not in those from healthy donors. They derive from monocyte and B cell lineages and circulate as peripheral blood mononuclear cells (PMBCs).125 Fibrocytes ordinarily comprise approximately 0.5% of circulating PBMCs and can infiltrate connective tissues at sites of injury.126 They participate in inflammation, wound healing, and tissue remodeling, and also are involved in fibrotic lung and kidney diseases.127,128 Fibrocytes synthesize collagen I (Col I), display CD34 and CXCR4, and traffic to tissues in response to multiple chemokines, including CXCL12.129 They become more numerous in GD (Fig. 4), and can differentiate into myofibroblasts and adipocytes, and, thus, may account for the characteristic tissue remodeling associated with TAO.130 The presence of fibrocytes in the TAO orbit may explain the divergent phenotypes observed in fibroblast populations.74,131
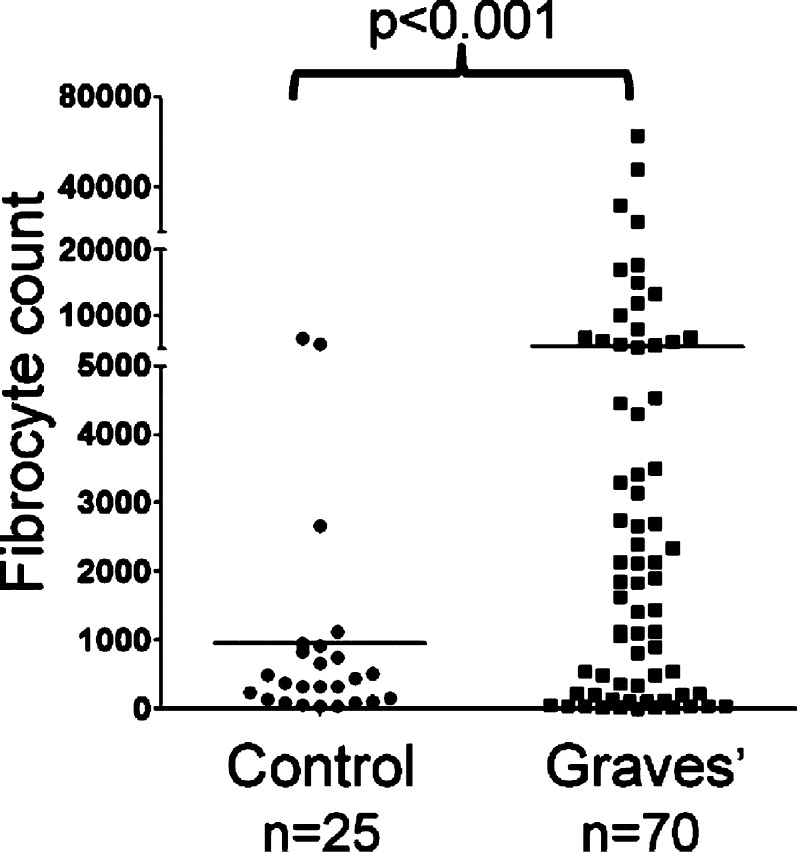
Figure 4.
Increased generation of fibrocytes from PBMCs of patients with GD. There was approximately 5-fold more fibrocytes in individuals with GD compared to controls (5268 ± 1260 fibrocytes per 106 PBMCs, n = 70 versus control, 954 ± 329 fibrocytes per 106 PBMCs, n = 25, mean ± SD, P < 0.001). Reprinted with permission from Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95:430–438. Copyright 2010 The Endocrine Society.
Fibrocytes unexpectedly express functional TSHR at levels comparable to those displayed on thyroid epithelial cells.74 A greater proportion of fibrocytes from donors with TAO express TSHR than do those from healthy donors.132 The levels of TSHR on fibrocytes are considerably higher than those on orbital fibroblasts, regardless of whether they derive from healthy tissues or those affected by TAO.132 When TSHR on fibrocytes is ligated with bTSH or monoclonal TSI (M22), production of several cytokines, including IL-6, IL-8, RANTES, MCP-1, IL-1β, and TNF-α, is upregulated dramatically.74,132 Further, fibrocytes are morphologically similar to orbital fibroblasts (Fig. 5A). The TAO orbital fat contains CD34+TSHR+CXCR4+Col1+ cells in situ, and the fibroblasts outgrowing these tissues display these markers74 (Figs. 5B, C). CD34+ orbital fibroblasts, like their circulating fibrocyte precursors,133 differentiated into either adipocytes or myofibroblasts, depending on the culture conditions to which they were subjected.103,104
Figure 5.
(A) Similar spindle-shaped phenotypes among orbital fibroblasts, dermal fibroblasts, and fibrocytes (hematoxylin and eosin, ×20). (B) Fibrocytes from individuals with GD display cell surface receptor CD34. 1, Immunofloresence staining of CD34 in TAO-derived tissue (inset as negative control). 2, Absence of CD34 expression in healthy orbital tissue (inset as positive control). (C) Orbital fibroblasts from individuals with and without TAO display similar receptors on fibrocytes, as shown by flow cytometric analysis with anti-CD34 and anti-Col I antibodies. Reprinted with permission from Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95:430–438. Copyright 2010 The Endocrine Society.
Adipogenesis and Hyaluronan Production by Orbital Fibroblasts: Reflections of Tissue Remodeling in TAO
Thyroid-associated ophthalmopathy is characterized by the gross enlargement of extraocular muscles.134 While this is due mostly to edema, the production of glycosaminoglycans (GAGs) by the orbital fibroblasts and hyperplasia of the adipose tissue also contribute to proptosis and can result in compression of the optic nerve.134–136 Once lymphocytes infiltrate and activate the orbital fibroblasts, these cells produce GAGs and differentiate into myofibroblasts or adipocytes.103,137–139
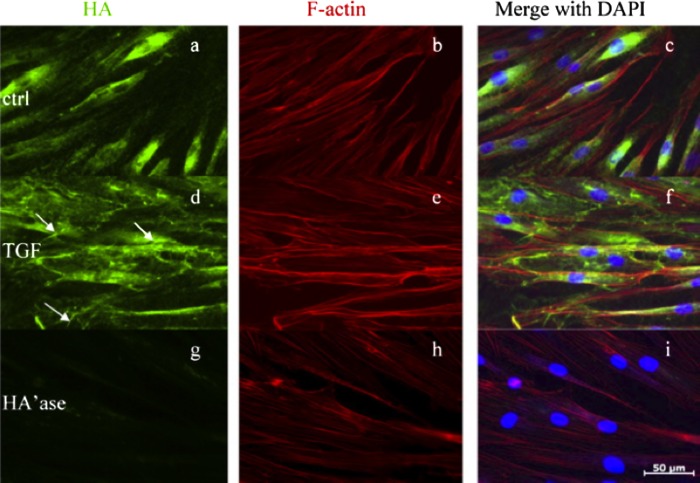
The cardinal feature of remodeling seen in TAO is the disordered accumulation of hyaluronan, a nonsulfated GAG. The extraordinary hydrophilic nature of hyaluronan causes volume expansion within orbital tissues.140 Orbital fibroblasts, as opposed to dermal fibroblasts, demonstrated a dramatic increase in hyaluronan production when exposed to leukoregulin, IFN-γ, and IL-1β through the induction of UDP-glucose dehydrogenase141 and the hyaluronan synthases.108,142 Further, when incubated with CD40L, they exhibited substantial coordinate increases in hyaluronan and PGE2 synthesis, with the latter being mediated through PGHS-2 and IL-1α synthesis.106 The robust response is due to low-level expression of sIL-1RA in orbital fibroblasts and subsequent poor inhibition of IL-1β.106,109,110 Also, TGF-β has been shown to regulate hyaluronan production (Fig. 6). Recently, PPARγ activation was shown to suppress TGF-β–induced activation of fibrosis-related processes.143–145 Guo et al.146 demonstrated that PPARγ ligands inhibited TGF-β–induced hyaluronan-dependent T cell adhesion to orbital fibroblasts. The same group reported that PGD2, a major prostanoid produced by mast cells, regulates hyaluronan production in orbital fibroblasts, actions mediated through PD1.147
Figure 6.
Immunofloresence of the induction of hyaluronan with TGF-β in human orbital fibroblasts. Cultures were treated with nothing (controls) or TGF-β1 for 24 hours. (a, d, g) Contain images of cells stained with biotinylated HABP and demonstrate hyaluronan. (b, e, h) Contain monolayers stained with phalloidin and demonstrate actin. (c, f, i) Show cultures stained with DAPI and disclose nuclei. (a–c) Untreated controls. Hyaluronan staining appears to be perinuclear. TGF-β1 induced hyaluronan staining and formation of microvillus-like projections. Streptomyces hyaluronidase-treated fibroblasts failed to exhibit hyaluronan staining, as in (g–i). Reprinted with permission from Guo N, Woeller CF, Feldon SE, Phipps RP. Peroxisome proliferator-activated receptor γ ligands inhibit transforming growth factor-β-induced, hyaluronan-dependent, T cell adhesion to orbital fibroblasts. J Biol Chem. 2011;286:18856–18867. Copyright 2011 The American Society for Biochemistry and Molecular Biology.
Crisp et al.134 examined the role of TSHR in the adipogenesis of orbital tissues and found that the receptor is expressed differently at several stages of orbital and nonorbital fat differentiation. Further, levels of TSHR become elevated in orbital fibroblasts undergoing adipogenesis. Supraphysiologic TSH concentrations stimulated TSHR expression in TAO orbital preadipocyte fibroblasts.134 In another study, PPARγ-expressing orbital fibroblasts underwent adipogenesis when co-cultured with activated T lymphocytes that produce PPARγ ligands. This activity could be attenuated by cyclooxygenase (COX) inhibitors.148 When Zhang et al.149 introduced TSHR harboring a gain-of-function mutation into orbital fibroblasts, cellular proliferation was slowed and the fibroblasts became refractory to PPARγ-induced adipogenesis. Other potentially important connections between TSHR and adipogenesis remain to be investigated thoroughly.
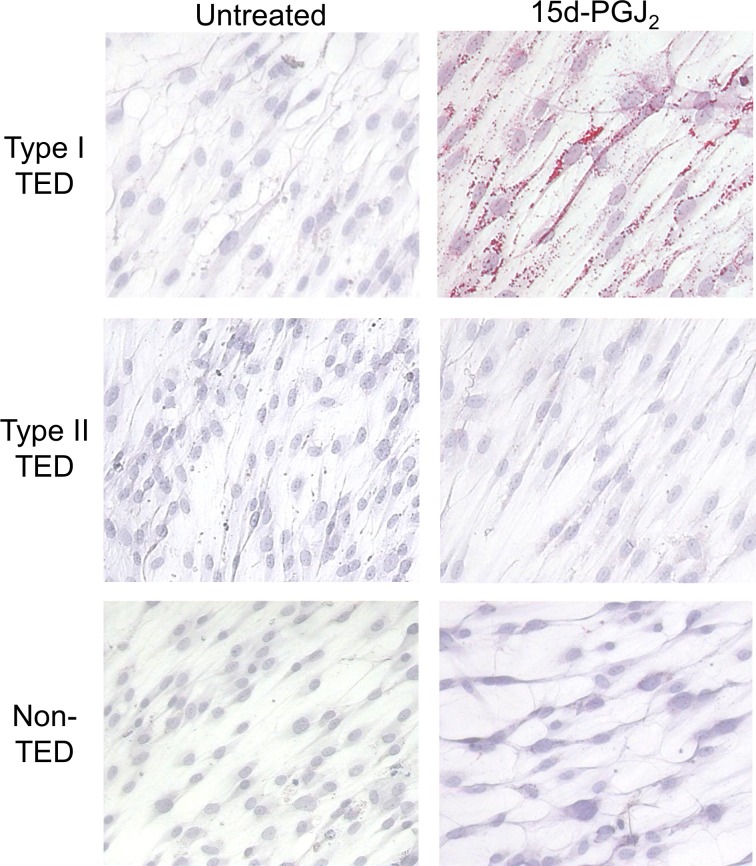
Individuals with TAO can be classified as manifesting either type I disease, which is characterized by expansion of adipose tissue, or type II, which is predominately extraocular muscle enlargement, or both.86 While ample evidence suggests the phenotypic divergence of orbital fibroblasts, Kuiryan et al.150 demonstrated that orbital fibroblasts from donors with type I TAO undergo adipogenesis more robustly than those from type II disease (Fig. 7). In contrast, type II fibroblasts exhibit a greater proliferative response to TGF-β. Therefore, it is possible that orbital fibroblast subtype determines clinical manifestation of TAO, as was suggested some time ago.102,139 Further, inhibition of PGHS-1 and PGHS-2 by indomethacin can attenuate 15-d-PGJ2 (a PPARγ ligand)-induced adipogenesis only in fibroblasts from type II donors.150 The mechanisms underlying this observation remain uncertain. Nonetheless, PGHS-2 inhibitors, such as celecoxib, may show promise in treating type II patients who prove unresponsive to corticosteroid treatment.151
Figure 7.
Treatment of orbital fibroblasts with 15d-PGJ2 from different subtypes of TAO. Orbital fibroblasts were grown in the presence of 5 μM 15d-PGJ2. Type I TAO orbital fibroblasts demonstrated more adipogenesis compared to type II or orbital fibroblasts from a healthy donor, as is evidenced by Oil Red O accumulation. TED, thyroid eye disease or TAO. Reprinted with permission from Kuriyan AE, Woeller CF, O'Loughlin CW, Phipps RP, Feldon SE. Orbital fibroblasts from thyroid eye disease patients differ in proliferative and adipogenic responses depending on disease subtype. Invest Ophthalmol Vis Sci. 2013;54:7370–7377. Copyright 2013 The Association for Research in Vision and Ophthalmology.
Thyroid Proteins in the Orbit? A Continuing Controversy
Detection of “thyroid-specific” proteins in the orbit was first reported by Konishi et al.,152 Kriss,153 and McDougall et al.,154 who detected Tg in tissues affected by TAO. This early report was followed by more recent work by Marino et al.,155 who also identified Tg in orbit and in TAO orbital fibroblasts. The investigators assumed its origin to be the thyroid.155 Fernando et al.156 subsequently reported finding Tg expression by human CD34+ fibrocytes and trace levels in TAO orbital fibroblasts. Their report suggested that fibrocytes express Tg as a consequence of substantial Tg gene promoter activity. This results in levels of Tg mRNA considerably below those found in thyroid tissue. Further, they found that the Tg was functional in that it could be iodinated in situ. Their studies suggest the potential for fibrocytes to generate iodothyronines, such as thyroid hormones. Further, they also raise the possibility that Tg might have some role as an orbital antigen.
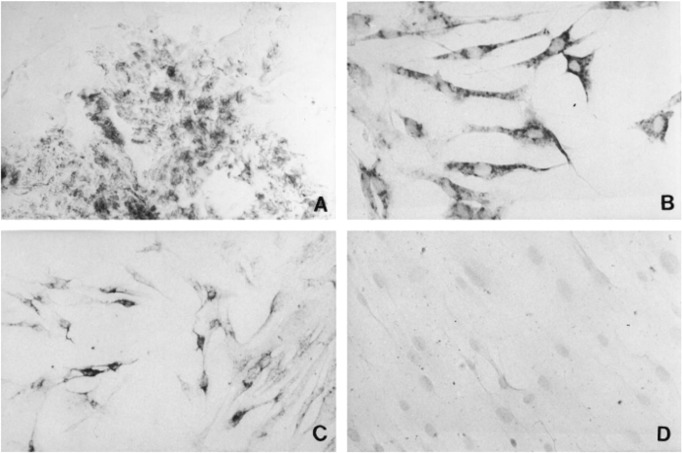
Mature TSHR mRNA was detected initially using PCR by Fenzi et al.72 in healthy orbital tissues and those affected by TAO. Their report soon was followed by that of Bahn et al.,157 who detected TSHR mRNA in orbital fibroblasts (Fig. 8). Subsequently, these investigators found even higher levels in fibroblasts from individuals with TAO, especially when the cells were incubated under culture conditions favoring adipogenic differentiation.157 Thus, orbital tissues and their derivative fibroblasts express at least two proteins that were believed previously to be restricted to the thyroid epithelium. Furthermore, considerably higher levels of Tg and TSHR were found in fibrocytes.156 Expression of these proteins in orbital fibroblasts localizes, albeit at considerably lower levels, to the CD34+ orbital fibroblasts, which are derived putatively from fibrocytes.156 This fibroblast subset is peculiar to cells derived from patients with TAO.156 Orbital fibroblasts from healthy donors are uniformly CD34−.74 It would appear that expression of Tg and TSHR is dampened as fibrocytes infiltrate the orbit and cross-talk with CD34− fibroblasts.156 The CD34− GD-orbital fibroblasts appear to downregulate Tg and TSHR expression.156 Taken together, we can conclude that circulating fibrocytes become more numerous in patients with GD and can traffic to the orbit where they participate in the ocular manifestations of the disease (Fig. 9).
Figure 8.
Immunohistochemical analysis of TSHR immunoreactivity on orbital connective tissue from a donor with TAO. The immunostaining was conducted with a monoclonal antibody directed against TSHR (amino acids 604-764). (A) Orbital connective tissue. (B) Passage one exhibits intense staining. (C) Passage three with reduced staining. (D) Passage 5 culture fails to show staining. Reprinted with permission from Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves' orbital adipose/connective tissues: potential autoantigen in Graves' ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. Copyright 1998 The Endocrine Society.
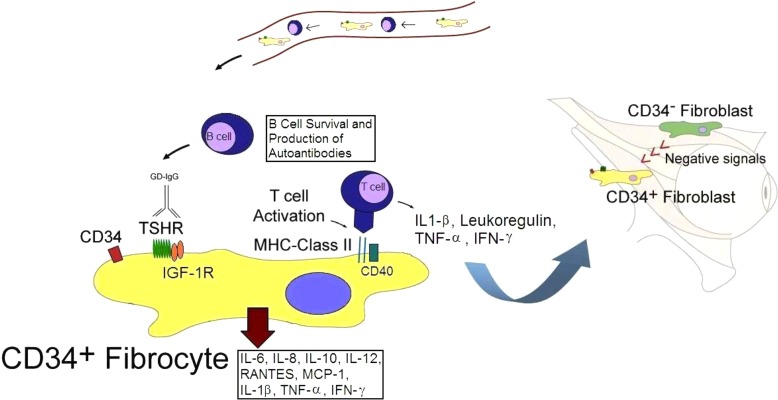
Figure 9.
Schematic illustrating the putative role of fibrocytes in the pathogenesis of TAO. CD34+ fibrocytes derive from the bone marrow and appear to be trafficked specifically to the orbit in TAO where they transition into CD34+ fibroblasts. Fibrocytes express relatively high levels of functional TSHR. Further, they can differentiate into either adipocytes or myofibroblasts in vitro. CD34+ orbital fibroblasts interact with the native residential CD34− orbital fibroblasts, resulting in dramatic reduction of expression of TSHR and other thyroid proteins. We postulate that the magnitude of this suppression may underlie susceptibility to TAO.
IGF-1R Pathway
Since Ingbar et al.158 first described the functional relationship between TSH and IGF-1 pathways, much evidence has evolved to reinforce that proposed connectivity. They demonstrated that IGF-1 promoted rat thyroid epithelial cell proliferation and enhanced the effect of TSH on DNA synthesis.158 Subsequently, substantial overlap between TSHR and insulin-like growth factor-1 receptor (IGF-1R) downstream signaling was reported. Both receptors extensively utilize the Akt/FRAP/mTOR/P70s6k pathway.159 Further, TSHR and IGF-1R form a functional and physical complex, suggesting a potential synergism that could promote abnormal signaling, such as that associated with GD.160 Monoclonal antibodies used to block IGF-1R signaling also attenuate that downstream signaling from TSHR, suggesting that IGF-1R may participate in physiological TSHR signaling.160
Although TSHR has been established as the central auto-antigen in GD, how it might participate in TAO remains less certain, as is the potential pathogenic involvement of other autoantigens. Insulin-like growth factor-1 influences several aspects of immunity, including thymic, B, and T cell development.161 Overexpression of IGF-1R has been demonstrated in autoimmune processes, such as those occurring in GD.98 The IGF-1 pathway was first implicated in GD when IgG from patients was found to displace radiolabeled IGF-1 from the surface of orbital fibroblasts.162
Anti–IGF-1R antibodies have been detected in sera from many individuals with GD, whereas they are absent in the vast majority of sera from healthy controls.123,124,163–167 At least a subset of these antibodies appear to activate IGF-1R and to initiate signaling that can be disrupted with a dominant negative IGF-1R, as well as with monoclonal anti–IGF-1R blocking antibodies.124 Moreover, IGF-1R levels are increased on TAO orbital fibroblasts compared to those from healthy tissues.124 When TAO orbital fibroblasts are treated with IGF-1 or IgG from patients, the cells produced hyaluronan165 and two powerful T-cell chemoattractants, namely IL-16 and RANTES.123,124 These actions are mediated through the Akt/FRAP/mTOR/P70s6k pathway.123 Furthermore, T cells and B cells from patients with GD also skew toward the IGF-1R+ phenotype.168,169 Display of IGF-1R may protect against Fas-mediated apoptosis in B cells and is associated with the production of anti-TSHR antibodies by these cells.169
Animal Models of TAO
Among the first animal models attempting to recapitulate GD experimentally was that created by Shimojo et al.170 These investigators immunized mice with human TSHR (hTSHR)-transfected fibroblasts also expressing MHC class II antigen.170 Hyperthyroidism was detected in 20% of the animals. Later, Costagliola et al.171 reported hyperthyroidism resulting from infection with an expression plasmid containing hTSHR cDNA. Nagayama et al.172 injected an adenoviral vector expressing hTSHR into mice. This strategy resulted in a greater proportion (30%–50%) of animals developing hyperthyroidism.172 When the free A-subunit of hTSHR was used for immunizations instead of the intact receptor, 65% to 80% of mice developed hyperthyroidism.70 This model has proven replicable and is widely used as an animal model for GD.173–176 More recent studies have combined TSHR plasmid injection with electroporation to enhance transfection efficacy.177 However, these earlier attempts at creating a complete model of GD, including the ocular features of TAO, were not completely successful.
In 2011, Zhao et al.178 attempted to induce hyperthyroidism and orbital pathology in mice by immunizing animals with plasmids encoding TSHR A and IGF-α1R. Deoxyribonucleic acid was delivered via skeletal muscle electroporation.178 Many mice developed hyperthyroidism and generated TSI. Surprisingly, animals immunized with plasmid harboring TSHR also developed antibodies directed against IGF-1Rα. Histopathologic examination of the orbits revealed fibrosis. The IGF-1R–immunized mice also developed a strong anti–IGF-1R antibody response, but failed to exhibit a phenotype resembling GD. This study suggested an association between IGF-1R and TSHR,160 although the basis of anti–IGF-1Rα antibody generation in TSHR A-immunized mice remains uncertain. Subsequently, Moshkelgosha et al.,179 using the same plasmid electroporation strategy, demonstrated extensive tissue infiltration and remodeling within the orbit. The animals exhibited signs of marked orbital congestion, such as edema and chemosis. The majority of immunized animals developed blocking anti-TSHR antibodies and manifested hypothyroidism. A feature of the ocular pathology found by this group was the dramatic infiltration of optic nerves, which is strikingly uncharacteristic of TAO. Unfortunately, no details concerning the status of intraocular tissues or the central nervous system following immunization were included in the report. Further, an explanation for the dramatically different hypothyroid phenotype and predominately blocking anti-TSHR antibody profile from this group's earlier report178 was not discussed in detail. Thus, greater definition of this model, including more careful and complete interrogation of the animals and their interesting phenotype, will be necessary before these findings can be evaluated critically. Nakahara et al.180 described successful induction of TSI in wild type mice that received splenocytes from TSHR-immunized TSHR-knockout mice. Although this study suggests a role for anti-TSHR immune response in the development of GD, a low percentage of mice (22%) were hyperthyroid. Some of these mice later became hypothyroid. Furthermore, orbital tissue from two of the nine recipient mice demonstrated modest macrophage infiltration without the presence of striking extraocular muscle or fat enlargement, or lymphocytic infiltration. Thus, while encouraging reports of preclinical mouse models for GD have appeared recently, there have been inconsistent results and a potentially confounding deviation from the human disease. Consequently, further study is required before the implications of these reports can be fully assessed.
Treatment Implications and Future Perspectives
Current medical therapy for active moderate to severe TAO is limited to corticosteroids and external beam radiotherapy.4 Surgical remediation usually awaits transition from active disease to the stable, chronic phase. This typically occurs over a course of a 36- to 48-month horizon.4 Unfortunately, none of these therapeutic approaches appears to alter the natural course of TAO, making development of new therapies critical to addressing an important unmet need. Thyroid-associated ophthalmopathy is a complex autoimmune condition that only now is being clarified. Greater definition of the molecular and immunological underpinnings of this condition should facilitate the process of therapy development. In addition, better animal models should allow critical preclinical testing of candidate therapies. Potential immunotherapies based on our current understanding of GD and TAO include depleting T cells with anti-CD3 antibodies or targeting CTLA-4, a regulator of T lymphocyte activation.181–184 Monoclonal antibodies against B cell surface antigen CD20, such as Rituximab, have demonstrated promising results in decreasing orbital inflammation in patients with TAO.185–187 However, the preliminary findings from the two recently completed controlled prospective studies of Rituximab suggest that its effectiveness may not be uniform.188,189 Alternative anti-B cell therapy might focus on anti-CD19, which would target plasmablasts and might provide a more complete response.169 Anti-cytokine therapy, such as Etanercept and Infliximab, has been associated with anecdotal improvement in a very limited cohort of patients with TAO.190–192 Controlled drug trials for these and related agents will be necessary before any conclusions can be drawn about their efficacy and safety in TAO. Anti-TSHR and anti-IGF-1R therapy also may prove to be effective. A trial of the latter strategy utilizing Teprotumumab as an IGF-1R blocking strategy currently is underway [available in the public domain at http://clinicaltrials.gov/show/NCT01868997].
Acknowledgments
Supported in part by National Institutes of Health Grants EY008976, EY011708, DK063121, Core Center for Vision Research through EY007003 from the National Eye Institute (NEI), and continued support from the Bell Charitable Foundation and Research to Prevent Blindness. TJS holds US patents 6936426, 7998681, 8153121, and 8178304.
Disclosure: Y. Wang, None; T.J. Smith, River Vision (C), P
References
- 1. Werner SC, Ingbar SH, Braverman LE, Utiger RD. Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text. 7th ed. Philadelphia, PA: Lippincott-Raven; 1996: 1124 [Google Scholar]
- 2. Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010; 362: 726–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stan MN, Garrity JA, Bahn RS. The evaluation and treatment of graves ophthalmopathy. Med Clin North Am. 2012; 96: 311–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Douglas RS, Gupta S. The pathophysiology of thyroid eye disease: implications for immunotherapy. Curr Opin Ophthalmol. 2011; 22: 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brix TH, Christensen K, Holm NV, Harvald B, Hegedus L. A population-based study of Graves' disease in Danish twins. Clin Endocrinol. 1998; 48: 397–400 [DOI] [PubMed] [Google Scholar]
- 6. Stan MN, Bahn RS. Risk factors for development or deterioration of Graves' ophthalmopathy. Thyroid. 2010; 20: 777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves' disease and ophthalmopathy. Endocr Rev. 2003; 24: 802–835 [DOI] [PubMed] [Google Scholar]
- 8. Kendler DL, Lippa J, Rootman J. The initial clinical characteristics of Graves' orbitopathy vary with age and sex. Arch Ophthalmol. 1993; 111: 197–201 [DOI] [PubMed] [Google Scholar]
- 9. Lim SL, Lim AK, Mumtaz M, Hussein E, Wan Bebakar WM, Khir AS. Prevalence, risk factors, and clinical features of thyroid-associated ophthalmopathy in multiethnic Malaysian patients with Graves' disease. Thyroid. 2008; 18: 1297–1301 [DOI] [PubMed] [Google Scholar]
- 10. Tellez M, Cooper J, Edmonds C. Graves' ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocrinol. 1992; 36: 291–294 [DOI] [PubMed] [Google Scholar]
- 11. Bartels ED, Aagesen E. Heredity in Graves' disease. Copenhagen, Denmark: E. Munksgaard; 1941: 384 [Google Scholar]
- 12. Ardley M, McCorquodale T, Lahooti H, Champion B, Wall JR. Eye findings and immunological markers in probands and their euthyroid relatives from a single family with multiple cases of thyroid autoimmunity. Thyroid Res. 2012; 5: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves' disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001; 86: 930–934 [DOI] [PubMed] [Google Scholar]
- 14. Farid NR, Stone E, Johnson G. Graves' disease and HLA: clinical and epidemiologic associations. Clin Endocrinol. 1980; 13: 535–544 [DOI] [PubMed] [Google Scholar]
- 15. Zamani M, Spaepen M, Bex M, Bouillon R, Cassiman JJ. Primary role of the HLA class II DRB1*0301 allele in Graves disease. Am J Med Genet. 2000; 95: 432–437 [DOI] [PubMed] [Google Scholar]
- 16. Yanagawa T, Hidaka Y, Guimaraes V, Soliman M, DeGroot LJ. CTLA-4 gene polymorphism associated with Graves' disease in a Caucasian population. J Clin Endocrinol Metab. 1995; 80: 41–45 [DOI] [PubMed] [Google Scholar]
- 17. Zhang J, Zahir N, Jiang Q, et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011; 43: 902–907 [DOI] [PubMed] [Google Scholar]
- 18. Tomer Y, Concepcion E, Greenberg DAA. A C/T single-nucleotide polymorphism in the region of the CD40 gene is associated with Graves' disease. Thyroid. 2002; 12: 1129–1135 [DOI] [PubMed] [Google Scholar]
- 19. Brand OJ, Lowe CE, Heward JM, et al. Association of the interleukin-2 receptor alpha (IL-2Ralpha)/CD25 gene region with Graves' disease using a multilocus test and tag SNPs. Clin Endocrinol. 2007; 66: 508–512 [DOI] [PubMed] [Google Scholar]
- 20. Gu LQ, Zhu W, Zhao SX, et al. Clinical associations of the genetic variants of CTLA-4, Tg, TSHR, PTPN22, PTPN12 and FCRL3 in patients with Graves' disease. Clin Endocrinol. 2010; 72: 248–255 [DOI] [PubMed] [Google Scholar]
- 21. Huber AK, Jacobson EM, Jazdzewski K, Concepcion ES, Tomer Y. Interleukin (IL)-23 receptor is a major susceptibility gene for Graves' ophthalmopathy: the IL-23/T-helper 17 axis extends to thyroid autoimmunity. J Clin Endocrinol Metab. 2008; 93: 1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hiratani H, Bowden DW, Ikegami S, et al. Multiple SNPs in intron 7 of thyrotropin receptor are associated with Graves' disease. J Clin Endocrinol Metab. 2005; 90: 2898–2903 [DOI] [PubMed] [Google Scholar]
- 23. Tomer Y, Greenberg DA, Concepcion E, Ban Y, Davies TF. Thyroglobulin is a thyroid specific gene for the familial autoimmune thyroid diseases. J Clin Endocrinol Metab. 2002; 87: 404–407 [DOI] [PubMed] [Google Scholar]
- 24. Zhernakova A, Alizadeh BZ, Bevova M, et al. Novel association in chromosome 4q27 region with rheumatoid arthritis and confirmation of type 1 diabetes point to a general risk locus for autoimmune diseases. Am J Hum Genet. 2007; 81: 1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hinks A, Eyre S, Ke X, et al. Association of the AFF3 gene and IL2/IL21 gene region with juvenile idiopathic arthritis. Genes Immun. 2010; 11: 194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Y, Helms C, Liao W, et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008; 4: e1000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adamovic S, Amundsen SS, Lie BA, et al. Association study of IL2/IL21 and FcgRIIa: significant association with the IL2/IL21 region in Scandinavian coeliac disease families. Genes Immun. 2008; 9: 364–367 [DOI] [PubMed] [Google Scholar]
- 28. Garner CP, Murray JA, Ding YC, Tien Z, van Heel DA, Neuhausen SL. Replication of celiac disease UK genome-wide association study results in a US population. Hum Mol Genet. 2009; 18: 4219–4225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Festen EA, Goyette P, Scott R, et al. Genetic variants in the region harbouring IL2/IL21 associated with ulcerative colitis. Gut. 2009; 58: 799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fedetz M, Ndagire D, Fernandez O, et al. Multiple sclerosis association study with the TENR-IL2-IL21 region in a Spanish population. Tissue Antigens. 2009; 74: 244–247 [DOI] [PubMed] [Google Scholar]
- 31. Brand OJ, Gough SC. Genetics of thyroid autoimmunity and the role of the TSHR. Mol Cell Endocrinol. 2010; 322: 135–143 [DOI] [PubMed] [Google Scholar]
- 32. Tomer Y, Hasham A, Davies TF, et al. Fine mapping of loci linked to autoimmune thyroid disease identifies novel susceptibility genes. J Clin Endocrinol Metab. 2013; 98: E144–E152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jia HY, Zhang ZG, Gu XJ, et al. Association between interleukin 21 and Graves' disease. Genet Mol Res. 2011; 10: 3338–3346 [DOI] [PubMed] [Google Scholar]
- 34. Zhang J, Xiao XW, Zhu FY, et al. Polymorphisms of interleukin-21 and interleukin-21-receptor genes confer risk for autoimmune thyroid diseases. BMC Endocr Disord. 2013; 13: 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Okada Y, Terao C, Ikari K, et al. Meta-analysis identifies nine new loci associated with rheumatoid arthritis in the Japanese population. Nat Genet. 2012; 44: 511–516 [DOI] [PubMed] [Google Scholar]
- 36. Whitson RH, Tsark W, Huang TH, Itakura K. Neonatal mortality and leanness in mice lacking the ARID transcription factor Mrf-2. Biochem Biophys Res Comm. 2003; 312: 997–1004 [DOI] [PubMed] [Google Scholar]
- 37. Heard-Costa NL, Zillikens MC, Monda KL, et al. NRXN3 is a novel locus for waist circumference: a genome-wide association study from the CHARGE Consortium. PLoS Genet. 2009; 5: e1000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yin X, Latif R, Bahn R, Davies TF. Genetic profiling in Graves' disease: further evidence for lack of a distinct genetic contribution to Graves' ophthalmopathy. Thyroid. 2012; 22: 730–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yin X, Latif R, Tomer Y, Davies TF. Thyroid epigenetics: X chromosome inactivation in patients with autoimmune thyroid disease. Ann N Y Acad Sci. 2007; 1110: 193–200 [DOI] [PubMed] [Google Scholar]
- 40. Chitnis S, Monteiro J, Glass D, et al. The role of X-chromosome inactivation in female predisposition to autoimmunity. Arthritis Res. 2000; 2: 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stefan M, Jacobson EM, Huber AK, et al. Novel variant of thyroglobulin promoter triggers thyroid autoimmunity through an epigenetic interferon alpha-modulated mechanism. J Biol Chem. 2011; 286: 31168–31179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Notkins AL. Molecular biology and viral pathogenesis: clinical spinoff. N Engl J Med. 1985; 312: 507–509 [DOI] [PubMed] [Google Scholar]
- 43. Fujinami RS, Oldstone MB. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985; 230: 1043–1045 [DOI] [PubMed] [Google Scholar]
- 44. Lahesmaa R, Skurnik M, Granfors K, et al. Molecular mimicry in the pathogenesis of spondyloarthropathies. A critical appraisal of cross-reactivity between microbial antigens and HLA-B27. Br J Rheumatol. 1992; 31: 221–229 [DOI] [PubMed] [Google Scholar]
- 45. Saegusa J, Prabhakar BS, Essani K, et al. Monoclonal antibody to coxsackievirus B4 reacts with myocardium. J Infect Dis. 1986; 153: 372–373 [DOI] [PubMed] [Google Scholar]
- 46. Lagaye S, Vexiau P, Morozov V, et al. Human spumaretrovirus-related sequences in the DNA of leukocytes from patients with Graves disease. Proc Natl Acad Sci U S A. 1992; 89: 10070–10074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schweizer M, Turek R, Reinhardt M, Neumann-Haefelin D. Absence of foamy virus DNA in Graves' disease. AIDS Res Hum Retroviruses. 1994; 10: 601–605 [DOI] [PubMed] [Google Scholar]
- 48. Yanagawa T, Ito K, Kaplan EL, Ishikawa N, DeGroot LJ. Absence of association between human spumaretrovirus and Graves' disease. Thyroid. 1995; 5: 379–382 [DOI] [PubMed] [Google Scholar]
- 49. Wick G, Trieb K, Aguzzi A, Recheis H, Anderl H, Grubeck-Loebenstein B. Possible role of human foamy virus in Graves' disease. Intervirology. 1993; 35: 101–107 [DOI] [PubMed] [Google Scholar]
- 50. Luo G, Fan JL, Seetharamaiah GS, et al. Immunization of mice with Yersinia enterocolitica leads to the induction of antithyrotropin receptor antibodies. J Immunol. 1993; 151: 922–928 [PubMed] [Google Scholar]
- 51. Luo G, Seetharamaiah GS, Niesel DW, et al. Purification and characterization of Yersinia enterocolitica envelope proteins which induce antibodies that react with human thyrotropin receptor. J Immunol. 1994; 152: 2555–2561 [PubMed] [Google Scholar]
- 52. Shenkman L, Bottone EJ. Antibodies to Yersinia enterocolitica in thyroid disease. Ann Intern Med. 1976; 85: 735–739 [DOI] [PubMed] [Google Scholar]
- 53. Weiss M, Rubinstein E, Bottone EJ, Shenkman L, Bank H. Yersinia enterocolitica antibodies in thyroid disorders. Isr J Medical Sci. 1979; 15: 553–555 [PubMed] [Google Scholar]
- 54. Heyma P, Harrison LC, Robins-Browne R. Thyrotrophin (TSH) binding sites on Yersinia enterocolitica recognized by immunoglobulins from humans with Graves' disease. Clin Exp Immunol. 1986; 64: 249–254 [PMC free article] [PubMed] [Google Scholar]
- 55. Weiss M, Ingbar SH, Winblad S, Kasper DL. Demonstration of a saturable binding site for thyrotropin in Yersinia enterocolitica. Science. 1983; 219: 1331–1333 [DOI] [PubMed] [Google Scholar]
- 56. Wenzel BE, Heesemann J, Wenzel KW, Scriba PC. Antibodies to plasmid-encoded proteins of enteropathogenic Yersinia in patients with autoimmune thyroid disease. Lancet. 1988; 1: 56 [DOI] [PubMed] [Google Scholar]
- 57. Wang Z, Zhang Q, Lu J, et al. Identification of outer membrane porin f protein of Yersinia enterocolitica recognized by antithyrotopin receptor antibodies in Graves' disease and determination of its epitope using mass spectrometry and bioinformatics tools. J Clin Endocrinol Metab. 2010; 95: 4012–4020 [DOI] [PubMed] [Google Scholar]
- 58. Hargreaves CE, Grasso M, Hampe CS, et al. Yersinia enterocolitica provides the link between thyroid-stimulating antibodies and their germline counterparts in Graves' disease. J Immunol. 2013; 190: 5373–5381 [DOI] [PubMed] [Google Scholar]
- 59. Bartalena L, Martino E, Marcocci C, et al. More on smoking habits and Graves' ophthalmopathy. J Endocrinol Invest. 1989; 12: 733–737 [DOI] [PubMed] [Google Scholar]
- 60. Prummel MF, Wiersinga WM. Smoking and risk of Graves' disease. JAMA. 1993; 269: 479–482 [PubMed] [Google Scholar]
- 61. Tallstedt L, Lundell G, Taube A. Graves' ophthalmopathy and tobacco smoking. Acta Endocrinol. 1993; 129: 147–150 [DOI] [PubMed] [Google Scholar]
- 62. Calkins BM. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci. 1989; 34: 1841–1854 [DOI] [PubMed] [Google Scholar]
- 63. Silman AJ. Smoking and the risk of rheumatoid arthritis. J Rheumatol. 1993; 20: 1815–1816 [PubMed] [Google Scholar]
- 64. Hagg E, Asplund K. Is endocrine ophthalmopathy related to smoking? BMJ. 1987; 295: 634–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pfeilschifter J, Ziegler R. Smoking and endocrine ophthalmopathy: impact of smoking severity and current vs lifetime cigarette consumption. Clin Endocrinol. 1996; 45: 477–481 [DOI] [PubMed] [Google Scholar]
- 66. Brix TH, Hansen PS, Kyvik KO, Hegedus L. Cigarette smoking and risk of clinically overt thyroid disease: a population-based twin case-control study. Arch Intern Med. 2000; 160: 661–666 [DOI] [PubMed] [Google Scholar]
- 67. Vestergaard P. Smoking and thyroid disorders--a meta-analysis. Eur J Endocrinol. 2002; 146: 153–161 [DOI] [PubMed] [Google Scholar]
- 68. Bartalena L, Marcocci C, Tanda ML, et al. Cigarette smoking and treatment outcomes in Graves ophthalmopathy. Ann Intern Med. 1998; 129: 632–635 [DOI] [PubMed] [Google Scholar]
- 69. Iyer S, Bahn R. Immunopathogenesis of Graves' ophthalmopathy: the role of the TSH receptor. Clin Endocrinol Metab. 2012; 26: 281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM. The thyrotropin receptor autoantigen in Graves disease is the culprit as well as the victim. J Clin Invest. 2003; 111: 1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Khoo DH, Eng PH, Ho SC, et al. Graves' ophthalmopathy in the absence of elevated free thyroxine and triiodothyronine levels: prevalence, natural history, and thyrotropin receptor antibody levels. Thyroid. 2000; 10: 1093–1100 [DOI] [PubMed] [Google Scholar]
- 72. Feliciello A, Porcellini A, Ciullo I, Bonavolonta G, Avvedimento EV, Fenzi G. Expression of thyrotropin-receptor mRNA in healthy and Graves' disease retro-orbital tissue. Lancet. 1993; 342: 337–338 [DOI] [PubMed] [Google Scholar]
- 73. Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves' ophthalmopathy and pretibial dermopathy. Thyroid. 1993; 3: 297–300 [DOI] [PubMed] [Google Scholar]
- 74. Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010; 95: 430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen B, Tsui S, Smith TJ. IL-1 beta induces IL-6 expression in human orbital fibroblasts: identification of an anatomic-site specific phenotypic attribute relevant to thyroid-associated ophthalmopathy. J Immunol. 2005; 175: 1310–1319 [DOI] [PubMed] [Google Scholar]
- 76. Forster G, Otto E, Hansen C, Ochs K, Kahaly G. Analysis of orbital T cells in thyroid-associated ophthalmopathy. Clin Exp Immunol. 1998; 112: 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tezuka H, Eguchi K, Fukuda T, et al. Natural killer and natural killer-like cell activity of peripheral blood and intrathyroidal mononuclear cells from patients with Graves' disease. J Clin Endocrinol Metab. 1988; 66: 702–707 [DOI] [PubMed] [Google Scholar]
- 78. Totterman TH, Andersson LC, Hayry P. Evidence for thyroid antigen-reactive T lymphocytes infiltrating the thyroid gland in Graves' disease. Clin Endocrinol. 1979; 11: 59–68 [DOI] [PubMed] [Google Scholar]
- 79. Pappa A, Lawson JM, Calder V, Fells P, Lightman S. T cells and fibroblasts in affected extraocular muscles in early and late thyroid associated ophthalmopathy. Br J Ophthalmol. 2000; 84: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aniszewski JP, Valyasevi RW, Bahn RS. Relationship between disease duration and predominant orbital T cell subset in Graves' ophthalmopathy. J Clin Endocrinol Metab. 2000; 85: 776–780 [DOI] [PubMed] [Google Scholar]
- 81. Reiner SL. Development in motion: helper T cells at work. Cell. 2007; 129: 33–36 [DOI] [PubMed] [Google Scholar]
- 82. Nanba T, Watanabe M, Inoue N, Iwatani Y. Increases of the Th1/Th2 cell ratio in severe Hashimoto's disease and in the proportion of Th17 cells in intractable Graves' disease. Thyroid. 2009; 19: 495–501 [DOI] [PubMed] [Google Scholar]
- 83. Peng D, Xu B, Wang Y, Guo H, Jiang Y. A high frequency of circulating th22 and th17 cells in patients with new onset graves' disease. PLoS One. 2013; 8: e68446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Smith TJ. Insights into the role of fibroblasts in human autoimmune diseases. Clin Exp Immunol. 2005; 141: 388–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Heufelder AE, Bahn RS. Detection and localization of cytokine immunoreactivity in retro-ocular connective tissue in Graves' ophthalmopathy. Eur J Clin Invest. 1993; 23: 10–17 [DOI] [PubMed] [Google Scholar]
- 86. Hiromatsu Y, Yang D, Bednarczuk T, Miyake I, Nonaka K, Inoue Y. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2000; 85: 1194–1199 [DOI] [PubMed] [Google Scholar]
- 87. Prummel MF, Wiersinga WM, Van der Gaag R, Mourits MP, Koornneef L. Soluble IL-2 receptor levels in patients with Graves' ophthalmopathy. Clin Exp Immunol. 1992; 88: 405–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Salvi M, Girasole G, Pedrazzoni M, et al. Increased serum concentrations of interleukin-6 (IL-6) and soluble IL-6 receptor in patients with Graves' disease. J Clin Endocrinol Metab. 1996; 81: 2976–2979 [DOI] [PubMed] [Google Scholar]
- 89. Molnar I, Balazs C. High circulating IL-6 level in Graves' ophthalmopathy. Autoimmunity. 1997; 25: 91–96 [DOI] [PubMed] [Google Scholar]
- 90. Wakelkamp IM, Gerding MN, Van Der Meer JW, Prummel MF, Wiersinga WM. Both Th1- and Th2-derived cytokines in serum are elevated in Graves' ophthalmopathy. Clin Exp Immunol. 2000; 121: 453–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kim SE, Yoon JS, Kim KH, Lee SY. Increased serum interleukin-17 in Graves' ophthalmopathy. Clin Exp Immunol. 2012; 250: 1521–1526 [DOI] [PubMed] [Google Scholar]
- 92. Busuttil BE, Frauman AG. Extrathyroidal manifestations of Graves' disease: the thyrotropin receptor is expressed in extraocular, but not cardiac, muscle tissues. J Clin Endocrinol Metab. 2001; 86: 2315–2319 [DOI] [PubMed] [Google Scholar]
- 93. Otto EA, Ochs K, Hansen C, Wall JR, Kahaly GJ. Orbital tissue-derived T lymphocytes from patients with Graves' ophthalmopathy recognize autologous orbital antigens. J Clin Endocrinol Metab. 1996; 81: 3045–3050 [DOI] [PubMed] [Google Scholar]
- 94. Wall JR, Bernard N, Boucher A, et al. Pathogenesis of thyroid-associated ophthalmopathy: an autoimmune disorder of the eye muscle associated with Graves' hyperthyroidism and Hashimoto's thyroiditis. Clin Immunol Immunopathol. 1993; 68: 1–8 [DOI] [PubMed] [Google Scholar]
- 95. Hiromatsu Y, Sato M, Inoue Y, et al. Localization and clinical significance of thyrotropin receptor mRNA expression in orbital fat and eye muscle tissues from patients with thyroid-associated ophthalmopathy. Thyroid. 1996; 6: 553–562 [DOI] [PubMed] [Google Scholar]
- 96. Kloprogge SJ, Busuttil BE, Frauman AG. TSH receptor protein is selectively expressed in normal human extraocular muscle. Muscle Nerve. 2005; 32: 95–98 [DOI] [PubMed] [Google Scholar]
- 97. Wall JR, Stachura I, Kennerdell JH. Mitochondrial abnormalities in eye muscle fiber from three cases of thyroid-associated ophthalmopathy. Thyroid. 2006; 16: 1181–1183 [DOI] [PubMed] [Google Scholar]
- 98. Naik VM, Naik MN, Goldberg RA, Smith TJ, Douglas RS. Immunopathogenesis of thyroid eye disease: emerging paradigms. Surv Ophthalmol. 2010; 55: 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Smith TJ. Orbital fibroblasts exhibit a novel pattern of responses to proinflammatory cytokines: potential basis for the pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2002; 12: 197–203 [DOI] [PubMed] [Google Scholar]
- 100. Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997; 151: 317–322 [PMC free article] [PubMed] [Google Scholar]
- 101. Smith TJ, Bahn RS, Gorman CA. Hormonal regulation of hyaluronate synthesis in cultured human fibroblasts: evidence for differences between retroocular and dermal fibroblasts. J Clin Endocrinol Metab. 1989; 69: 1019–1023 [DOI] [PubMed] [Google Scholar]
- 102. Smith TJ, Sempowski GD, Wang HS, Del Vecchio PJ, Lippe SD, Phipps RP. Evidence for cellular heterogeneity in primary cultures of human orbital fibroblasts. J Clin Endocrinol Metab. 1995; 80: 2620–2625 [DOI] [PubMed] [Google Scholar]
- 103. Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003; 163: 1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1- subpopulations exhibit distinct phenotypes. Eur J Immunol. 2002; 32: 477–485 [DOI] [PubMed] [Google Scholar]
- 105. Smith TJ, Padovani-Claudio DA, Lu Y, et al. Fibroblasts expressing the thyrotropin receptor overarch thyroid and orbit in Graves' disease. J Clin Endocrinol Metab. 2011; 96: 3827–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps RP, Smith TJ. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998; 273: 29615–29625 [DOI] [PubMed] [Google Scholar]
- 107. Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-1beta in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J Biol Chem. 2002; 277: 16355–16364 [DOI] [PubMed] [Google Scholar]
- 108. Wang HS, Cao HJ, Winn VD, et al. Leukoregulin induction of prostaglandin-endoperoxide H synthase-2 in human orbital fibroblasts. An in vitro model for connective tissue inflammation. J Biol Chem. 1996; 271: 22718–22728 [PubMed] [Google Scholar]
- 109. Cao HJ, Han R, Smith TJ. Robust induction of PGHS-2 by IL-1 in orbital fibroblasts results from low levels of IL-1 receptor antagonist expression. Am J Physiol Cell Physiol. 2003; 284: C1429–C1437 [DOI] [PubMed] [Google Scholar]
- 110. Li B, Smith TJ. Divergent expression of IL-1 receptor antagonists in CD34(+) fibrocytes and orbital fibroblasts in thyroid-associated ophthalmopathy: contribution of fibrocytes to orbital inflammation. J Clin Endocrinol Metab. 2013; 98: 2783–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Smith TJ, Wang HS, Hogg MG, Henrikson RC, Keese CR, Giaever I. Prostaglandin E2 elicits a morphological change in cultured orbital fibroblasts from patients with Graves ophthalmopathy. Proc Natl Acad Sci U S A. 1994; 91: 5094–5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang HS, Keese CR, Giaever I, Smith TJ. Prostaglandin E2 alters human orbital fibroblast shape through a mechanism involving the generation of cyclic adenosine monophosphate. J Clin Endocrinol Metab. 1995; 80: 3553–3560 [DOI] [PubMed] [Google Scholar]
- 113. Brown DM, Warner GL, Ales-Martinez JE, Scott DW, Phipps RP. Prostaglandin E2 induces apoptosis in immature normal and malignant B lymphocytes. Clin Immunol Immunopathol. 1992; 63: 221–229 [DOI] [PubMed] [Google Scholar]
- 114. Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991; 146: 108–113 [PubMed] [Google Scholar]
- 115. Hu ZQ, Asano K, Seki H, Shimamura T. An essential role of prostaglandin E on mouse mast cell induction. J Immunol. 1995; 155: 2134–2142 [PubMed] [Google Scholar]
- 116. Hwang CJ, Afifiyan N, Sand D, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci. 2009; 50: 2262–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Smith TJ. The putative role of prostaglandin endoperoxide H synthase-2 in the pathogenesis of thyroid-associated orbitopathy. Exp Clin Endocrinol Diabetes. 1999; 107 (suppl 5): S160–S163 [DOI] [PubMed] [Google Scholar]
- 118. Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998; 16: 249–284 [DOI] [PubMed] [Google Scholar]
- 119. Diehl S, Chow CW, Weiss L, et al. Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. J Exp Med. 2002; 196: 39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chen MH, Chen MH, Liao SL, Chang TC, Chuang LM. Role of macrophage infiltration in the orbital fat of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf). 2008; 69: 332–337 [DOI] [PubMed] [Google Scholar]
- 121. Gianoukakis AG, Douglas RS, King CS, Cruikshank WW, Smith TJ. Immunoglobulin G from patients with Graves' disease induces interleukin-16 and RANTES expression in cultured human thyrocytes: a putative mechanism for T-cell infiltration of the thyroid in autoimmune disease. Endocrinology. 2006; 147: 1941–1949 [DOI] [PubMed] [Google Scholar]
- 122. Sciaky D, Brazer W, Center DM, Cruikshank WW, Smith TJ. Cultured human fibroblasts express constitutive IL-16 mRNA: cytokine induction of active IL-16 protein synthesis through a caspase-3-dependent mechanism. J Immunol. 2000; 164: 3806–3814 [DOI] [PubMed] [Google Scholar]
- 123. Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves' disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002; 168: 942–950 [DOI] [PubMed] [Google Scholar]
- 124. Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003; 170: 6348–6354 [DOI] [PubMed] [Google Scholar]
- 125. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994; 1: 71–81 [PMC free article] [PubMed] [Google Scholar]
- 126. Chesney J, Bucala R. Peripheral blood fibrocytes: mesenchymal precursor cells and the pathogenesis of fibrosis. Curr Rheumatol Rep. 2000; 2: 501–505 [DOI] [PubMed] [Google Scholar]
- 127. Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007; 82: 449–456 [DOI] [PubMed] [Google Scholar]
- 128. Sakai N, Wada T, Matsushima K, et al. The renin-angiotensin system contributes to renal fibrosis through regulation of fibrocytes. J Hypertens. 2008; 26: 780–790 [DOI] [PubMed] [Google Scholar]
- 129. Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007; 117: 549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Smith TJ, Hegedus L, Douglas RS. Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves' orbitopathy. J Clin Endo Metab. 2012; 26: 291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Smith TJ. Potential role for bone marrow-derived fibrocytes in the orbital fibroblast heterogeneity associated with thyroid-associated ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2010; 162: 24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gillespie EF, Papageorgiou KI, Fernando R, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012; 97: E740–E746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007; 282: 22910–22920 [DOI] [PubMed] [Google Scholar]
- 134. Crisp M, Starkey KJ, Lane C, Ham J, Ludgate M. Adipogenesis in thyroid eye disease. Invest Ophthalmol Vis Sci. 2000; 41: 3249–3255 [PubMed] [Google Scholar]
- 135. Hansen C, Fraiture B, Rouhi R, Otto E, Forster G, Kahaly G. HPLC glycosaminoglycan analysis in patients with Graves' disease. Clin Sci. 1997; 92: 511–517 [DOI] [PubMed] [Google Scholar]
- 136. Sorisky A, Pardasani D, Gagnon A, Smith TJ. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab. 1996; 81: 3428–3431 [DOI] [PubMed] [Google Scholar]
- 137. Gabbiani G. The myofibroblast: a key cell for wound healing and fibrocontractive diseases. Prog Clin Biol Res. 1981; 54: 183–194 [PubMed] [Google Scholar]
- 138. Kumar S, Coenen MJ, Scherer PE, Bahn RS. Evidence for enhanced adipogenesis in the orbits of patients with Graves' ophthalmopathy. J Clin Endocrinol Metab. 2004; 89: 930–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002; 87: 385–392 [DOI] [PubMed] [Google Scholar]
- 140. Smith TJ, Bahn RS, Gorman CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev. 1989; 10: 366–391 [DOI] [PubMed] [Google Scholar]
- 141. Spicer AP, Kaback LA, Smith TJ, Seldin MF. Molecular cloning and characterization of the human and mouse UDP-glucose dehydrogenase genes. J Biol Chem. 1998; 273: 25117–25124 [DOI] [PubMed] [Google Scholar]
- 142. Smith TJ, Bahn RS, Gorman CA, Cheavens M. Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991; 72: 1169–1171 [DOI] [PubMed] [Google Scholar]
- 143. Burgess HA, Daugherty LE, Thatcher TH, et al. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005; 288: L1146–L1153 [DOI] [PubMed] [Google Scholar]
- 144. Guo B, Koya D, Isono M, Sugimoto T, Kashiwagi A, Haneda M. Peroxisome proliferator-activated receptor-gamma ligands inhibit TGF-beta 1-induced fibronectin expression in glomerular mesangial cells. Diabetes. 2004; 53: 200–208 [DOI] [PubMed] [Google Scholar]
- 145. Yamanaka O, Miyazaki K, Kitano A, Saika S, Nakajima Y, Ikeda K. Suppression of injury-induced conjunctiva scarring by peroxisome proliferator-activated receptor gamma gene transfer in mice. Invest Ophthalmol Vis Sci. 2009; 50: 187–193 [DOI] [PubMed] [Google Scholar]
- 146. Guo N, Woeller CF, Feldon SE, Phipps RP. Peroxisome proliferator-activated receptor gamma ligands inhibit transforming growth factor-beta-induced, hyaluronan-dependent, T cell adhesion to orbital fibroblasts. J Biol Chem. 2011; 286: 18856–18867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Guo N, Baglole CJ, O'Loughlin CW, Feldon SE, Phipps RP. Mast cell-derived prostaglandin D2 controls hyaluronan synthesis in human orbital fibroblasts via DP1 activation: implications for thyroid eye disease. J Biol Chem. 2010; 285: 15794–15804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Feldon SE, O'Loughlin CW, Ray DM, Landskroner-Eiger S, Seweryniak KE, Phipps RP. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol. 2006; 169: 1183–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhang L, Baker G, Janus D, Paddon CA, Fuhrer D, Ludgate M. Biological effects of thyrotropin receptor activation on human orbital preadipocytes. Invest Ophthalmol Vis Sci. 2006; 47: 5197–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kuriyan AE, Woeller CF, O'Loughlin CW, Phipps RP, Feldon SE. Orbital fibroblasts from thyroid eye disease patients differ in proliferative and adipogenic responses depending on disease subtype. Invest Ophthalmol Vis Sci. 2013; 54: 7370–7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kuriyan AE, Phipps RP, O'Loughlin CW, Feldon SE. Improvement of thyroid eye disease following treatment with the cyclooxygenase-2 selective inhibitor celecoxib. Thyroid. 2008; 18: 911–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Konishi J, Herman MM, Kriss JP. Binding of thyroglobulin and thyroglobulin-antithyroglobulin immune complex to extraocular muscle membrane. Endocrinology. 1974; 95: 434–446 [DOI] [PubMed] [Google Scholar]
- 153. Kriss JP. Radioisotopic thyroidolymphography in patients with Graves' disease. J Clin Endocrinol Metab. 1970; 31: 315–323 [DOI] [PubMed] [Google Scholar]
- 154. McDougall IR, Kriss JP. New thoughts about the cause and treatment of the severe ocular manifestations of Graves' disease. Scott Med J. 1974; 19: 165–169 [DOI] [PubMed] [Google Scholar]
- 155. Marino M, Lisi S, Pinchera A, et al. Identification of thyroglobulin in orbital tissues of patients with thyroid-associated ophthalmopathy. Thyroid. 2001; 11: 177–185 [DOI] [PubMed] [Google Scholar]
- 156. Fernando R, Atkins S, Raychaudhuri N, et al. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci U S A. 2012; 109: 7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves' orbital adipose/connective tissues: potential autoantigen in Graves' ophthalmopathy. J Clin Endocrinol Metab. 1998; 83: 998–1002 [DOI] [PubMed] [Google Scholar]
- 158. Tramontano D, Cushing GW, Moses AC, Ingbar SH. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves'-IgG. Endocrinology. 1986; 119: 940–942 [DOI] [PubMed] [Google Scholar]
- 159. Cass LA, Meinkoth JL. Differential effects of cyclic adenosine 3′,5′-monophosphate on p70 ribosomal S6 kinase. Endocrinology. 1998; 139: 1991–1998 [DOI] [PubMed] [Google Scholar]
- 160. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol. 2008; 181: 4397–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev. 2010; 62: 199–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity. 1993; 16: 251–257 [DOI] [PubMed] [Google Scholar]
- 163. Minich WB, Dehina N, Welsink T, et al. Autoantibodies to the IGF1 receptor in Graves' orbitopathy. J Clin Endocrinol Metab. 2013; 98: 752–760 [DOI] [PubMed] [Google Scholar]
- 164. Smith TJ. Is IGF-I receptor a target for autoantibody generation in Graves' disease? J Clin Endocrinol Metab. 2013; 98: 515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Smith TJ, Hoa N. Immunoglobulins from patients with Graves' disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004; 89: 5076–5080 [DOI] [PubMed] [Google Scholar]
- 166. Varewijck AJ, Boelen A, Lamberts SW, et al. Circulating IgGs may modulate IGF-I receptor stimulating activity in a subset of patients with Graves' ophthalmopathy. J Clin Endocrinol Metab. 2013; 98: 769–776 [DOI] [PubMed] [Google Scholar]
- 167. Wiersinga WM. Autoimmunity in Graves' ophthalmopathy: the result of an unfortunate marriage between TSH receptors and IGF-1 receptors? J Clin Endocrinol Metab. 2011; 96: 2386–2394 [DOI] [PubMed] [Google Scholar]
- 168. Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves' disease may carry functional consequences for disease pathogenesis. J Immunol. 2007; 178: 3281–3287 [DOI] [PubMed] [Google Scholar]
- 169. Douglas RS, Naik V, Hwang CJ, et al. B cells from patients with Graves' disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol. 2008; 181: 5768–5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Shimojo N, Kohno Y, Yamaguchi K, et al. Induction of Graves-like disease in mice by immunization with fibroblasts transfected with the thyrotropin receptor and a class II molecule. Proc Natl Acad Sci U S A. 1996; 93: 11074–11079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Costagliola S, Rodien P, Many MC, Ludgate M, Vassart G. Genetic immunization against the human thyrotropin receptor causes thyroiditis and allows production of monoclonal antibodies recognizing the native receptor. J Immunol. 1998; 160: 1458–1465 [PubMed] [Google Scholar]
- 172. Nagayama Y, Kita-Furuyama M, Ando T, et al. A novel murine model of Graves' hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol. 2002; 168: 2789–2794 [DOI] [PubMed] [Google Scholar]
- 173. Chen CR, Pichurin P, Chazenbalk GD, et al. Low-dose immunization with adenovirus expressing the thyroid-stimulating hormone receptor A-subunit deviates the antibody response toward that of autoantibodies in human Graves' disease. Endocrinology. 2004; 145: 228–233 [DOI] [PubMed] [Google Scholar]
- 174. Land KJ, Gudapati P, Kaplan MH, Seetharamaiah GS. Differential requirement of signal transducer and activator of transcription-4 (Stat4) and Stat6 in a thyrotropin receptor-289-adenovirus-induced model of Graves' hyperthyroidism. Endocrinology. 2006; 147: 111–119 [DOI] [PubMed] [Google Scholar]
- 175. Mizutori Y, Saitoh O, Eguchi K, Nagayama Y. Adenovirus encoding the thyrotropin receptor A-subunit improves the efficacy of dendritic cell-induced Graves' hyperthyroidism in mice. J Autoimmun. 2006; 26: 32–36 [DOI] [PubMed] [Google Scholar]
- 176. Wu L, Xun L, Yang J, et al. Induction of murine neonatal tolerance against Graves' disease using recombinant adenovirus expressing the TSH receptor A-subunit. Endocrinology. 2011; 152: 1165–1171 [DOI] [PubMed] [Google Scholar]
- 177. Kaneda T, Honda A, Hakozaki A, Fuse T, Muto A, Yoshida T. An improved Graves' disease model established by using in vivo electroporation exhibited long-term immunity to hyperthyroidism in BALB/c mice. Endocrinology. 2007; 148: 2335–2344 [DOI] [PubMed] [Google Scholar]
- 178. Zhao SX, Tsui S, Cheung A, Douglas RS, Smith TJ, Banga JP. Orbital fibrosis in a mouse model of Graves' disease induced by genetic immunization of thyrotropin receptor cDNA. J Endocrinol. 2011; 210: 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Moshkelgosha S, So PW, Deasy N, Diaz-Cano S, Banga JP. Cutting edge: retrobulbar inflammation, adipogenesis, and acute orbital congestion in a preclinical female mouse model of Graves' orbitopathy induced by thyrotropin receptor plasmid-in vivo electroporation. Endocrinology. 2013; 154: 3008–3015 [DOI] [PubMed] [Google Scholar]
- 180. Nakahara M, Johnson K, Eckstein A, et al. Adoptive transfer of antithyrotropin receptor (TSHR) autoimmunity from TSHR knockout mice to athymic nude mice. Endocrinology. 2012; 153: 2034–2042 [DOI] [PubMed] [Google Scholar]
- 181. Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002; 346: 1692–1698 [DOI] [PubMed] [Google Scholar]
- 182. Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005; 352: 2598–2608 [DOI] [PubMed] [Google Scholar]
- 183. Kremer JM, Genant HK, Moreland LW, et al. Results of a two-year followup study of patients with rheumatoid arthritis who received a combination of abatacept and methotrexate. Arthritis Rheum. 2008; 58: 953–963 [DOI] [PubMed] [Google Scholar]
- 184. Viglietta V, Bourcier K, Buckle GJ, et al. CTLA4Ig treatment in patients with multiple sclerosis: an open-label, phase 1 clinical trial. Neurology. 2008; 71: 917–924 [DOI] [PubMed] [Google Scholar]
- 185. Khanna D, Chong KK, Afifiyan NF, et al. Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology. 2010; 117: 133–139 e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Mitchell AL, Gan EH, Morris M, et al. The effect of B cell depletion therapy on anti-TSH receptor antibodies and clinical outcome in glucocorticoid-refractory Graves' orbitopathy. C Endocrinol. 2013; 79: 437–442 [DOI] [PubMed] [Google Scholar]
- 187. Silkiss RZ, Reier A, Coleman M, Lauer SA. Rituximab for thyroid eye disease. Ophthal Plast Reconstr Surg. 2010; 26: 310–314 [DOI] [PubMed] [Google Scholar]
- 188. Salvi M. Rituximab in Graves' Ophthalmopathy. San Juan, Puerto Rico: American Thyroid Association; 2013. [Google Scholar]
- 189. Stan MN, Garrity JA, Thapa P, Bradley EA, Bahn RS. Randomized double-blind placebo-controlled trial of rituximab for treatment of Graves' ophthalmopathy. Paper presented at: 83rd Annual Meeting of the American Thyroid Association; October 16–20, 2013; San Juan, Puerto Rico. Available at: http://www.thyroid.org/wp-content/uploads/2013_83rd_annualmeeting/slides/stan_abstract_2013_ata.pdf [Google Scholar]
- 190. Durrani OM, Reuser TQ, Murray PI. Infliximab: a novel treatment for sight-threatening thyroid associated ophthalmopathy. Orbit. 2005; 24: 117–119 [DOI] [PubMed] [Google Scholar]
- 191. Paridaens D, van den Bosch WA, van der Loos TL, Krenning EP, van Hagen PM. The effect of etanercept on Graves' ophthalmopathy: a pilot study. Eye. 2005; 19: 1286–1289 [DOI] [PubMed] [Google Scholar]
- 192. Prummel MF, Mourits MP, Berghout A, et al. Prednisone and cyclosporine in the treatment of severe Graves' ophthalmopathy. N Engl J Med. 1989; 321: 1353–1359 [DOI] [PubMed] [Google Scholar]