Abstract
Disgust, an emotion related to avoiding harmful substances, has been linked to moral judgments in many behavioral studies. However, the fact that participants report feelings of disgust when thinking about feces and a heinous crime does not necessarily indicate that the same mechanisms mediate these reactions. Humans might instead have separate neural and physiological systems guiding aversive behaviors and judgments across different domains. The present interdisciplinary study used functional magnetic resonance imaging (n = 50) and behavioral assessment to investigate the biological homology of pathogen-related and moral disgust. We provide evidence that pathogen-related and sociomoral acts entrain many common as well as unique brain networks. We also investigated whether morality itself is composed of distinct neural and behavioral subdomains. We provide evidence that, despite their tendency to elicit similar ratings of moral wrongness, incestuous and nonsexual immoral acts entrain dramatically separate, while still overlapping, brain networks. These results (i) provide support for the view that the biological response of disgust is intimately tied to immorality, (ii) demonstrate that there are at least three separate domains of disgust, and (iii) suggest strongly that morality, like disgust, is not a unified psychological or neurological phenomenon.
INTRODUCTION
Disgust is an emotion traditionally defined as an aversive state that motivates withdrawal from offensive substances, such as animal products and certain foods (Rozin & Fallon, 1987). Recently, researchers from a variety of disciplines have taken interest in the relationship between disgust and moral behavior. For example, social psychologists have argued that disgust is an emotion underpinning moral judgment (Haidt, Rozin, McCauley, & Imada, 1997), adaptation-minded researchers have proposed that disgust is a mechanism co-opted from its original function as a pathogen avoidance system to also guide decision-making in the moral domain (Lieberman, Tooby, & Cosmides, 2003), clinical cognitive neuroscientists have addressed how impairments in disgust might underlie psychopathic behavior (Blair, Marsh, Finger, Blair, & Luo, 2006), and lawyers and policy-makers have considered how disgust affects the judgment and sentencing of criminals (Kahan, 1998). Despite the purported role disgust plays in mediating the avoidance of harmful substances and regulating sociomoral judgments, the fact that subjects across a variety of experiments report feelings of disgust when considering both feces and a heinous crime (Miller, 1997) does not necessarily indicate that the same mechanisms mediate these reactions. Humans might instead have separate neural and physiological systems guiding aversive judgments and behaviors across distinct types of nonsocial and social domains, all of which precipitate a feeling we subjectively label “disgust” for lack of a more refined linguistic term (Bloom, 2004).
Given the growing number of publications across academic disciplines that cite a relationship between disgust and moral disapprobation, it seems critical to determine whether our reactions toward items such as vomit, feces, and rotten food are indeed biologically homologous to our reactions toward acts such as incest, theft, and murder, or whether they are instead only linguistically (but not biologically) analogous. Accordingly, the first goal of the present interdisciplinary study was to use functional magnetic resonance imaging (fMRI; n = 50) and behavioral assessment to begin characterizing the proposed homology between pathogen-related and sociomoral disgust. Drawing upon theoretical insights and recent discoveries from evolutionary psychology, moral psychology, and clinical cognitive neuroscience (Koenigs et al., 2007; Schaich Borg, Hynes, Van Horn, Grafton, & Sinnott-Armstrong, 2006; Heekeren et al., 2005; Greene, Nystrom, Engell, Darley, & Cohen, 2004), we converged upon another hypothesis: Like feelings of disgust, perhaps feelings of moral wrongness do not arise from a singular mechanism. Despite the fact that moral wrongness is usually conceptualized as a singular feeling or judgment, some separate neural and physiological systems may guide negative decisions and aversive behaviors in response to different classes of social transgressions. Thus, the second goal of the present study was to investigate the shared and distinct neural correlates of two different domains of sociomoral transgressions: sexual immoral behaviors (incest) and nonsexual immoral behaviors (such as cheating, stealing, and killing).
A combination of behavioral and clinical/imaging investigations inspired the present collaboration. Cross-culturally, disgust is the emotion most correlated with appraisals of immorality (Scherer, 1997). For instance, when respondents are asked to nominate acts eliciting disgust, the majority of acts mentioned are moral offenses (Haidt et al., 1997). Furthermore, conventional transgressions (rule violations that would not be considered wrong in the absence of a cultural prohibition) are more often judged to be moral violations (violations that are considered wrong regardless of local conventions) if the violations are disgusting. Additionally, the more disgust-sensitive an individual is, the more likely he or she is to judge a disgusting conventional transgression as morally wrong (Nichols, 2002). It has also been shown that American participants hypnotized to feel a flash of disgust upon reading an arbitrary word judged acts described using that word to be more morally wrong than control participants, even if the act was judged by control participants to have no moral content (Wheatley & Haidt, 2005). Most recently, it has been demonstrated that recalling unethical deeds inspires participants to buy and use more cleaning products, a pattern interpreted as an attempt to wash away feelings of impurity and disgust (Zhong & Liljenquist, 2006). In sum, behavioral studies demonstrate that, across cultures, reports of disgust and moral disapproval often coincide.
Investigations constructed around evolutionary principles also link disgust and morality. Adaptation-minded researchers have described disgust as a mechanism that evolved to motivate the avoidance of substances associated with disease-causing agents in ancestral environments (Curtis & Biran, 2001). In support, pictures of objects cuing pathogen presence (via slimy, moist surfaces or colors reminiscent of body fluids) are rated as more disgusting than the same objects presented without pathogen-related cues (via furry surfaces or bright colors rarely associated with animate substances; Curtis, Aunger, & Rabie, 2004). Disgust is proposed to have been co-opted for other purposes as well, in particular, for the regulation of social judgments and behaviors. Adaptationist logic states that these new and separate functions would have evolved in ways specific to the environmental pressures disgust was being co-opted to solve. Through analyzing these pressures, adaptation-minded researchers have predicted that disgust evolved to solve the problem of incest, a sexual behavior with deleterious consequences (Lieberman, Tooby, & Cosmides, 2007; Fessler & Navarrete, 2004). Counterintuitively, although incest is generally considered to be immoral in most contemporary human societies, disgust’s function in incest avoidance is argued to be distinct from disgust’s function in nonsexual social domains, where disgust is thought to mediate behaviors and judgments toward sociomoral harms such as theft, violence, cheating, and deception (Haidt et al., 1997). Therefore, in nuanced contrast to past descriptions of disgust in morality (ranging from Haidt et al., 1997 to Zhong & Liljenquist, 2006), an adaptationist view predicts that there should be separate sexual and nonsexual moral-related disgust systems. In total, there should be pathogen-, sexual moral– and nonsexual moral–related disgust systems that all share a common disgust avoidance mechanism, but that are also dissociable according to the unique collection of information processing systems required by the specific adaptive problem each type of disgust evolved to solve (e.g., systems that detect odors or colors to detect pathogen presence, systems that estimate genetic relatedness to avoid incest, and systems that assess social costs to evaluate sociomoral harms). Recent behavioral evidence supports this novel prediction (Lieberman et al., 2007; Simpson, Carter, Anthony, & Overton, 2006; Fessler & Navarrete, 2003), and provides the framework for the neural hypotheses investigated in the current study.
Although psychologists have made progress in understanding how self-reports of disgust can be parsed, clinical and cognitive neuroscientists have made progress in understanding how disgust is represented in the brain. The brain regions associated with disgust have been identified through a growing number of clinical populations with selective disgust impairments (Suzuki, Hoshino, Shigemasu, & Kawamura, 2006; Schienle et al., 2003; Sprengelmeyer et al., 1996), indicating that disgust is a unique biological response differentiable from other responses, such as fear (Williams et al., 2005). Disgust is dissociable in healthy human populations as well, and has been correlated with activity in the thalamus, basal ganglia, visual cortex, and sometimes, the amygdala, anterior insula, and medial prefrontal cortex (Moll, de Oliveira-Souza, et al., 2005; Schafer, Schienle, & Vaitl, 2005; Stark et al., 2003, 2005; Fitzgerald et al., 2004; Wicker et al., 2003; Zald, 2003; Phan, Wager, Taylor, & Liberzon, 2002; Calder, Keane, Manes, Antoun, & Young, 2000; Phillips et al., 1997, 1998).
Little work has been done to investigate the neural correlates of disgust in the context of social interactions, but one study showed that a patient with lesions in the left insula and the putamen scored lower than control subjects on most pathogen-related subscales of the Disgust Scale (Haidt, McCauley, & Rozin, 1994), but higher than controls on the sex subscale (Calder et al., 2000). Another recent study showed that Huntington’s disease patients scored higher than controls on the sex and hygiene subscales of the Disgust Scale, but scored equally on all other subscales, including the food, animal nature, and body products subscales (Hayes, Stevenson, & Coltheart, 2007). Huntington’s disease patients were also more able to produce scenarios describing moral disgust violations than pathogen disgust violations. These last sets of data suggest that different types of disgust are neurally dissociable, but this possibility is not often discussed in the neuroscience literature, even in the studies in which the data were originally published.
Two recent fMRI studies provide preliminary data that common brain regions are entrained by certain types of disgust and sociomoral sentiment (Sambataro et al., 2006; Moll, de Oliveira-Souza, et al., 2005). However, in these studies, the authors investigated the emotion of “indignation” (Moll, de Oliveira-Souza, et al., 2005) and facial expressions of contempt (Sambataro et al., 2006), and it is not clear how “indignation” or “contempt” maps onto other taxonomies of disgust or relates specifically to ratings of moral wrongness. Furthermore, the stimuli used by Moll, de Oliveira-Souza, et al. (2005) conflated pathogen and moral disgust by using only moral stimuli that referenced pathogens (such as intentionally putting a spider on a baby’s face or spotting a cockroach in a restaurant). The authors also did not differentiate incest from other types of moral transgressions, which might be problematic given previous work demonstrating that the predictors of moral sentiments toward incest are not the same as the predictors of moral sentiments toward other moral transgressions (Lieberman et al., 2007). Thus, it has still not been tested whether pathogen and moral disgust entrain common neural systems, despite the number of disciplines that assume their biological commonalities. It has also not been tested whether equally intense sentiments of moral wrongness associated with different classes of moral transgressions are correlated with a singular set of psychological or neurological processes, despite the accruing number of fMRI and lesion studies published on moral processing. The present study was designed to address these two main issues.
Here we investigate the common and distinct neural and behavioral signatures of reactions toward pathogen-related substances and sociomoral violations. Fifty male participants were given a memory/recognition task involving pathogen-related acts (pathogen), pathogen-unrelated sociomoral acts (sociomoral), and neutral acts (neutral) while being scanned in a 3-T magnetic resonance imaging scanner. Sociomoral acts were divided into incestuous acts (incest) and nonsexual sociomoral acts (nonsexual moral). After being scanned, participants provided reports of disgust, appeal, and moral wrongness for each act they saw (see Methods). In light of previous theoretical and empirical work, we made the following predictions: (i) Pathogen-related acts and sociomoral acts would be rated as more disgusting than neutral acts, and memorizing and recalling phrases describing these disgusting acts would activate common neural regions including those previously shown to be involved in disgust processing. (ii) Further, if cognitive processing of these disgust domains also requires the use of distinct information processing systems in addition to their common disgust system, then pathogen-related acts and sociomoral acts should be dissociable both in the self-report ratings they provoke and in the brain regions they entrain. (iii) Finally, despite eliciting similar ratings of moral wrongness and activating common regions of the brain previously shown to be involved in moral processing—including mainly the medial prefrontal cortex and areas around the temporal–parietal junction (Schaich Borg et al., 2006; Heekeren et al., 2005; Moll, Zahn, de Oliveira-Souza, Krueger, & Grafman, 2005; Greene et al., 2004; Heekeren, Wartenburger, Schmidt, Schwintowski, & Villringer, 2003; Greene & Haidt, 2002)—we drew from adaptationist principles to predict that incestuous acts and nonsexual immoral acts would, themselves, be behaviorally and neurally dissociable. That is, morality, like disgust, might not be a unified psychological or neurological phenomenon in the way it is traditionally understood and operationalized in behavioral and fMRI studies.
METHODS
Subjects
Subjects were recruited via advertisements in the Fall of 2004. Following previous investigations of disgust that recruited subjects of one sex only (Schienle et al., 2002, 2006; Wicker et al., 2003; Phillips et al., 1998), we chose to study men only (a similar study with all women is being prepared for future publication). Fifty healthy men (age = 25 ± 6 years) provided written, informed, IRB-approved consent at Hartford Hospital and Yale University and were compensated $20/hr for their participation. All participants were right-handed on self-report and were able to perform the task successfully during practice sessions prior to scanning.
Experimental Task
To test implicit processing of our experimental conditions, we intentionally used a task that preoccupied subjects with mental operations irrelevant to our conditions of interest. We selected a simple memory task shown previously to effectively probe affective processing (Kiehl et al., 2005). During scanning, subjects were presented with memorize–recall blocks of short statements from one of four conditions: pathogen (P), incest (I), nonsexual moral (M), or neutral (N) (see below). Each block contained statements from only one condition. Subjects memorized four statements in the “memorize” phase of each block and reported in the “recall” phase whether each of four subsequent statements was one shown in the previous memorize section or a new statement (Figure 1). Two of the statements in each “memorize” phase were randomly chosen to appear in random positions in the subsequent “recall” phase (participants were not told what proportion of the statements in the “recall” phase would be ones they had just seen). In total, each block had six condition-related statements: two presented in both the “memorize” and “recall” phases, two presented only in the “memorize” phase, and two presented only in the “recall” phase.
Figure 1.
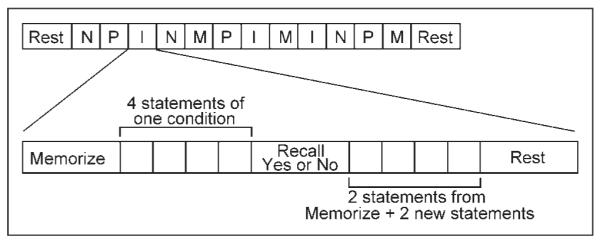
An illustration of the organization of the 12 blocks in each run: 3 neutral (N), 3 pathogen (P), 3 incest (I ), and 3 nonsexual moral (M).
Statements were presented one at a time for 2500 msec and had an intertrial interval of 500 msec. All statements were equated for length and complexity. Subjects responded with their right hand, and were instructed to press the button underneath their index finger if the displayed statement was one they had just seen in the “memorize” phase or the button underneath their middle finger if the displayed statement was one they had not seen before. Upon completion of each “recall” phase, the word “REST” was displayed continuously on the screen for 5000 msec.
Two runs of 12 randomized memorize–recall–rest blocks were presented on a visual display projected from the back of the scanner with 30 sec of rest at the beginning and end of each run. Of these 12 blocks, three blocks included statements about physically repulsive acts performed on/with a sibling of the opposite sex (Pathogen: You sipping your sister’s urine, You eating your sister’s scab), three included statements about incestuous acts performed on/with a sibling of the opposite sex (Incest: You watching your sister masturbate, You fondling your sister’s nipples), three included statements about nonsexual immoral actions performed against a sibling of the opposite sex (Nonsexual moral: You killing your sister’s child, You burglarizing your sister’s home), and three included neutral statements about actions performed on/with a sibling of the opposite sex (Neutral: You holding your sister’s groceries, You walking with your sister). In total, six blocks each of pathogen, incest, nonsexual moral, and neutral statements were presented to participants by the end of the second run. All stimuli across conditions were equated for sibling presence. We analyzed all male data together, independent of family composition given unpublished work showing that men with sisters (n = 205) and men without sisters (n = 87) do not provide significantly different disgust ratings of subsets of pathogen-related ( p = .22, two-tailed) or incestuous acts ( p = .54, two-tailed) described in the second person (DL, unpublished data). Two-sample t tests confirmed that brain activity of men with sisters did not differ significantly from brain activity of men without sisters in pathogen versus neutral, incest versus neutral, or nonsexual moral versus neutral contrasts.
After the scanning session, subjects completed a short survey in which they rated each of the acts presented during the scanning session on levels of disgust, appeal, and moral wrongness.
Imaging
fMRI data were collected on a Siemens Allegra 3-T head-dedicated scanner equipped with 40 mT/m gradients and a standard quadrature head coil at the Olin Neuropsychiatry Research Center at the Institute of Living. Participants viewed all experimental stimuli via a mirror on top of the head coil that reflected a screen at the rear entrance of the magnet bore. Stimuli were displayed on the screen using a computer-controlled projection system. A custom visual presentation package (VAPP; http://nilab.psychiatry.ubc.ca/vapp) controlled the timing of the experimental stimuli and recorded behavioral data. The functional scans were acquired using gradient-echo echo-planar imaging (EPI) (scanning parameters: repeat time [TR] = 1.50 sec, echo time [TE] = 27 msec, field of view = 24 cm, acquisition matrix = 64 × 64, flip angle = 70°, voxel size = 3.75 × 3.75 × 4 mm, gap = 1 mm, 29 slices, ascending acquisition). Six “dummy” scans were performed at the beginning of each functional run to allow for longitudinal equilibrium and were discarded before image analysis. Behavioral responses were recorded using an MRI-compatible fiber-optic response device (Lightwave Medical, Vancouver, BC).
Analysis
Preprocessing
fMRI data were preprocessed using Statistical Parametric Mapping (SPM2, Wellcome Department of Imaging Neuroscience). Functional images were reconstructed offline, and the two runs were separately realigned to the first scan of the session using INRIalign, a motion correction algorithm unbiased by local signal changes (Freire, Roche, & Mangin, 2002). Translation and rotation corrections did not exceed 2.5 mm and 2.5°, respectively, for any of the participants. After realignment, a mean functional (EPI) image was computed for each run and was subsequently matched to the SPM2 EPI template. Data were transformed into standard Montreal Neurological Institute space using a tailored algorithm with both linear and nonlinear components, and this transformation was then applied to all other corresponding functional images (Friston et al., 1995). Finally, data were spatially smoothed four times the voxel dimensions (3 × 3 × 3 mm) with a full width at half maximum Gaussian kernel (Kiehl et al., 2005), and submitted to a fifth-order infinite impulse response Butterworth low-pass filter of 0.16 Hz to remove any high-frequency noise.
Data Analysis
Each individual participant’s first-level analysis modeled the canonical hemodynamic response with temporal derivative to each condition of interest in a block design. The amplitude of the hemodynamic response was calculated from both its nonderivative and derivative terms (Calhoun, Stevens, Pearlson, & Kiehl, 2004) to reduce the impact of spatially varying hemodynamic delays and delays due to slice timing differences. First-order motion parameters obtained from realignment were included as confounds in each participant’s model to remove possible residual task-related motion effects. A high-pass filter (cutoff period = 256 sec) was also incorporated into the model to remove noise associated with low-frequency confounds (e.g., respiratory artifact, scanner drift). No within-session scaling (also called proportional scaling) was used. Contrasts from these first-level analyses were subsequently entered into group-level random effects models.
Contrasts and conjunction analyses were performed according to previously published guidelines (Nichols, Brett, Andersson, Wager, & Poline, 2005). The conjunction analysis (using the conjunction null hypothesis) was calculated using a within-subject three-way analysis of variance of the pathogen versus neutral, incest versus neutral, and nonsexual moral versus neutral contrasts, corrected for nonsphericity, as were subsequent analyses comparing individual disgust conditions. All contrasts used in these analyses were conservatively performed on the memorize and recall phases combined together in one block (following Kiehl et al., 2005), as we had no a priori hypotheses about how each condition might interact with experimental phase and there was no significant precedent in the literature for analyzing one phase over the other (but see Supplementary Information: www.disgustandmorality.com). We also had no a priori evidence proving incest acts would have different neural correlates than nonsexual moral acts. For this reason, all initial contrasts between pathogen disgust (P) and sociomoral disgust (S–M) were performed using an average of the incest (I) and nonsexual moral (M) conditions (as would be the case if other studies included incest-related stimuli in their moral stimuli). We believed this would be the most conservative a priori model for our data, as well as the model most similar to what has been applied in previous studies of disgust and moral processing.
All data reported in this study are significant at p < .05, FDR-corrected, or less. Figures often display contrast maps at lower p values to facilitate visualization of discernible clusters. Additional figures illustrating effects at multiple p levels are posted in the Supplementary Information.
RESULTS
Behavioral Results
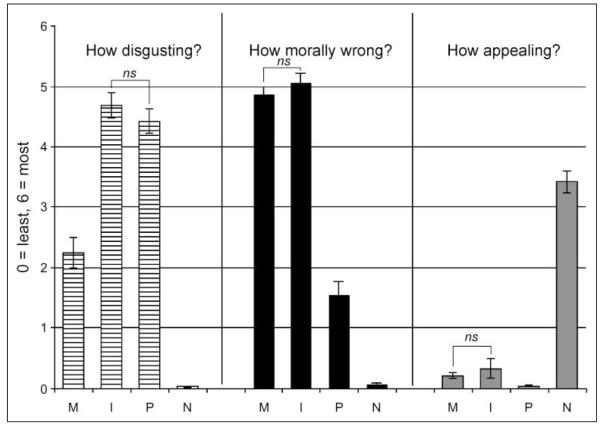
Participants correctly classified an average of 91.5% of the phrases they saw in the “recall” phase as ones they had seen in the previous “memorize” phase, and each classification took about 1.2 sec. There were no significant differences in accuracy or reaction time between conditions (Table 1). Five participants did not fill out the postscanning surveys. From the 45 subjects who did, pathogen acts and sociomoral acts were rated as more disgusting than neutral acts by self-report, and within sociomoral acts, incest acts were rated as more disgusting than nonsexual moral acts. Still, incest and nonsexual moral acts were rated as equally immoral. Neutral acts were the only acts to be rated as appealing (Figure 2).
Table 1.
Accuracy and Reaction Times for the Task Presented in the Scanner
| Type of Act | Percent Correctly Recognized |
Mean Reaction Time (sec) |
|---|---|---|
| Pathogen | 93.3 | 1.21 |
| Incest | 91.5 | 1.23 |
| Nonsexual moral | 92.0 | 1.16 |
| Neutral | 89.3 | 1.16 |
Figure 2.
Self-report ratings. M = nonsexual moral acts; I = incest acts; P = pathogen acts; N = neutral acts. ns = not significant.
Pathogen and Sociomoral Disgust Activate Common Neural Regions
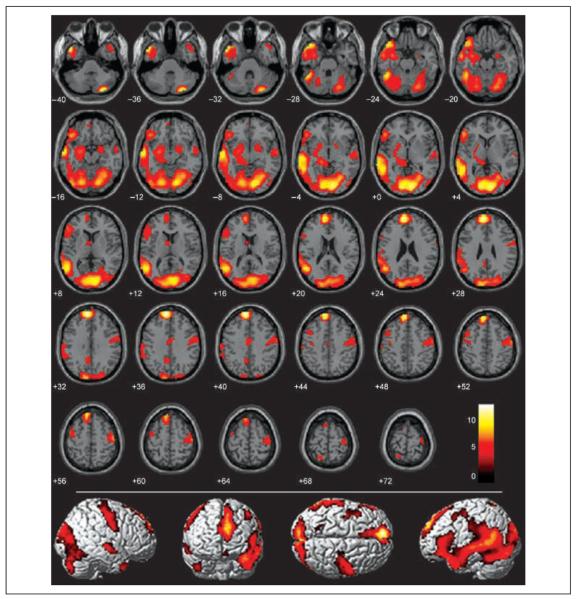
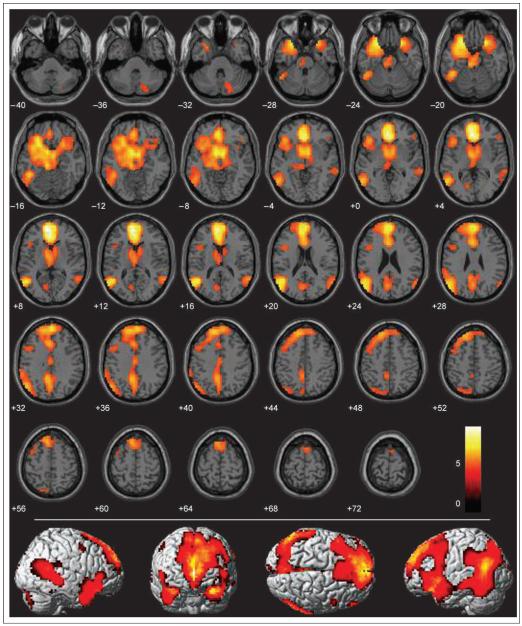
We performed a conjunction analysis to identify brain regions that were significantly more active during the memorizing and recognizing of both pathogen and sociomoral acts than to neutral acts (see Methods). A large network of brain regions was common to processing both of these disgust-eliciting stimuli (Figure 3, Table 2). Regions in this network included the basal ganglia (covering the globus pallidus, putamen, and caudate head), the amygdala, the thalamus, the para-hippocampal gyrus, the dorsal anterior cingulate (BA 24), the precuneus, the visual cortex (BA 17/18), and both the precentral (BA 6) and postcentral gyri (BA 3/4/7). No significant voxels appeared in the anterior insula.
Figure 3.
Conjunction analysis of all disgust > neutral conditions (pathogen + incest + nonsexual moral > neutral) overlaid on SPM2 canonical T1 brain. (FDR corrected p < .001, cluster minimum = 10 voxels, neurological convention).
Table 2.
Conjunction Analysis of All Disgust > Neutral Contrasts
| Conjunction: Pathogen + Incest + Nonsexual Moral vs. Neutral (FDR Corrected, p < .001) | ||||||
|---|---|---|---|---|---|---|
| MNI Coordinates |
||||||
| Brain Region | BA | x | y | z | Z | t |
| Frontal Lobes | ||||||
| Medial/Superior frontal gyrus | 10/9/(8, 6) | −9 | 54 | 36 | (B)876 | 12.83 |
| Medial frontal gyrus | 11 | −6 | 54 | −15 | (K)19 | 4.50 |
| Left middle frontal gyrus | 6 | −45 | 9 | 54 | (H)130 | 5.02 |
| Precentral gyrus | 6 | 63 | −9 | 42 | (C)480 | 6.51 |
| −42 | −6 | 60 | (H)130 | 5.62 | ||
| −36 | −12 | 51 | (H)130 | 4.44 | ||
| Temporal Lobes | ||||||
| Bilateral temporal pole (into inferior frontal gyrus on left side) |
38 | −48 | 12 | −42 | (A)9017 | 12.85 |
| 47 | −42 | 32 | −14 | (A)9017 | ||
| 38 | 48 | 12 | −42 | (E)93 | 6.16 | |
| Bilateral middle/superior temporal gyrus (anterior and posterior regions) |
21/22 | −69 | −36 | 0 | (M)2001 | 10.52 |
| −60 | −12 | −12 | (M)2001 | 10.5 | ||
| 66 | −9 | −6 | (D)146 | 6.62 | ||
| Parietal Lobes | ||||||
| Postcentral gyrus | 4 | 54 | −12 | 54 | (C)480 | 7.74 |
| 3 | 51 | −18 | 60 | (C)480 | 7.70 | |
| 7 | −21 | −57 | 72 | ( J)18 | 5.04 | |
| Precuneus/post cingulate | 31 | −6 | −51 | 33 | (G)59 | 5.69 |
| Occipital Lobes | ||||||
| Bilateral lingual gyrus/cuneus | 17/18 | 9 | −87 | 0 | (A)9017 | 11.59 |
| −13 | −84 | 0 | (A)9017 | |||
| Cingulate/Subcortical | ||||||
| Anterior cingulate (dorsal) | 24 | −3 | −12 | 39 | (I)57 | 5.34 |
| Right putamen/globus pallidus | 21 | 0 | −12 | (F)122 | 6.08 | |
| Parahippocampal gyrus/thalamus (into left putamen/globus pallidus) |
−15 | −30 | −3 | (N)59 | 6.65 | |
| Caudate | −6 | 0 | 12 | (L)27 | 4.39 | |
| Bilateral amygdala | 24 | −3 | −15 | (F)122 | ||
| −24 | −5 | −15 | (N)59 | |||
Brodmann’s area (BA), MNI coordinates, number of voxels (Z, letters indicate same cluster), and t value (t) of each cluster > 10 voxels are reported.
As illustrated in Figure 3, the most significant and extensive activity in the conjunction analysis appeared in the medial prefrontal cortex and the left temporal lobe. The medial prefrontal cortex activations consisted of one extensive dorsal cluster (BA 9/10 extending dorsally to BA 8/6) and one smaller, more ventral cluster (BA 11), both slightly left-lateralized. Left temporal lobe activations spanned from the middle into the superior temporal gyrus, and extended rostrally from the most posterior regions of BA 21/22 all the way to the left temporal pole (BA 38). Isolated regions of the right posterior middle temporal gyrus and temporal pole were also more active, but not to the same degree or extent as the same regions in the left hemisphere. In sum, a large collection of brain areas is active during implicit processing of both pathogen-related and sociomoral acts, supporting behavioral evidence that reactions toward disgusting objects feel subjectively similar to reactions toward sociomoral transgressions.
Pathogen Disgust and Sociomoral Disgust Activate Distinct Neural Regions
The conjunction analysis demonstrated that many brain regions are commonly activated by pathogen and sociomoral disgust. The following contrasts, however, demonstrate that pathogen and sociomoral disgust are not represented identically in the brain.
Pathogen > Sociomoral
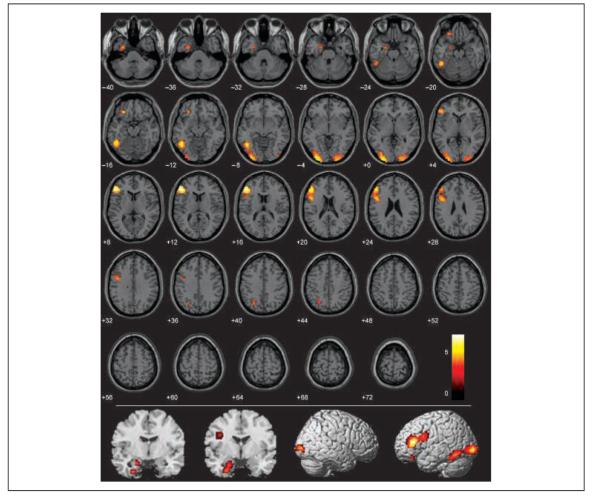
This contrast (P > S–M) was performed to determine what regions of the brain were uniquely active when processing pathogen acts compared to sociomoral acts. Activity in the left amygdala (Figure 4, Table 3) was more significant in response to pathogen acts than to sociomoral acts. In the occipital lobes, the left fusiform gyrus (BA 37) and the bilateral lingual gyrus (BA 18) were most active in response to pathogen acts as well. In the frontal lobes, a large cluster in the left inferior frontal gyrus (BA 46) and a separate smaller cluster in the more ventral region of the orbito-frontal cortex (BA 11) were identified. Finally, a region of the precuneus (BA 7) more rostral and dorsal to the region identified in the conjunction analysis (BA 31) was uniquely associated with the pathogen condition.
Figure 4.
Regions more active in pathogen condition than in the sociomoral and neutral conditions (pathogen > sociomoral) overlaid on SPM2 canonical T1 image. (FDR corrected p < .05, cluster minimum = 10 voxels, neurological convention).
Table 3.
Regions More Activated in Pathogen Condition Compared to Sociomoral (Incest and Nonsexual Moral) and Neutral Conditions
| Pathogen > Sociomoral (FDR Corrected, p < .05) | ||||||
|---|---|---|---|---|---|---|
| MNI Coordinates |
||||||
| Brain Region | BA | x | y | z | Z | t |
| Frontal Lobes | ||||||
| Left inferior frontal gyrus (into the dorsolateral prefrontal cortex) |
46 | −48 | 36 | 12 | (A)444 | 6.39 |
| 9 | −45 | 6 | 27 | (A)444 | 4.63 | |
| Left middle frontal gyrus |
11 | −27 | 33 | −15 | (B)35 | 4.61 |
| Parietal Lobes | ||||||
| Precuneus/Superior parietal lobule |
7 | −24 | −66 | 42 | (C)80 | 4.38 |
| Occipital Lobes | ||||||
| Left fusiform gyrus | 37 | −48 | −51 | −15 | (D)446 | 5.42 |
| Bilateral lingual gyrus |
18 | −27 | −99 | −3 | (D)446 | 5.12 |
| 27 | −99 | −3 | (E)132 | 4.30 | ||
| Cingulate/Basal Ganglia | ||||||
| Left amygdala | −24 | −3 | −24 | (F)45 | 3.56 | |
| Uncus | 20/36 | −30 | −3 | −39 | (G)27 | 3.58 |
Brodmann’s area (BA), MNI coordinates, number of voxels (Z, letters indicate same cluster), and t value (t) of each cluster > 10 voxels are reported.
All of the brain regions identified in the P > S–M contrast were preferentially active during processing of disgusting acts without high levels of moral content. The following analysis determined what brain regions were preferentially active during processing of disgusting acts with high levels of moral content. It was predicted that these brain regions should include the medial prefrontal cortex and regions around the temporo-parietal junction, two regions consistently shown to be involved in moral processing (Schaich Borg et al., 2006; Heekeren et al., 2003, 2005; Moll, Zahn, et al., 2005; Greene et al., 2004).
Sociomoral > Pathogen
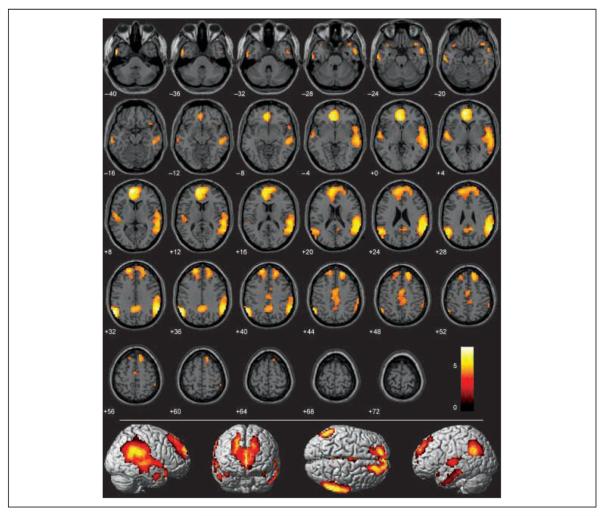
Compared to pathogen and neutral acts, sociomoral acts (incest and nonsexual moral) received very high ratings of moral wrongness (Figure 2). We examined which brain regions were more active during the unique processing of sociomoral acts compared to that of pathogen acts (S–M > P). As predicted, significantly more activity in large clusters of the medial prefrontal cortex (BA 10, extending into the anterior cingulate) and bilateral temporo-parietal junction was elicited by morally wrong acts than by acts rated not to be morally wrong (Figure 5, Table 4). Two other regions previously identified in fMRI studies on moral processing, the temporal poles (BA 38) and the precuneus (BA 31), were identified as well (Greene & Haidt, 2002). Areas of the superior, middle, and inferior temporal gyri were more active bilaterally in response to sociomoral acts than to pathogen acts, as were bilateral regions of the dorsolateral prefrontal cortex (BA 9) and a region of the dorsal anterior cingulate (BA 24). These areas have also previously been implicated in some types of moral processing (but have not been identified in all morality studies; Schaich Borg et al., 2006; Heekeren et al., 2003, 2005; Greene et al., 2004). The only brain region that appeared in the S–M > P contrast that is not commonly found in morality studies was the right posterior insula (which is anatomically and functionally distinct from the anterior insula mentioned earlier). In one study, however, this region was found to be more active in the left hemisphere when participants read phrases that elicited moral indignation than when they read neutral phrases (Moll, de Oliveira-Souza, et al., 2005). Overall, then, the regions identified in the S–M > P contrast are consistent with those identified previously to be involved in moral processing, even though the present study uses a different task than any other published study.
Figure 5.
Regions more active in the sociomoral condition than in the pathogen and neutral conditions (sociomoral > pathogen) overlaid on SPM2 canonical T1 image. (FDR corrected p < .001, cluster minimum = 10 voxels, neurological convention).
Table 4.
Regions More Activated in Sociomoral (Incest and Nonsexual Moral) Conditions Compared to Pathogen and Neutral Conditions
| Sociomoral > Pathogen (FDR Corrected, p < .001) | ||||||
|---|---|---|---|---|---|---|
| MNI Coordinates |
||||||
| Brain Region | BA | x | y | z | Z | t |
| Frontal Lobes | ||||||
| Bilateral medial frontal gyrus (extends into bilateral anterior cingulate) |
10 | −9 | 54 | 6 | (A)2137 | 6.62 |
| 32 | −6 | 42 | 0 | (A)2137 | 6.02 | |
| Bilateral superior frontal gyrus (dorsolateral prefrontal cortex) |
9 | −18 | 51 | 36 | (A)2137 | 5.11 |
| Temporal Lobes | ||||||
| Bilateral temporal pole | 38 | −36 | 18 | −21 | (B)19 | 4.19 |
| 42 | 21 | −21 | (C)48 | 4.3 | ||
| Left inferior temporal gyrus | 20 | −63 | −18 | −24 | (D)129 | 5.23 |
| Middle temporal gyrus | 21 | −51 | 3 | −39 | (D)129 | 5.1 |
| 54 | 3 | −27 | (E)61 | 4.65 | ||
| Bilateral superior temporal gyrus (extends into transverse temporal gyrus) |
22 | −54 | −12 | 6 | (F)191 | 4.76 |
| 41 | −51 | −30 | 15 | (F)191 | 3.77 | |
| 57 | −12 | 3 | (G)2044 | 4.94 | ||
| Bilateral supramarginal/angular gyrus, inferior parietal lobule (includes temporo-parietal junction) |
39/40 | −57 | −60 | 36 | (H)434 | 6.55 |
| 57 | −51 | 33 | (G)2044 | 6.24 | ||
| (right extends into middle temporal gyrus, postcentral gyrus, posterior insula) |
21 | 54 | −27 | −6 | (G)2044 | 4.9 |
| 2/3 | (G)2044 | |||||
| 13 | (G)2044 | |||||
| Occipital Lobes | ||||||
| Bilateral precuneus | 31 | −6 | −51 | 33 | (I)669 | 4.77 |
| 6 | −51 | 33 | (I)669 | 4.68 | ||
| Limbic System | ||||||
| Anterior cingulate (dorsal) | 24 | 0 | −6 | 48 | (I)669 | 4.47 |
Brodmann’s area (BA), MNI coordinates, number of voxels (Z, letters indicate same cluster), and t value (t) of each cluster > 10 voxels are reported.
Sociomoral Disgust: A Unified Phenomenon?
The results of the S–M > P contrast illustrate that pathogen and sociomoral disgust are two separate (but related) physiological processes. The next experimental question was whether sociomoral disgust, itself, is composed of multiple distinct but related physiological processes. We examined whether incest acts and nonsexual moral acts entrain unique brain regions in addition to their common brain networks by comparing directly the results of an incest > neutral and nonsexual moral > neutral contrast (see Methods), even though incest and nonsexual moral acts are rated as equally morally wrong.
Incest > Nonsexual Moral
The incest manipulation was very powerful in this study and the results of the incest > nonsexual moral (I > M) contrast were highly statistically significant and extended across many anatomical boundaries (Figure 6). The anatomical labels in Table 5 represent our best approximation of the regions implicated in the I > M contrast, including regions referenced by the reported coordinates and the proximate brain regions the reported clusters expanded into.
Figure 6.
Regions more active in the incest condition than in the nonsexual moral condition (incest > nonsexual moral) overlaid on SPM2 canonical T1 image. (FDR corrected p < .0001, cluster minimum = 10 voxels, neurological convention).
Table 5.
Regions More Activated in Incest Condition Compared to the Nonsexual Moral Condition
| Incest > Nonsexual Moral (FDR Corrected, p < .0001) | ||||||
|---|---|---|---|---|---|---|
| MNI Coordinates |
||||||
| Brain Region | BA | x | y | z | Z | t |
| Frontal Lobes | ||||||
| Medial frontal gyrus, superior frontal gyrus | 10/9 | −9 | 54 | 9 | (A)4499 | 9.67 |
| Bilateral inferior frontal gyrus (into insula on left side) |
47 | 27 | 21 | −21 | (A)4499 | 7.28 |
| 13 | −27 | 21 | −21 | (A)4499 | 7.28 | |
| Temporal Lobes | ||||||
| Bilateral angular gyrus | 39 | −51 | −60 | 18 | (B)820 | 6.82 |
| (includes bilateral temporo-parietal junction) | 57 | −66 | 24 | (C)73 | 5.13 | |
| (extends into superior/middle temporal gyri) | 22 | 63 | −57 | 15 | (C)73 | 5.36 |
| Parietal Lobes | ||||||
| Left supramarginal gyrus | 40 | −57 | −42 | 30 | (D)11 | 4.89 |
| Precuneus/posterior cingulate | 7/31 | −6 | −54 | 36 | (E)291 | 5.73 |
| Occipital Lobes | ||||||
| Left fusiform gyrus | 37 | −45 | −48 | −18 | (F)820 | 6.15 |
| Limbic System/Basal Ganglia | ||||||
| Anterior cingulate (ventral) | 32/24 | 0 | 36 | 3 | (A)4499 | 9.43 |
| Anterior cingulate (dorsal) | 24 | 0 | −15 | 36 | (G)22 | 5.15 |
| Left amygdala | (A)4499 | |||||
| Left hippocampus | (A)4499 | |||||
| Bilateral caudate/globus pallidus/putamen | (A)4499 | |||||
| Bilateral thalamus | (A)4499 | |||||
Brodmann’s area (BA), MNI coordinates, number of voxels (Z, letters indicate same cluster), and t value (t) of each cluster > 10 voxels are reported.
The ventral bilateral anterior cingulate (BA 32, 24), extending into the medial and superior frontal gyri (BA 10 and 9, respectively), and the bilateral inferior frontal gyri (BA 47), extending into the anterior insulae (much less so on the right side, BA 13), were more active during the processing of statements describing incestuous acts than nonsexual immoral acts (Figure 6, Table 5). In the temporal lobes, a small region of the right anterior superior temporal gyrus, and larger regions around the temporo-parietal junction (extending dorsally and posteriorly, stronger on the left side than on the right) were identified in the incest > nonsexual moral (I > M) contrast. The left fusiform gyrus was preferentially active in response to incest actions, as was the dorsal anterior cingulate (BA 24), the precuneus/posterior cingulate (BA 7/31), and the bilateral amygdalae. Finally, medial areas of the basal ganglia, the thalamus, and the midbrain were more active in response to incest acts than to nonsexual moral acts.
It is common in fMRI studies with many participants to see clusters of activation that expand across anatomical boundaries, such as those identified in the I > M contrast, especially in deep regions of the brain (see Figure 2 or Figure 4 in Kiehl et al., 2005, n = 100, for examples). Still, it may seem surprising that such dramatic differences can be observed between two types of moral stimuli that are rated as equally moral wrong. To demonstrate the robustness of the results from the I > M contrast, we plotted histograms of each subject’s parameter estimates for each anatomical region reported in Table 4 as taken from their derivative-boosted I > M contrast map (Calhoun et al., 2004). These histograms (see Supplementary Information) demonstrated clearly that the data for the I > M group contrast follow normal distributions and that many subjects have very strong responses to the incest condition compared to the nonsexual moral condition. These results suggest strongly that the dramatic effects we see in the I > M contrast are not due to methodological or mechanical complications with data acquisition, and support the interpretation that incest acts are indeed processed very differently in the brain than nonsexual moral acts.
To identify the brain regions in the I > M contrast that were either unique to the incest condition or just more active in the incest than in the nonsexual moral condition, the results of the I > M contrast were compared to those of the nonsexual moral > neutral (M > N) contrast (not shown). Voxels active in only the I > M and not the M > N contrast were interpreted as unique to incest. The ventral bilateral anterior cingulate, anterior insulae, right anterior superior temporal gyrus, and medial basal ganglia, thalami and midbrain regions identified in the I > M contrast were all uniquely associated with the incest condition. The anterior cingulate cluster extended into the medial and superior frontal gyri and the left insula cluster extended into the inferior frontal gyrus, but these regions were active in the nonsexual moral condition as well, just to a lesser extent than in the incest condition. The rest of the brain regions identified in the I > M contrast, including the left fusiform gyrus, the posterior cingulate, the dorsal anterior cingulate, the extended bilateral temporo-parietal junction, and the amygdalae, were all active in the nonsexual moral condition as well as the incest condition.
Nonsexual Moral > Incest
No voxels withstood multiple comparison corrections in the nonsexual moral > incest (M > I) contrast.
Pathogen, Incest, and Nonsexual Moral Acts Activate Distinct Neural Regions
The results of the I > M and M > I contrasts show that incestuous acts are processed differently in the brain than nonsexual immoral acts. This raises the question, is the pathogen condition distinct from both the incest and the nonsexual moral conditions, or just from one of the conditions? The answer is that the pathogen condition is unique. Although the incest and pathogen acts were rated as equally disgusting, the incest > pathogen contrast yielded a large network of brain areas very similar to those identified in the sociomoral > pathogen contrast. Additional significant voxels were identified in the lateral temporal poles (lt = −33, 18, −28; rt = 36, 18, −27), the right inferior frontal gyrus extending into the anterior insula (39, 21, −21), bilateral middle temporal gyrus (lt = −61, −27, −16; rt = 63, −36, 0), and superior temporal gyrus (lt = −54, −18, 6; rt = 63, −18, 3), right supramarginal gyrus (60, −54, 27) extending into inferior regions all around the temporo-parietal junction. Fewer significant voxels were found in the basal ganglia, and unlike the sociomoral > pathogen contrast, no voxels were found in the brainstem and left fusiform gyrus. The reverse pathogen > incest contrast did not yield any significant results, probably because the incest manipulation was so powerful. However, the pathogen > nonsexual moral contrast yielded results very similar to the P > S–M contrast with added significant activity in both amygdalae (lt amygdala = 24, −3, −24; rt amygdala = 23, −1, −21), brainstem (lt brainstem = − 6, −29, −15; rt brainstem = 3, −27, −18), and ventromedial basal ganglia (lt = −6, 0, −9; rt = 6, −2, −6), and slightly less significant activity in the visual cortex (see Supplementary Information for figures). The reverse nonsexual moral > pathogen contrast did not yield any significant voxels. In sum, pathogen acts invoke more activity in many brain regions than do nonsexual moral acts, and incest acts invoke more activity in many brain regions than do either pathogen or nonsexual moral acts, despite the fact that incest acts are equally disgusting as pathogen acts and equally immoral as nonsexual moral acts.
DISCUSSION
This study is the first investigation of the common and unique neural correlates of three separate domains of disgust. The present results provide evidence that: (i) common brain regions are active during processing of both pathogen-related and sociomoral acts, (ii) pathogen-related and sociomoral acts each entrain unique brain regions, (iii) despite their tendency to elicit similar ratings of moral disapproval, incest-related acts and nonsexual immoral acts entrain different, but overlapping, brain networks, and (iv) despite their tendency to elicit similar ratings of disgust, pathogen-related acts and incest-related acts entrain different, but again, overlapping brain networks.
These findings call attention to a number of issues. First, some researchers argue that the insula and amygdala have dissociable, nonoverlapping functions with the insula serving as the seat of disgust processing and the amygdala the seat of fear processing (Phillips et al., 1998, 2004; Calder, 2003). The data reported here challenge this view. The insula was only preferentially active in response to incest acts (not pathogen or nonsexual moral acts), whereas the amygdala was more active in response to all three types of disgusting acts than to neutral acts. A large area of the left inferior frontal gyrus (BA 47) was identified in the conjunction analysis, but it did not extend into the anterior insular cortex discussed in previous studies of disgust (Schafer et al., 2005; Wright, He, Shapira, Goodman, & Liu, 2004; Krolak-Salmon et al., 2003; Shapira et al., 2003; Phillips et al., 1997, 1998). Although these results may seem surprising to some, a growing number of studies have shown that the insula and the amygdala are often, but not always, involved in disgust processing (Schafer et al., 2005; Stark et al., 2003, 2005; Phillips et al., 2004; Schienle et al., 2002). This is consistent with a recent meta-analysis of imaging studies that found the anterior insula to be no more active during disgust than other emotions (Feldman Barrett & Wager, 2007).
Nonetheless, it is possible that the lack of insula activity in our conjunction analysis was a result of induced tonic insula activity in the neutral condition due to carryover effects from our highly provocative stimuli. Exploratory analyses suggest that the insula was, indeed, active during the neutral condition, but not as a result of carryover effects (see Supplementary Information). Insula activity was present bilaterally when the neutral condition was compared to baseline. This activity remained when the analysis was restricted to just the first blocks of neutral stimuli, a result that is immune to carryover effects because the first blocks of neutral stimuli were the first acts presented to subjects. These data suggest that the lack of insular activity in our conjunction analyses was most likely due to an equal presence of insular activity in the neutral condition as in the disgust conditions, and not due to a lack of insula activity in any condition.
If this interpretation is correct, why would the insula respond to neutral stimuli? One possibility is that the insula is involved in language processing (Ogar et al., 2006; Nestor et al., 2003) and may have a second function in mapping visceral states associated with conscious, personal emotional experience (Damasio, 2003). Our stimuli were short phrases describing acts in the second person, so they required language processing and likely elicited imagery of performing the acts described. If the insula’s role in language or in self-monitoring trumps its role in processing information associated with disgust, perhaps the neutral acts activated the insula as much as the disgusting acts because all the acts elicited the same amount of language processing and imaginative introspection. In support of this explanation, the only other study using written acts rather than faces or pictures as disgust stimuli did not find the anterior insula to be more active in disgusting versus neutral statements either (Moll, de Oliveira-Souza, et al., 2005). Furthermore, patients with Huntington’s disease, a clinical population believed to have decreased insular volume compared to healthy individuals (Kassubek et al., 2004), have been shown to have intact disgust recognition in response to linguistic stimuli, but impaired disgust recognition in response to both facial and nonfacial visual stimuli (Hayes et al., 2007). Therefore, the verbal nature of our stimuli may provide the most plausible explanation for the equivalent insula activity across all our conditions.
Alternatively, perhaps the task demands rather than the stimuli modulated insula activity. The insula (and the amygdala) results reported here may be due to the fact that participants were engaged in a memory task in this study and were not required to explicitly evaluate how disgusting each act was until after the scanning session. A previous study demonstrated that the amygdala—not the insula—was preferentially active in response to unattended pictures of disgusted faces while the insula was only preferentially active in response to attended pictures of disgusted faces (Anderson, Christoff, Panitz, De Rosa, & Gabrieli, 2003). Furthermore, although the insula is active in response to consciously perceived disgusted facial expressions, it is not preferentially active in response to subliminally presented disgusted facial expressions (Phillips et al., 2004). Thus, perhaps the absence of insula activity in our conjunction analysis is due to the fact that participants were too preoccupied with our implicit task to actively perceive the disgusting nature of the stimuli. However, this interpretation is unlikely because participants’ voluntary comments after scanning suggested that participants actively detected the stimuli’s disgusting nature.
One additional possibility is that the anterior insula is simply not more involved in disgust processing than any other type of emotional processing (Feldman Barrett & Wager, 2007). Perhaps the insula activity identified in past disgust studies represents emotion-related activity in general rather than disgust-specific activity, and the neutral stimuli used in the present study were well matched for overall emotional engagement. Until more research evaluates these proposed interpretations and explanations, the present data support the view that the insula is not always preferentially involved in disgust processing, and the amygdala, a structure commonly associated with fear processing, can also be preferentially involved in disgusting processing (see also Kiehl et al., 2005 on amygdala activation to salient, not just fear-inducing, stimuli).
A second issue raised by the results of this study is the powerful nature of the incest manipulation. The incest condition activated almost the entire brain (see Supplementary Information), making it difficult to detect any significant effects in either the moral > incest or the pathogen > incest contrasts. One account could be that the unique neural correlates of the incest condition are due mostly to the fact that the incest acts are more emotionally arousing (emotionally intense) than other acts. A separate group of healthy volunteers (n = 33) rated a subset of the incest acts to be slightly more arousing than a similar subset of the pathogen ( p < .01), nonsexual moral ( p < .001), and neutral acts ( p < .001). Still, it unlikely that the magnitude and pervasiveness of the incest condition’s neural effects are due solely to increased arousal because the hemodynamic signature of the incest condition does not mimic typical arousal effects observed in other fMRI studies. In particular, the amygdala has been shown to be correlated specifically with arousal (Lewis, Critchley, Rotshtein, & Dolan, 2007; Cunningham, Raye, & Johnson, 2004), and the amygdala was not significantly more active while processing incest acts compared to pathogen acts. Moreover, the other regions identified in the I > M contrast have not been shown to be correlated with arousal ratings. It is also useful to note that pathogen and nonsexual moral acts were rated as equally arousing ( p = .22), suggesting that the amygdala activation in the P > M contrast cannot be due to differences in arousal. Nevertheless, if part of the neural signature of the incest condition was due to an arousal interaction, the propensity of incest acts to arouse more than nonsexual moral acts is a surprising inherent difference between these two similarly immoral conditions that may have implications for the design of moral stimuli in future studies.
Another possible explanation for the pattern of activity in the incest condition is that our stimuli were written in the second rather than third person. Adaptation-minded researchers investigating the levels of disgust associated with incestuous acts have encountered ceiling effects when asking individuals to imagine engaging in sexual acts with a family member compared to imagining third-party incestuous acts (Lieberman et al., 2007; Fessler & Navarrete, 2004). Perhaps incest stimuli written in the third person would not have elicited such dramatic neural activity compared to nonsexual moral and pathogen stimuli.
As a third issue, data presented here raise the possibility that the typical operationalization of “disgust” and “morality” in experimental investigations may require revision. Neuroscientific investigations often treat disgust as a unified psychological and neurological construct by using disgusted faces or pictures to represent all domains of disgust (Phillips et al., 1998, 2004; Calder, 2003). However, the data presented here show that although incest acts and pathogen acts are rated as equally disgusting, incest acts are rated as more immoral and elicit dramatically more hemodynamic activity than pathogen acts. These data, combined with data from other recent multimodal disgust studies, suggest that disgust is likely best conceived of as a set of heterogeneous responses overlaying a unified psychological and neurological response, and therefore, most likely will not be fully understood through studying only one sensory modality or one type of disgusting stimulus.
Similarly, neuroscientific investigations have often treated morality as a unified psychological and neurological construct (e.g., Heekeren et al., 2003; Moll et al., 2002), heterogeneous only insomuch as it invokes different proportions of activity in common “emotional” versus “cognitive” systems (Schaich Borg et al., 2006; Greene et al., 2004). The field has benefited profoundly from these studies because they have identified a set of brain regions including the medial prefrontal cortex and areas around the temporo-parietal junction that is reliably more active during processing of a broad range of moral stimuli compared to nonmoral stimuli. These regions appeared robustly in the results of the sociomoral > pathogen contrast in the data presented here as well. Together, previous and present results support the hypothesis that the medial prefrontal cortex and areas around the temporo-parietal junction are likely to be involved in general moral processing across many different situations. However, the results reported here also illustrate that despite the fact that incest acts and nonsexual moral acts were rated as equally immoral, incest acts elicit dramatically more activity in an extensive expanse of brain regions ranging from the medial prefrontal cortex itself to the basal ganglia. This additional pattern of results suggests that it is important to acknowledge the possibility that not all morality or immorality is necessarily “treated equally” by the brain.
This possibility is starting to be recognized by neuroscientists interested in morality. One study has examined whether the presence or absence of direct bodily harm affects the brain networks involved in moral decision making (Heekeren et al., 2005). However, the moral stimuli that included direct bodily harm were rated as significantly more morally wrong than moral stimuli without bodily harm, so it is not clear whether the neurological differences identified in that study were due to sentiments of moral wrongness or to systems specific for detecting bodily harm. Recently, it was shown that neural activity associated with processing justice-based moral transgressions differs from that associated with processing care-based moral transgressions. However, this study did not test whether justice- and care-based moral transgressions are rated as equally morally wrong, so again, it is not clear whether the differences they report can be explained by sentiments of moral wrongness (Robertson et al., 2007). The data reported here, then, are the first to show that equally immoral acts can be represented differently in the brain.
Heterogeneity of moral stimuli, such as that between sexual and nonsexual moral transgressions, likely extends to functional domains beyond those explored in this study, especially those that require specific types of information processing such as theory of mind or cheater-detection (Young, Cushman, Hauser, & Saxe, 2007; Ermer, Guerin, Cosmides, Tooby, & Miller, 2006). We suspect that future studies examining explicit moral judgments made in the scanner, as opposed to after scanning as investigated here, will evidence similar heterogeneity as well. Although the data we provide here do not speak to whether brain regions differentially involved in moral processing are necessary for that moral processing, they do suggest that future morality studies might opt to use stimuli that are distributed across the different moral domains in a controlled manner, and interpret data in light of the possibility that some reported results may be specific to only some moral domains. In other words, the results of reported moral > nonmoral contrasts will differ depending on the types of moral transgressions used as stimuli.
Appreciating the functional heterogeneity within disgust and morality and learning more about the commonalities and differences of neural systems that process distinct disgusting stimuli or moral transgressions could lead to better treatment and diagnosis of clinical populations whose symptoms may reflect impairments in specific moral and/or disgust domains, such as sexual offenders, psychopaths, drug-abusers, Huntington’s disease patients, and obsessive–compulsive disorder patients. Within psychology and cognitive neuroscience, appreciating disgusts’ heterogeneity may help us uncover the true neural building blocks of basic emotions. Similarly, exploring if and how different types of moral transgressions can invoke different brain networks will become increasingly important as neuroscientific data become more influential in legal and ethical decisions. If lawyers and ethicists continue to debate whether lesion patients or psychiatric patients with functional deficits should be considered culpable for their immoral actions (Mobbs, Lau, Jones, & Frith, 2007), it will be helpful to acknowledge that some brain regions might be involved in only specific subsets of moral processing because patients could conceivably be held culpable for some types of immoral actions but not for others. Although there is still much to explore, the data reported here lay the groundwork for many future interdisciplinary investigations which promise to advance our knowledge about the structure of various psychopathologies, the nature of disgust, and our own moral behavior.
Supplementary Material
REFERENCES
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JDE. Neural correlates of the automatic processing of threat facial signals. Journal of Neuroscience. 2003;23:5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J, Marsh AA, Finger E, Blair KS, Luo J. Neuro-cognitive systems involved in morality. Philosophical Explorations. 2006;9:13–27. [Google Scholar]
- Bloom P. Descartes’ baby: How the science of child development explains what makes us human. Basic Books; New York: 2004. [Google Scholar]
- Calder AJ. Disgust discussed. Annals of Neurology. 2003;53:427–428. doi: 10.1002/ana.10565. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nature Neuroscience. 2000;3:1077–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Stevens MS, Pearlson GD, Kiehl KA. fMRI analysis with the General Linear Model: Removal of latency-induced amplitude bias by incorporation of hemodynamic derivative terms. Neuroimage. 2004;22:252–257. doi: 10.1016/j.neuroimage.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: fMRI correlates of valence, emotional intensity, and control in the processing of attitudes. Journal of Cognitive Neuroscience. 2004;16:1717–1729. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Curtis V, Aunger R, Rabie T. Evidence that disgust evolved to protect from risk of disease. Proceedings of the Royal Society of London, Series B, Biological Sciences. 2004;271:s131–s133. doi: 10.1098/rsbl.2003.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis V, Biran A. Dirt, disgust, and disease. Is hygiene in our genes? Perspectives in Biology and Medicine. 2001;44:17–31. doi: 10.1353/pbm.2001.0001. [DOI] [PubMed] [Google Scholar]
- Damasio A. Feelings of emotion and the self. Annals of the New York Academy of Sciences. 2003;1001:253–261. doi: 10.1196/annals.1279.014. [DOI] [PubMed] [Google Scholar]
- Ermer E, Guerin S, Cosmides L, Tooby J, Miller M. Theory of mind broad and narrow: Reasoning about social exchange engages ToM areas, precautionary reasoning does not. Social Neuroscience. 2006;1:196–219. doi: 10.1080/17470910600989771. [DOI] [PubMed] [Google Scholar]
- Feldman Barrett L, Wager T. The neural reference space for emotion: New meta-analytic insights; Paper presented at the Neural Systems of Social Behavior; Austin, TX. 2007.2007. [Google Scholar]
- Fessler DMT, Navarrete CD. Domain-specific variation in disgust sensitivity across the menstrual cycle. Evolution and Human Behavior. 2003;24:406–417. [Google Scholar]
- Fessler DMT, Navarrete CD. Third-party attitudes toward sibling incest: Evidence for Westermarck’s hypotheses. Evolution and Human Behavior. 2004;25:277–294. [Google Scholar]
- Fitzgerald DA, Posse S, Moore GJ, Tancer ME, Nathan PJ, Phan KL. Neural correlates of internally-generated disgust via autobiographical recall: A functional magnetic resonance imaging investigation. Neuroscience Letters. 2004;370:91–96. doi: 10.1016/j.neulet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin J-F. What is the best similarity measure for motion correction in fMRI time series? IEEE Transactions on Medical Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;3:165–189. [Google Scholar]
- Greene J, Haidt J. How (and where) does moral judgment work? Trends in Cognitive Sciences. 2002;6:517–523. doi: 10.1016/s1364-6613(02)02011-9. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Haidt J, McCauley C, Rozin P. Individual differences in sensitivity to disgust: A scale sampling seven domains of disgust elicitors. Personality and Individual Differences. 1994;16:701–713. [Google Scholar]
- Haidt J, Rozin P, McCauley C, Imada S. Body, psyche, and culture: The relationship between disgust and morality. Psychology and Developing Societies. 1997;9:107–131. [Google Scholar]
- Hayes CJ, Stevenson RJ, Coltheart M. Disgust and Huntington’s disease. Neuropsychologia. 2007;45:1135–1151. doi: 10.1016/j.neuropsychologia.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Prehn K, Schwintowski HP, Villringer A. Influence of bodily harm on neural correlates of semantic and moral decision-making. Neuroimage. 2005;24:887–897. doi: 10.1016/j.neuroimage.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Schwintowski HP, Villringer A. An fMRI study of simple ethical decision-making. NeuroReport. 2003;14:1215–1219. doi: 10.1097/00001756-200307010-00005. [DOI] [PubMed] [Google Scholar]
- Kahan DM. “The anatomy of disgust” in criminal law. Michigan Law Review. 1998;96:1621–1657. [Google Scholar]
- Kassubek J, Juengling FD, Kioschies T, Henkel K, Karitzky J, Kramer B, et al. Topography of cerebral atrophy in early Huntington’s disease: A voxel based morphometric MRI study. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:213–220. [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: Supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. Neuroimage. 2005;25:899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, et al. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolak-Salmon P, Henaff M-A, Isnard J, Tallon-Baudry C, Guenot M, Vighetto A, et al. An attention modulated response to disgust in human ventral anterior insula. Annals of Neurology. 2003;53:446–453. doi: 10.1002/ana.10502. [DOI] [PubMed] [Google Scholar]
- Lewis P, Critchley H, Rotshtein P, Dolan R. Neural correlates of processing valence and arousal in affective words. Cerebral Cortex. 2007;17:742–748. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman D, Tooby J, Cosmides L. Does morality have a biological basis? An empirical test of the factors governing moral sentiments related to incest. Proceedings of the Royal Society of London, Series B, Biological Sciences. 2003;270:819–826. doi: 10.1098/rspb.2002.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman D, Tooby J, Cosmides L. The architecture of human kin detection. Nature. 2007;445:727–731. doi: 10.1038/nature05510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WI. The anatomy of disgust. Harvard University Press; Cambridge, MA: 1997. [Google Scholar]
- Mobbs D, Lau HC, Jones OD, Frith CD. Law, responsibility, and the brain. PLoS Biology. 2007;5 doi: 10.1371/journal.pbio.0050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, Bramati IE, Mourao-Miranda J, Andreiuolo PA, et al. The neural correlates of moral sensitivity: A functional magnetic resonance imaging investigation of basic and moral emotions. Journal of Neuroscience. 2002;22:2730–2736. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Moll FT, Ignacio FA, Bramati IE, Caparelli-Daquer EM, et al. The moral affiliations of disgust: A functional MRI study. Cognitive and Behavioral Neurology. 2005;18:68–78. doi: 10.1097/01.wnn.0000152236.46475.a7. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. The neural basis of human moral cognition. Nature Reviews Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Nichols S. Norms with feeling: Towards a psychological account of moral judgment. Cognition. 2002;84:221–236. doi: 10.1016/s0010-0277(02)00048-3. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain and Language. 2006;97:343–350. doi: 10.1016/j.bandl.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, et al. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage. 2004;21:1484–1496. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. Proceedings of the Royal Society of London, Series B, Biological Sciences. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Robertson D, Snarey J, Ousley O, Harenski K, Bowman F, Gilkey R, et al. The neural processing of moral sensitivity to issues of justice and care. Neuropsychologia. 2007;45:755–766. doi: 10.1016/j.neuropsychologia.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Rozin P, Fallon AE. A perspective on disgust. Psychological Review. 1987;94:23–41. [PubMed] [Google Scholar]
- Sambataro F, Dimalta S, Di Giorgio A, Taurisano P, Blasi G, Scarabino T, et al. Preferential responses in amygdala and insula during presentation of facial contempt and disgust. European Journal of Neuroscience. 2006;24:2355–2362. doi: 10.1111/j.1460-9568.2006.05120.x. [DOI] [PubMed] [Google Scholar]
- Schafer A, Schienle A, Vaitl D. Stimulus type and design influence hemodynamic responses towards visual disgust and fear elicitors. International Journal of Psychophysiology. 2005;57:53–59. doi: 10.1016/j.ijpsycho.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Schaich Borg J, Hynes C, Van Horn J, Grafton S, Sinnott-Armstrong W. Consequences, action, and intention as factors in moral judgments: An fMRI investigation. Journal of Cognitive Neuroscience. 2006;18:803–817. doi: 10.1162/jocn.2006.18.5.803. [DOI] [PubMed] [Google Scholar]
- Scherer KR. The role of culture in emotion-antecedent appraisal. Journal of Personality and Social Psychology. 1997;73:902–922. [Google Scholar]
- Schienle A, Schafer A, Hermann A, Walter B, Stark R, Vaitl D. fMRI responses to pictures of mutilation and contamination. Neuroscience Letters. 2006;393:174–178. doi: 10.1016/j.neulet.2005.09.072. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Stark R, Walter B, Franz M, Vaitl D. Disgust sensitivity in psychiatric disorders: A questionnaire study. Journal of Nervous and Mental Disease. 2003;191:831–834. doi: 10.1097/01.nmd.0000100928.99910.2d. [DOI] [PubMed] [Google Scholar]
- Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, et al. The insula is not specifically involved in disgust processing: An fMRI study. NeuroReport. 2002;13:2023–2026. doi: 10.1097/00001756-200211150-00006. [DOI] [PubMed] [Google Scholar]
- Shapira NA, Liu Y, He AG, Bradley MM, Lessig MC, James GA, et al. Brain activation by disgust-inducing pictures in obsessive–compulsive disorder. Biological Psychiatry. 2003;54:751–756. doi: 10.1016/s0006-3223(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Simpson J, Carter S, Anthony SH, Overton PG. Is disgust a homogeneous emotion? Motivation and Emotion. 2006;30:31–41. [Google Scholar]
- Sprengelmeyer R, Young AW, Calder AJ, Karnat A, Lange H, Homberg V, et al. Loss of disgust: Perception of faces and emotions in Huntington’s disease. Brain. 1996;119:1647–1665. doi: 10.1093/brain/119.5.1647. [DOI] [PubMed] [Google Scholar]
- Stark R, Schienle A, Sarlo M, Palomba D, Walter B, Vaitl D. Influences of disgust sensitivity on hemodynamic responses towards a disgust-inducing film clip. International Journal of Psychophysiology. 2005;57:61–67. doi: 10.1016/j.ijpsycho.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Stark R, Schienle A, Walter B, Kirsch P, Sammer G, Ott U, et al. Hemodynamic responses to fear and disgust-inducing pictures: An fMRI study. International Journal of Psychophysiology. 2003;50:225–234. doi: 10.1016/s0167-8760(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Hoshino T, Shigemasu K, Kawamura M. Disgust-specific impairment of facial expression recognition in Parkinson’s disease. Brain. 2006;129:707–717. doi: 10.1093/brain/awl011. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Haidt J. Hypnotic disgust makes moral judgments more severe. Psychological Science. 2005;16:780–784. doi: 10.1111/j.1467-9280.2005.01614.x. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet J-P, Gallese V, Rizzolatti G. Both of us disgusted in my insula: The common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Williams LMCA, Das P, Liddell B, Olivieri G, Peduto A, Brammer MJ, et al. BOLD, sweat and fears: fMRI and skin conductance distinguish facial fear signals. NeuroReport. 2005;16:49–52. doi: 10.1097/00001756-200501190-00012. [DOI] [PubMed] [Google Scholar]
- Wright P, He G, Shapira N, Goodman W, Liu Y. Disgust and the insula: fMRI responses to pictures of mutilation and contamination. NeuroReport. 2004;15:2347–2351. doi: 10.1097/00001756-200410250-00009. [DOI] [PubMed] [Google Scholar]
- Young L, Cushman F, Hauser M, Saxe R. The neural basis of the interaction between theory of mind and moral judgment. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:8235–8240. doi: 10.1073/pnas.0701408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zhong C, Liljenquist K. Washing away your sins: Threatened morality and physical cleansing. Science. 2006;313:1451–1452. doi: 10.1126/science.1130726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.