Abstract
Background
Persistent or recurrent papillary thyroid carcinoma (PTC) occurs in some patients after initial thyroid surgery and often, radioactive iodine treatment. Here, we identify the efficacy, safety, and long-term outcome of our current surgical management paradigm for persistent/recurrent PTC in the central compartment in an interdisciplinary thyroid cancer clinical and research program at a tertiary thyroid cancer referral center.
Methods
We retrospectively analyzed our standardized approach of comprehensive bilateral level VI/VII lymph node dissection (SND [VI, VII]) for cytologically confirmed PTC in the central compartment.
Results
From 1994 to 2004, 210 patients, median age 42 (range 12–82) underwent SND (VI, VII). Most patients (106, 51%) had already undergone ≥2 surgical procedures for persistent or recurrent disease, and 31 (15%) had distant metastases at presentation. Postoperatively, 104 (71%) of the 146 patients who were thyroglobulin (Tg) positive had no evidence of disease. Anti-Tg antibodies were present in 38 patients (18%), 17 of whom (53%) did not have anti-Tg antibodies postoperatively. Fourteen patients (7%) were hypoparathyroid at presentation, and 2 more (1%) became permanently hypoparathyroid after surgery. Four patients (2%) experienced recurrent laryngeal nerve paralysis (RLNP) of a previously functioning nerve. Unanticipated RLNP was observed in only one nerve at risk. External beam radiation was given to 33 patients (17%). An additional 17 patients (8%) developed distant metastases during follow-up. At the last follow-up, 130 (66%) of the 196 patients had no detectable Tg; of these, 99 (76%) had no further evidence of disease. A median of 7.25 years after surgery, 167 (90%) of the 185 patients were without evidence of central disease, and 18 (10%) had developed central compartment recurrences within a median interval of 24.3 months. Of those with recurrence, 16 out of 18 patients (89%) underwent a subsequent surgical procedure, thus resulting in an overall 98% central compartment control rate. Kaplan–Meier disease-specific survival at 10 years was 98.9% for patients <45 years old and 77.9% for those ≥45 years old (log-rank p<0.00001). The only predictor of central compartment recurrence was malignancy in a thyroid remnant noted within the central compartment surgical specimen.
Conclusions
Bilateral comprehensive level VI/VII dissections are safe and effective for long-term control of recurrent/persistent PTC in the central lymphatic compartment.
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of differentiated thyroid malignancy. Overall, survival is generally considered excellent for most patients with PTC, but this depends on histologic type and on well-defined prognostic factors such as age, sex, soft-tissue extension, lymph node metastasis, and distant metastasis (1–4). Persistent disease after definitive surgical excision for early stage I and II PTC has been reported to be as high as 11%–30% (5). In a prospective national thyroid cancer registry, recurrent disease occurred in 10%–30% of patients with stage I–III PTC (6). Local and regional recurrences within the central compartment may add considerable risk to long-term morbidity, including loss of or change in quality of voice and swallowing. Local/central compartment recurrences may also carry considerable risk for tumor-related death among patients above age 45.
Many issues remain unresolved regarding the treatment of recurrent or persistent PTC. Over the past several decades, The University of Texas M.D. Anderson Cancer Center has established a dedicated program for the interdisciplinary management of patients with thyroid malignancies. As a result, a standardized surgical approach has been developed for patients with persistent/recurrent PTC within the central compartment. We sought to critically examine and determine the efficacy of our institutional approach to the surgical management of these patients with regard to long-term control and outcome.
Materials and Methods
Patients
We conducted a retrospective, single-institution, longitudinal study of patients undergoing bilateral paratracheal and anterior superior mediastinal dissection between September 15, 1994 and July 9, 2004. Patients were identified by a search of the Physician Referral Service database of surgical records at The University of Texas M.D. Anderson Cancer Center that identified all patients who underwent selective neck dissection including both central compartment and anterior/superior mediastinal lymph nodes, referred to as SND(VI,VII) (7,8).
Inclusion criteria consisted of a diagnosis of any histologic type of PTC followed by a later recurrence or persistent disease and referral to M.D. Anderson. Recurrence was determined by fine-needle aspiration in all patients; surgical disease was defined as at least one lesion >1 cm in greatest dimension in patients <45 years old and greater than 6 mm in patients ≥45 years old. Our approach for patients <45 years old is to observe nonbulky disease (<1 cm) until evidence of progression of disease before recommending surgical intervention. No distinction was made between recurrent and persistent disease. All patients had undergone at least a previous thyroidectomy with curative intent 6 months to 48 years before their documented recurrence/persistence. Exclusion criteria included a diagnosis of follicular or medullary thyroid carcinoma, only soft-tissue or visceral involvement of the central compartment structures, and administration of previous external beam radiation therapy. The study was approved by our institutional review board.
Patient demographic data, including sex, date of birth, and self-reported race/ethnicity (United States Office of Management and Budget Categories), were identified from the electronic medical record for each patient. The primary medical evaluation, consisting of medical records from referral sources, surgery reports, and pathologic reports, was reviewed to establish age and the AJCC TNM classification at initial diagnosis, and extent and number of initial surgical procedures. When available, pathologic slides from surgery before referral were independently reviewed by a thyroid pathologist at our institution. The number and total millicurie delivered of previous radioactive iodine treatments were also obtained.
Surgical evaluations, approach, and postoperative care
On referral to our institution, all patients were evaluated by an interdisciplinary team including the primary surgeon and endocrinologist. Baseline laboratory values for serum thyroglobulin (Tg), anti-Tg antibody, thyroid-stimulating hormone, free T4, and routine blood counts and serum chemistry were obtained. Intact parathyroid hormone (iPTH) levels were obtained for patients with a history of hypoparathyroidism. A complete oncologic restaging was performed, and additional investigations were performed when there was clinical suspicion of metastatic disease.
The preoperative evaluations were searched for complaints of symptoms referable to the head and neck. The physical examination documented by the head and neck surgeon was assessed for findings of locoregional metastatic disease. All patients underwent preoperative indirect or flexible fiberoptic laryngoscopy to determine the status of recurrent and possible superior laryngeal nerve function. Any abnormal findings or symptoms were evaluated further with videostroboscopy. The surgeon's follow-up notes were reviewed for complaints of dysphagia and wound morbidity. Postoperative recurrent laryngeal nerve function was determined on the basis of laryngoscopy findings.
The surgeon's operative dictation was reviewed to determine the extent of lymphadenectomy in addition to levels VI and VII, the autotransplantation of parathyroid tissue, and additional procedures. The content of the central compartment lymphadenectomy was categorized by the lymph-node levels, number of nodes, and locations of disease. In all cases, the goal of surgery was complete resection of all grossly evident disease with functional preservation of speech, swallowing, protective airway mechanisms, and parathyroid preservation (when feasible).
Surgeries were performed by a surgeon experienced in thyroid cancer management utilizing microsurgical techniques ranging from 3.5 to 5-fold loupe magnification for binocular microscopic dissection. The surgical method for comprehensive central compartment dissection has been previously described (7,8). These dissections may extend well beyond the sites and location of paratracheal and superior mediastinal lymph nodes associated with empiric or “prophylactic” dissections. Intraoperative ultrasonographic facilitation of surgery was used when necessary. To minimize the risk of permanent hypoparathyroidism, healthy parathyroid tissue was pathologically confirmed and autotransplanted whenever feasible.
After surgery, calcium and intact PTH levels were monitored immediately and during short-term follow-up to assess the integrity of parathyroid function. Follow-up clinical surveillance included history and physical examination, determination of serum Tg and anti-Tg antibody levels, and high-resolution ultrasound. Assays for anti-Tg antibodies were not routinely available at our institution until 1996. High-resolution computed tomography imaging and fine-needle aspiration cytology were performed as indicated to determine the status of control or disease.
Pathologic findings
SND(VI,VII) specimens were evaluated by a thyroid pathologist to determine the histologic subtype of PTC, the number of lymph nodes removed, and the number and size of lymph nodes containing metastatic disease. All specimens were oriented for the pathologist by a member of the surgical team to identify the lymph-node levels submitted. A resection was considered oncologically complete only if the surgeon removed all gross disease. The pathologic records were searched to determine the levels in which metastatic disease was found. Additional pathologic features obtained from the pathology report included lymph nodes with extracapsular spread, soft-tissue metastasis, lymphovascular invasion, perineural invasion, the presence of remnant thyroid tissue, visceral invasion (trachea, larynx, or esophagus invasion), and intrathymic metastasis.
Postoperative parameters after SND(VI,VII)
Hospital discharge summaries were evaluated to determine the length of stay after surgery. The period of follow-up was measured from the date of surgery to the date of last contact. Death was determined from follow-up notes dictated by the primary physician and telephone contact with referring and follow-up physicians.
Normal preoperative parathyroid function was defined by a serum intact PTH level within the laboratory reference range and/or no requirement for oral calcium and vitamin D to maintain normal serum calcium levels. Postoperative parathyroid function was defined as normal in patients with a normal postoperative intact PTH level and no postoperative serum ionized calcium levels <1.13 mmol/L (normal range, 1.13–1.32 mM). Transient hypocalcemia was defined as postoperative serum ionized calcium levels <1.13 mmol/L or the presence of symptoms, which resolved without the need for supplemental calcium and calcitriol for >6 months. Permanent hypoparathyroidism was defined as persistent serum iPTH levels <10 pg/mL and/or the need for supplemental calcium and calcitriol for >6 months after surgery. The lowest postoperative serum ionized calcium level was recorded as the lowest measured level of ionized calcium during the inpatient perioperative period.
Data collection and statistical analysis
Patient characteristics, pathologic findings, and postoperative parameters were independently collected without the knowledge of other variables during data acquisition. Correlations between parameters and endpoints were assessed by Pearson's χ2 test or, when there were <10 subjects in any cell of a 2×2 grid, by the two-tailed Fisher's exact test. Curves describing overall and disease-specific survival were generated by the Kaplan–Meier product limit method. The statistical significance of differences between the actuarial curves was tested by the log-rank test. Follow-up time for survival measurements was the time from the first surgery for central compartment recurrence at M.D. Anderson Cancer Center until the date of last contact or death. Proportional hazard ratios and multivariate models were assessed by Cox regression analysis as appropriate. Statistical tests were performed by using Statistica (StatSoft, Inc., Tulsa, OK) and SPSS (IBM Corporation, Somers, NY) software. p-values of <0.05 were considered statistically significant.
Results
Characteristics of patients before undergoing SND (VI, VII)
Demographic, initial staging, previous surgical, and radioactive iodine treatment characteristics of patients who underwent SND(VI,VII) for metastatic PTC are shown in Table 1. In the cohort of 210 patients, the median age of patients at their incident (initial) surgery for their malignancy was 39 years (range, 9–79 years age). Nearly two-thirds of patients were ≥45 years old at the time of incident surgery.
Table 1.
Patient Demographics
| Characteristic | No. (%) of patients |
|---|---|
| Age at initial presentation | |
| <45 years | 135 |
| ≥45 years | 75 |
| Sex | |
| Female | 125 |
| Male | 85 |
| Ethnicity | |
| White | 156 (74%) |
| Hispanic | 42 (20%) |
| Asian | 6 (4%) |
| Black | 4 (2%) |
| Filipino | 2 (1%) |
| Incident disease stage | |
| I | 120 |
| II | 6 |
| III | 12 |
| IV | 63 |
| Unable to determine | 9 |
| Initial thyroid surgery | |
| Total thyroidectomy | 172 |
| Near-total thyroidectomy | 23 |
| Completion thyroidectomy | 1 |
| Lobectomy or subtototal | 14 |
| Initial or subsequent neck surgery | |
| Central neck dissection (VI,VII) | 68 (36%) |
| Central and lateral neck dissection | 65 (34%) |
| Lateral neck dissection | |
| SNDII–V | 121 |
| Modified radical neck dissection | 12 |
| Radical neck dissection | |
| Radioactive iodine ≥100 millicuries | 152 (72%) |
| Prior surgeries for “recurrent” disease | |
| 1 | 34 |
| ≥2 | 106 |
SND, selective neck dissection.
Of 205 patients with information available regarding initial overall AJCC TNM staging, 59% were classified as having stage I disease at their initial evaluation. Somewhat fewer primary tumors were staged T1, T2, or T3 (66/194; 34%) than T4 (128/194; 66%). Patients <45 years old were more likely to present with T1 tumors (18/112; 16%) than patients ≥45 years old (7/82; 9%), but this difference was not significant (p=0.135). In 10 patients, 2<45 years old and 8≥45 years old, the primary tumor stage could not be determined from available information from clinical or pathologic reports from the outside institutions where they had undergone initial evaluation. Most patients exhibited central compartment nodal disease at their initial presentation (133/201; 66%) with similar proportions among the younger (68%) and older (63%) patients (p=0.453). Seven patients (3%) had distant metastatic disease on initial staging, 1/119 (1%) of the younger patients and 6/86 (7%) of the older ones (p=0.023). Patients <45 years old mostly had stage I disease (118/119; 99%), compared with only 2 patients (2%) ≥45 years old (p<0.00001). Most patients ≥45 years old (63/82; 77%) had stage IV disease at presentation (p<0.00001).
Fifty-four patients (28% of 190) underwent only thyroidectomy before SND(VI,VII). However, 68 patients (36%) underwent dissection of the central compartment, and 65 patients (34%) underwent dissection of the central compartment and one or both lateral neck compartments. Therefore, 133 out of 210 patients (63%) had undergone at least one surgical procedure that included dissection of the central compartment lymphatics. One hundred six patients (51%) underwent ≥2 surgical procedures for recurrent or persistent disease before presenting to our institution. The median time from initial thyroidectomy to SND(VI,VII) was 21 months (range, 3–580 months). The interval was <10 months for 73 patients (35%), 10–40 months for 71 patients (34%), and >40 months for 66 patients (31%).
Adjuvant or initial postoperative therapy with radioactive iodine was given to 152 (72%) of the patients in the cohort. An additional 11 (5%) received radioactive iodine after another surgery for recurrent/persistent disease but before presentation for central compartment surgery. The median dose of radioactive iodine among these 163 patients was 100 millicuries. Fifty-four (26%) patients received multiple treatment doses of radioactive iodine in the subsequent course of therapy before undergoing SND(VI,VII) for metastatic disease. The median dose of radioactive iodine among the latter group of patients was 299 millicuries (range, 100–760 millicuries).
Characteristics of patients at the time of SND(VI,VII)
The median age at the time of SND(VI,VII) was 42 years (range, 12–82 years). A slight majority of patients (116/210; 55%) were <45 years old at the time of this study's surgery for persistence/recurrence, and the remainder (94/210; 45%) were ≥45 years old. The ratio of women to men in the <45 year-old group was very close to 2:1 (78:38). In the group of patients aged 45 or older, there were the same numbers of men and women (47:47).
Among the 106 patients who had undergone radioactive iodine scanning to evaluate the disease ultimately treated by SND(VI,VII), 37 (34%) demonstrated no evidence of radioiodine uptake, and 69 (65% of those scanned, 33% of the entire cohort) had evidence of radioiodine uptake.
Serum Tg levels
Among the 163 patients without detectable Tg antibodies, serum Tg was detectable during thyroid hormone suppression in 146 (89%) of them before surgery, of whom 22 (15%) also exhibited evidence of distant disease. (For those with distant metastases, surgery was felt to provide a benefit of attempted central compartment control in the light of stable or nonthreatening distant disease.) In contrast, Tg was not detectable in 17 (10%) patients, of whom two also had evidence of distant metastases. Postoperatively, 104 (71%) of the 146 patients who were Tg informative exhibited no biochemical evidence of disease. Anti-Tg antibodies that had been present preoperatively in 38 patients (18%) became nondetectable in 17 (53%) of them postoperatively.
At the last available follow-up, 130 (66%) of the 196 patients had no detectable serum Tg; of these, 99 (76%) had no further evidence of disease by ultrasound and chest imaging. Five (4%) patients were living with disease.
Extent of surgery for recurrent/persistent disease
In addition to SND(VI,VII), the extent of surgery for metastatic papillary thyroid cancer varied with the extent of disease found on preoperative imaging. Bilateral SND(VI,VII) alone was performed in 51 patients (24.0%). Lateral compartment(s) of the neck (levels II through V anteriorly) were dissected in addition to levels VI and VII in 159 patients (76%). Of these patients, 104 out of 159 (66%) underwent bilateral dissection of the lateral compartment in addition to levels VI and VII.
Pathologic findings of SND(VI,VII) specimens
Positive lymph nodes were found in all 210 patients. In 59 patients (29%), pathologic adenopathy was found in 2–4 lymph nodes. In 132 patients (65%), disease was found in >4 lymph nodes. The largest central compartment lymph node was 1–2 centimeters in 124 (60%) of 203 patients and 2–6 cm in 79 patients (40%). Soft-tissue metastasis or extracapsular spread in lymph nodes was present in 117 (56%) patients. Perineural invasion of the recurrent laryngeal nerve was found in 3 patients. Lymphovascular invasion was identified in an additional 3 patients. Soft-tissue invasion of the visceral central compartment organs—defined as the trachea, larynx, esophagus, or constrictors of the pharynx—was pathologically confirmed in 9 (4%) patients. Thyroid remnant tissue was identified in 44 patients (21%).
Location of pathologically confirmed disease within the central compartment
Table 2 shows the locations of disease pathologically confirmed within the central compartment. The most frequent location of disease was medial to the carotid artery and lateral and/or dorsal to the recurrent laryngeal nerve in (124 patients [59%]). Metastatic disease medial to the nerve and inferior to the nerve inlet was identified in 122 patients (58%). Of 44 thyroid remnants removed, 39 contained residual malignancy along with nonmalignant thyroid tissue. In addition, 47 patients had metastatic lymph nodes in the recurrent laryngeal nerve inlet, either in the suspensory ligament area or interposed between or beneath the recurrent laryngeal nerve branches. Pretracheal disease within level VI and anterior superior mediastinal disease were commonly co-associated and were found in 95 (45%) and 91 (43%) patients, respectively. Delphian lymph nodes, subfascial within the cricothyroid muscle or ventral to membrane, were found in 23 patients (11%).
Table 2.
Pathologic Location of Sites of Disease within Central Compartment
| Location | n | % |
|---|---|---|
| Medial and ventral (anterior) to recurrent laryngeal nerve | 122 | 58.1 |
| Lateral and/or dorsal (posterior) to recurrent laryngeal nerve | 124 | 59.0 |
| Both medial and posterior or lateral to nerve | 80 | 38.1 |
| Nodal tissue at recurrent laryngeal nerve inlet | 47 | 22.4 |
| Thyroid Remnant | 46 | 21.9 |
| Cricothyroid membrane/Delphian area | 23 | 11.0 |
| Pretracheal (inferior level VI central) | 95 | 45.2 |
| Level VII | 91 | 43.3 |
| Both pretracheal and Level VII | 56 | 26.7 |
| Thymic | 7 | 3.3 |
| Constrictor at pharynx | 12 | 5.7 |
| Subdigastric/superior thyroid artery takeoff | 20 | 9.5 |
| External jugular | 5 | 2.4 |
| Medial to vertebral artery | 18 | 8.6 |
| Strap muscle | 8 | 3.8 |
Fewer than ten percent of patients exhibited disease within the thymus gland, medial to the vertebral artery, subdigastric takeoff of the superior thyroid artery, external jugular lymphatics, within strap musculature, or cephalad to the recurrent laryngeal nerve inlet in the area of the constrictors of the pharynx.
Postoperative laryngeal function and swallowing
Despite the preoperative presence of unilateral vocal fold paralysis in 37 patients (17.6%), there was only a single planned temporary tracheostomy performed electively during surgery, which remained in place during the patient's postoperative external beam radiation therapy and then was decannulated. Four patients (2%) experienced recurrent laryngeal paralysis of a previously functioning nerve; of these, 3 had been sacrificed due to gross tumor infiltration. Unanticipated RLNP was observed in one at-risk nerve (0.2%).
Although swallowing was maintained among all patients in this series, 5 patients who subsequently underwent external beam irradiation required nutritional support during the course of their adjuvant treatment.
Parathyroid function
Fourteen patients (7%) were hypoparathyroid on presentation with recurrent/persistent disease. An additional 2 patients (1%) became permanently hypoparathyroid after surgery.
Additional radioactive iodine therapy after SND(VI,VII)
Postoperatively, 106 patients underwent radioactive iodine uptake studies, of which 70 (66%) suggested abnormal uptake and resulted in therapeutic radioactive dosing. The median dose was 150 millicuries (range 75–225). Four of these individuals received additional radioactive iodine during the course of their follow-up.
Adjuvant external beam radiation therapy
Postoperative external beam radiation therapy was administered to 33 patients (16%) based on operative and pathologic findings that indicated visceral involvement of the central compartment. Eight of these patients (24%) died of disease during the follow-up period, all of distant disease.
Disease control
Central compartment control of disease
With a median follow-up period of 7.25 years after surgery, 137 (74%) of the 185 patients for whom information was available were without evidence of disease. Forty-eight (26%) patients developed recurrences, 18 of which (10%) involved the central compartment. Of those with central compartment recurrence, 16 (89%) underwent a subsequent surgical procedure. None of the 18 had died of disease at the time of last contact. Eight had positive Tg levels, and 4 were living with disease. Overall, central compartment control was obtained in 98% of the cohort of 210 patients.
Distant metastases
Thirty-one patients (15%) exhibited evidence of distant metastases at the time of their initial presentation or at presentation with recurrent or persistent disease. Four of these patients were <45 years old, and 27 (87%) were ≥45 years old. An additional 17 patients (8%) developed evidence of distant pulmonary metastases during the follow-up period. Bone, brain, and other visceral metastases were noted in 5 (2%), 2 (1%), and 7 (3.5%) patients, respectively. Kaplan–Meier disease-specific survival at 10 years for patients ≥45 years old who presented with distant metastases was 55%. In contrast, for patients without distant metastases at initial evaluation, disease-specific survival at 10 years was 95% (p<0.00001).
Factors affecting recurrence in the central compartment after definitive surgery
The only risk factor predicting central compartment recurrence after SND(VI,VII) was the presence of a thyroid remnant (p<0.034). The number or size of lymph nodes and extracapsular extension were not risk factors and were not correlated with the administration of adjuvant radiation therapy. Both soft-tissue invasion into central visceral organs and perineural invasion were associated with administration of external beam radiation therapy, and, therefore, their potential effect on central compartment recurrence may have been mitigated with this adjuvant approach.
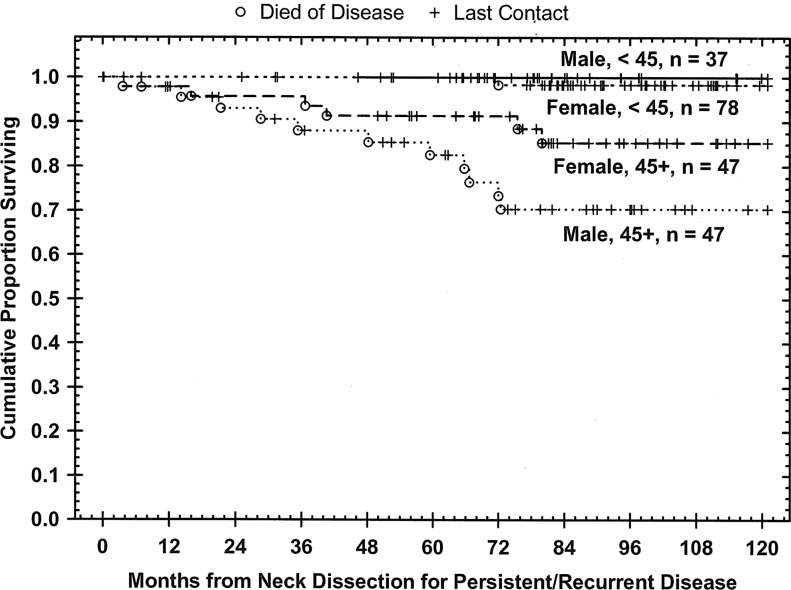
Kaplan–Meier cumulative survival plots
The 10-year predicted disease-specific survival in this study for all patients, based on age at initial presentation and sex, is shown in Figure 1. Table 3 depicts log-rank testing and reveals that age at presentation ≥45 years, sex, the presence of distant metastases, clinical and pathologic indications for postoperative adjuvant radiation therapy, central compartment lymph nodes >3 centimeters in size, and Tg levels of ≥10, ≥50, and ≥100 were all correlated with an increased risk of dying of the disease. Nevertheless, since all these factors were also directly correlated with age ≥45 years at initial diagnosis, their ultimate significance cannot be determined.
FIG. 1.
Kaplan–Meier disease-specific survival curve for all patients, n=210. A significant difference was observed between women <45 years versus >45 years of age (p=0.006), women <45 versus men >45 (p=0.00002), men <45 versus women >45 (p=0.02), and men <45 versus men >45 (p=0.0005). Women >45 versus men >45 was not significant (p=0.097).
Table 3.
Log-Rank Testing of Risk Factors of Overall and Disease-Specific Survival
| Overall survival | Disease-specific survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Log-rank-p | Wald p | Risk ratio | Lower 95% CI | Upper 95% CI | Log-rank-p | Wald p | Risk ratio | Lower 95% CI | Upper 95% CI |
| Male sex | 0.00926 | 0.011800 | 2.729953 | 1.249367 | 5.965132 | 0.04442 | 0.050387 | 2.57665 | 0.99844 | 6.649496 |
| Age ≥45 at Level VI and VII dissection | <0.00001 | 0.000057 | 19.33731 | 4.572785 | 81.773200 | <0.00001 | 0.001731 | 25.13465 | 3.344524 | 188.890900 |
| M1 on presentation | <0.00001 | 0.000008 | 9.346025 | 3.511948 | 24.871710 | <0.00001 | 0.000025 | 10.98626 | 3.603866 | 33.491280 |
| ≥2 previous surgeries | 0.19911 | 0.193737 | 1.904468 | 0.720856 | 5.031513 | 0.95013 | 0.948494 | 1.049644 | 0.24133 | 4.565341 |
| Thyroglobulin >10 | 0.00525 | 0.011737 | 4.846018 | 1.420168 | 16.536032 | 0.00092 | 0.011400 | 13.569626 | 1.800102 | 102.291326 |
| Thyroglobulin >50 | 0.00013 | 0.000599 | 5.341095 | 2.051710 | 13.904170 | 0.00004 | 0.000445 | 7.456039 | 2.430113 | 22.876500 |
| Thyroglobulin >100 | 0.00004 | 0.000229 | 5.63063 | 2.245818 | 14.116890 | 0.00001 | 0.000193 | 7.282063 | 2.564559 | 20.677400 |
| Thyroid remnant | 0.86779 | 0.872798 | 1.073077 | 0.452544 | 2.544493 | 0.44648 | 0.464541 | 0.629342 | 0.181951 | 2.176803 |
| Distant metastases at time of Level VI/VII surgery | <0.00001 | 0.000009 | 6.072237 | 2.743882 | 13.437920 | <0.00001 | 0.000001 | 10.47164 | 4.031839 | 27.197729 |
| Extracapsular nodal extension | 0.01697 | 0.022995 | 2.865263 | 1.156295 | 7.100004 | 0.05163 | 0.063614 | 2.862623 | 0.942192 | 8.697391 |
| Largest node ≥1.5 cm | 0.00019 | 0.000872 | 1.078606 | 1.031609 | 1.127745 | 0.00622 | 0.012009 | 1.067466 | 1.014452 | 0.123251 |
| Largest node >3 cm | 0.00328 | 0.004963 | 1.139263 | 1.040217 | 1.247741 | 0.00216 | 0.003776 | 1.171961 | 1.052646 | 1.304798 |
| Soft-tissue invasion | 0.35794 | 0.340586 | 2.01552 | 0.476861 | 8.518878 | 0.13535 | 0.138817 | 3.036802 | 0.697685 | 13.218540 |
| Postoperative radiation therapy | <0.00001 | 0.000002 | 6.383369 | 2.997181 | 13.595240 | 0.00055 | 0.001186 | 4.662541 | 1.838545 | 11.823190 |
| Distant metastases during surveillance | <0.00001 | 0.000001 | 7.245101 | 3.265083 | 16.076630 | <0.00001 | 0.000003 | 18.994220 | 5.487747 | 65.742940 |
| Second primary malignancies | 0.57560 | 0.551182 | 1.549852 | 0.366869 | 6.547399 | 0.88468 | 0.874330 | 1.176784 | 0.156536 | 8.846667 |
Boldface indicates statistical significance
CI, confidence interval.
Having undergone >2 previous thyroid cancer surgeries before central compartment dissection, pathologic evidence of extracapsular nodal extension or soft tissue invasion, second primary cancers, and a thyroid remnant were not associated with any significant effect on disease-specific survival.
Discussion
The beginning of this study marked an era of transition from aggressive surgical intervention for recurrent or persistent differentiated thyroid cancer to that of one focusing on surgically “disease-appropriate” intervention, including interdisciplinary evaluation and care and comprehensive surgical management of disease sparing organs and function. Our results show that, in experienced hands, bilateral paratracheal and mediastinal dissection is a reproducible and safe procedure in patients with recurrent or persistent PTC, despite the multiple interventions that these patients may have previously undergone (7,9–11). Frequently, the gravest concern of such patients is tracheostomy or laryngeal loss. However, even in the circumstances of multiple recurrence of disease along the only functioning recurrent laryngeal nerve, this surgery can almost always be safely performed without significant morbidity to laryngeal function, including voice and swallowing.
This study also clearly establishes the long-term efficacy of surgery in controlling PTC within the central compartment. The incidence of central compartment recurrence after surgery for persistent/recurrent PTC was 10%, as determined by routine high-resolution ultrasound surveillance and Tg monitoring. Even among these relatively uncommon instances of recurrence, ultimate disease control in the central compartment was achieved in 98% of patients. However, given that nearly two thirds of patients in our study were <45 years old, long-term disease control should be tempered by the inherent potential risk of long-term hypoparathyroidism and nerve integrity.
Even though approximately 23% of patients exhibited or developed distant metastases in this study, nearly 70% of patients who were Tg informative (patients with serum Tg concentrations suggestive of persistent or recurrent PTC) demonstrated both radiographic and serologic disease control after their surgical intervention. Disease-specific survival among young patients was predictably outstanding, at >99%, with single death occurring in a patient who had diffuse multiorgan metastases. However, even among patients ≥45 years old at initial diagnosis, disease-specific survival exceeded 77% at 10 years. This result agrees with those of a study from Waseem et al., which showed a significant reduction in disease-specific survival among older patients in a similar patient population with multiple recurrences (9).
External beam radiotherapy was administered to only 2 patients <45 years old, despite being administered to 16% of the patients in this study, which suggests that many of the older patients had advanced central compartment disease. Indications for adjuvant radiotherapy include extensive soft-tissue metastases in locations where disease recurrence would prevent surgical salvage without sacrificing laryngotracheal, hypopharyngeal, or esophageal organs (12,13). In these instances, the long-term adverse effects of radiotherapy are tempered by the desire to preserve quality of life and functional organs. The success of this approach has been previously published by our program as well as others (14).
The most common adverse event associated with central compartment surgery is permanent hypoparathyroidism. In most circumstances, this outcome may be expected given the extent of disease (ranging from extensive nodal and soft-tissue extension throughout the central compartment as well as involvement of the trachea, thyroid cartilage, esophagus, or pharyngeal structures). Autotransplantation can reduce the risk of long-term hypoparathyroidism. Since it cannot be determined how many parathyroid glands are functioning in a patient, each parathyroid gland should be treated as the patient's only one. Since PTC will recur more than once in some of these patients, the potential for further surgery within the central compartment during their lifetime should be considered. Pathologically confirmed healthy parathyroid tissue (at least a component) should, therefore, be autotransplanted even if the surgeon is confident of sparing the vascular supply or parathyroid tissue.
We found our approach of observing young patients with subcentimeter central neck disease until disease progression is substantiated by the excellent outcomes observed in this study. It is possible that observation would be useful even among patients ≥45 years old. We found that survival and recurrence were not compromised and that surgery was facilitated by making intraoperative metastatic node recognition easier. However, masses >1 cm should still be promptly removed, especially among patients ≥45 years old.
More than 60% of patients in our study had undergone at least one surgical procedure that included elective or therapeutic dissection of the central compartment before their diagnosis of persistent/recurrent disease. This suggests that prophylactic central compartment dissection may not significantly reduce the incidence of persistent/recurrent PTC. More study in a larger cohort is clearly needed.
The only predictor of central compartment recurrence was the presence of malignancy in a thyroid remnant noted in the surgical specimen. This underlies the importance of comprehensive total thyroidectomy among patients at high risk, including those with larger masses, poorly differentiated pathology, and T3 and T4 disease and those ≥45 years old at presentation. Other pathologic factors may also have contributed to central compartment recurrence, but their effect may have been mitigated by the administration of radioactive iodine and external beam radiotherapy, so further study is needed.
Our results should be interpreted in the context of a major tertiary referral center. The consecutive patients in this series were all undergoing surgical treatment of recurrent or persistent central compartment disease. Other patients presented with central compartment disease during the study period but did not undergo surgical intervention for control owing to many factors, most commonly extensive progressing disease at multiple sites, especially diffuse visceral, bone, or brain metastatic foci, which would now be considered appropriate indications for prospective clinical trials of systemic agents or systemic therapy.
In summary, we found that comprehensive central compartment dissection for recurrent/persistent PTC provides long-term control of disease for most patients. Patients tolerated the procedure well, and the most significant predictable morbidity was long-term hypoparathyroidism, which occurred in a small subset. Among experienced surgeons, this surgery can be safely and effectively performed within the central compartment even if disease is present along an only functioning recurrent laryngeal nerve. Although new therapies should still be developed to manage distant and extensive disease, surgical management of recurrent/persistent disease in the central compartment should be considered the standard for comparison.
Disclosure Statement
G.C. is an NIH-funded researcher, although this study was not funded by the NIH. S.I.S. has relevant financial activities outside the submitted work: board membership—Veracyte; consultancies—AstraZeneca, Bayer, Eisai, Exelixis, Genzyme, Lilly, NovoNordisk, Pfizer, Plexxikon, Roche; grants pending or received—Amgen, Genzyme, Pfizer; payment for lectures and/or educational presentations—Angiogenesis Foundation, Exelixis. All other authors report no competing financial interests.
References
- 1.American Joint Committee on Cancer. AJCC Cancer Staging Manual. Seventh. Springer Press; New York: 2009. [Google Scholar]
- 2.Cady B. Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–953. [PubMed] [Google Scholar]
- 3.Shaha AR. Loree TR. Shah JP. Intermediate-risk group for differentiated carcinoma of the thyroid. Surgery. 1994;116:1036–1041. [PubMed] [Google Scholar]
- 4.Hay ID. Bergstralh EJ. Goellner JR. Ebersold JR. Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1058. [PubMed] [Google Scholar]
- 5.Hundahl SA. Cady B. Cunningham MP. Mazzaferri E. McKee RF. Rosai J. Shah JP. Fremgen AM. Stewart AK. Holzer S. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer. 2000;89:202–217. doi: 10.1002/1097-0142(20000701)89:1<202::aid-cncr27>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Sherman SI. Angelos P. Ball DW. Beenken SW. Byrd D. Clark OH. Daniels GH. Dilawari RA. Ehya H. Farrar WB. Gagel RF. Kandeel F. Kloos RT. Kopp P. Lamonica DM. Loree TR. Lydiatt WM. McCaffrey J. Olson JA., Jr. Ridge JA. Robbins R. Shah JP. Sisson JC. Thompson NW National Comprehensive Cancer Network. Thyroid carcinoma. J Natl Compr Cancer Netw. 2005;3:404–457. doi: 10.6004/jnccn.2005.0021. [DOI] [PubMed] [Google Scholar]
- 7.Clayman GC. Shellenberger TD. Ginsberg LE. Edeiken BS. El-Naggar AK. Sellin RV. Waguespack SG. Roberts DB. Mishra A. Sherman SI. Approach and safety of comprehensive central compartment dissection in patients with recurrent thyroid cancer. Head Neck. 2009;9:1152–1163. doi: 10.1002/hed.21079. [DOI] [PubMed] [Google Scholar]
- 8.Carty SE. Cooper DS. Doherty GM. Duh QY. Kloos RT. Mandel SJ. Randolph GW. Stack BC., Jr. Steward DL. Terris DJ. Thompson GB. Tufano RP. Tuttle RM. Udelsman R. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19:1153–1158. doi: 10.1089/thy.2009.0159. [DOI] [PubMed] [Google Scholar]
- 9.Waseem Z. Palme CE. Walfish P. Freeman JL. Prognostic implications of site of recurrence in patients with recurrent well-differentiated thyroid cancer. J Otolaryngol. 2004;33:339–344. doi: 10.2310/7070.2004.04013. [DOI] [PubMed] [Google Scholar]
- 10.Kim MK. Mandel SH. Balock A. Livolsi VA. Langer JE. Didanato L. Fish S. Weber RS. Morbidity following central compartment reoperation for recurrent or persistent thyroid cancer. Arch Otolaryngol Head Neck Surg. 2004;130:1214–1216. doi: 10.1001/archotol.130.10.1214. [DOI] [PubMed] [Google Scholar]
- 11.Schuff KG. Weber SM. Givi B. Samuels MH. Andersen PE. Cohen JI. Efficacy of nodal dissection for treatment of persistent/recurrent papillary thyroid cancer. Laryngoscope. 2008;118:768–775. doi: 10.1097/MLG.0b013e318162cae9. [DOI] [PubMed] [Google Scholar]
- 12.Samaan NA. Schulz PN. Hickey RC. Goepfert H. Haynie TP. Johnston DA. Ordonex NG. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714–720. doi: 10.1210/jcem.75.3.1517360. [DOI] [PubMed] [Google Scholar]
- 13.Breaux GP. Guillamondegui OM. Treatment of locally invasive carcinoma of the thyroid: how radical? Am J Surg. 1980;140:514–517. doi: 10.1016/0002-9610(80)90202-0. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz DL. Lobo MJ. Ang KK. Morrison WH. Rosenthal DI. Ahamad A. Evans DB. Clayman G. Sherman SI. Garden AS. Postoperative external beam radiotherapy for differentiated thyroid cancer: outcomes and morbidity with conformal treatment. Int J Radiat Oncol Biol Phys. 2009;74:1083–1091. doi: 10.1016/j.ijrobp.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]