Abstract
Poor medication adherence is a well-known problem, particularly in patients with chronic conditions, and is associated with significant morbidity, mortality, and health care costs. Multi-faceted and personalized interventions have shown the greatest success. Pharmacogenetic (PGx) testing may serve as another tool to boost patients’ confidence in the safety and efficacy of prescribed medications. Here we consider the potential impact (positively or negatively) of PGx testing on medication-taking behavior.
Pharmacogenetic (PGx) testing provides information about a patient’s likelihood to have an adverse response and/or a therapeutic response to a medication, enabling the potential for a tailored and personalized approach to medication therapy. Genetic variation has been estimated to account for 20 to 95 percent of the variation in individual responses to medications1 A total of 113 drug labels approved by the US Food and Drug Administration (FDA) include information about variability in patient response secondary to genetic variability (http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm), a handful including information about more than one gene. Although the primary focus of PGx testing has been on improving drug selection and dosing in patient populations or individuals, a secondary potential benefit of testing may be the improvement of medication adherence. Poor medication adherence is a well-known problem, particularly in patients with chronic conditions, resulting in greater morbidity, mortality, and health care costs2–5. Although the potential benefit of PGx testing to improve adherence has been recognized for chronic diseases such as attention-deficit/hyperactivity disorder6 and diabetes,7 it is just beginning to be evaluated as an intervention, either alone or in combination with other interventions8. Successful implementation of PGx testing into clinical practice, which assists patients and providers with therapy decisions, is consistent with current national goals to improve health care, engage patients, and reduce health care costs. In this paper, we discuss the potential mechanisms by which PGx testing may affect medication-taking behavior – either positively or negatively.
The Continuing Challenge of Medication Adherence
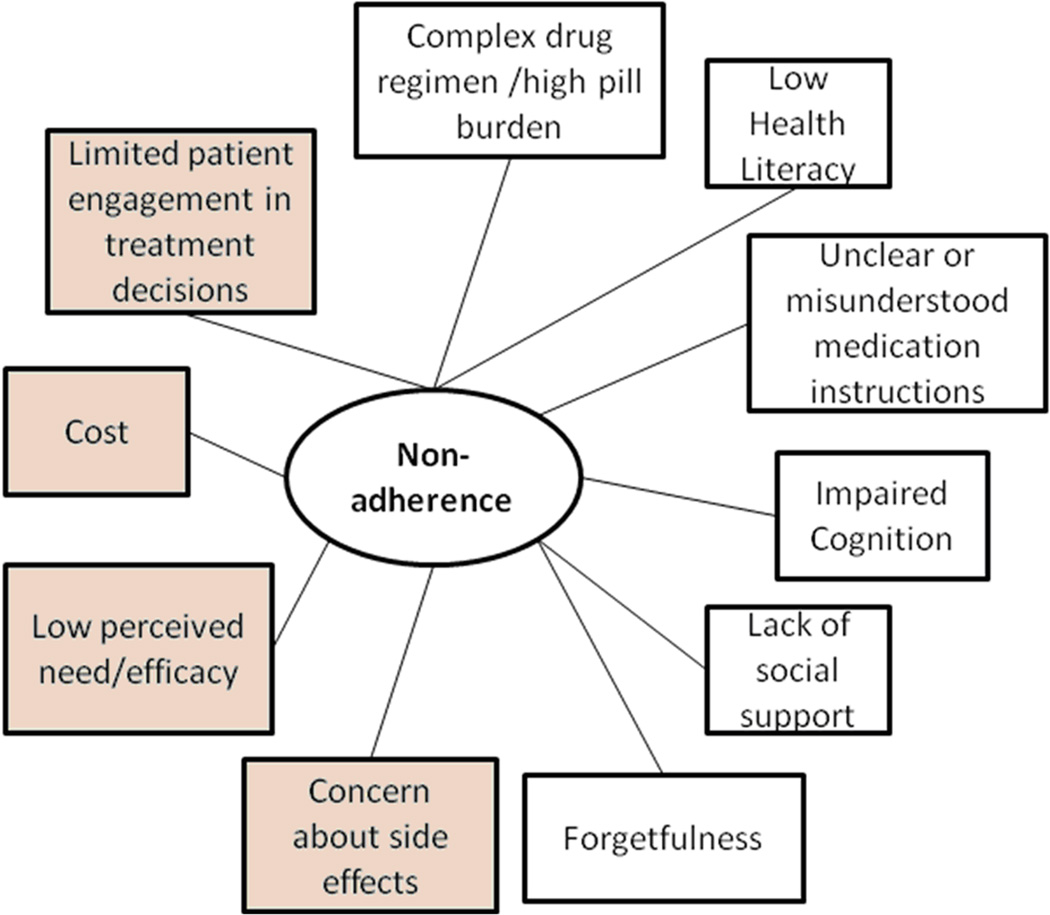
It is estimated that the annual economic burden of non-adherence is about $300 billion in the United States2, and that about one-third to one-half of all patients in the U.S. do not take their medications as directed by their health care providers.3 However, due to inconsistent definitions and measures of adherence in the literature and in clinical practice, the exact prevalence of non-adherence and associated cost is unknown.3 The World Health Organization broadly defines patient medication adherence as the extent to which a person’s medication taking behavior corresponds with agreed recommendations from a health care provider.9 As shown in the Figure, many factors, often in combination, have been reported to be associated with medication non-adherence including side effects, perception that the medication is unnecessary10, low health literacy11, and cost or lack of insurance coverage12. In particular, poor medication adherence has been well-documented for chronic conditions given that effective management typically requires long-term use of one or more medications13.
Figure.
Factors affecting patient medication adherence. Factors highlighted in gray may potentially be addressed with pharmacogenetic testing.
Improving Medication Adherence
Effective strategies to promote adherence to a prescribed regimen(s) are desperately needed to reduce this ongoing problem in health care. However, given the complexity and heterogeneity of factors influencing medication adherence, it has been challenging to develop effective interventions to improve adherence. In general, the success rate of tested interventions has been relatively low14, though even a small increment in adherence may result in substantial cost-savings15 Multifaceted interventions and/or personalized interventions may be necessary to reduce medication non-adherence in the US.9, 15, 16, The most promising types of interventions to enhance medication adherence involve simplification of the drug regimen and addressing barriers to adherence such as improving convenience of care and providing information, counseling, reminders, self-monitoring, and reinforcement and follow-up.16 Patient-tailored interventions that explicitly address patients’ concerns and perceived necessity, and engage them in treatment decisions may be more likely to succeed in improving adherence17.
Potential Positive Consequences of PGx Testing on Medication Adherence
In addition, a few studies have suggested some positive impact with PGx testing in improving medication adherence and clinical outcomes.7, 8 The inherent personal nature of learning of one’s genetic likelihood of having a positive therapeutic response or not having an adverse effect from a medication may increase perceived efficacy/necessity or decrease patient concern, respectively, ultimately improving medication adherence. The act of testing alone may reduce anxiety about the medication, contributing to increased adherence.18 Having patients engaged in medication selection or dosing decisions based upon the results of PGx testing may not only improve patient knowledge of their disease and treatment options, but improve patient trust resulting in improved medication adherence 19. Patient confidence in a physician’s recommendations contribute to likelihood of drug initiation20 and discussion of therapeutic options provides a personal risk assessment and perhaps a greater sense of shared decision-making. Even if patients decline to have PGx testing after discussing it with their physician, the discussion about testing may improve patient satisfaction and feelings of shared decision-making. PGx-informed therapeutic decisions may result in reduced patient burden and costs associated with trial and error of medications for treatment and follow-up care required for management of adverse effects. The reduced burden and cost to the patient could positively influence medication adherence and thereby improve long-term outcomes and health costs.
Potential Adverse Consequences of PGx Testing on Medication Adherence
As is the case for many types of genetic tests, PGx testing may result in beneficial or harmful outcomes, depending on the interpretation and communication of the test results. Thus, PGx testing may yield its own adverse effects that need to be carefully weighed. For example, while a PGx test result predicting that a patient will benefit from a given treatment or have a low risk of adverse events may improve adherence, prediction that the guideline-recommended treatment may not be as effective or associated with a higher potential risk of side effects may negatively influence adherence by both the provider and patient. In particular, reduction in initial or long-term dose based on a PGx test result may raise doubt and reduce confidence about the safety and/or effectiveness of the treatment. Patients’ familiar with or having a preference for a particular drug may be disappointed if the PGx test result indicates other drugs may be safer or more effective. Similarly, patients may perceive that a “targeted” drug (designed to target specific genetic aberrations) is more effective, and thus, use of a non-targeted drug is less effective, negatively impacting adherence to the alternatively prescribed drug. Furthermore, patients’ anxiety may be heightened following receipt of an ‘unfavorable’ result resulting in non-adherence and increased concerns about their health and provider’s ability to effectively care for them.
In addition, there are potential negative consequences for providers. Specifically, if a guideline recommended medication is not used due to results of a PGx test demonstrating lack of efficacy or high risk of adverse effects, the clinician may be required to provide justification for their decision and potential negative impact on his/her performance measures or the performance measures of his/her clinic or hospital. In addition, depending upon the strength of evidence demonstrating the anticipated lack of benefit or increased risk, the clinician may be opening himself/herself up to potential litigation if outcomes are not as expected.
A number of other factors associated with non-adherence may be exacerbated by PGx testing. For instance, studies of public attitudes toward PGx testing have reported concerns that genetically tailored drugs would be more costly and result in greater health disparities21. Therefore, patients and clinicians may be concerned that PGx testing may result in greater health care costs and health disparities. Some patients may interpret PGx test results as determinant of an outcome, a concern if PGx test results are perceived to be unfavorable. Even tests that indicate no increased risk for an adverse event, however, may not reduce patient concerns and even raise anxiety in some patients.
Readiness of PGx Testing for Clinical Care
Despite the FDA’s efforts toward providing information for health professionals about the impact of genetic variation on drug response or risk of adverse responses through revised drug labeling, there is substantial debate about the clinical utility of PGx testing, due in part to the lack of evidence. Cost-effective data for some tests are ambiguous22 and insurance coverage is not wide-spread 23, further limiting uptake. However, several studies have reported that the public is generally supportive of PGx testing,24–26 and physician and patient demand may override concerns of the lack of clinical evidence for PGx testing27. Furthermore, implementation of PGx testing would fit well with the current movement toward patient-centered care model supported by recent healthcare reform in the U.S. (Affordable Care Act). The scope of clinical utility should be expanded to include medication adherence and the design of clinical trials to assess utility of PGx testing should also include measures of the impact of PGx test results on adherence28.
While the reporting of results and communication between patient and provider about the interpretation and implications of the results is consistent with a patient-centered care model, full implementation in the clinical practice setting may be challenging. For example, some clinical practices may have policies on only reporting abnormal laboratory findings. As such, “normal” results, such as the results of a PGx test that does not indicate the need for a drug or dose adjustment may not be automatically reported to the patient. However, it is precisely this type of result that should be reported in order to potentially boost patient confidence in the efficacy of the drug or reduce concerns about adverse effects, thereby, potentially increasing adherence. Likewise. if the ‘normal’ result is reported simply as ‘normal’ without some explanation as to its significance to the patient’s likelihood to respond favorably to the prescribed drug or have a lower risk to experience an adverse effect, the meaning of the result may not be clear to the patient and the potential benefit of increased adherence will also be lost.
Understanding PGx test results will be a challenge for all patients, particularly those with lower health literacy. Millions of Americans have inadequate health literacy29. PGx testing typically provides risk estimates or categorical risks (e.g., increased likelihood) instead of definitive results, which may also be challenging to understand for many individuals, particularly those with low numeracy skills30. Educational materials designed for patients with low health literacy about PGx testing and the results should be presented in a manner understandable for patients of varying levels of literacy to minimize confusion and misinterpretation. As PGx testing can inform future therapeutic decision-making, it is particularly important to understand the meaning and significance of the results when they are first ordered to increase likelihood that the results will be disclosed or shared with future healthcare providers.
Conclusion
Much of the uncertainty surrounding the clinical use of PGx testing at this time may be a reflection of the novelty of testing as it remains a heavily investigated area of research. Although it is unlikely that any recommendations for PGx testing will be based on its impact on medication adherence, this potential benefit should not be overlooked. PGx testing in combination with other inventions may serve to improve adherence in combination with other interventions. A small proportion of patients demonstrating improved medication adherence due to PGx testing could result in substantial health-savings. With the movement toward patient-centered care, it appears to be an opportune time to investigate the benefit of PGx testing for medication adherence and how testing can be smoothly integrated into various health delivery systems to realize such potential benefit. Understanding the relationship between PGx test results and medication adherence will be useful in other areas of research such as risk communication, cost-effectiveness, analyses of interventions, and patient acceptance and engagement in self-care.
Acknowledgement
This work was supported by funding from the U.S. National Institutes of Health 5R01-GM081416-05.
References
- 1.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. The New England journal of medicine. 2011;364(12):1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NEHI. Thinking Outside the Pillbox: A System-wide Approach to Improving Patient Medciation Adherence for Chronic Disease. 2011 http://www.nehi.net/uploads/full_report/pa_issue_brief__final.pdf. [Google Scholar]
- 3.Osterberg L, Blaschke T. Adherence to medication. The New England journal of medicine. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 4.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 5.Fischer MA, Stedman MR, Lii J, Vogeli C, Shrank WH, Brookhart MA, et al. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. Journal of general internal medicine. 2010;25(4):284–290. doi: 10.1007/s11606-010-1253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGough JJ. Attention-deficit/hyperactivity disorder pharmacogenomics. Biol Psychiatry. 2005;57(11):1367–1373. doi: 10.1016/j.biopsych.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Grant RW, Hivert M, Pandiscio JC, Florez JC, Nathan DM, Meigs JB. The clinical application of genetic testing in type 2 diabetes: a patient and physician survey. Diabetologia. 2009;52(11):2299–2305. doi: 10.1007/s00125-009-1512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charland SL, Agatep BC, Epstein RS, Frueh FW, Herrera V, Devlin J, et al. Patient Knowledge of Pharmacogenetic Information Improves Adherence to Statin Therapy: Results of the Additional Kif6 Risk Offers Better Adherence to Statins (Akrobats) Trial. Journal of the American College of Cardiology. 2012;59(13):E1848–E1848. [Google Scholar]
- 9.WHO. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003. http://www.who.int/chp/knowledge/publications/adherence_report/en/ [Google Scholar]
- 10.Kreps GL, Villagran MM, Zhao X, McHorney CA, Ledford C, Weathers M, et al. Development and validation of motivational messages to improve prescription medication adherence for patients with chronic health problems. Patient education and counseling. 2011;83(3):375–381. doi: 10.1016/j.pec.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Kripalani S, Gatti ME, Jacobson TA. Association of age, health literacy, and medication management strategies with cardiovascular medication adherence. Patient education and counseling. 2010;81(2):177–181. doi: 10.1016/j.pec.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 12.Piette JD, Heisler M, Wagner TH. Cost-related medication underuse: do patients with chronic illnesses tell their doctors? Archives of internal medicine. 2004;164(16):1749–1755. doi: 10.1001/archinte.164.16.1749. [DOI] [PubMed] [Google Scholar]
- 13.Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: a meta-analysis. Can J Cardiol. 2012;28(5):574–580. doi: 10.1016/j.cjca.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Archives of internal medicine. 2007;167(6):540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 15.Bosworth HB, Granger BB, Mendys P, Brindis R, Burkholder R, Czajkowski SM, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412–424. doi: 10.1016/j.ahj.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database Syst Rev. 2004;(2):CD004804. doi: 10.1002/14651858.CD004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Bokhoven MA, Koch H, van der Weijden T, Grol RP, Kester AD, Rinkens PE, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints. Ann Fam Med. 2009;7(2):112–120. doi: 10.1370/afm.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trachtenberg F, Dugan E, Hall MA. How patients' trust relates to their involvement in medical care. J Fam Practice. 2005;54(4):344–352. [PubMed] [Google Scholar]
- 20.Dowell J, Hudson H. A qualitative study of medication-taking behaviour in primary care. Fam Pract. 1997;14(5):369–375. doi: 10.1093/fampra/14.5.369. [DOI] [PubMed] [Google Scholar]
- 21.Bevan JL, Lynch JA, Dubriwny TN, Harris TM, Achter PJ, Reeder AL, et al. Informed lay preferences for delivery of racially varied pharmacogenomics. Genetics in medicine : official journal of the American College of Medical Genetics. 2003;5(5):393–399. doi: 10.1097/01.gim.0000087989.12317.3f. [DOI] [PubMed] [Google Scholar]
- 22.Shah RR, Shah DR. Personalized medicine: is it a pharmacogenetic mirage? British journal of clinical pharmacology. 2012;74(4):698–721. doi: 10.1111/j.1365-2125.2012.04328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hresko A, Haga S. Insurance Coverage Policies for Personalized Medicine. Journal of Personalized Medicine. 2012;2(4):201–216. doi: 10.3390/jpm2040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almarsdottir AB, Bjornsdottir I, Traulsen JM. A lay prescription for tailor-made drugs--focus group reflections on pharmacogenomics. Health policy. 2005;71(2):233–241. doi: 10.1016/j.healthpol.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Issa AM, Tufail W, Hutchinson J, Tenorio J, Baliga MP. Assessing patient readiness for the clinical adoption of personalized medicine. Public health genomics. 2009;12(3):163–169. doi: 10.1159/000189629. [DOI] [PubMed] [Google Scholar]
- 26.Rogausch A, Prause D, Schallenberg A, Brockmoller J, Himmel W. Patients' and physicians' perspectives on pharmacogenetic testing. Pharmacogenomics. 2006;7(1):49–59. doi: 10.2217/14622416.7.1.49. [DOI] [PubMed] [Google Scholar]
- 27.Trosman JR, Van Bebber SL, Phillips KA. Coverage policy development for personalized medicine: private payer perspectives on developing policy for the 21-gene assay. J Oncol Pract. 2010;6(5):238–242. doi: 10.1200/JOP.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorgensen AL, Hughes DA, Hanson A, van Eker D, Toh CH, Pirmohamed M, et al. Adherence and variability in warfarin dose requirements: assessment in a prospective cohort. Pharmacogenomics. 2013;14(2):151–163. doi: 10.2217/pgs.12.199. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen-Bohlman L. Health literacy : a prescription to end confusion. Washington, D.C.: National Academies Press; 2004. Institute of Medicine (U.S.). Committee on Health Literacy; p. xix.p. 345. [PubMed] [Google Scholar]
- 30.Galesic M, Garcia-Retamero R. Statistical numeracy for health: a cross-cultural comparison with probabilistic national samples. Archives of internal medicine. 2010;170(5):462–468. doi: 10.1001/archinternmed.2009.481. [DOI] [PubMed] [Google Scholar]