SUMMARY
We measured LFP and BOLD fMRI in the medial temporal lobes of monkeys and humans, respectively, as they performed the same conditional motor associative learning task. Parallel analyses were used to examine both datasets. Despite significantly faster learning in humans relative to monkeys, we found equivalent neural signals differentiating new versus highly familiar stimuli, first stimulus presentation, trial outcome and learning strength in the entorhinal cortex and hippocampus of both species. Thus, the use of parallel behavioral tasks and analyses in monkeys and humans revealed conserved patterns of neural activity across the medial temporal lobe during an associative learning task.
Keywords: human, monkey, medial temporal lobe, associative learning, fMRI, LFP
INTRODUCTION
The striking homologies of the macaque monkey and human brain makes the macaque model system one of the most powerful animal models of human brain function available today (Nakahara et al., 2007; Passingham, 2009). For example, lesion studies (Mishkin, 1978; Zola-Morgan et al., 1989; Zola-Morgan and Squire, 1985) and neuroanatomical studies (Insausti et al., 1987; Suzuki and Amaral, 1994) in monkeys have been successful in either confirming or identifying brain areas important for declarative/relational memory in humans. Less is known about the neurophysiological underpinnings of memory in humans or about the precise homology between memory-related neural activity across primate species. In early visual areas, studies comparing monkey and human functional magnetic resonance imaging (fMRI) signals have reported strong parallels, though stronger differences have been seen in both mid- and higher order visual areas (Orban et al., 2004). In the medial temporal lobe, parallels between single unit activity in monkeys and blood oxygen level dependent (BOLD) fMRI signals in humans have been noted, however, these comparisons remain superficial both because of the differences in the nature of the physiological signals measured as well as because of the differences in the behavioral tasks typically used across species (Nakahara et al., 2007; Orban et al., 2004; Passingham, 2009).
One striking parallel in the memory signals seen across the monkey and human medial temporal lobe is significantly stronger responses to novel relative to familiar visual stimuli in the perirhinal cortex (Brown et al., 1987; Brozinsky et al., 2005; Fahy et al., 1993; Gonsalves et al., 2005; Henson et al., 2003; Kohler et al., 1998; Li et al., 1993; Montaldi et al., 2006). Beyond this signal of relative stimulus novelty, however, the parallels between memory-related physiological signals in monkeys and humans are less striking. For example, in the monkey perirhinal cortex, Miller and Desimone (1994) reported stimulus-selective enhancement to a behaviorally relevant matching stimuli (match enhancement) as well as stimulus-selective suppression to non-relevant matching stimuli (match suppression) during a delayed match to sample task. Reports of match enhancement in the human perirhinal cortex, however, have been mixed (Dudukovic et al., 2011; Duncan et al., 2009) though the tasks used in humans differed in numerous respects from the task used in monkeys. In the hippocampus, several human fMRI studies (Dudukovic et al., 2011; Duncan et al., 2009) as well as a human single unit study in epileptic patients (Fried et al., 1997) reported strong match enhancement signals. By contrast, in the monkey hippocampus, several recent reports have described decrements but not enhancements in neural responses associated with repeated stimulus presentations (Jutras and Buffalo, 2010; Yanike et al., 2009).
While many previous studies have mapped early visual areas in monkeys and humans performing the same perceptual task, few studies have compared medial temporal lobe activity across species as subjects perform the same memory task. One exception is Law et al. (2005), who developed a conditional motor associative learning task for humans based on one used in a previously published monkey physiology study (Wirth et al., 2003). Law et al. (2005) reported clear increases in the BOLD fMRI signals across the medial temporal lobe structures as human subjects learned new conditional motor associations. These findings appeared to parallel the single unit findings in the monkey hippocampus described by Wirth et al. (2003) that showed either increases or decreases in hippocampal single unit activity that were correlated with the animal’s learning curve. However, it remained unclear how the increases and decreases in single unit activity seen in individual monkey hippocampal cells corresponded to the global pattern of increased BOLD activity seen in humans.
To better characterize the precise correspondence between the patterns of neural activity in the medial temporal lobe in monkeys and humans performing the same behavioral task, we compare local field potential (LFP) signals measured with low impedance sharp tetrodes in monkeys (gathered specifically for these experiments) to BOLD fMRI signals measured in humans from a previous study (Kirwan et al., 2007; Law et al., 2005). Previous studies in the primate visual cortex using simple perceptual paradigms suggested that LFP signals in the gamma band correspond best to the BOLD fMRI signals (Goense and Logothetis, 2008; Logothetis, 2002). We analyzed neural activity in the hippocampus and the entorhinal cortex using parallel analytic tools in both monkeys and humans. We report equivalent neural signals across the entorhinal cortex and hippocampus in monkeys and humans for all major learning and memory-related signals examined. Moreover, in two cases, learning or memory-related signals initially seen either only in humans (immediate novelty effect) or only in monkeys (trial outcome signal) were queried in the data from the other species. In both cases, this strategy revealed novel mnemonic signals not previously observed in the other species.
RESULTS
Behavior
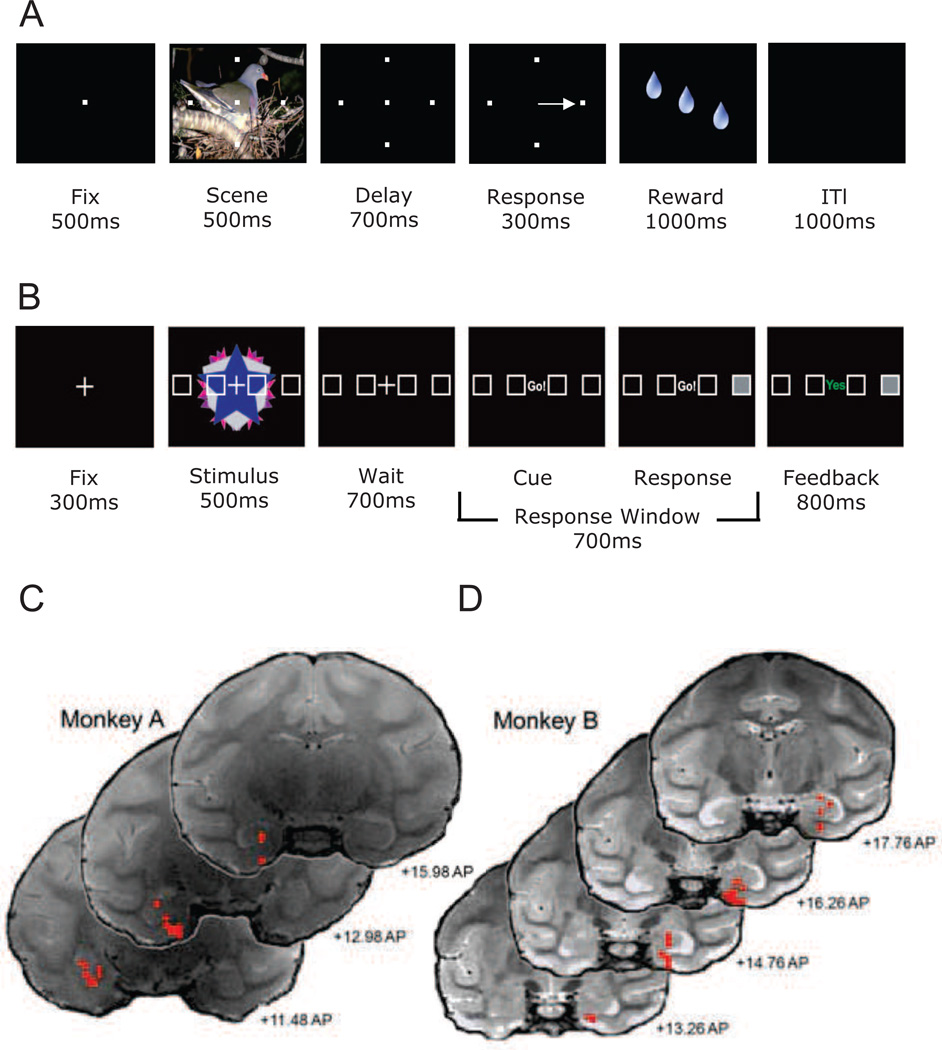
Monkey and human subjects performed a conditional motor associative learning task in which they learned to match one of four target locations presented on a computer screen with novel complex visual stimuli for either juice reward (monkeys; Figure 1A) or positive feedback (humans; Figure 1B). Highly familiar “reference” stimulus-target associations were also randomly presented throughout the task. Trials started with subjects briefly fixating a central point before the stimulus and targets appeared. After 500 ms, the stimulus disappeared, leaving the targets on the screen for a 700 ms delay period. The subjects were then cued to respond with either an eye movement (monkeys) or a touch response (humans) to one of the possible targets. Correct responses were followed immediately by either juice reward or positive feedback. The start of the next trial was preceded by an inter-trial-interval (ITI). Before each new learning session, monkeys performed a “fixation only” task during which the novel complex visual stimuli to be presented during the learning trials for that day were shown. Animals received juice reward simply for maintaining fixation during the stimulus presentation. For similar baseline purposes, human subjects performed a challenging, non-mnemonic, perceptual baseline condition randomly interspersed throughout learning.
Figure 1. Schematic diagram depicting the within trial sequence of the conditional motor association tasks used for monkeys and humans.
(A) Monkeys initiated trials by fixating on a central spot for 500ms. Fixation was followed by a 500ms scene period, during which four targets were shown superimposed over a large complex visual scene that took up much of the computer monitor. The scene period was followed by a 700ms delay period during which the scene disappeared but the four targets remained. At the end of the delay period the monkeys were cued by the disappearance of the fixation spot, and had 300ms to make a saccade to one of the four targets. If correct, the monkeys received several drops of juice as a reward over approximately 1000ms. The reward period was followed by an inter-trial interval (ITI) period of 1000ms after which the next trial was started. If the monkeys made an error, the ITI period began immediately. (B) Human subjects initiated each trial by fixating on a centrally located “+” for 300ms.Fixation was followed by a 500ms stimulus period during which four targets placed in a horizontal array appeared superimposed across abstract kaleidoscopic images. The stimulus period was followed by a 700ms wait period during which the kaleidoscopic images were removed, but the targets remained. At the end of the delay period the subjects were cued by replacing the fixation cross with the cue (“Go!”), subsequent to which they had 700ms to press one of 4 button response keys that matched the horizontal array. Directly following their response the selected box was filled on the screen and feedback was presented for 800 ms: “yes” (shown in green) if they were correct, and “no” (shown in red) if they made an error. Panels C and D: Selected T2 weighted coronal MRI sections displaying the entorhinal and hippocampal locations (red circles) of the LFP recording sessions from monkeys A and B. In this illustration, the left side of the MRI image reflects the left hemisphere and the right side reflects the right hemisphere. Anterior-posterior locations of the slices are based on the surgical coordinates for centering the grid.
Monkeys A and B were given between 2–4 or 1–2 new visuomotor associations to learn concurrently in each recording session, respectively. Thirty-one human subjects were tested with 4, 8, or 12 visuomotor associations run concurrently, dependent on individual performance during a pre-scan training session. The total number of associations presented in each session varied across human subjects as new associations would replace learned associations during training (after >6 correct responses in a row).
To characterize the behavioral learning of visuomotor associations in both species, we used a logistic regression algorithm (Smith et al., 2004) to generate learning curves based on binary responses (Law et al., 2005; Wirth et al., 2003). Typical learning curves consisted of a variable number of predominantly incorrect responses, followed by a sharp transition to predominantly correct responses. Associations were considered learned once the lower bound 95% confidence interval of the logistic regression became greater than would be expected by chance. The trial on which the learning passed this criterion was considered the “learning trial”. An analysis of the learning trial indicated that the curves initially presented within a set could be ordered, identifying “fast”, “medium”, and “slow” learned conditions, a pattern observed both in monkeys (F(3,21)=17.92; p<.001) and humans (F(3,87)=34.91; p<.0005) that was linear in nature (F(1,36)=115.97; p<.0005). A similar analysis of the maximum learning curve slopes reinforced the idea that the curves could be ordered linearly (F(1,36)=52.45; p<.0005). Overall, the pattern suggests that a common strategy was adopted by both monkeys and humans during which only one association was “worked on” at a time (Hadj-Bouziane and Boussaoud, 2003).
While the overall learning strategy appeared remarkably similar across species, not surprisingly, both the speed of learning and number of learned associations were superior in humans compared to monkeys. Human subjects had steeper learning curves than monkeys, as evidenced by differences in the average maximum slope of learned visuomotor associations (t(125)=13.81; p<.0001) and a smaller number trials to criterion (Humans: mean 4.67, range, 2–28, SEM 0.68; Monkeys: mean 17.14, range 2–39 SEM 0.69; t(30)=5.483; p<.0001). As a consequence, humans learned significantly more associations per session than monkeys (monkeys = 1.73, humans = 20.26; t(30)=13.64; p<.0001). Of the 152 visuomotor associations presented during the 74 recording sessions, monkeys learned a total of 56.56% (86) associations. Conversely, of the 924 stimulus-location associations presented in 31 scanning sessions, human subjects learned a total of 67.96% (628) associations. Thus, overall, humans also learned a significantly greater percentage of conditions than did the monkeys (Chisquare(1)=7.58; p<.01).
Neurophysiology
Analysis Strategy
To identify homologies between the neurophysiological responses in the monkeys and human hippocampus and entorhinal cortex during the performance of the same behavioral task, we measured LFP recordings from two monkeys (Figure 1C and D) and BOLD fMRI from 31 human subjects focused on these two regions (Goense and Logothetis, 2008; Kirwan et al., 2007; Law et al., 2005; Logothetis, 2002). The BOLD activity was analyzed with a traditional general linear model (GLM) approach using multiple linear regression to estimate β weights that correspond to activity for each trial type of interest relative to a perceptual baseline condition (Kirwan et al., 2007; Kutner et al., 2004; Law et al., 2005). We analyzed the monkey LFP data using the same multiple linear regression β weight analysis used on the human BOLD fMRI signals, examining non-overlapping frequency bandwidths in the gamma (30–100Hz) and beta (10–25Hz) ranges derived from spectral analyses (Figure S2A and B). In some cases where there was not enough data available to carry out a multiple regression analysis, we used standard parametric statistics to analyze the LFP data. The results of each analysis were compared across species to identify similarities as well as differences in the neurophysiological responses.
Differentiating New from Highly Familiar Stimuli
A common finding from the monkey entorhinal cortex has been strong responses to relatively novel stimuli (stimuli seen for the first time in the current session) compared to highly familiar stimuli (significant exposure over multiple days to months; Brown et al., 1987; Suzuki et al., 1997; Xiang and Brown, 1998). Few if any such signals have been reported in the hippocampus (Brown et al., 1987; Xiang and Brown, 1998). We first asked whether differences in responses to new versus highly familiar stimuli could be found in the monkey LFP signals. LFP sweeps were converted to frequency spectra and the mean log power from both the beta and gamma bandwidths of a 1100 ms epoch spanning the scene and delay periods were derived. The spectral power values from the selected bandwidths were then analyzed with multiple regressions for each session to generate β values for both the new and the highly familiar reference stimuli. These β values were then compared across sessions using parametric tests.
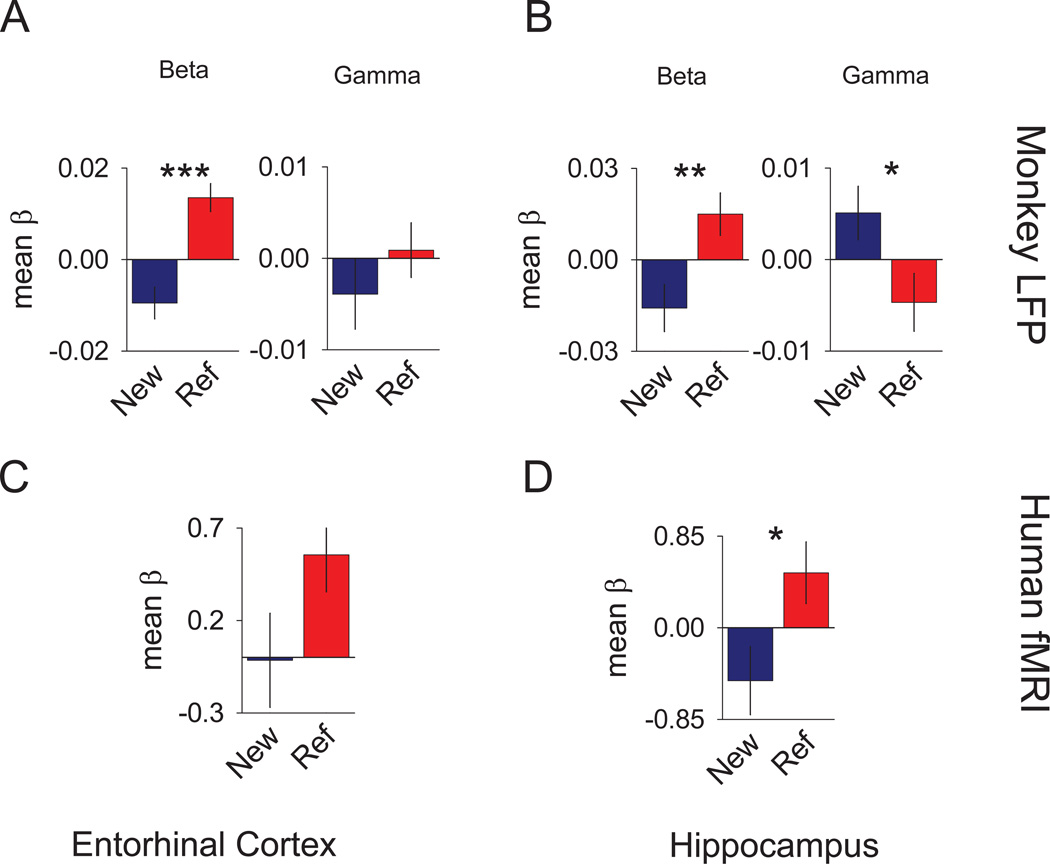
For the monkey entorhinal cortex, significant differences between new and reference β values were found for the beta bandwidth (t(52)=5.69; p<.0005), but not the gamma bandwidth (t(52)=0.323; p=ns; Figure 2A). The direction of the effect in the beta bandwidth favored reference over new trial spectra. During separate recording sessions in the monkey hippocampus, significant differences in β values were found for both the beta (t(39)=3.15; p<.003) and the gamma (t(39)=2.35; p<.024) bandwidths. Additional analyses done to examine the detailed structure of signal showed that the differential signals we observed arose from a transient decrease during the scene/delay period relative to the fixation period that was larger (more negative) for the new conditions than for the reference conditions. (Figure S2C–F). In humans, we applied a multiple regression analysis of the fMRI data calculating coefficients for the new and reference trial responses for each subject. The β values reflected the difference in activity between mnemonic and non-mnemonic tasks for each voxel. To parallel the LFP monkey data, we used an anatomically-defined region of interest (ROI) approach, hand-segmenting the entorhinal cortex and hippocampus for each subject. These segmentations, collapsed across hemispheres were used to assess average activity within each region. The resulting estimates of activity (mean β values) were then subjected to group analyses to determine whether the novelty of the trial reliably affected the fMRI signal in these regions. Results indicated a significant difference for the hippocampal ROI (t(30)=3.46; p<.0017; Figure 2D) and a trend towards significance for the entorhinal ROI (t(30)=2.0; p=.055), as depicted in Figure 2C. The direction of the differences for the hippocampal and entorhinal ROIs both favored the reference trials over the new trials. Thus, parallel signals of relative stimulus novelty/familiarity are seen in the entorhinal cortex and hippocampus of both monkeys and humans.
Figure 2. New versus highly familiar signals of the monkey LFP and human fMRI.
Individual bar graphs depict the comparisons between mean reference (red) versus mean new (blue) trials (± SEM of the differences). Top row of graphs show the results of the multiple regression analyses for different bandwidth spectra in monkeys, while the lower row of graphs show the results of the multiple regression analyses of the mean β values for the same bandwidth spectra for humans. (A) Results for the monkey entorhinal LFP signal multiple regression analyses for different bandwidth spectra comparing the mean β values. (B) Results for the monkey hippocampal LFP signal multiple regression analyses for different bandwidth spectra in monkeys comparing the mean β values. (C) Results of the human entorhinal fMRI BOLD signal multiple regression analyses comparing the mean β values. (D) Results of the human hippocampal fMRI BOLD signal multiple regression analyses comparing the mean β values. *** = p <0.005, ** = p <0.01, and * = p <0.05. See also Figure S2.
Immediate Novelty
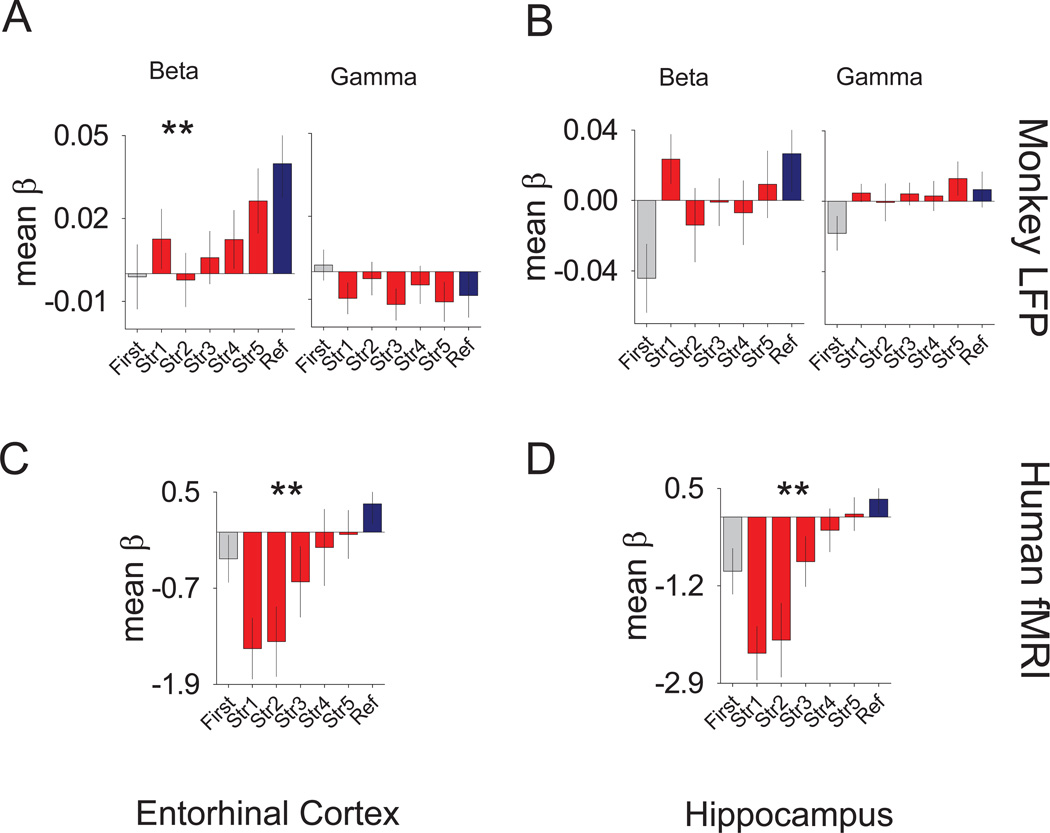
One of the more prominent findings in the human fMRI study of Law et al. (2005) was an immediate novelty effect in which the initial presentation of new stimuli was followed by a drop in BOLD activity on successive presentations. This immediate novelty effect is common in the neuroimaging literature and is thought to provide a novelty detection signal (Schacter and Wagner, 1999). When the human fMRI data were re-analyzed using the methods here, the results were consistent with the original Law et al. (2005) finding for both the entorhinal (t(30)=2.5; p<.016) and hippocampal (t(30)=2.16; p<.03) ROIs (Figure 6E and F).
Figure 6. Learning and immediate novelty signals of the monkey LFP and human fMRI.
(A) Results for the monkey entorhinal LFP parametric analyses of the mean log power of the first five (blue) pre-learning trials compared to the last five (red) post learning trials (± SEM of the differences) of the different bandwidth spectra. (B) Results for the monkey hippocampal LFP analyses using the same panel organization as described for (A). (C) Bar graphs depicting the results of the monkey entorhinal LFP multiple regression analyses comparing the mean β values across the different learning strengths (red), reference (blue), and initial presentation (grey) trials for the different bandwidth spectra. The panel shows the beta bandwidth comparison, while the right panel shows the gamma bandwidth comparison. (D) Results for the monkey hippocampal LFP multiple regression analyses comparing the mean β values across the different learning strengths (red), reference (blue), and initial presentation (grey) trials for the different bandwidth spectra using the same organization as described for (C). (E) Results of the human entorhinal fMRI BOLD signal multiple regression analyses comparing the mean β values across the different learning strengths (red), reference (blue), and initial presentation (grey) trials. (F) Results of the human hippocampal fMRI BOLD signal multiple regression analyses comparing the mean β values as in (C). *** = p <0.005 and * = p <0.05. See also Figures S6 and S2–6.
While relative stimulus familiarity has been examined throughout the monkey medial temporal lobe (Brown and Aggleton, 2001; Li et al., 1993; Riches et al., 1991; Zhu et al., 1995), the question of whether monkey hippocampal or entorhinal activity provide a similarly prominent signal the very first time a novel stimulus is shown, has never, to our knowledge been examined. One difference between the monkey and human testing was that while the humans saw the novel visual images for the first time during the associative learning task, the monkeys were habituated to the novel visual images for 15–20 trials of simple fixation before the learning trials started. Since these fixation trials were the very first time the animals saw these novel stimuli, we focused our analysis on these trials. Since the initial presentation only occurred once for a small number of stimuli per session, a parametric analysis of the bandwidth power was used.
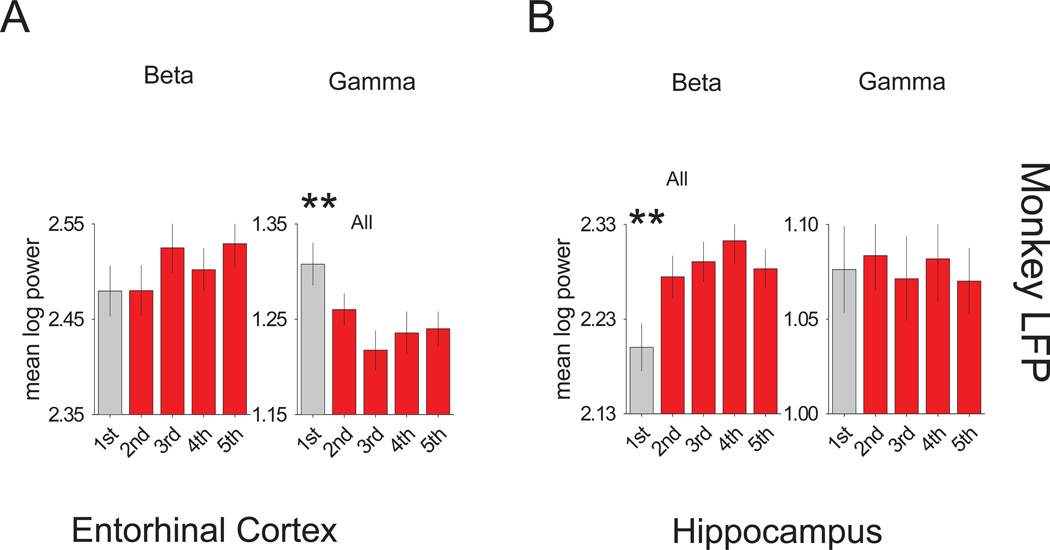
For each fixation only session, frequency spectra averages for the initial presentation of new stimuli were analyzed across a 400 ms epoch during the scene period for gamma and beta bandwidths, contrasting them to spectra averages of the successive presentations. Results for the entorhinal monkey LFP spectra averages indicated a difference favoring the gamma bandwidth power of the first presentation over subsequent presentations (all t(29) >2.9; p<.0069), but no differences for the beta bandwidth power (Figure 3A). The converse was true of the hippocampal spectra averages with the beta bandwidth exhibiting a difference favoring all successive presentations over the initial presentation (all t(25) >3.4; p<.0025), and the gamma bandwidth exhibiting no differences (Figure 3B). Thus, while the polarity of the responses differed between the monkey entorhinal cortex and hippocampus, both structures signaled immediate novelty similar to the prominent signal seen in humans.
Figure 3. Immediate novelty signals of the monkey LFP.
Individual bar graphs depict the fixation task comparisons between first (grey) versus the second, third, fourth and fifth (red) trials (± SEM of the differences). (A) Results for the monkey entorhinal LFP signal analyses showing the mean log power for the beta (left) and gamma (right) bandwidth spectra. (B) Results for the monkey hippocampal LFP signal analyses showing the mean log power for the beta (left) and gamma (right) bandwidth spectra. *** = p <0.005 and ** = p <0.01. See also Figure S2–6.
Trial Outcome
One of the most prominent task-related signals we have seen in the monkey hippocampus from single cell recording was a strong differentiation between correct and error trials (trial outcome) during the reward and ITI periods of an object-place associative learning task (Wirth et al., 2009). Similar trial outcome signals have also been reported by us in the entorhinal cortex during the location-scene association task used in the present study (Hargreaves et al., 2006; Hargreaves et al., 2007). This information can be used to strengthen correct/rewarded associations and modify incorrect/unrewarded ones during learning.
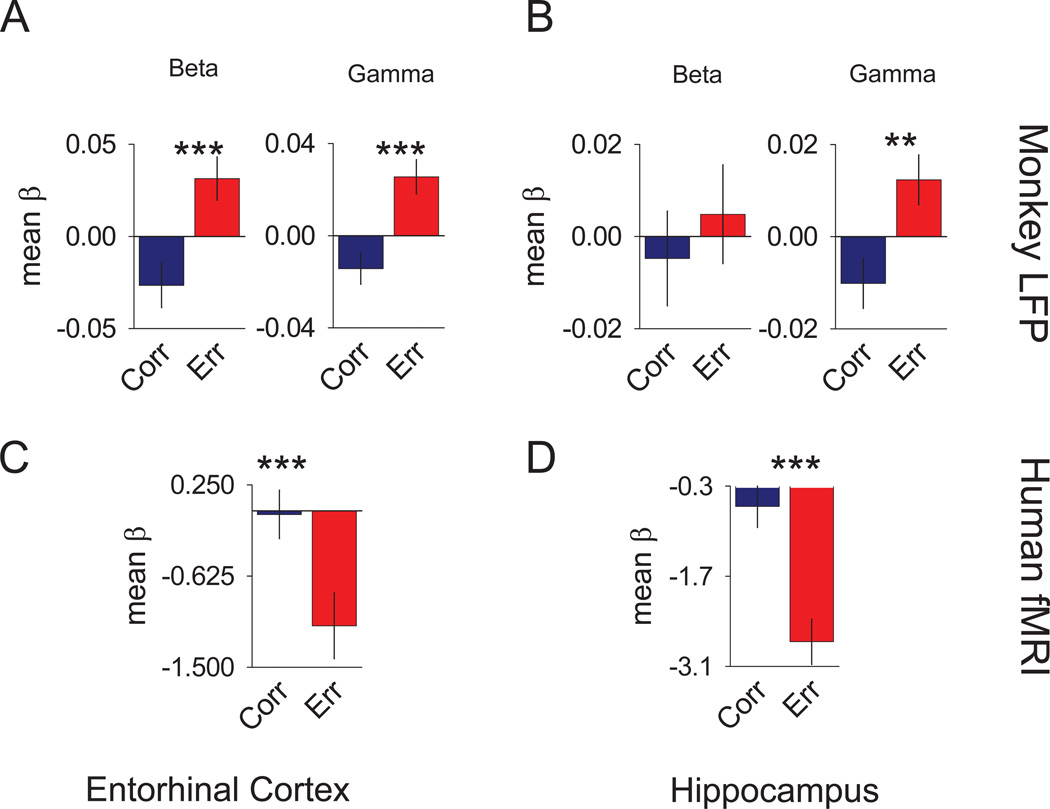
We first asked whether the prominent outcome signals seen at the single unit level of analysis in monkeys were also reflected in the LFP. For all new stimuli, frequency spectra averages of the “correct” and “error” trials were analyzed during a post-response trial epoch spanning 1500 ms across the reward and ITI periods. Multiple regressions generated β values for power of both the gamma and beta bandwidths, which were then compared in group analyses using parametric statistics (Figure 4A and B). An additional exclusion criterion was applied to these analyses requiring that sessions had a minimum of seven error responses for adequate weighting of the β coefficients. For the entorhinal cortex, significant differences between correct and error trials were seen for both the gamma (t(41)=4.25; p<.0001) and beta (t(41)=3.63; p<.0007) bands (Figure 4A). The direction of the difference for both bandwidths favored the error trials with positive β values contrasted to the correct trials negative β values. Consistent with our single unit findings in the hippocampus (Wirth et al., 2009), significant differences between correct and error trial β values were seen for the gamma band (t(24)=3.09; p<.0036), but not the beta band. Like the entorhinal cortex, the gamma band difference in the hippocampus favored the error trials with positive β values (Figure 4B).
Figure 4. Trial Outcome signals of the monkey LFP and human fMRI.
Individual bar graphs depict the comparisons between mean correct (blue) versus mean error (red) trials (± SEM of the differences). (A) Results for the monkey entorhinal LFP signal multiple regression analyses comparing the mean β values . (B) Results for the monkey hippocampal LFP signal multiple regression analyses comparing the mean β values (C) Results of the human entorhinal fMRI BOLD signal multiple regression analyses comparing the mean β values. (D) Results of the human hippocampal fMRI BOLD signal multiple regression analyses comparing the mean β values. *** = p <0.005. See also Figure S2–6.
To examine trial outcome signals in the human MTL, we analyzed the entorhinal and hippocampal ROIs using the same multiple regression to generate β values for the correct and error trial responses to new stimuli for each subject. We observed significant differences in both the entorhinal cortex (t(30)=3.19; p<.0034; Figure 4C) and hippocampal (t(30)=4.75; p<.0001; Figure 4D) ROIs. The direction of the differences was consistent across both ROIs, favoring the error trials with greater negative β values than the correct trials. While the polarity of the responses differed between monkeys and humans, the signals in both species clearly differentiate correct from error trials. We address possible reasons underlying the difference in polarity in the discussion.
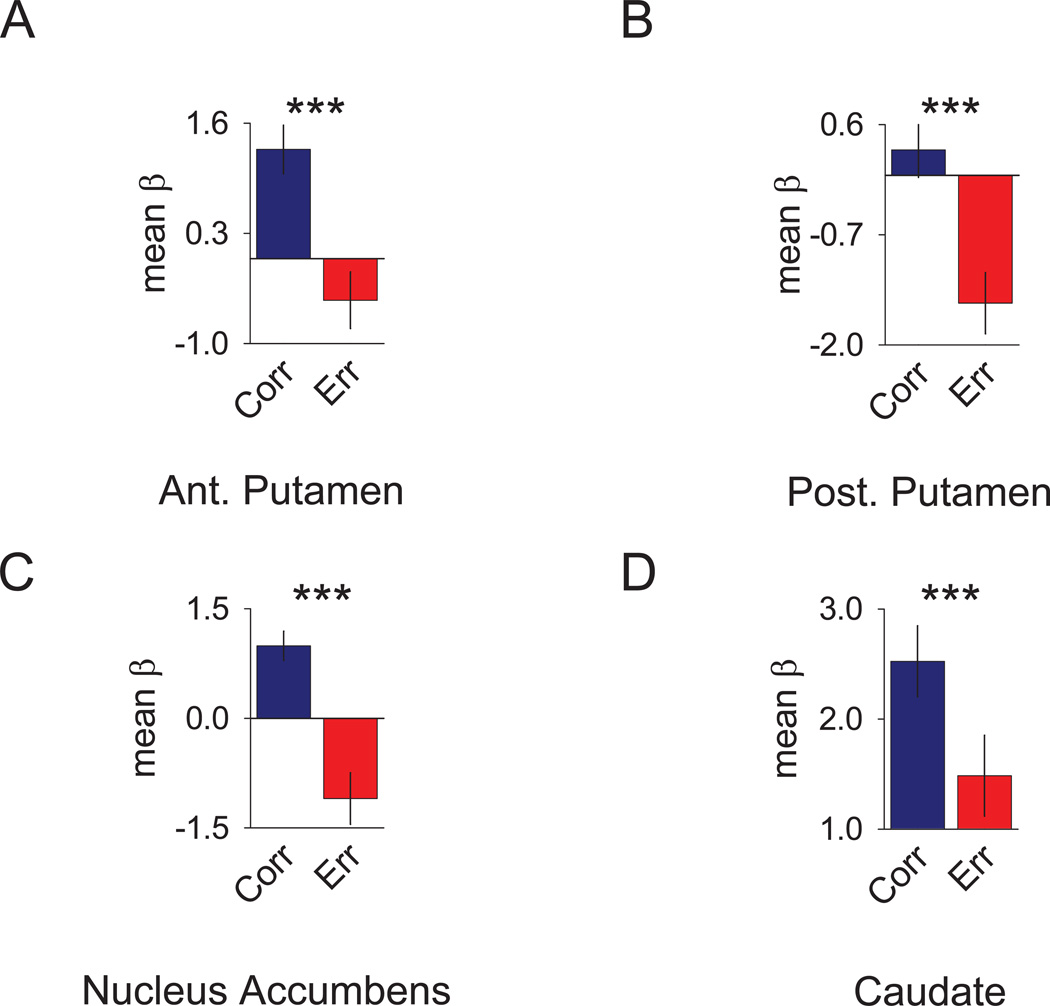
One advantage of functional neuroimaging over electrophysiological recording is the ability to acquire neurophysiological responses from a large number of regions simultaneously. The strong trial outcome signals observed in the entorhinal cortex and hippocampus in both species suggests that perhaps regions such as the striatum – traditionally thought to play an important role in reward learning and memory – may also be correlated with trial outcome. To address this possibility we compared the responses to correct and error trials for new stimuli in the human caudate, anterior putamen, posterior putamen and nucleus accumbens (Figure 5). This analysis showed similarly robust trial outcome signals in these areas (caudate: t(30)=3.08; p<.0045; anterior putamen: t(30)=5.55; p<.0001; nucleus accumbens: t(30)=6.80; p<.0001; posterior putamen: t(30)=6.45; p<.0001). These results suggest that the striatum and medial temporal lobe may work in a synergistic way to signal information about trial outcome during the learning process.
Figure 5. Trial Outcome in the Human Striatum.
Bar graphs depict the mean response of striatal ROIs to correct (blue) versus error (red) trials (± SEM of the differences). The striatum was anatomically divided into the (A) anterior (ant.) putamen, (B) posterior (post.) putamen, (C) nucleus accumbens, and (D) caudate. Results reflect the mean β values obtained by multiple linear regression averaged across all voxels defined by the anatomical mask. *** = p <0.005. See also Figure S2–6.
Associative Learning
Wirth et al. (2003) reported that during the acquisition of new location-scene associations, 28% of hippocampal neurons responded selectively to individual new stimuli, either increasing or decreasing their stimulus selective activity correlated with the learning of individual associations. We have seen similar results in the entorhinal cortex (Hargreaves et al., 2006; Hargreaves et al., 2007). Law et al. (2005) reported gradually increasing BOLD fMRI signal with increasing learning strength across multiple MTL areas in humans. We next asked if this same gradual learning signal were seen at the level of the LFP in monkeys. To address this question, β values were generated for the gamma and beta frequency spectra bandwidths of an 1100 ms epoch spanning the scene and delay periods that were associated with one of five learning strengths. Learning strengths were derived from breaking down the continuous learning curve estimates into five successive likelihood categories. Additional β values for the same epoch and bandwidths were generated separately for the first presentation of a new scene and for reference scenes. Results from the entorhinal β values revealed a significant linear patterns of increases across the learning strengths for the beta bandwidth (F(1,48)=10.767; p<.002; Figure 6A). To ensure that this learning signal was not due to non-specific changes over time, we performed an additional multiple regression analysis in which trials were coded by presentation order broken down into 20% increments (quintiles). Significant linear trends were seen across quintiles across both bandwidths and in both areas (Figure S6A and B). However, when we regressed time out from the learning strength signal by pitting the presentation order predictors against the learning strength predictors in the same analysis, we found the β values in the beta band of the entorhinal cortex retained a statistically significant linear trend ((F(1,48)=5.01; p<.03;Figure S6C, left), suggesting a selective learning effect. None of the other learning strength patterns in either the entorhinal cortex (gamma band) or the hippocampus (beta or gamma band) remained reliable once any non-specific effect of time was regressed out (Figure S6C, right and S6D).
The original report of Law et al. (2005) in humans functionally defined regions in the MTL bilaterally by isolating clusters in of voxels within ROIs in which activity varied in some manner by memory strength. Here, to parallel the monkey methodology more closely, all voxels within anatomically defined ROIs were collapsed bilaterally. Consistent with the original Law et al. (2005) report, the resulting mean β values showed significant linear increases across the successive learning strengths for both the hippocampal (F(1,30)=25.283; p<0.0001) and entorhinal (F(1,30)=11.618; p<0.002) ROIs (Figure 6C and D). No general effect of time was present in this or any other of the fMRI analyses. As is typical in fMRI data analysis, regressors are already included to model low frequency drift in the scanner signal. Thus, if there were a global effect of time masquerading as an effect of memory strength (which would also require a correlation between time and memory strength – something explicitly disrupted by the replacement of stimuli as they are learned), it would have been removed by these low-frequency regressors.
DISCUSSION
Despite the superior learning abilities of humans relative to monkeys during a conditional motor associative learning task, the information conveyed by neural activity in the medial temporal lobe was equivalent across all major categories of learning- and memory-related signals examined. Activity in the hippocampus and/or entorhinal cortex in both species provided a signal of relative stimulus novelty/familiarity, immediate novelty, trial outcome and associative learning (Figure S2–6 shows an overall comparison of all monkey and human signals across all comparisons using the same scale). These findings suggest a more precise homology of electrophysiological signals in high level association areas than has been previously demonstrated. These findings also highlight the similarity between the learning- and memory-related signals seen across the hippocampus and entorhinal cortex in both primate species. These latter findings are consistent with our previous reports in monkeys showing similar patterns of single unit activity in the hippocampus (Wirth et al., 2003), entorhinal cortex (Hargreaves et al., 2006; 2005) and perirhinal cortex (Yanike et al., 2009) during the same conditional motor associative learning task used here. The findings do not show a simple one-to-one equivalence across species and techniques, but analogous signals conveying the same information are extensively present. Thus, in monkeys and humans, both the hippocampus and entorhinal cortex provide similar learning- and memory-related neural signals during tasks of new association learning.
Novelty Response
We report that in monkeys and humans both the hippocampus and entorhinal cortex signal the very first time a novel stimulus is presented with a differential BOLD fMRI or LFP signal relative to subsequent presentations of that stimulus, though the polarity of the signal differed across species. These findings are consistent with previous findings in the human literature (Law et al., 2005; Tulving et al., 1996), and with single unit studies in the rodent hippocampus (Cheng and Frank, 2008; Fyhn et al., 2002), though to our knowledge have not been reported before in the monkey entorhinal cortex or hippocampus. The signals previously reported in humans have commonly been linked to memory encoding strength and may provide an initial measure of how well that stimulus or event may be remembered. These findings suggest that the hippocampal novelty effects are highly conserved across species.
New and Highly Familiar Stimuli
We also show that the monkey and human hippocampus and entorhinal cortex differentiate between novel stimuli seen for the first time during that recording session and highly familiar stimuli seen daily for many months with increased LFP and BOLD fMRI responses, respectively to the familiar stimuli. A similar differential familiarity signal has also been reported in the perirhinal cortex at the level of single unit responses, though the latter responses are opposite in polarity with enhanced responses to novel relative to familiar stimuli (Fahy et al., 1993; Li et al., 1993; Xiang and Brown, 1998). Enhanced single unit activity to familiar stimuli relative to novel stimuli has been described in the macaque prefrontal cortex (Xiang and Brown, 2004) and was interpreted as playing a role in the process of long-term memory retrieval. Another common familiarity signal seen at the single unit level of analysis is a decremental response as initially novel stimuli are repeated. Early studies in monkeys reported no such decremental signal in the hippocampus relative to the perirhinal cortex (Brown and Aggleton, 2001; Li et al., 1993; Riches et al., 1991; Zhu et al., 1995). However, more recently, several studies have described such decremental signals in the monkey (Jutras and Buffalo, 2010; Yanike et al., 2009) or human (Pedreira et al., 2010) hippocampus. These findings suggest that the monkey and human hippocampus and entorhinal cortex exhibit a wider range of familiarity signals than previously appreciated and support the much debated view in the literature that the hippocampus not only contributes to recollection (Brown and Aggleton, 2001; Eichenbaum et al., 2007), but also to familiarity (Wixted and Squire, 2010).
Trial Outcome
We previously showed that different populations of cells in the monkey hippocampus monitored information about trial outcome including both success (correct up cells) and failure (error up cells; Wirth et al., 2009). Here we confirm that this trial outcome signal is also present at the level of the LFP in monkeys and show for the first time that this signal is also seen at the level of BOLD fMRI signals in humans. We also show prominent trial outcome signals in the human striatum including the caudate, putamen and nucleus accumbens. Previous studies in monkeys have shown associative learning signals in the anterior caudate and putamen using tasks very similar to the one used here (Pasupathy and Miller, 2005; Williams and Eskandar, 2006). How might the trial outcome and associative learning signals seen in both the medial temporal lobe (Wirth et al., 2003; Wirth et al., 2009) and striatum (Pasupathy and Miller, 2005; present findings; Williams and Eskandar, 2006) interact? Lisman and Grace (2005) hypothesized that activity in a hippocampal-VTA loop, connected via projections through the nucleus accumbens, may control the entry of new information into long-term memory. Our findings suggest that a similar functional loop may also underlie the development of new conditional motor associations. Future studies recording both single-units and LFP activity simultaneously in the medial temporal lobe and striatum during new conditional motor learning in monkeys will be a powerful model system to test important unanswered questions about the nature, timing and direction of the learning signals across these areas suggested by the Lisman and Grace (2005) model.
Another striking feature of the trial outcome signal was that the polarity of the LFP signals seen in monkeys (error trials > correct trials) was opposite to the BOLD fMRI pattern observed in humans (correct trials > error trials). Polarity differences were also seen in some of the areas and bandwidths for the new versus reference comparison (Figure 2B) and the novelty response (Figure 3B). There are a number of possible explanations for these polarity differences. One possibility is that the underlying differential neural signals across species are equivalent and the polarity differences reflect the complex translation between LFP measures in monkeys and BOLD fMRI signals in humans. Alternatively, the polarity differences may reflect differences in behavioral strategy across species. For example, in the case of trial outcome, while both species use trial outcome data to solve the task, humans may focus on correct trials while monkeys may focus more on error trials. Further studies will be needed to differentiate between these possibilities.
Associative Learning
Our previous study in humans reported clear increases in BOLD fMRI signals across the medial temporal lobe as humans gradually learned new conditional motor associations (Law et al., 2005). Here we showed similar patterns of learning-related LFP signals in the monkey entorhinal cortex. Similar to humans, the beta band in the monkey entorhinal cortex showed clear increases across performance levels. Surprisingly, a similar learning signal was not seen in either LFP frequency band of the monkey hippocampus, a structure that exhibits strong associative-learning related signals at the single cell level in the same task (Wirth et al., 2003). This may be due to a number of different factors. For example, the presence of similar number of increasing and decreasing responses at the single cell level with learning in the hippocampus might have masked the LFP signal. alternatively this absence of learning signal in the monkey hippocampal LFP may be due to the broad sensitivity of the LFP signal. For example, recent reports from population analyses in monkeys and rodents revealed that hippocampal neurons convey significant information about incremental timing both within a trial (MacDonald et al., 2011; Naya and Suzuki, 2011) as well as across the entire recording session (Manns et al., 2007). These findings may relate to our observation that striking changes over the time course of the trial were observed in both the beta and gamma bands of the monkey hippocampus (Figure S6B) and may have overwhelmed the associative learning signals in this region.
Our findings show that for associative learning signals, the pattern of beta band activity in the monkey entorhinal cortex corresponded best to the BOLD fMRI signal in humans. However it is tempting to ask the more general question of which LFP frequency band in monkeys corresponds best to BOLD fMRI signals seen in humans across all signals examined. Our findings show mixed results and that there may be neither a simple one-to-one equivalence nor even a consistently superior mapping (Supplementary Table 1). When considering examples where the polarity was identical across species or all examples in which significant differential signals were observed irrespective of polarity, there are cases of beta band, gamma band and in some cases both frequency bands corresponding to the BOLD fMRI signal. However, there is a slight numerical advantage for the beta band to correspond in more cases. These findings differ from the reports of Logothetis and colleagues in area V1 where they saw the best correspondence between the gamma band and the BOLD fMRI signal (Goense and Logothetis, 2008; Logothetis, 2002). Together, these suggest that the relationship between LFP and BOLD, while clearly present, is not a simple one and that details of the underlying neural signals, representations, neurotransmitters, and other differences across brain regions may affect the relationships between LFP and BOLD fMRI signals.
Conclusions
A major goal in neuroscience research is to understand how the detailed neurophysiological underpinnings of higher cognitive functions, often measured in nonhuman primates, correspond to human neurophysiology. While previous studies have tried to span this gap with BOLD fMRI studies in both species (Nakahara et al., 2007; Orban et al., 2004), here we provide evidence that LFP signals measured in monkeys and BOLD fMRI signals measured in humans both performing the same associative learning task are conserved. These findings validate the analogous nature of LFP signals measured in monkeys and BOLD fMRI signals measured in humans. Moreover, because LFP signals in monkeys can be easily recorded in parallel with single unit activity, this opens the door to a wide range of new studies that will allow us to compare single unit data from monkeys more directly with related studies using BOLD fMRI in humans in all areas of cognitive neuroscience. We also showed that despite differences in the speed of learning, magnitude of learning and response modality (eye movements in monkeys versus finger movements in humans) across species, the learning and memory related patterns of activity were conserved across all major task-related signals measured. This suggests that we are tapping into fundamental and homologous learning signals that do not depend on the precise levels of accuracy or modality of motor output. It is also important to note that while conserved signals were observed across species, there was not a one-to one match between the monkey LFP signals and human BOLD fMRI signals. In a number of cases differences in polarity were seen and while striking learning signals were seen in human BOLD fMRI signals in both the entorhinal cortex and hippocampus, only entorhinal and not hippocampal LFP signaled associative learning in monkeys. These findings emphasize the idea that the relationship between LFP and BOLD fMRI is complex and highlight the need for further studies using both a wider range of behavioral tasks and a larger set of brain areas to further specify the relationship between LFP signals in monkeys and BOLD fMRI signals in humans.
EXPERIMENTAL PROCEDURES
Monkey LFPs
Subjects
We analyzed LFP recordings from two male macaque monkeys, one rhesus (monkey A; 11.5 kg) and one bonnet (monkey B; 7.8 kg). Following behavioral training the animals were implanted with a head post and recording chamber (Crist Instruments, Damascus, MD) under isofluorane anesthesia using sterile surgical techniques. Animals received post-operative analgesics and antibiotics. All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. During training and recording the monkey’s head was fixed in position by the implanted headpost, while the animal was seated comfortably in a primate chair (Crist Instruments).
Recording Locations and Techniques
The positioning of the recording chambers was determined from pre-surgical MRI images. Monkey A had the chamber positioned over the left anterior hippocampus, and overlying entorhinal cortex, while monkey B had the chamber positioned over the right anterior hippocampus and entorhinal cortex. The same images were used during recording to estimate the depth of the recording electrode tip along the target trajectory, as well as the medial-lateral, and anterior-posterior positioning of the recording trajectory itself. For each session a tetrode, consisting of four platinum/tungsten core channels embedded in a quartzite probe with a triangular/center configuration (Thomas Recording imped: 500KΩ – 1.4MΩ) was inserted through a stainless steel guide tube positioned in a grid system (Crist Instruments) within the recording chamber. The recording tip of the tetrode was physiologically monitored as it was driven down to target by a microdrive (Nan Drives Inc. Israel).
LFP Signal Processing
Continuous LFP recordings were drawn from one of four tetrode channels. Signals were pre-amplified with unity gain (Plexon headstage), and then amplified (5,000–20,000X), and bandpassed (0.7Hz-170Hz) using the Plexon Muti-channel Acquisition Processor (MAP) system. Signals were digitized at 1KHz, and saved to disk for offline analysis. Offline analyses were conducted using Matlab scripts developed for the current project, and incorporated the Chronux toolbox (P. Mitra at Cold Spring Harbor Laboratories). The LFP signals for each session were separated into 4 s sweeps coinciding with the trial onsets and offsets. Individual LFP sweeps were inspected for noise and artifacts that saturated the amplifiers, with the sweeps that violated these criteria being removed from further analyses. 60Hz line noise was digitally removed using Butterworth filters (Matlab signal processing toolbox). Each sweep was converted into an individual spectra of frequency and power across the 4 s duration, using 5 Discrete Prolate Spheroidal Sequence (DPSS) data tapers applied to a 300 ms sliding window, stepped at 50 ms intervals, giving a 10Hz aggregate resolution. Specific trial type comparisons of the LFP spectra were made for the non-overlapping spectra bandwidths of gamma (30–100Hz) and beta (10–25Hz) across pre-determined epochs based on the previous single unit findings of Wirth et al., (2003 and 2009).
Human BOLD fMRI
Subjects
Human BOLD fMRI data were pooled from two studies that employed the same conditional-motor-associative learning task for a total of 31 subjects (Kirwan et al., 2007; Law et al., 2005). Subjects were solicited from the John Hopkins community and paid for their participation. Thirteen of the subjects were male, 18 were female and all subjects were right handed with a mean age of 26.7 years (range 18–33).
BOLD fMRI Imaging Parameters, Locations and Techniques
Imaging data were collected using a Phillips 3.0 Tesla scanner (Best, The Netherlands) equipped with a SENSE (Sensitivity Encoding) head coil. Functional echoplanar images were collected via a high-speed single-shot pulse sequence with an 80×80 acquisition matrix size, a 30ms echo time, a 70° flip angle, a SENSE factor of 2, and a 3 × 3 mm in-plane acquisition resolution. Two acquisitions per trial for 132 trials per run made for a total of 264 whole-brain three-dimensional volumes that were acquired with a repetition time (TR) of 1.5 s for each run. Functional volumes were aligned to the principle axis of the hippocampus and consisted of 30 triple oblique axial slices. To allow for MR signal stabilization data acquisition began after the fourth image. To facilitate anatomical localization and cross-participant alignment, a standard whole-brain, three-dimensional magnetization-prepared rapid gradient echo (MP-RAGE) scan was acquired (150 oblique axial slices, echoplanar with the fMRI data, 1 × 1 × 1 mm voxels).
A region of interest alignment (ROI-AL) approach developed in the Stark laboratory (e.g., Stark and Okado, 2003) was used to align both the structural and functional data. This entailed aligning all structural and functional scans to the Talairach atlas (Talairach and Tournoux, 1988). The Talairach transformed MP-RAGE (1 mm3) structural images were then used to hand segment the bilateral hippocampus, and entorhinal cortices according to the boundaries outlined by Insausti et al. (1998).
A model for the fine tuned transformation calculations was then constructed by choosing a single participant (number 29) to serve as the initial model for the transformation calculation for all the other participants. The ROI-AL approach uses high dimensionality diffeomorphic techniques (ROI-Demons) (Stark and Okado, 2003; Yassa and Stark, 2009) to map the transformation between an individual's ROI segmentations and the model's segmentation. ROI-Demons generate a smooth three-dimensional vector field that is used to transform images between coordinate systems. This or related techniques have been used successfully to align across participants the structures of the MTL and the substructures of the hippocampus (Bakker et al., 2008; Kirwan et al., 2007; Kirwan and Stark, 2007; Law et al., 2005; Miller et al., 2005; Stark and Okado, 2003), and have been extended here to the striatum. After each participant's structural image was aligned to the model the resulting transformation matrices were applied to align the functional images.
BOLD fMRI Signal Processing
GLM analyses of the human BOLD fMRI data were performed to estimate activity of selected trial types. Nuisance regressors - coding for scanner drift and offset - were also included in the GLM analyses. The resulting estimates of activity (β values) for the trial types of interest were subjected to our anatomical ROI analyses.
Statistical Analyses
Matched comparisons between the different trial types and regions for the LFP and fMRI sessions were performed using paired t-tests, regardless, of whether the analyses were performed upon the average log power of the selected bandwidths and epochs of the monkey LFP spectra, or performed upon the derived multiple regression β values from either the same monkey LFP spectra or human BOLD fMRI ROIs. For the analyses of learning strengths, repeated measures analysis of variance examining linear trends was used, regardless of being performed upon the monkey LFP or the human fMRI data.
HIGHLIGHTS.
Humans and monkeys performed the same visuomotor associative learning task.
Parallel analyses revealed strong similarities in human fMRI and monkey LFP signals.
Primate MTL showed familiarity, first presentation, outcome and learning signals.
Neural signals during associative learning tasks are conserved across species.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat.Rev.Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 3.Brown MW, Wilson FAW, Riches IP. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Res. 1987;409:158–162. doi: 10.1016/0006-8993(87)90753-0. [DOI] [PubMed] [Google Scholar]
- 4.Brozinsky CJ, Yonelinas AP, Kroll NE, Ranganath C. Lag-sensitive repetition suppression effects in the anterior parahippocampal gyrus. Hippocampus. 2005;15:557–561. doi: 10.1002/hipo.20087. [DOI] [PubMed] [Google Scholar]
- 5.Cheng S, Frank LM. New experiences enhance coordinated neural activity in the hippocampus. Neuron. 2008;57:303–313. doi: 10.1016/j.neuron.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudukovic NM, Preston AR, Archie JJ, Glover GH, Wagner AD. High-resolution fMRI reveals match enhancement and attentional modulation in the human medial temporal lobe. J.Cogn Neurosci. 2011;23:670–682. doi: 10.1162/jocn.2010.21509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan K, Curtis C, Davachi L. Distinct memory signatures in the hippocampus: intentional States distinguish match and mismatch enhancement signals. J.Neurosci. 2009;29:131–139. doi: 10.1523/JNEUROSCI.2998-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichenbaum H, Yonelinas AR, Ranganath C. The Medial Temporal Lobe and Recognition Memory. Annu.Rev.Neurosci. 2007 doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahy FL, Riches IP, Brown MW. Neuronal activity related to visual recognition memory: long- term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp.Brain Res. 1993;96:457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- 10.Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 11.Fyhn M, Molden S, Hollup S, Moser MB, Moser EI. Hippocampal neurons responding to first-time dislocation of a target object. Neuron. 2002;35:555–566. doi: 10.1016/s0896-6273(02)00784-5. [DOI] [PubMed] [Google Scholar]
- 12.Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr.Biol. 2008;18:631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 13.Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Hadj-Bouziane F, Boussaoud D. Neuronal activity in the monkey striatum during conditional visuomotor learning. Exp.Brain Res. 2003;153:190–196. doi: 10.1007/s00221-003-1592-4. [DOI] [PubMed] [Google Scholar]
- 15.Hargreaves EL, Naya Y, Suzuki WA. Unit and local field potential (LFP analysis of trial outcome in primate entorhinal cortex during location-scene associative learning. Soc.for Neurosci. 2007 [Google Scholar]
- 16.Hargreaves EL, Smith AC, Brown EN, Suzuki WA. Tetrode recordings of learning related activity in the primate entorhinal cortex during a location-scene associative learning task. Soc.for Neurosci.Abstr. 2006 [Google Scholar]
- 17.Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- 18.Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents. J.Comp.Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- 19.Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am.J.Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- 20.Jutras MJ, Buffalo EA. Recognition memory signals in the macaque hippocampus. Proc.Natl.Acad.Sci U S A. 2010;107:401–406. doi: 10.1073/pnas.0908378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirwan CB, Jones CK, Miller MI, Stark CE. High-resolution fMRI investigation of the medial temporal lobe. Hum.Brain Mapp. 2007;28:959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirwan CB, Stark CE. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn.Mem. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohler S, McIntosh AR, Moscovitch M, Winocur G. Functional interactions between the medial temporal lobes and posterior neocortex related to episodic memory retrieval. Cereb.Cortex. 1998;8:451–461. doi: 10.1093/cercor/8.5.451. [DOI] [PubMed] [Google Scholar]
- 24.Kutner MH, Neter J, Nachtsheim CJ, Li W. Applied Statistical Linear Models. New York: McGraw-Hill; 2004. [Google Scholar]
- 25.Law JR, Flanery MA, Wirth S, Yanike M, Smith AC, Frank LM, Suzuki WA, Brown EN, Stark CE. Functional magnetic resonance imaging activity during the gradual acquisition and expression of paired-associate memory. J.Neurosci. 2005;25:5720–5729. doi: 10.1523/JNEUROSCI.4935-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. J.Neurophys. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- 27.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos.Trans.R.Soc.Lond B Biol.Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald CJ, Legage KQ, Eden UT, Eichenbaum H. Hippocampal "time cells" bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- 32.Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proc.Natl.Acad.Sci U.S.A. 2005;102:9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- 34.Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- 35.Nakahara K, Adachi Y, Osada T, Miyashita Y. Exploring the neural basis of cognition: multi-modal links between human fMRI and macaque neurophysiology. Trends Cogn Sci. 2007;11:84–92. doi: 10.1016/j.tics.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- 37.Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Passingham R. How good is the macaque monkey model of the human brain? Curr.Opin.Neurobiol. 2009;19:6–11. doi: 10.1016/j.conb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- 40.Pedreira C, Mormann F, Kraskov A, Cerf M, Fried I, Koch C, Quiroga RQ. Responses of human medial temporal lobe neurons are modulated by stimulus repetition. J.Neurophysiol. 2010;103:97–107. doi: 10.1152/jn.91323.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riches IP, Wilson FA, Brown MW. The effects of visual stimulation and memory on neurons of the hippocampal formation and the neighboring parahippocampal gyrus and inferior temporal cortex of the primate. J.Neurosci. 1991;11:1763–1779. doi: 10.1523/JNEUROSCI.11-06-01763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 43.Smith AC, Frank LM, Wirth S, Yanike M, Hu D, Kubota Y, Graybiel AM, Suzuki WA, Brown EN. Dynamic analysis of learning in behavioral experiments. J.Neurosci. 2004;24:447–461. doi: 10.1523/JNEUROSCI.2908-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stark CE, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J.Neurosci. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. J.Comp.Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J.Neurophys. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- 47.Talairach J, Tournoux P. A Co-Planar Steres Jotactic Atlas of the Human Brain. Stuttgard, Germany: Thieme; 1988. [Google Scholar]
- 48.Tulving E, Markowitsch HJ, Craik FIM, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb.Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 49.Williams ZM, Eskandar EN. Selective enhancement of associative learning by microstimulation of the anterior caudate. Nat.Neurosci. 2006;9:562–568. doi: 10.1038/nn1662. [DOI] [PubMed] [Google Scholar]
- 50.Wirth S, Avsar E, Chiu CC, Sharma V, Smith AC, Brown E, Suzuki WA. Trial outcome and associative learning signals in the monkey hippocampus. Neuron. 2009;61:930–940. doi: 10.1016/j.neuron.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300:1578–1581. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- 52.Wixted JT, Squire LR. The role of the human hippocampus in familiarity-based and recollection-based recognition memory. Behav.Brain Res. 2010;215:197–208. doi: 10.1016/j.bbr.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology. 1998;37:657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 54.Xiang JZ, Brown MW. Neuronal responses related to long-term recognition memory processes in prefrontal cortex. Neuron. 2004;42:817–829. doi: 10.1016/j.neuron.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 55.Yanike M, Wirth S, Smith AC, Brown EN, Suzuki WA. Comparison of associative learning-related signals in the macaque perirhinal cortex and hippocampus. Cereb.Cortex. 2009;19:1064–1078. doi: 10.1093/cercor/bhn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yassa MA, Stark CE. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. Neuroimage. 2009;44:319–327. doi: 10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 57.Zhu XO, Brown MW, Aggleton JP. Neuronal signaling of information important to visual recognition memory in rat rhinal and neighboring cortices. Euro.J.Neurosci. 1995;7:753–765. doi: 10.1111/j.1460-9568.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 58.Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behav.Neurosci. 1985;99:22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]
- 59.Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J.Neurosci. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.