Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common type of chronic liver disease in the United States, affecting an estimated 70 million Americans. The histologic spectrum of NAFLD ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), fibrosis, and eventually cirrhosis. Patients with NASH and significant fibrosis seen on liver biopsy have an increased risk for liver-related morbidity and mortality compared to patients with simple steatosis. Due to the high prevalence of NAFLD, there has been an urgent need to develop reliable noninvasive markers and tests that can accurately predict the presence of advanced disease without the need for liver biopsy. These tests can be divided into 2 groups: those that predict the presence of NASH (such as markers of hepatocyte apoptosis, oxidative stress, and inflammation, as well as predictive models based on clinical variables) and those that predict the presence of fibrosis (such as simple and complex predictive models). This paper provides an overview of various noninvasive methods for detecting NAFLD and suggests a diagnostic algorithm that can be used in clinical practice.
Keywords: Nonalcoholic ratty liver disease, nonalcoholic steatohepatitis, fibrosis, oxidative stress, apoptosis, noninvasive markers, liver biopsy
Obesity, metabolic syndrome (MetS), and type 2 diabetes have reached epidemic proportions, and these conditions are strongly associated with nonalcoholic fatty liver disease (NAFLD), which is currently the most common type of chronic liver disease in both adults and children.1-3 In the United States, 1 in 3 adults and 1 in 10 children or adolescents have simple steatosis (SS), a histologic subtype within the spectrum of NAFLD, which is characterized by triglyceride accumulation in liver cells.4,5 Nonalcoholic steatohepatitis (NASH) is defined, as lipid accumulation with evidence of cellular damage, inflammation, and different degrees of scarring or fibrosis.6,7 NASH has been shown to be present in more than 25% of severely obese patients, 40% of whom have advanced stages of fibrosis.8
NASH is a serious condition that can progress to cirrhosis and its feared complications of portal hypertension, liver failure, and hepatocellular carcinoma.9-11 Cirrhosis has been shown to develop in 21-28% of NASH patients compared to only 3% of SS patients.10 Researchers do not yet know why steatosis is nonprogressive in some patients, while other patients develop liver injury and cirrhosis. The presence and extent of fibrosis, in association with NASH, are important factors in the prognosis of NAFLD. In fact, a recent meta-analysis demonstrated that the hazard ratio for liver-related mortality was 5.7 for patients with NASH and 10 for those with advanced fibrosis compared to patients with SS.12 This finding clearly indicates that the natural history of NAFLD-related liver morbidity and mortality depends on histologic severity, as determined by the presence of NASH and the stage of fibrosis.
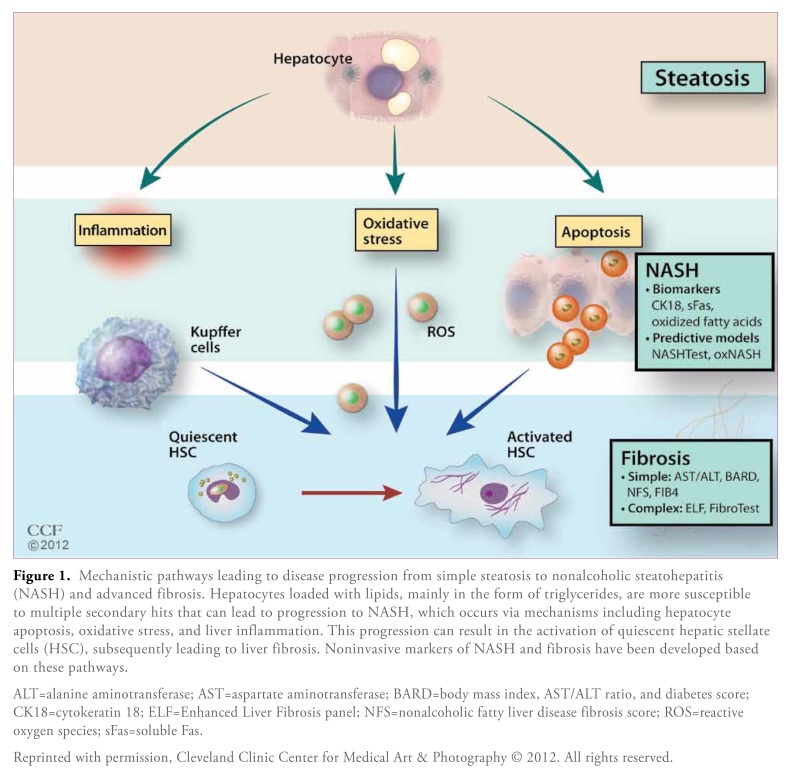
Histologic staging and grading via liver biopsy is the only method currently available for differentiating SS from NASH and for assessing the severity of liver fibrosis in patients with NAFLD. However, given the disease burden of NAFLD, liver biopsy is not a logistically feasible diagnostic method. In addition, liver biopsy is prone to sampling errors, and it is an invasive procedure that is associated with significant potential complications; the mortality rate following percutaneous liver biopsy is approximately 1 in 10,000 patients.13,14 Furthermore, because biopsies are also used to determine patient response to therapeutic interventions and to monitor disease progression, some patients may need to undergo multiple liver biopsies in their lifetime.15 Therefore, in recent years, there has been increasing interest in the possibility of identifying liver damage via noninvasive surrogate markers that can be measured in peripheral blood (Figure 1). Many of these tests have been described in the literature.16 A common way of comparing the diagnostic accuracy of different tests is by performing a receiver operating characteristics (ROC) analysis, which is the graphical representation of the compromise between false-negative and false-positive rates for every possible cutoff, and then calculating the area under the ROC curve (AUROC). A diagnostic test is considered to be ideal when it has an AUROC of 1.
Figure 1.
Mechanistic pathways leading to disease progression from simple steatosis to nonalcoholic steatohepatitis (NASH) and advanced fibrosis. Hepatocytes loaded with lipids, mainly in the form of triglycerides, are more susceptible to multiple secondary hits that can lead to progression to NASH, which occurs via mechanisms including hepatocyte apoptosis, oxidative stress, and liver inflammation. This progression can result in the activation of quiescent hepatic stellate cells (HSC), subsequently leading to liver fibrosis. Noninvasive markers of NASH and fibrosis have been developed based on these pathways.
ALT=alanine aminotransferase; AST=aspartate aminotransferase; BARD=body mass index, AST/ALT ratio, and diabetes score; CK18=cytokeratin 18; ELF=Enhanced Liver Fibrosis panel; NFS=nonalcoholic fatty liver disease fibrosis score; ROS=reactive oxygen species; sFas=soluble Fas.
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2012. All rights reserved.
This paper will review noninvasive methods of diagnosing the presence of NASH and advanced fibrosis within the spectrum of NAFLD.
Noninvasive Diagnosis of Nonalcoholic Steatohepatitis
Biomarkers of Nonalcoholic Steatohepatitis
Markers of Apoptosis Increased cell death in the liver has emerged as an important mechanism that contributes to disease progression to NASH.17 Apoptosis, or programmed cell death, is a highly organized process that can occur via 2 fundamental pathways: extrinsic mediation by death receptors (such as Fas) or intrinsic mediation by organelles (such as mitochondria). Both pathways can lead to the activation of effector caspases (mainly caspase 3), which cleave different intracellular substrates, including cytokeratin 18 (CK18), which is the major intermediate filament protein in hepatocytes. Caspase-generated CK18 fragment levels can be measured in plasma using the M30 monoclonal antibody enzyme-linked immunosorbent assay (ELISA), and these levels have been found to be significantly higher in NASH patients than SS patients.18,19 CK18 fragments have been extensively validated as a marker of NASH in multiple studies, with a pooled AUROC of 0.82 (95% confidence interval [CI], 0.76-0.88), and they have been recognized by the recent NAFLD guidelines as the most promising noninvasive test for diagnosing and managing NAFLD.12,20 However, this assay is not yet commercially available, and there is no established CK18 cutoff value for identifying NASH because each study has utilized its own cutoff value. Building on this research, our group recently found significantly higher levels of the death receptor Fas measured in plasma (soluble Fas [sFas]) in NASH patients than in SS patients or control patients.21 We developed a prediction model for NASH that included CK18 fragment levels and sFas levels, with AUROCs of 0.93 in the training set and 0.79 in the validation set. However, this model must be validated externally and in larger studies before it can be used in clinical practice.
In contrast to the M30 ELISA—which only detects caspase-cleaved CK18 (CK18 fragments)—the M65 ELISA can detect both caspase-cleaved and uncleaved CK18 (total CK18), and this assay is used as a marker of overall hepatocyte death, including both apoptosis and necrosis. In addition, this assay has shown some promise for detecting steatosis, steatohepatitis, and liver fibrosis in a cohort of patients with chronic liver disease.22
Markers of Oxidative Stress Oxidative stress plays a central role in hepatocyte injury and disease progression from SS to NASH, but precise molecular species have not yet been identified.23-27 Several oxidation pathways may play a role in the overproduction of lipid peroxidation products in NASH patients, including enzymatic and nonenzymatic free radical-mediated processes. Each of these pathways may generate different oxidation products that could potentially be quantified. Chalasani and colleagues measured systemic lipid peroxidation in patients with biopsy-confirmed NASH and control patients matched by age, gender, and body mass index (BMI); the researchers found that levels of both oxidized low-density lipoprotein and thiobarbituric acid-reacting substances were significantly higher in NASH patients.28 On the other hand, Başkol and associates investigated levels of the antioxidant enzyme paraoxonase 1 and found significantly lower levels in the NASH cohort.29 However, there was no correlation between the level of this enzyme and the grade or stage of NAFLD. Progressive decreases in the hepatic tissue levels of superoxide dismutase, catalase, and gluta-thione peroxidase—as well as the antioxidant capacity of plasma—have been shown across the histologic spectrum of NAFLD.26 Via mass spectrometry, our group demonstrated that products of free radical-mediated oxidation of linoleic acid (9- and 13-hydroxy octadecadienoic acid and 9- and 13-oxo-octadecadienoic acid) measured in plasma were significantly elevated in NASH patients compared to patients with SS or patients with normal biopsies.30
Markers of Inflammation The well-recognized chronic inflammatory state that exists in obesity and NAFLD may contribute to disease progression to NASH. Levels of proinflammatory cytokines, such as tumor necrosis factor-α and interleukin (IL)-6, have been shown to be higher in NASH patients than SS patients, but the differences have not been significant enough to allow the use of these cytokines as noninvasive markers for predicting the presence of NASH.31,32 Many other cytokines (IL-1B and macrophage inflammatory proteins) and adipokines (resistin, visfatin, and retinol-binding protein 4) have been studied as potential biomarkers, with conflicting results. The blood neutrophil to lymphocyte (N/L) ratio is a simple indicator of the overall inflammatory status of the body that has been used to predict outcomes in patients with cancer and coronary artery disease. In a recent study, we identified the N/L ratio as a novel noninvasive marker of NAFLD severity, and we demonstrated that this ratio was higher in patients with NASH than those with SS.33 Furthermore, we found that the N/L ratio correlated with the main histologic features of NAFLD, including inflammation and fibrosis.
Serum ferritin is an acute-phase reactant that can be induced in the setting of chronic systemic inflammation, and it has been observed to be elevated in patients with obesity-related complications such as diabetes and MetS. Recently, Kowdley and coauthors demonstrated that ferritin levels more than 1.5 times the upper limit of normal were associated with the diagnosis of NASH and advanced fibrosis in a large cohort of biopsy-confirmed NAFLD patients who were enrolled in the NASH Clinical Research Network (CRN).34
Predictive Models
Predictive models combine routinely assessed clinical variables with laboratory tests and biomarkers (such as hepatocyte apoptosis markers, oxidative stress markers, and inflammatory cytokines) to accurately predict the presence of NASH on liver biopsy. Examples of predictive models that use a combination of clinical and laboratory data are the HAIR score (which is based on hypertension, alanine aminotransferase [ALT] level, and insulin resistance) and the NASH predictive index (which is based on age, gender, BMI, homeostatic model assessment of insulin resistance, and log [aspartate aminotransferase {AST} x ALT]).8,35 Although the accuracy of these models for predicting the presence of NASH is promising (AUROCs of 0.90 and 0.87, respectively), these models have not been externally validated in other cohorts in a prospective manner. The NASHTest (BioPredictive) was developed in a set of 160 patients by combining 13 clinical and biochemical variables: age; gender; weight; height; and serum levels of cholesterol, triglycerides, α2 macroglobulin, apolipoprotein A1, haptoglobin, gamma glutamyl-transferase (GGT), ALT, AST, and bilirubin.36 The AUROC was 0.78 for pathologist-diagnosed NASH. The NASHTest has been validated in a cohort of 97 patients from different centers.
The NASH CRN recently developed progressive models based on readily available clinical and laboratory variables for predicting histologic diagnoses on liver biopsy (including the presence of NASH).37 A model based on AST level, ALT level, AST/ALT ratio, demographics (age, race, gender, and ethnicity), comor-bidities (hypertension, type 2 diabetes, BMI, waist circumference, waist/hip ratio, and acanthosis nigricans), and other laboratory tests yielded an AUROC of 0.79 (95% CI, 0.76-0.83) for predicting NASH on liver biopsy; in addition, this model yielded a similar AUROC for predicting the presence of ballooning degeneration, which is a main histologic feature of NASH.
Several predictive models include NASH biomarkers in addition to clinical variables. For example, CK18 has been combined with ALT levels and the presence of MetS in a new composite scoring system (known as the Nice model) designed to diagnose NASH in morbidly obese patients; this system has yielded promising results (AUROCs of 0.83-0.88).38 Younossi and colleagues recently developed a NAFLD diagnostic panel (with a NASH prediction model) based on diabetes, gender, BMI, triglycerides, M30 (CK18 fragments as a marker of apoptosis), and M65 plus M30 (total CK18 and CK18 fragments as a marker of necrosis); this model yielded an AUROC of 0.81 (95% CI, 0.70-0.89), which was higher than the AUROC of CK18 fragments alone (0.71; 95% CI, 0.60-0.81).39
The risk score oxNASH was developed by Feldstein and associates based on multivariable modeling and the finding that products of free radical-mediated oxidation of linoleic acid were significantly higher in patients with NASH.30 This score was calculated from age, BMI, AST level, and the ratio of 13-hydroxy octadecadienoic acid to linoleic acid. Patients with oxNASH scores over 72 were 10 times more likely to have NASH than patients with oxNASH scores less than 47.30 In a sample of 122 patients with biopsy-confirmed NAFLD, we recently demonstrated that oxNASH scores correlated with his-tologic features that define NASH, including steatosis, ballooning, and inflammation.40
Noninvasive Diagnosis of Liver Fibrosis
The presence and extent of fibrosis may be the most important factors in the prognosis of NAFLD and in the prediction of the risk of progression to cirrhosis and its complications.41 Factors that predict the development of progressive fibrosis and cirrhosis include obesity, type 2 diabetes, age older than 45 years, an elevated AST/ALT ratio, hypertension, and hyperlipidemia.8,42,43 Over the past decade, many noninvasive strategies have been developed to predict the stage of liver fibrosis in this patient population. Nonradiologic tests can be grouped into simple bedside models (which use a combination of age, BMI, AST/ALT ratio, and other clinical variables) or more complex models such as the Enhanced Liver Fibrosis (ELF) panel (which use serum markers of fibrosis).43-45 The stages of NASH-associated fibrosis range from absent (stage F0) to cirrhosis (stage 4), with stages F2—F4 considered to be clinically significant and stages F3—F4 considered to be advanced fibrosis. When interpreting studies of predictive models and markers of fibrosis, it is important to determine whether the primary objective is the identification of any fibrosis, clinically significant fibrosis, or advanced fibrosis. In this paper, discussion will be limited to the prediction of advanced fibrosis, which mandates close monitoring for the development of cirrhosis and its complications. Recently developed imaging techniques—such as the measurement of liver stiffness via transient elastography—have shown promising results for staging fibrosis in NAFLD patients; however, describing the utility of these methods is beyond the scope of this paper.
Simple Predictive Models for Advanced Fibrosis
AST/ALT Ratio ALT levels are usually higher than AST levels in NAFLD patients; however, an AST/ALT ratio greater than 1 is suggestive of an advanced fibrotic form of the disease. This ratio is the simplest predictive model for advanced fibrosis, and it can be calculated using readily available liver function tests. Despite its simplicity, this ratio has a good negative predictive value and can be used to rule out the presence of advanced fibrosis, as demonstrated in a study conducted by McPherson and coauthors.46 The AST/ALT ratio has also been incorporated into other models, including the BMI, AST/ALT ratio, and diabetes (BARD) score and the NAFLD fibrosis score (NFS).
BMI, AST/ALT Ratio, and Diabetes Score Developed in a study of 827 NAFLD patients, this score combines variables in a weighted sum in order to generate an easily calculated composite score for predicting advanced fibrosis (BMI ≥28=1 point; AST/ALT ratio ≥0.8=2 points; and the presence of diabetes=1 point).44 A BARD score of at least 2 was associated with an odds ratio of 17 (95% CI, 9.2—31.9) and an AUROC of 0.81 for detecting stages 3—4 fibrosis. In recent studies, use of the BARD score has been associated with lower AUROCs, ranging from 0.70 to 0.77.46,47
Nonalcoholic Fatty Liver Disease Fibrosis Score The NFS was developed by Angulo and associates in a large cohort of patients with NAFLD that was confirmed on biopsy.48 The NFS is based on age, hyperglycemia, BMI, platelet count, albumin level, and AST/ALT ratio. The score has 2 cutoff values: A score less than —1.455 predicts the absence of advanced fibrosis, whereas a score greater than 0.675 predicts the presence of advanced fibrosis. The NFS has been validated in multiple studies, and a recent meta-analysis revealed an AUROC of 0.85 (95% CI, 0.81—0.90).12 The recent NAFLD guidelines acknowledged that the NFS is a clinically useful tool for identifying advanced fibrosis in patients with NAFLD.20 This score is available online at http://nafldscore.com/, and it can be easily calculated during patient visits. However, a major drawback of this score is that a large percentage of patients fall in the indeterminate category and cannot be classified as having a high or low probability of advanced fibrosis.
FIB4 Index The FIB4 index was originally developed to stage liver fibrosis in patients with hepatitis C virus infection; this index is based on age, platelet count, ALT level, and AST level.49 The FIB4 index has been used in NAFLD patients with promising results. Using a cutoff value less than 1.3, the FIB4 index has a negative predictive value of 90—95% for ruling out advanced fibrosis.46,47 Interestingly, when the FIB4 index was compared to other noninvasive markers of fibrosis—including the AST/ALT ratio, BARD score, and the NFS—it had the highest AUROC for predicting advanced fibrosis (0.80—0.86).
Nonalcoholic Steatohepatitis Clinical Research Network Model The NASH CRN model is based on AST level, ALT level, AST/ALT ratio, demographic factors, comorbidities, and other laboratory test results.37 This model had an AUROC of 0.85 (95% CI, 0.82—0.89) for predicting advanced fibrosis (stages F3—F4) and an AUROC of 0.96 (95% CI, 0.93—0.98) for predicting cirrhosis (stage F4) on liver biopsy. Applying other predictive fibrosis scores to this data set did not show better diagnostic accuracy than the NASH CRN model, although the other scores were much easier to calculate and required fewer clinical and laboratory variables.
Complex Predictive Models Using Biomarkers of Fibrosis
Enhanced Liver Fibrosis Panel This panel is based on the idea that liver fibrosis is a dynamic process that results in increased plasma levels of markers of extracellular matrix turnover. The panel includes 3 biomarkers of fibrosis (hyal-uronic acid, tissue inhibitor of metalloproteinase 1, and amino-terminal peptide of procollagen III), and the panel is excellent at detecting advanced fibrosis, with an AUROC of 0.90 (95% CI, 0.84-0.96).45 Combining the ELF panel with the NFS increased diagnostic accuracy, yielding an AUROC of 0.98 for fibrosis stages F3—F4. Interestingly, the ELF panel was shown to be a good predictor of clinical outcomes (liver-related morbidity and mortality) in a group of patients with chronic liver disease, including 44 patients with NAFLD, making the panel a promising prognostic tool.50
FibroTest FibroTest (BioPredictive) is a panel that uses 5 biomarkers—haptoglobin, α2-macroglobulin, apolipoprotein A1, total bilirubin, and GGT—to predict the presence of fibrosis. Ratziu and colleagues assessed the diagnostic value of FibroTest in a large cohort of NAFLD patients and demonstrated that it can reliably predict advanced fibrosis, with an AUROC of 0.88 (95% CI, 0.82-0.92).51 However, a recent study showed that FibroTest was less accurate than the FIB4 index for predicting advanced fibrosis in 242 patients who were undergoing liver biopsy (AUROCs of 0.80 and 0.86, respectively); this finding demonstrates that complex predictive models are not necessarily more accurate than the simple models that can be derived from clinical parameters at no extra cost to the patient.52 Doctors should be cautious when interpreting FibroTest results in patients with Gilbert syndrome, cholestasis, or acute inflammation, as these conditions result in elevated levels of bilirubin or haptoglobin. To provide a more comprehensive evaluation of liver injury in NAFLD patients, NASH FibroSURE (LabCorp.) combines FibroTest (for assessment of fibrosis), SteatoTest (BioPredictive; for assessment of steatosis), and NASHTest (for assessment of NASH).
Future Avenues of Research
Recent advances in the “-omics” technologies—such as genomics, proteomics, and metabolomics—have offered opportunities for unbiased investigation of changes in metabolic and signaling pathways and their interactions in complex diseases such as NAFLD. Genome-wide association studies have identified a new single nucleotide polymorphism in the PNPLA3 gene that is strongly associated with hepatic fat content and the histologic features of NAFLD.53,54 By using a global, unbiased, metabolomic profiling platform, Kalhan and associates were able to identify several metabolites in multiple pathways that were increased in patients with NASH.55 These new technologies have the ability to generate vast amounts of raw data, which can be used to attempt to unravel the pathogenesis of NAFLD and to develop new noninvasive markers of disease severity.
Conclusion
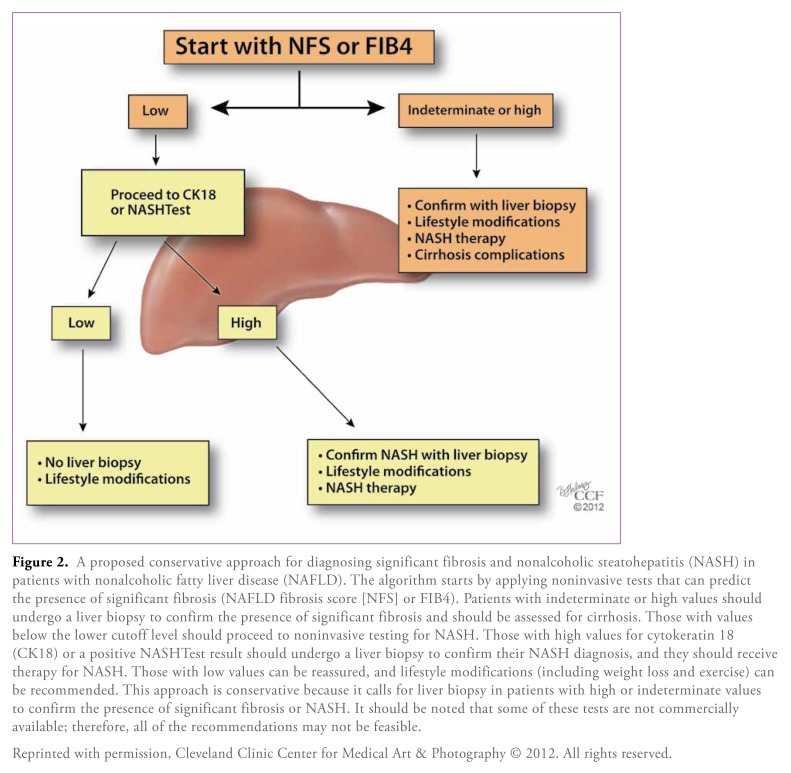
Clearly, there is no shortage of biomarkers and predictive models of NASH and advanced fibrosis; however, none of these noninvasive modalities can completely replace liver biopsy at this time. According to the current NAFLD guidelines, a liver biopsy should be considered in patients at high risk for NASH and advanced fibrosis, such as patients with MetS, and when noninvasive methods cannot exclude competing etiologies for fatty liver or coexisting chronic liver disease. We suggest that clinicians first use a simple panel to rule out the presence of advanced fibrosis. If the result indicates advanced fibrosis or is indeterminate, a biopsy is warranted to confirm this finding and to determine the need for long-term monitoring for cirrhosis and its complications. If there is no evidence of advanced fibrosis, then a test that can identify the presence of NASH is recommended, and a biopsy should be obtained if the test result is positive. This management approach is summarized in Figure 2, which shows an algorithm for clinical decision-making. In our practice, we tend to use the NFS or FIB4 index to determine the presence of advanced fibrosis, and we usually use CK18 levels or the NASHTest to determine the presence of NASH, as these noninvasive markers have good accuracy and have been validated in multiple studies.12
Figure 2.
A proposed conservative approach for diagnosing significant fibrosis and nonalcoholic steatohepatitis (NASH) in patients with nonalcoholic fatty liver disease (NAFLD). The algorithm starts by applying noninvasive tests that can predict the presence of significant fibrosis (NAFLD fibrosis score [NFS] or FIB4). Patients with indeterminate or high values should undergo a liver biopsy to confirm the presence of significant fibrosis and should be assessed for cirrhosis. Those with values below the lower cutoff level should proceed to noninvasive testing for NASH. Those with high values for cytokeratin 18 (CK18) or a positive NASHTest result should undergo a liver biopsy to confirm their NASH diagnosis, and they should receive therapy for NASH. Those with low values can be reassured, and lifestyle modifications (including weight loss and exercise) can be recommended. This approach is conservative because it calls for liver biopsy in patients with high or indeterminate values to confirm the presence of significant fibrosis or NASH. It should be noted that some of these tests are not commercially available; therefore, all of the recommendations may not be feasible.
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2012. All rights reserved.
References
- 1.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 2.Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005;17:636–641. doi: 10.1097/01.mop.0000172816.79637.c5. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 6.Brunt EM, Neuschwander-Tetri BA, Oliver D, Wehmeier KR, Bacon BR. Nonalcoholic steatohepatitis: histologic features and clinical correlations with 30 blinded biopsy specimens. Hum Pathol. 2004;35:1070–1082. doi: 10.1016/j.humpath.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445–451. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 9.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 11.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 12.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of noninvasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 13.Ratziu V, Poynard T. NASH: a hidden and silent fibroser finally revealed? J Hepatol. 2005;42:12–14. doi: 10.1016/j.jhep.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. American Association for the Study of Liver Diseases. Liver biopsy Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 15.Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28:386–395. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 17.Alkhouri N, Carter-Kent C, Feldstein AE. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2011;5:201–212. doi: 10.1586/egh.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 20.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 21.Tamimi TI, Elgouhari HM, Alkhouri N, et al. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J Hepatol. 2011;54:1224–1229. doi: 10.1016/j.jhep.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joka D, Wahl K, Moeller S, et al. Prospective biopsy-controlled evaluation of cell death biomarkers for prediction of liver fibrosis and nonalcoholic steatohepatitis. Hepatology. 2012;55:455–464. doi: 10.1002/hep.24734. [DOI] [PubMed] [Google Scholar]
- 23.Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:663–678. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira CP, da Costa Gayotto LC, Tatai C, et al. Oxidative stress in the patho-genesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J Cell Mol Med. 2002;6:399–406. doi: 10.1111/j.1582-4934.2002.tb00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roskams T, Yang SQ, Koteish A, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic Biol Med. 2004;37:1499–1507. doi: 10.1016/j.freeradbiomed.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 27.Gao D, Wei C, Chen L, Huang J, Yang S, Diehl AM. Oxidative DNA damage and DNA repair enzyme expression are inversely related in murine models of fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1070–G1077. doi: 10.1152/ajpgi.00228.2004. [DOI] [PubMed] [Google Scholar]
- 28.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 29.Başkol M, Başkol G, Deniz K, Ozbakir O, Yücesoy M. A new marker for lipid peroxidation: serum paraoxonase activity in non-alcoholic steatohepatitis. Turk J Gastroenterol. 2005;16:119–123. [PubMed] [Google Scholar]
- 30.Feldstein AE, Lopez R, Tamimi TA, et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abiru S, Migita K, Maeda Y, et al. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int. 2006;26:39–45. doi: 10.1111/j.1478-3231.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 32.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic ste-atohepatitis. Am J Gastroenterol. 2008;103:1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 33.Alkhouri N, Morris-Stiff G, Campbell C, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:297–302. doi: 10.1111/j.1478-3231.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 34.Kowdley KV, Belt P, Wilson LA, et al. NASH Clinical Research Network. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zein CO, Edmison JM, Schluchter M, Feldstein AE, Zein NN, McCullough A. A NASH predictive index (NPI) for use in patients with nonalcoholic fatty liver disease. Hepatology. 2007;46:747A. [Google Scholar]
- 36.Poynard T, Ratziu V, Charlotte F, et al. LIDO study group; CYTOL study group. Diagnostic value of biochemical markers (NashTest) for the prediction of nonalcoholic steatohepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. doi: 10.1186/1471-230X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anty R, Iannelli A, Patouraux S, et al. A new composite model including metabolic syndrome, alanine aminotransferase and cytokeratin-18 for the diagnosis of non-alcoholic steatohepatitis in morbidly obese patients. Aliment Pharmacol Ther. 2010;32:1315–1322. doi: 10.1111/j.1365-2036.2010.04480.x. [DOI] [PubMed] [Google Scholar]
- 39.Younossi ZM, Page S, Rafiq N, et al. A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg. 2011;21:431–439. doi: 10.1007/s11695-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 40.Alkhouri N, Berk M, Lopez R, et al. OxNASH score correlates with histologic features and severity of nonalcoholic fatty liver disease. Gastroenterology. 2011;140:S905. doi: 10.1007/s10620-014-3031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51:373–375. doi: 10.1002/hep.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 43.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 44.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 45.Guha IN, Parkes J, Roderick P, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European liver fibrosis panel and exploring simple markers. Hepatology. 2008;47:455–460. doi: 10.1002/hep.21984. [DOI] [PubMed] [Google Scholar]
- 46.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 47.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. NASH Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 49.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and Fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 50.Parkes J, Roderick P, Harris S, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59:1245–1251. doi: 10.1136/gut.2009.203166. [DOI] [PubMed] [Google Scholar]
- 51.Ratziu V, Massard J, Charlotte F, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with nonalcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams LA, George J, Bugianesi E, et al. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J Gastro-enterol Hepatol. 2011;26:1536–1543. doi: 10.1111/j.1440-1746.2011.06774.x. [DOI] [PubMed] [Google Scholar]
- 53.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valenti L, Al-Serri A, Daly AK, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–1217. doi: 10.1002/hep.23622. [DOI] [PubMed] [Google Scholar]
- 55.Kalhan SC, Guo L, Edmison J, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60:404–413. doi: 10.1016/j.metabol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]