Abstract
Background
Ultrasound (US) of the central neck compartment (CNC) is considered of limited sensitivity for nodal spread in papillary thyroid cancer (PTC); elective neck dissection is commonly advocated even in the absence of sonographic abnormalities. We hypothesized that US is an accurate predictor for long-term disease-free survival, regardless of the use of elective central neck dissection in patients with PTC.
Methods
A retrospective chart review of 331 consecutive PTC patients treated with total thyroidectomy at M.D. Anderson Cancer Center between 1996 and 2003 was performed. Information retrieved included preoperative sonographic status of the CNC, surgical treatment of the neck, demographics, cancer staging, histopathological variables and use of adjuvant treatment. The endpoints for the study were nodal recurrence and survival.
Results
There were 112 males and 219 females with a median age of 44 years (range 11–87). The median follow-up time for the series was 71.5 months (range 12.7–148.7). There were 151 (45.6%) patients with a T1, 58 (17.5%) with a T2, 70 (21.1%) with a T3, and 52 (15.7%) with a T4. Preoperative sonographic abnormalities were present in the CNC in 79 (23.9%) patients. During the surveillance period, 11 (3.2%) patients recurred in the central neck, with an average time for recurrence of 22.8 months. Advanced T stage (T3/T4) and abnormal US were independent prognostic factors for recurrence in the central neck (p=0.013 and p=0.005 respectively). There were 119 (35%) patients with a sonographically negative central compartment who underwent elective central neck dissection; 85 of them (71.4%) were found to be histopathologically N(+) while 34 (28.6%) were pN0. There were no differences in overall survival (p=0.32), disease specific survival (DSS; p=0.49), and recurrence-free survival (p=0.32) between these two groups. Preoperative US of the CNC was an age-independent predictor for overall survival (p<0.001), DSS (p=0.0097), and disease-free survival (p=0.0005) on bivariate Cox regression.
Conclusions
US of the central compartment is an age-independent predictor for survival and CNC recurrence-free survival in PTC. Prophylactic neck dissection of the central compartment does not improve long-term disease control, regardless of the histopathological status of the lymph nodes retrieved. Our findings emphasize the ability of US to clinically detect relevant nodal disease and support conservative management of the CNC in the absence of abnormal findings.
Introduction
The incidence of thyroid cancer in the United States has increased faster than any other malignancy, potentially contributed to by improvements in diagnostic tools such as high resolution ultrasound (US) and US-guided fine-needle aspiration biopsy (FNA) (1). Sonographic assessment of the neck has become the standard of care for preoperative staging of thyroid malignancies and long-term surveillance; the 2009 guidelines of the American Thyroid Association (ATA) favor routine preoperative imaging with US over other imaging modalities for all patients with thyroid malignancies (2).
Lymph node metastases can be present in 80% of patients with papillary thyroid cancer (PTC) (3) but their impact on the oncologic outcomes is still a matter of debate, especially with regard to younger patients. Regional disease has been largely considered an indicator more than a governor of the disease and its oncologic impact is usually thought to be restricted to the older population. This notion has been challenged by recent series that identify lymphatic metastases and soft tissue extension as independent risk factors (4).
While there is consensus on the surgical management of the central and lateral neck compartments in the presence of documented disease, in the absence of abnormal findings the surgical management of the central compartment remains controversial. Proponents of elective central dissection argue that the procedure improves locoregional control (5), disease specific survival (DSS) (6), reduces reoperations (7), and is not associated with a higher complication rate in high volume centers (8). Alternatively, prophylactic central compartment dissection leads to an increase in pN1 stage that does not usually progress to clinical recurrent disease (9) and does not consistently improve survival (10). It is also argued that nodal recurrence is higher in patients who undergo a prophylactic versus therapeutic central compartment dissection (11).
The primary objective of the study was to assess the predictive value of US of the central neck compartment (CNC) for long-term recurrence and survival in PTC. The secondary objective was to evaluate the impact of elective neck dissection of the CNC in oncologic outcomes. We hypothesized that US is an accurate predictor of long-term recurrence and survival and that elective dissection does not affect oncologic outcomes in patients with nonultrasonographically or clinically detectable PTC.
Methods
The database of the University of Texas M.D. Anderson Cancer Center (MDACC) was queried to identify 505 patients who underwent definitive surgical treatment for previously untreated PTC at our institution between January 1996 and December 2003. Of these patients, 331 met the inclusion criteria which were defined as: (1) Papillary thyroid carcinoma including all histological variants, (2) preoperative US of the head and neck performed at our institution, (3) detailed description of ultrasonographic findings in the central and lateral neck compartments, and (4) initial surgical treatment performed at MDACC.
US scanning of the soft tissues of the neck was performed with a high resolution scanner (Sequoia [Acuson, Mountain View, CA], Elegra [Siemens, Issaquah, WA], HDI 5000 [Phillips-ATL, Bothell, WA], and Powervision 7000 [Toshiba, Tokyo, Japan]) and a high frequency linear-array transducer with frequencies from 7 to 13 MHz. All these studies were performed by radiologists specializing in cervical ultrasonography at our institution. The central compartment was defined as the conjunction of neck levels VI and VII as described by the American Joint Committee on Cancer (AJCC) (12), with its boundaries being the hyoid bone superiorly, the innominate artery inferiorly and common carotid arteries laterally. Lymph nodes located lateral to the common carotid artery were characterized as pertaining to the lateral neck compartment. The preoperative status of the neck compartments was determined from the sonographic appearance of the lymph nodes and specifically recorded for each lateral and for the central compartment. Each specific compartment was deemed sonographically abnormal in the presence of at least one of the following findings in a lymph node: increased size (>1 cm), rounded shape, absence of a visible hilum, irregular reflection pattern, unsharp borders, cystic change, calcifications, and nonhilar vasculature. The size cutoff value used in the US assessment of potentially suspicious lymph nodes was 1 cm (13). In the absence of any of these characteristics the compartment was considered sonographically normal.
According to our practice, a FNA of will be routinely obtained for suspicious lymph nodes if technically feasible. A biopsy of a lateral neck lymph node is preferred in patients who present with lateral and central sonographic abnormalities. Based on positive sonographic identification, localization, and histological confirmation of nodal disease, we may routinely perform a therapeutic neck dissection at the time of the thyroidectomy encompassing the contents of levels VI-VII with a compartment-oriented dissection of levels IIa, III, IV, and V based upon the levels of involvement. In contrast, for patients with a sonographically negative neck, the surgical approach reflects individual practice patterns and can be dichotomized as elective dissection based upon intraoperative findings versus prophylactic central compartment dissection (levels VI and VII).
The pathology reports were reviewed and all patients were staged according to the most current AJCC staging system based on the documented histopathological findings. Adjuvant treatment with radioactive iodine (RAI) was routinely given depending upon the postoperative whole body scan uptake among those patients possessing soft tissue extension or lymph node metastases. For patients who underwent additional RAI interventions, the cumulative RAI dose utilized was considered. Baseline suppressed and stimulated, and follow-up thyroglobulin (TG) levels were recorded in addition to the detection of antithyroglobulin antibodies. Adjuvant external beam radiation was used for patients with poorly differentiated carcinomas with extensive extrathyroidal spread, particularly when surgical management of a local recurrence would not be able to spare the laryngotracheal/esophageal organ system.
The clinical course was determined and all patients who presented with recurrent disease were identified. Cervical recurrence was defined as metastatic involvement of any cervical lymph node developing 6 months or more after surgery. The timing and location of distant disease was documented when indicated; patients with persistently elevated serum TG and negative imaging one year after treatment were considered alive with disease for all statistical analyses.
This study was approved by the MDACC Institutional Review Board. The data analysis was generated using Version 8 of the SAS System®, SAS Institute Inc, Cary, NC. Categorical patient characteristics were summarized as proportions while continuous characteristics were summarized as medians and quartiles. A recurrence event was defined as a clinical or radiological evidence of central nodal disease after surgery. The time to recurrence was measured from the date of surgery to the recurrence date, with censoring at last follow-up if no recurrence event occurred by then. Central compartment recurrence was analyzed for association with other variables, initially by Kaplan–Meier plots with log-rank test, and subsequently by Cox regression. Because only 11 recurrences were observed among 331 patients, the Cox regressions were limited to univariate analysis to avoid overfitting and monotone likelihoods (14). The combined effect of age and sonographic findings on survival and other outcome measures was assessed with Interaction test on bivariate Cox regression.
Results
The clinicopathological characteristics of the study group are summarized in Table 1. There were 112 males and 219 females with a median age of 44.0 years (range 11 to 87). TNM staging at presentation was: 151 patients with a T1, 58 patients with a T2, 70 patients with a T3, and 52 patients with a T4. The tumor was located in the right thyroid lobe in 117 cases, left thyroid lobe in 73 cases, thyroid isthmus in 8, and it was multicentric in 133 cases. A total thyroidectomy or completion thyroidectomy was performed in every instance.
Table 1.
Summary of Clinicopathological Characteristics for the Cohort (n=331)
| Variable | Number of patients | % |
|---|---|---|
| Age 45 or older | ||
| Yes | 162 | 48.9 |
| No | 169 | 51.1 |
| Sex | ||
| Female | 219 | 66.2 |
| Male | 112 | 33.8 |
| T Stage | ||
| T1 | 151 | 45.6 |
| T2 | 58 | 17.5 |
| T3 | 70 | 21.1 |
| T4 | 52 | 15.7 |
| N (+) | ||
| Yes | 172 | 52.0 |
| No | 159 | 48.0 |
| M (+) | ||
| Yes | 21 | 6.3 |
| No | 310 | 93.7 |
| Central US abnormality | ||
| Yes | 79 | 23.9 |
| No | 252 | 76.1 |
| Poor differentiation | ||
| Yes | 78 | 23.6 |
| No | 253 | 76.4 |
| Multicentricity | ||
| Yes | 133 | 40.2 |
| No | 198 | 59.8 |
| Radioactive iodine ablation | ||
| Yes | 252 | 76.1 |
| No | 79 | 23.9 |
| Radiation therapy | ||
| Yes | 33 | 10.0 |
| No | 298 | 90.0 |
TNM stage according to the American Joint Committee on Cancer TNM staging system, 7th edition.
US, ultrasound.
An ablative dose of RAI was initially used in 252 (76.1%) cases and eventually repeated based on clinical criteria. The total ablative dose utilized ranged from 28 to 455 mCi of iodine-131 with an average of 115.7 mCi. External beam radiation therapy was utilized as part of the initial treatment scheme in 33 cases (10%), likely reflecting to some degree our referral pattern. The median follow-up time for the series was 71.5 months with a range of 3.6 to 146.1 months.
Preoperative sonographic abnormalities were present in the central compartment in 79 patients (23.9%), right neck in 44 (13.3%), left neck in 41 (12.3%), and bilateral neck in 37 (11.2%) patients. The presence of nodal disease was preoperatively documented with FNA in 82.5% of the patients. Overall, 190 patients (57.4%) had a negative US in all three compartments at presentation. A therapeutic central neck dissection was performed in all 79 patients with US abnormalities and in all patients who presented with lateral neck disease. The 5-and 10-year overall survival for the cohort was 97.6% and 82.5% respectively, with a 5- and 10-year DSS of 99% and 89.1% respectively.
Central neck recurrence
During the surveillance period, 11 of the 331 (3.2%) patients recurred in the CNC; four of them with a synchronous recurrence in the lateral neck. Additionally, there were five recurrences in the right neck and nine in the left neck, with bilateral neck recurrences in three cases. The average time for central compartment recurrence was 22.8 months with a range of 6 to 55.3 months. The relationship between clinicopathological variables and central compartment recurrence-free survival (CCRFS) is presented in Table 2. Since there were no recurrences among patients who presented with distant metastasis and those treated with external beam radiation, the hazard ratios (HRs) were not calculated for these variables.
Table 2.
Univariate Cox Proportional Hazards Model for Central Compartment Recurrence-Free Survival Stratifying by Clinical Variables
| Variable | Coefficient | SE | HR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Gender (male vs. female) | 0.53 | 0.60 | 1.71 | (0.52 | 5.61) | 0.79 |
| Age ≥ 45 | −0.47 | 0.62 | 0.62 | (0.18 | 2.12) | 0.45 |
| Multicentricity (yes vs. no) | −1.04 | 0.78 | 0.35 | (0.01 | 1.63) | 0.18 |
| Poor differentiation (yes vs. no) | 0.22 | 0.67 | 1.26 | (0.33 | 4.74) | 0.74 |
| Tumor size (T3–T4 vs. T1–T2) | 1.17 | 0.62 | 3.23 | (1.15 | 11.04) | 0.012 |
| pN status (pN+ vs. pN0) | 0.95 | 0.67 | 2.59 | (0.68 | 9.79) | 0.16 |
| RAI (yes vs. no) | 1.12 | 1.04 | 3.08 | (0.39 | 24.11) | 0.28 |
M-status is not presented because there were no events in the M1 category. External radiation data is not presented because there were no events among radiated patients.
SE, standard error; HR, hazard ratio; CI, confidence interval; pN0, histopathologically negative neck nodes; pN+, histopathologically positive neck nodes; RAI, radioactive iodine.
US and neck recurrence
Preoperative central neck US was an outcome predictor for central neck recurrence; the 5- and 10-year CCRFS was (93.3%/86.2%), respectively for patients with sonographic abnormalities, versus (97.9%/97.9%), respectively for patients with a negative US (p=0.0106). Among patients with a sonographically abnormal central compartment, the presence of US abnormalities in the lateral compartments did not further increase the risk of central neck recurrence (p=0.15).However, in patients with a sonographically normal central compartment, the presence of US abnormalities in a lateral neck compartment increased the risk of central neck recurrence from 0.52% to 6.4% (p=0.0136).
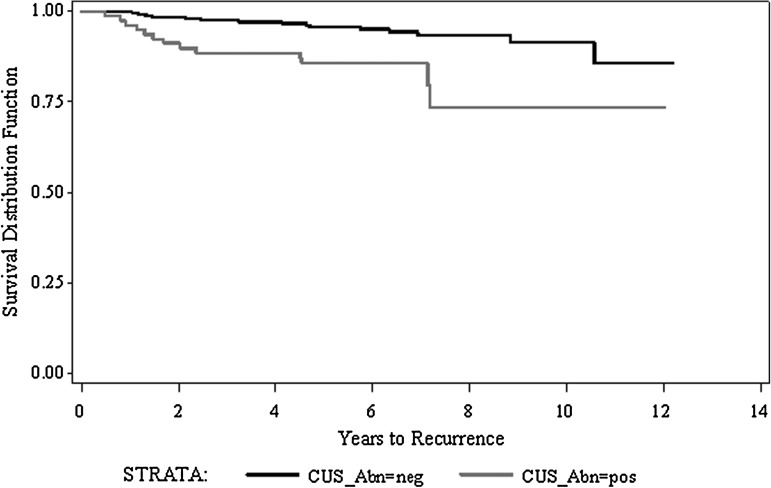
Central compartment US was also a predictor for nodal recurrence-free survival, defined as years to recurrence in any neck compartment. Figure 1 shows the Kaplan–Meier estimate stratifying by sonographic status of the central compartment at presentation. The 5- and 10-year RFS was 95.6% and 91.4%, respectively for the sonographically negative group, versus 85.7% and 73.5% for the sonographically abnormal group (p=0.0005).
FIG. 1.
Kaplan–Meier estimate for nodal recurrence-free survival (years to recurrence in any neck compartment) stratifying by sonographic status of the central neck compartment (p=0.0005).
US and survival
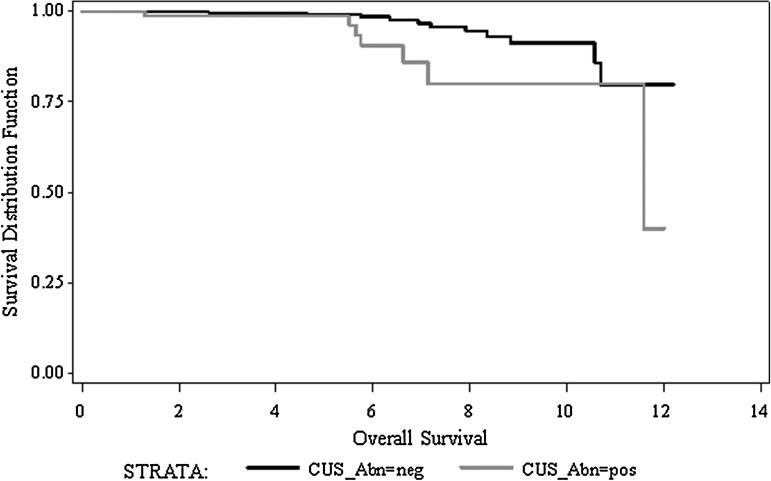
The sonographic status of the CNC was an outcome predictor for both overall and DSS. Sonographic abnormalities were associated with a reduction in the 5- and 10-year overall survival from (97.7%/86.2%) to (97.5%/69.1%; p<0.0001) and a reduction in the 5- and 10-year DSS from (99.1%/91.2%) to (98.7%/79.8%; p=0.0097), respectively as presented in Figure 2. Given the impact of age on survival in PTC, a bivariate Cox regression with interaction analysis was performed for the variables age and sonographic status of the CNC (Table 3). The predictive value of central compartment US for overall, disease-specific, and recurrence-free survival was found to be statistically significant regardless of the age at presentation.
FIG. 2.
Kaplan–Meier estimate for disease-specific survival stratifying by sonographic status of the central neck compartments (p=0.0097).
Table 3.
Bivariate Regression for Age and Central Compartment Sonographic Abnormalities on Survival and Recurrence-Free Survival
| Coefficient | SE | p-value | |
|---|---|---|---|
| Overall survival | |||
| Age >45 | 0.275 | 0.519 | 0.60 |
| Abnormal CCUS | 1.760 | 0.515 | 0.0006 |
| Age >45+abnormal CCUS | −0.802 | 0.796 | 0.31 |
| Disease-specific survival | |||
| Age >45 | −0.004 | 0.607 | 0.99 |
| Abnormal CCUS | 1.264 | 0.660 | 0.06 |
| Age >45+abnormal CCUS | −0.095 | 0.978 | 0.92 |
| Recurrence-free survival | |||
| Age >45 | 0.203 | 0.519 | 0.70 |
| Abnormal CCUS | 1.569 | 0.516 | 0.0023 |
| Age >45+abnormal CCUS | −0.741 | 0.796 | 0.35 |
Values in boldface are statistically significant.
CCUS, central compartment ultrasound; SE, standard error.
Prophylactic neck dissection
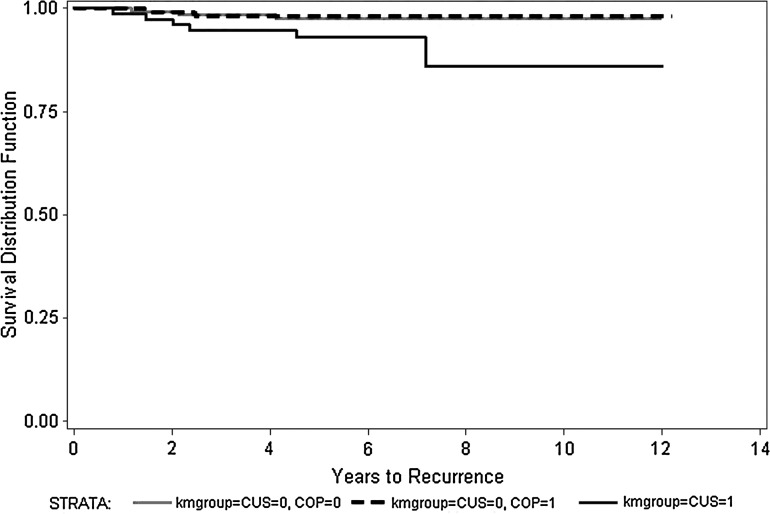
Of the 252 patients with a sonographically negative central compartment, 119 (47.2%) underwent an elective neck dissection while 133 were conservatively managed. The proportion of patients treated with adjuvant RAI was very similar in these two groups (92/119=77.3% vs. 98/133=73.6%, p=0.55); they were also comparable in age (p=0.15), T stage (p=0.27), and differentiation (p=0.09). The long-term oncologic outcomes of these groups are presented in Table 4. Overall, there were no statistically significant differences in recurrence or survival regardless of the addition of elective surgery to the treatment. Figure 3 shows a Kaplan–Meier estimate for CCRFS stratifying by the sonographic status of the central compartment and addition of elective neck dissection.
Table 4.
Long-Term Effect of Prophylactic Central Compartment Dissection in the Absence of Abnormal Sonographic Findings at Presentation
| Central compartment RFSa | Overall RFSb | Disease-specific survival | Overall survival | |||||
|---|---|---|---|---|---|---|---|---|
| Prophylactic neck dissection | 5-year | 10-year | 5-year | 10-year | 5-year | 10-year | 5-year | 10-year |
| Yes (n=119) | 98.2% | 98.2% | 95.6% | 89% | 99.2% | 90.4% | 98.1% | 82.6% |
| No (n=133) | 97.6% | 97.6% | 95.9% | 94.6% | 99.1% | 92.7% | 97.4% | 91.1% |
| p-value | 0.78 | 0.79 | 0.89 | 0.93 | ||||
Central compartment RFS: central compartment recurrence-free survival, defined as time to recurrence in the central compartment only.
Overall RFS: overall recurrence-free survival, defined as time to recurrence in any neck compartment.
FIG. 3.
Kaplan–Meier estimate for central compartment recurrence-free survival stratifying by sonographic status of the central compartments and prophylactic neck dissection. Solid black line: abnormal CCUS (p=0.0106 vs. negative CCUS); gray line: negative CCUS, no prophylactic neck dissection; dotted black line: negative CCUS, prophylactic neck dissection. CCUS, central compartment ultrasound.
Histopathological status of the central neck lymph nodes
To assess the potential effect of microscopic nodal disease in long-term oncologic outcomes, the group that underwent a prophylactic central neck dissection (n=119) was further stratified based on the histopathologic status of the nodes retrieved. Histopathologic analysis confirmed nodal metastases (pN+) in 85 (71.4%) of these specimens, while 34 (28.6%) were found to be negative (pN0). The differences in recurrence and survival between these two groups were not statistically significant. Furthermore, in patients with normal US of all three neck compartments, both disease-specific and disease-free survival reached 100%, regardless of the histopathologic status of the lymph node removed in elective dissections (Table 5).
Table 5.
Long-Term Impact of Histopathological Nodal Status in Patients with Sonographically Negative Neck Ultrasound Who Underwent Elective Central Neck Dissection
| 10-year OS | 10-year DSS | 10-year CCRFS | |
|---|---|---|---|
| Sonographically negative central compartment (n=119) | |||
| pN0 (n=34) | 97.1% | 97.1% | 97.1% |
| pN+ (n=85) | 91.8% | 94.1% | 91.8% |
| p-value | 0.32 | 0.49 | 0.32 |
| Sonographically negative central and lateral compartments (n=76) | |||
| pN0 (n=29) | 100% | 100% | 100% |
| pN+ (n=47) | 97.9% | 100% | 97.9% |
| p-value | 0.44 | — | 0.44 |
OS, overall survival; DSS, disease-specific survival; CCRFS, central compartment recurrence-free survival.
Discussion
The incidence of papillary thyroid carcinoma has almost doubled in the last decades, predominantly in those with Caucasian ancestor background. Since the mortality for the disease has remained stable, it is frequently assumed that the incremental changes in incidence are, in part, due to improved detection (15). Papillary thyroid carcinoma has a remarkable tendency to invade regional lymph nodes with incidences reported as high as 80% when microscopic disease is accounted for (16).
Since its introduction, US has been playing an ever more prominent role in the evaluation and management of patients with thyroid cancer proving to affect the surgical planning in up to 30% of patients (17–19). The current ATA guidelines recommend preoperative neck US as the main staging tool in all patients undergoing thyroidectomy for malignant cytological findings on biopsy (20). Despite the wide acceptance of the technique, it is well known that the sensitivity of US in the diagnosis of nodal disease in the central compartment is lower than in the lateral neck. In a recent series, Hwang and Orloff concluded that the sensitivity and specificity of US for detection of PTC metastasis in the central neck was 30% and 87% respectively, versus 93.8% and 80% for the lateral neck (21). In another communication, Leboulleux et al. reported that US only identified half of lymph nodes found during surgery (18), which he attributed to overlying thyroid tissue concealing lymph nodes located in the tracheoesophageal groove and deep paratracheal regions. US also has limitations to identify and characterize lymph nodes located low in the neck or in the superior mediastinum (Level VII), where the presence of bony structures represents a major obstacle for adequate sonographic exposure.
In spite of its limitations preoperative US identification of suspicious lymph nodes by US is not uncommon. In the present series, we found that 23.9% of the patients had central compartment abnormalities at presentation which is within the range of 20%–31% frequently cited in the literature (22,23).
There is little debate regarding surgical indication in patients that present with abnormal clinical or sonographic findings in the central compartment. The ATA guidelines recommend a therapeutic level VI and VII central neck dissection for patients with clinically involved central lymph nodes based on evidence suggesting that this procedure could improve overall survival and decrease recurrence rates (9,17,24). According to our interdisciplinary program recommendations, all patients in the series who presented with a suspicious US underwent a therapeutic central neck compartmental dissection.
Whereas there is consensus regarding the surgical indication of patients with proven neck metastasis at the time of diagnosis, the ideal management of the CNC in the absence of clinical or radiological findings remains highly debated. The initial ATA Guidelines, published in 2006, recommended a prophylactic central dissection (2). In the 2009 ATA guidelines (20), recommendation for a prophylactic central neck dissection was restricted to high risk patients, such as those with large tumors, but the recommendation was made in a less emphatic fashion.
Unequivocally, the most relevant finding in this study is that in a cohort routinely treated with thyroidectomy and adjuvant RAI, the presence of macroscopic disease—as detected by US—was a significant prognostic indicator, whereas the presence of microscopic disease, as identified among patients who underwent elective neck dissection, was not.
In terms of locoregional control we found that US was a predictor for central neck recurrence, with a reduction in 10-year CCRFS from 97.9% to 86.2% among patients who presented with an abnormal CNC US test. This difference becomes even more relevant when we take into account that all these patients underwent a therapeutic central neck dissection and the vast majority of them received adjuvant RAI. The relationship between lymphatic nodal extension and regional recurrence has been documented in the literature. In a study by Wada et al., patients with histopathologically positive lymph nodes had 16.3% recurrence rate versus 0% for those with negative lymph nodes (25). Interestingly, the recurrence rate was higher in patients with palpable nodal disease than those with microscopic disease alone; the author suggested that this could reflect a difference in biological behavior between macro- and microscopic nodal disease. Such a difference in biologic behavior might also explain the lack of benefit in regional control rates we observed in the group treated with elective neck dissection. Approximately one third of patients with a normal US underwent elective dissection, based primarily on individual practice patterns of the attending surgeons; most importantly, these groups were comparable in the initial staging and use of RAI. We found no differences in regional control rate or survival between these groups in long-term follow-up, regardless of the high proportion of microscopic metastatic disease (71.4%) found on the surgical specimens of those surgically treated.
The rationale for prophylactic neck dissection comes from a combination of the high rate of micrometastasis identified on specimens, limited sensitivity of preoperative imaging, and increased morbidity of reoperation for recurrent disease in the central compartment (26). The evidence supporting an improvement in regional control with such a procedure is not consistent; only two of the six retrospective trials that have examined this issue support lower recurrence rates and there are no prospective trials in the literature (27). Our findings are consistent with this data and support a conservative approach to the central compartment in the absence of macroscopic nodal involvement, as detected by US.
Since the elevated rate of micrometastasis found on prophylactic dissection specimens is usually considered one of the strongest arguments in favor of the procedure, we compared the outcomes of this subgroup of patients stratifying by the histopathological nodal status. We found no statistically significant differences in the 10-year recurrence-free survival between patients with histopathologically positive versus negative lymph nodes. Moreover, the 10-year recurrence-free survival among patients with positive nodes increased from 91.8% to 97.9% when all three neck compartments were negative versus the central compartment alone, emphasizing the importance of a comprehensive sonographic examination of the neck upon presentation. These findings can be interpreted in light of the pattern of lymphatic dissemination in PTC, where the central compartment lymph nodes represent the first echelon for lymphatic drainage. As such, the presence of lateral compartment nodes should be considered as an indicator for subclinical central compartment disease. This notion is reinforced by Machens et al. (28) who reported that at least 80% of patients with lateral nodal disease will have central compartment metastasis on histopathological examination.
Several studies have reached the conclusion that while lymphatic extension is associated with an increased risk of regional recurrence, there appears to be a significant difference between histopathological disease and macroscopic nodal disease (11,29). Our findings further support this notion since microscopic nodal involvement was not a predictor for recurrence-free survival in this series. While US is limited in terms of absolute sensitivity for diagnosing nodal disease at the microscopic level (21), it appears to be an effective tool to document macroscopic nodal extension which is essentially clinically-significant lymphatic disease. More importantly, the preoperative distinction between histopathological and macroscopic nodal extension may have deep implications in risk stratification and treatment algorithms in the future. Our data suggest that high resolution US can be a valuable tool to guide the clinician in the decision-making process that follows the diagnosis of PTC.
Besides a positive US, a large primary tumor size was also a predictor for CCRFS. In this series, patients with large primaries (T3–T4) had a statistically significant higher risk (HR=3.23) for central neck recurrence versus patients with earlier stage tumors (T1–T2). Tumor size has been previously identified as a risk factor for neck recurrence in PTC. In a recent publication, Giles Senyurek et al. (30) found that the rate of neck recurrence was higher among patients with large primary tumors, gross extrathyroidal extension, and adverse histological features. One of the accepted hypotheses for this association has been proposed by Noguchi et al. (31), who proposed that this could be secondary to the increased rate of soft tissue extension observed in larger tumors. The current ATA guidelines (20) acknowledge primary tumor size as a risk factor for nodal recurrence and recommend elective dissection of the CNC in this subset of patients.
The impact of nodal involvement upon survival is still a matter of debate. In the present series we found that macroscopic involvement in the central compartment, as documented by preoperative US, had a statistically significant impact in DSS among patients >45 years of age. In a study based on the SEER database, Podnos et al. reported that the presence of lymphatic extension was associated with a limited but statistically significant reduction in 10-year survival from 82% to 79% (32). In a similar way, a large Swedish case-control study reported that the presence of lymph node metastasis was one of their main predictors of death (odds ratio=1.9) (4).
In this series US was a predictor for survival; the presence of sonographic abnormalities in the central compartment was associated with a significant decrease in 10-year DSS from 91.2% to 79.8%. Interestingly, bivariate analysis indicates that the preoperative US is an age-independent predictor of survival. In fact, age at presentation was not an outcome predictor per se in this series. Of the 10 patients younger than 45 years who died of the disease, 5 presented with metastatic disease and 3 had poorly differentiated carcinomas with extensive soft tissue extension. The unusual mortality in this subset of patients can be, at least partially, explained by these findings and the bias of being a tertiary referral center.
There are some limitations of this study that are inherent to its design. It is well known that US is an operator-dependent test and its sensitivity for the detection of lymphatic abnormalities will not only be influenced by technical factors, but most importantly by the experience of the ultrasonographer. The department of diagnostic radiology at MDACC performs ∼10,000 cervical sonograms/year, the vast majority of them are for initial evaluation or surveillance for thyroid cancer. In this regard, the findings of the present series must be interpreted in the context of a highly specialized environment from both a diagnostic and therapeutic standpoint. Further, the quality of this specialized unit also plays a part in the detection of recurrences that may otherwise remain undetected among less experienced diagnosticians.
A second limitation of the study is that it does not allow us to evaluate the impact of RAI on the oncologic outcomes. The proportion of patients treated with RAI was almost exactly the same in the group who underwent elective neck dissection and those conservatively managed (77.3% vs. 77.6% respectively); even more, in the group treated with surgery, 78.8% received RAI in the histopathologically positive group while 75.8% in the negative group (p=0.80). Since there were no statistically significant differences in long-term recurrence or survival between these groups, a plausible explanation is that RAI effectively ablated microscopic disease in the group conservatively treated. As such, the high regional control rates herein presented must be viewed in light of a highly specialized diagnostic environment and aggressive use of adjuvant therapy in which they were produced. Finally, the retrospective nature of the study must also be considered as an inherent limitation given the potential bias in the selection of both surgical and adjuvant treatment.
Despite its limitations, we believe that one of the unique strengths of this study is it reliance on the direct comparison of patient groups that primarily differed on their management of central compartment lymph nodes. In contrast to most studies analyzing the role of elective central neck dissection, in this cohort the groups were contemporary, comparable, and had the exact same baseline evaluation and long-term follow-up. This provides a unique perspective to compare oncologic outcomes based primarily on the differential management of the CNC.
Local or regional disease is rarely the cause of death in these patients; most of them will succumb to uncontrolled distant disease, which may be present at the time of diagnosis in up to one third of patients. If regional lymphatic metastases are viewed as indicators of biologic behavior and distant metastatic potential of PTC, central neck US could be regarded as a reliable surrogate of macroscopic nodal involvement and be utilized for risk stratification. Based on our findings, we conclude that there is no indication for surgical management of the central neck in the absence of sonographically or intraoperatively defined evidence of nodal disease.
Conclusions
US findings in the CNC are age-independent predictors of outcome for DSS and recurrence-free survival in patients with PTC. Prophylactic central compartment dissection does not improve long-term regional disease control or survival, regardless of the histopathological status of the lymph nodes retrieved. US is a reliable tool to identify clinically-relevant nodal disease in the central compartment and should be considered to have a more relevant role in risk stratification and treatment planning in patients with PTC.
Addendum
Readers may be interested in the authors' previous report concerning the long-term prognostic value of lateral neck ultrasound in papillary thyroid cancer. In that study, preoperative ultrasound was found to be an excellent outcome predictor for lateral neck disease-free interval and disease-specific survival (33).
Acknowledgments
The authors would like to acknowledge Rolando De Luna, C-PA, and Patricia Lopez-Rojas, B.S.N. for their valuable assistance in data collection.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Heller KS. Do all cancers need to be treated? The role of thyroglobulin in the management of thyroid cancer: the 2006 Hayes Martin lecture. Arch Otolaryngol Head Neck Surg. 2007;133:639–643. doi: 10.1001/archotol.133.7.639. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Sherman SI. Tuttle RM. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–559. doi: 10.1056/NEJM199302253280807. [DOI] [PubMed] [Google Scholar]
- 4.Lundgren CI. Hall P. Dickman PW. Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106:524–531. doi: 10.1002/cncr.21653. [DOI] [PubMed] [Google Scholar]
- 5.Kupferman ME. Patterson DM. Mandel SJ. LiVolsi V. Weber RS. Safety of modified radical neck dissection for differentiated thyroid carcinoma. Laryngoscope. 2004;114:403–406. doi: 10.1097/00005537-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Gimm O. Thyroid cancer. Cancer Lett. 2001;163:143–156. doi: 10.1016/s0304-3835(00)00697-2. [DOI] [PubMed] [Google Scholar]
- 7.Buhr HJ. Mann B. [Thyroidectomy and lymphadenectomy] Chirurg. 1999;70:987–998. doi: 10.1007/s001040050756. [DOI] [PubMed] [Google Scholar]
- 8.Palazzo FF. Gosnell J. Savio R. Reeve TS. Sidhu SB. Sywak MS. Robinson B. Delbridge LW. Lymphadenectomy for papillary thyroid cancer: changes in practice over four decades. Eur J Surg Oncol. 2006;32:340–344. doi: 10.1016/j.ejso.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Gemsenjager E. Perren A. Seifert B. Schuler G. Schweizer I. Heitz PU. Lymph node surgery in papillary thyroid carcinoma. J Am Coll Surg. 2003;197:182–190. doi: 10.1016/S1072-7515(03)00421-6. [DOI] [PubMed] [Google Scholar]
- 10.Hughes CJ. Shaha AR. Shah JP. Loree TR. Impact of lymph node metastasis in differentiated carcinoma of the thyroid: a matched-pair analysis. Head Neck. 1996;18:127–132. doi: 10.1002/(SICI)1097-0347(199603/04)18:2<127::AID-HED3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Wada N. Duh QY. Sugino K. Iwasaki H. Kameyama K. Mimura T. Ito K. Takami H. Takanashi Y. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237:399–407. doi: 10.1097/01.SLA.0000055273.58908.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Joint Committee on Cancer. AJCC Cancer Staging Manual. Seventh. Springer; New York: 2010. [Google Scholar]
- 13.Ahuja AT. Chow L. Chick W. King W. Metreweli C. Metastatic cervical nodes in papillary carcinoma of the thyroid: ultrasound and histological correlation. Clin Radiol. 1995;50:229–231. doi: 10.1016/s0009-9260(05)83475-0. [DOI] [PubMed] [Google Scholar]
- 14.Hosmer DW LS. Numerical Problems. John Wiley & Sons; New York: 1999. [Google Scholar]
- 15.Iyer NG. Shaha AR. Silver CE. Devaney KO. Rinaldo A. Pellitteri PK. Ferlito A. Thyroid incidentalomas: to treat or not to treat. Eur Arch Otorhinolaryngol. 2010;267:1019–1026. doi: 10.1007/s00405-010-1207-1. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez HE. Cruz F. O'Brien A. Goni I. Leon A. Claure R. Camus M. Dominguez F. Mosso L. Arteaga E. Gonzalez G. Lopez JM. Rodriguez JA. Carrasco C. Fardella C. Impact of preoperative ultrasonographic staging of the neck in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133:1258–1262. doi: 10.1001/archotol.133.12.1258. [DOI] [PubMed] [Google Scholar]
- 17.Kouvaraki MA. Shapiro SE. Fornage BD. Edeiken-Monro BS. Sherman SI. Vassilopoulou-Sellin R. Lee JE. Evans DB. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134:946–954. doi: 10.1016/s0039-6060(03)00424-0. [DOI] [PubMed] [Google Scholar]
- 18.Leboulleux S. Girard E. Rose M. Travagli JP. Sabbah N. Caillou B. Hartl DM. Lassau N. Baudin E. Schlumberger M. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:3590–3594. doi: 10.1210/jc.2007-0444. [DOI] [PubMed] [Google Scholar]
- 19.Marshall CL. Lee JE. Xing Y. Perrier ND. Edeiken BS. Evans DB. Grubbs EG. Routine pre-operative ultrasonography for papillary thyroid cancer: effects on cervical recurrence. Surgery. 2009;146:1063–1072. doi: 10.1016/j.surg.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 20.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 21.Hwang HS. Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011;121:487–491. doi: 10.1002/lary.21227. [DOI] [PubMed] [Google Scholar]
- 22.Solorzano CC. Carneiro DM. Ramirez M. Lee TM. Irvin GL., 3rd Surgeon-performed ultrasound in the management of thyroid malignancy. Am Surg. 2004;70:576–580. discussion 580–572. [PubMed] [Google Scholar]
- 23.Shimamoto K. Satake H. Sawaki A. Ishigaki T. Funahashi H. Imai T. Preoperative staging of thyroid papillary carcinoma with ultrasonography. Eur J Radiol. 1998;29:4–10. doi: 10.1016/s0720-048x(97)00184-8. [DOI] [PubMed] [Google Scholar]
- 24.Ito Y. Tomoda C. Uruno T. Takamura Y. Miya A. Kobayashi K. Matsuzuka F. Kuma K. Miyauchi A. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J Surg. 2004;28:498–501. doi: 10.1007/s00268-004-7192-z. [DOI] [PubMed] [Google Scholar]
- 25.Wada N. Suganuma N. Nakayama H. Masudo K. Rino Y. Masuda M. Imada T. Microscopic regional lymph node status in papillary thyroid carcinoma with and without lymphadenopathy and its relation to outcomes. Langenbecks Arch Surg. 2007;392:417–422. doi: 10.1007/s00423-007-0159-4. [DOI] [PubMed] [Google Scholar]
- 26.Gopalakrishna Iyer N. Shaha AR. Complications of thyroid surgery: prevention and management. Minerva Chir. 2010;65:71–82. [PubMed] [Google Scholar]
- 27.Iyer NG. Shaha AR. Central compartment dissection for well differentiated thyroid cancer…and the band plays on. Curr Opin Otolaryngol Head Neck Surg. 2011;19:106–112. doi: 10.1097/MOO.0b013e328343af58. [DOI] [PubMed] [Google Scholar]
- 28.Machens A. Hauptmann S. Dralle H. Lymph node dissection in the lateral neck for completion in central node-positive papillary thyroid cancer. Surgery. 2009;145:176–181. doi: 10.1016/j.surg.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Ito Y. Uruno T. Nakano K. Takamura Y. Miya A. Kobayashi K. Yokozawa T. Matsuzuka F. Kuma S. Kuma K. Miyauchi A. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003;13:381–387. doi: 10.1089/105072503321669875. [DOI] [PubMed] [Google Scholar]
- 30.Giles Senyurek Y. Tunca F. Boztepe H. Alagol F. Terzioglu T. Tezelman S. The long term outcome of papillary thyroid carcinoma patients without primary central lymph node dissection: expected improvement of routine dissection. Surgery. 2009;146:1188–1195. doi: 10.1016/j.surg.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi S. Yamashita H. Uchino S. Modified radical neck dissection is better than partial dissection of lymph nodes. World J Surg. 2009;33:394–396. doi: 10.1007/s00268-008-9813-4. [DOI] [PubMed] [Google Scholar]
- 32.Podnos YD. Smith D. Wagman LD. Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005;71:731–734. doi: 10.1177/000313480507100907. [DOI] [PubMed] [Google Scholar]
- 33.Moreno MA. Agarwal G. de Luna R. Siegel ER. Sherman SI. Edeiken-Monroe BS. Clayman GL. Preoperative lateral neck ultrasonography as a long-term outcome predictor in papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. 2011;137:157–162. doi: 10.1001/archoto.2010.254. [DOI] [PubMed] [Google Scholar]