Abstract
Background
Nitric oxide (NO) is an intercellular messenger that plays a critical role in learning and memory processes. Effects of nitric oxide synthase (NOS) inhibitors and guanylate cyclase (GC) inhibitors on cognitive function remain controversial.
Material/Methods
The aim of this study was to investigate effects of an NOS inhibitor, 7-nitroindazole (7-NI), and a GC inhibitor, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), on different aspects of memory in passive avoidance (PA), novel object recognition (NOR), and social transmission of food preference (STFP) tests. Male Balb-c mice were treated intraperitoneally with 7-NI (15 mg/kg), ODQ (3,10 mg/kg), L-arginine (100 mg/kg) + 7-NI (15 mg/kg), or physiological saline.
Results
ODQ (10 mg/kg) and 7-NI (15 mg/kg) significantly decreased second-day latency in PA test. 7-NI (15 mg/kg) and ODQ (10 mg/kg) significantly decreased the ratio index in the NOR test. 7-NI and ODQ (10 mg/kg) decreased cued/non-cued food eaten in STFP test. Amount of time spent in center zone significantly increased in ODQ (10 mg/kg) and 7-NI (15 mg/kg) groups in open field test, but there was no effect on total distance moved and speed of animals. ODQ (10 mg/kg) significantly increased number of entries into new compartments in exploratory activity apparatus, while 7-NI had no effect. Administration of L-arginine (100 mg/kg) before 7-NI reversed 7-NI-induced effects, supporting the role of NO in cognition.
Conclusions
Our results confirm that inhibition of NO/cGMP/GS pathway might disturb emotional, visual, and olfactory memory in mice. Also, 7-NI and ODQ had anxiolytic effects in open field test, and ODQ also enhanced exploratory activity.
Keywords: 7-NI, ODQ, Memory, Exploratory Activity, Mice
Background
Nitric oxide (NO) is a free-radical gas that is synthesized from L-arginine by NO synthase (NOS). Activation of N-methyl- D -aspartate (NMDA), non-NMDA, or metabotropic glutamate receptors result in NO formation through NOS activation. Activation of NMDA receptors greatly increases the outflow of cyclic guanosine monophosphate (cGMP), whereby the enhanced cGMP outflow is inhibited by NOS inhibitors and soluble guanylate cyclase [1]. Thus, stimulation of excitatory amino acid transmission promotes NO synthesis, which increases cGMP synthesis via guanylate cyclase activation. This pathway plays an important role in learning and memory. NO has been implicated in the regulation of various behavioral, cognitive, and emotional processes, including aggression, anxiety, depression, and locomotion [2–4]. Recently, it was suggested that NO plays a role in modulating learning and memory [5], although the role of NO in learning is not completely understood.
Studies on the modulation of synaptic function by cGMP indicate that multiple mechanisms modulate synaptic efficacy and its actions, including the regulation of synaptic plasticity [6]. Multiple studies have also provided evidence of sGC activation in memory formation [7–9]. Activation of soluble guanylyl cyclase may be a major pathway regulating NO messenger function in the brain [10,11], as it has been reported that the induction of long-term potentiation (LTP) in hippocampal slices can be blocked with soluble guanylyl cyclase inhibitors [12,13].
In different rodent models, many studies using several drugs were performed to investigate the effect of NO on learning and memory, but very controversial results have been reported. Some of these studies found that compounds blocking NOS disturbed learning [14,15], while other studies did not support these findings [16,17]. In the Morris water maze test, systemic inhibition of NO had disturbing effects in some studies [18,19], although systematic or local inhibition of hippocampal NO synthase did not exert any effect in other studies [17,20]. In addition to this, LTP which is considered to have a role in NO-mediated learning, was blocked completely after the hippocampal injection of NOS inhibitors in some studies [21,22], whereas others have found a partial inhibition [13,23] and some have concluded that no effect was seen [24,25]. Several studies of NOS inhibitors demonstrated that 7- nitroindazole (7-NI), a nonselective NOS inhibitor, impaired learning and memory in different tasks, such as the Morris water maze, radial maze, passive avoidance, and elevated plus maze tests [26–28].
The aim of the present study was to further evaluate the effects of 7-NI, a nonselective inhibitor of NOS; L-arginine, a NO precursor combined with 7-NI; and [1H-[1,2,4]-oxadiazole[4,3a]-quinoxaline-1-one] (ODQ), a highly selective, irreversible inhibitor of soluble guanylate cyclase (sGC), on different aspects of memory in the passive avoidance (PA), novel object recognition (NOR), and social transmission of food preference (STFP) tests, as well as investigating the role of NO in learning and memory since there is a controversy about this in the literature. We also investigated the effect of these drugs on locomotion, anxiety and exploratory activity in the open field and free exploratory activity tests.
Material and Methods
Animals
We used 7-week-old male inbred BALB/c ByJ mice (MAM TUBİTAK, Gebze, Kocaeli, Turkey). Animals (4–5 per cage) were kept in the laboratory at 21±1.5°C with 60% relative humidity under a 12 h light/dark cycle (light on at 8.00 p.m.) for 2 weeks prior to experimentation. Tap water and food pellets were available ad libitum. All procedures involving animals were performed in compliance with the European Community Council Directive of 24 November 1986, and ethics approval was granted by the Kocaeli University Ethics Committee (Number: AEK 9/4-2010, Kocaeli, Turkey). All animals were naive to the experimental apparatus, and different animals were used for each test.
Passive avoidance test
Animals were trained in a one-trial, step-through, PA apparatus to evaluate memory based on contextual fear conditioning and instrumental learning [29]. A decrease in retention latency indicates memory impairment in the PA task. The apparatus consists of a box with an illuminated area (L 7×12.5×h 14 cm) and a dark area (L 24×12.5×h 14 cm), both equipped with a grid floor composed of steel bars (0.3 cm diameter) spaced 0.9 cm apart. The inhibitory avoidance task consisted of 2 trials. On the first day of training, mice were placed individually into the light compartment and allowed to explore the boxes. The intercompartment door was opened after a 60 s acclimation period. In the acquisition trial, each mouse was placed in the illuminated compartment, which was lighted by a bright bulb (2000 lux). The animals received drugs prior to acquisition training. If the mouse stepped into the dark compartment (2/3 of the tail in the dark compartment), the door was closed by the experimenter, and an inescapable foot shock (0.25 mA/1 s) was delivered through the grid floor of the dark compartment. A cutoff time of 5 min was selected. The time taken to enter the dark compartment (training latency) was recorded. Immediately after the shock, the mouse was returned to the home cage. The retention trial started 24 h after the end of the acquisition trial. Each mouse was placed in the illuminated compartment, in the same manner as the training trial. The door was opened after a 30 s acclimation period. The step-through latency in the retention trial (with a maximum 300 s cutoff time) was used as the index of retention of the learned experience. No shock was applied during the retention trial.
Novel object recognition test (NOR)
The NOR was performed according to the protocol of Ennaceur and Delacour [30], with slight modification. The apparatus consisted of a circular open field (40 cm diameter and 30 cm height) made of PVC with a black and white-striped cardboard pattern (30×20 cm) nailed to one of the walls. A bulb provided constant illumination (approximately 100 lux) above the central section. The novel object recognition task procedure consisted of 3 trials: habituation, training, and retention. Each mouse was individually habituated to the apparatus for 5 min in the absence of objects (habituation trial). Thirty min after the habituation trial, the mouse was placed in the apparatus for the training trial (T1), and 2 identical objects (moon or butterfly) were placed in a symmetrical position 10 cm above the side wall. The order of objects used per subject per trial was determined randomly. The total time spent exploring the 2 objects was recorded for 5 min by the experimenter. Exploration of an object was defined as directing the nose to the object and/or touching it with the nose. After a predetermined retention interval of 1 h, the mouse was placed in the apparatus for the retention trial (T2), but with 2 dissimilar objects, a familiar one and a new one. The object not used in the training trial was used as the novel object in the retention trial. The animals were then allowed to explore freely for 5 min, and the time spent exploring each object was recorded. If recognition memory was intact, the mice were expected to spend more time exploring the novel object [30]. A ratio index (RI) was calculated as the time spent exploring the new object (N) divided by the total time exploring the objects (N+R) multiplied by 100. A higher ratio index reflects greater memory retention.
Social transmission of food preference test (STFP)
Hippocampus-dependent non-spatial olfactory memory [31] was studied using the STFP. The experiment was conducted in 3 phases: (i) habituation to flavored food, (ii) interaction between ‘demonstrator’ and ‘observer’ mice, and (iii) test of the food preference in the ‘observer’ mice. Mice were housed at a ratio of 3–4 observer mice to 1 demonstrator. In the habituation phase, a demonstrator mouse was chosen from each cage. Demonstrators were housed alone in a cage separate from the colony for 3 h with free access to water but not food. After 3 h, each demonstrator was allowed to eat powdered ground chow scented with either cinnamon (1% w/w) or cocoa (2% w/w) for 2 h. The criterion for inclusion in the experiment was consumption of 0.2 g chow. Each demonstrator was then returned to its observer cagemates for 30 min. Observers interacted with the demonstrator, sniffing the scent around the muzzle and on the breath of the demonstrator mouse. After the interaction period, the demonstrator mouse was removed from the interaction cage and returned to its individual cage. The food preference of the observer mice was tested 24 h after the end of the interaction with the demonstrator. Five hours before the preference test, observer mice were caged individually, and food was removed from the observer’s cage for 3 h prior to the test. The preference test consisted of presenting each observer with a pair of weighed food pellets in the individual cage for 2 h. Half the observers were tested with the cinnamon-flavored cued food eaten by their demonstrator versus the novel cocoa-flavored food, whereas the other half were tested with the cocoa-flavored cued food eaten by their demonstrator versus the novel cinnamon-flavored food.
The ratio of the weight of the cued food eaten and the total weight of food eaten was used as a measure of food preference.
Open field test
Effects of treatment on animal locomotor activity were measured using the open field test. This test is also used to examine anxiety-like behaviors, and it is used to evaluate anxiolytic treatment [32]. This experiment was performed as previously described [33]. Briefly, the testing apparatus consisted of a wooden box (33×33×30 cm) with an indirect red light. An animal was placed in the center of the test box, and the total distance moved throughout the area, the speed of locomotion, and the time spent in the center zone were recorded using the Ethovision-XT (Noldus) for 5 min. The center zone of the open field was a circle with a 12-cm diameter.
Free exploratory paradigm (Hughes Box)
The apparatus consisted of a polyvinyl chloride box (30×20×20 cm) covered with Plexiglas and subdivided into 6 identical square exploration units, all interconnected by small doors [34]. A temporary partition divided the apparatus lengthwise in half. Approximately 24 h before testing, each subject was placed in one half of the apparatus, with the temporary partition in place, for familiarization. The floor was covered with sawdust, and the animal was given unlimited access to food and water. The next day, the same mouse was exposed to both the familiar and novel environments after the temporary partition was removed, without removing the animal from the box. The subject was observed under a red light for 10 min, and the number of units entered and the time spent in the novel side were recorded.
Drug administration
7-Nitroindazol (7-NI), ODQ and L-Arginine were purchased from Sigma Chemical Company (Sigma, St. Louis, MO). ODQ and L-Arginine were dissolved in saline, and 7-NI was dissolved in saline supplemented with 10% DMSO. All drugs were freshly prepared and administered in a volume of 0.1 ml per 10 g body weight. The control groups received the same volume of vehicle. 7-NI (15 mg/kg), ODQ (3 and 10 mg/kg), L-arginine or vehicle were administered intraperitoneally (i.p.) 30, 30, and 60 min, respectively, before the first session (acquisition session, day 1) of the passive avoidance test, retention trials of NOR and STFP tests and the open field and free exploratory paradigm (Hughes box) tests. The number of animals per group was 6. The effective dose and route of administration of each drug was selected according to previous behavioral and neurochemical studies [3,35–37].
Statistics
The two-way analysis of variance (ANOVA) post hoc Tukey test was used to analyze passive avoidance, NOR, STFP, and open field tests. The Wilcoxon signed rank test was used to compare differences between the first and the second day latencies in the passive avoidance test. Data are expressed as the mean values ±SEM. p<0.05 was considered statistically significant.
Results
Effects of 7-NI, ODQ and 7-NI+L-Arginine on learning and memory in the passive avoidance test
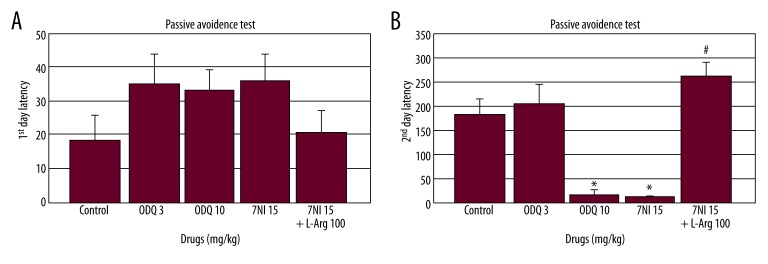
When 7-NI (15 mg/kg), ODQ (3 and 10 mg/kg), and 7-NI (15 mg/kg) + L-arginine (100 mg/kg) were administered before the acquisition session (training; day 1), no significant effects were observed for the drugs alone [F(3.29)=1.25, p=0.31, Figure 1A] or for the combination [F(1.29)=2.15, p=0,31; Figure 1A] in the passive avoidance test. The individual drugs [F(3.29)=16.97 p<0.001, Figure 1B] and the combination of drugs [F(1.29)=53.44, p<0,001, Figure 1B] significantly affected retention latency in the passive avoidance test. ODQ (10 mg/kg) and 7-NI (15 mg/kg) significantly shortened the second-day latency compared to the control group when the drugs were administered before the acquisition session (p<0.001, Figure 1B). L-arginine (100 mg/kg) combined with 7-NI (15 mg/kg) significantly prolonged second-day latency compared to the 7-NI (15 mg/kg) alone group (p<0.001; Figure 1B).
Figure 1.
(A, B) Effects of ODQ (3 and 10 mg/kg), 7-NI (15 mg/kg), and L-arginine (100 mg/kg) on learning and memory (n=6) (ODQ, 7-NI and L-Arginine were administered 30, 30, and 60 min before the acquisition, respectively) in the passive avoidance test. The data are expressed as the mean ±SEM. * p<0.001 compared to the control group. # p<0.001 compared to the 7-NI alone group.
Effects of 7-NI, ODQ, and 7-NI ± L-Arginine on visual memory in the NOR test
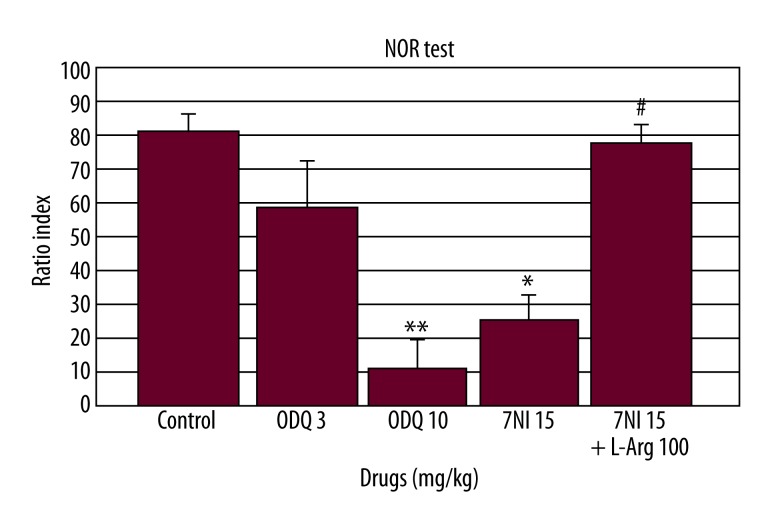
When 7-NI (15 mg/kg), ODQ (3 and 10 mg/kg), and 7-NI (15 mg/kg) + L-arginine (100 mg/kg) were administered before the retention trial in the NOR test, the individual drugs [F(3.29)=12.50, p<0.001; Figure 2] and the combination [F(1.29)=16.59, p<0.001; Figure 2] had a significant effect on the ratio index. ODQ (10 mg/kg) and 7-NI (15 mg/kg) significantly decreased the ratio index compared to the control group (p<0.001, p<0.01, respectively; Figure 2). L-Arginine (100 mg/kg) combined with 7-NI (15 mg/kg) significantly increased the ratio index compared to the 7-NI (15 mg/kg) alone group (p<0.001; Figure 2).
Figure 2.
Effect of ODQ (3 and 10 mg/kg), 7-NI (15 mg/kg) and L-arginine (100 mg/kg) on the ratio index (n=6) in the novel object recognition (NOR) test in mice. ODQ, 7-NI, and L-arginine were administered 30, 30, and 60 min before the retention trial, respectively. The data are expressed as the mean ±SEM * p<0.01, ** p<0.001 compared to the control group. # p<0.001 compared to the 7-NI alone group.
Effects of 7-NI, ODQ, and 7-NI ± L-Arginine on olfactory memory in the STFP test
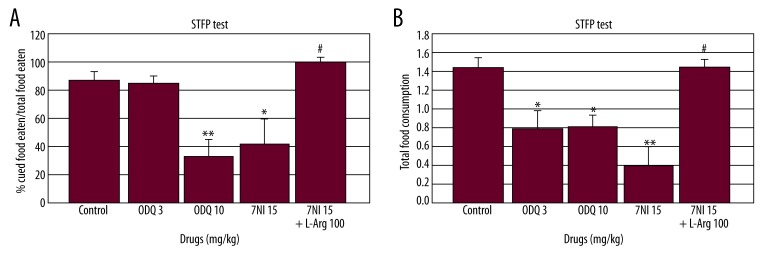
When 7-NI (15 mg/kg), ODQ (3 and 10 mg/kg), and 7-NI (15 mg/kg)+ L-arginine (100 mg/kg) were administered before the retention session of the STFP test, the individual drugs [F(3.29)=10.08, p<0.001; Figure 3A] and the combination [F(1.29)=20.09, p<0.001; Figure 3A] significantly effected the percent cued food/total food eaten. ODQ (10 mg/kg) and 7-NI (15 mg/kg) significantly decreased percent cued food/total food eaten compared to the control group (p<0.01 and p<0.001; respectively, Figure 3A). L-arginine (100 mg/kg) combined with 7-NI (15 mg/kg) significantly increased the percent cued food/total food eaten compared to the 7-NI (15 mg/kg) alone group (p<0,001; Figure 3A).
Figure 3.
Effect of ODQ (3 and 10 mg/kg), 7-NI (15 mg/kg), and L-arginine (100 mg/kg) (n=6). (A) On percent cued/non-cued food eaten and (B) total food consumption in the social transmission of food preference (STFP) test. ODQ, 7-NI and L-arginine were administered 30, 30, and 60 min before the retention trial, respectively. The data are expressed as the mean ±SEM. * p<0.01, ** p<0.001 compared to the control group. # p<0.001 compared to the 7-NI alone group.
The individual drugs [F(3.29)=8.72, p<0.001; Figure 3B] and the combination [F(1.29)=24.79, p<0.001; Figure 3B] significantly affected total food consumption. ODQ (10 mg/kg) and 7-NI (15 mg/kg) significantly decreased total food consumption compared to the control group (p<0.01 and p<0.001; respectively, Figure 3B). L-arginine (100 mg/kg) combined with 7-NI (15 mg/kg) significantly increased total food consumption compared the effect of 7-NI (15 mg/kg) alone (p<0.001; Figure 3B).
Effects of 7-NI, ODQ, and 7-NI ± L-Arginine on exploratory activity in the Hughes box
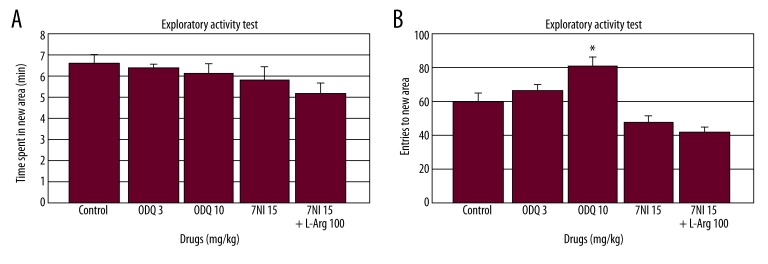
The drugs [F(3.29)=0.69, p=0.56; Figure 4A] and combination [F(1.29)=1.14, p=0.29; Figure 4A] did not significantly affect total time spent in the novel side of the Hughes box.
Figure 4.
Effect of ODQ (3 and 10 mg/kg), 7-NI (15 mg/kg) and L-arginine (100 mg/kg) (n=6) administration on exploratory activity in the free exploratory paradigm (Hughes box). The drugs were injected 30, 30, and 60 min prior to testing, respectively. The data are expressed as the mean ±SEM. (A) Total time spent in the novel side and (B) total number of entries to the novel side. * p<0.05 compared to the control group.
However, the drugs [F(3.29)=7.87, p<0.001; Figure 4B] significantly affected the total number of entries into the novel side in the Hughes box. ODQ (10 mg/kg) significantly increased the total number of entries into the novel side compared to the control group (p<0.05; Figure 4B). The combination [F(1.29)=0.55, p=0.46; Figure 4B] did not significantly alter the total number of entries into the novel side.
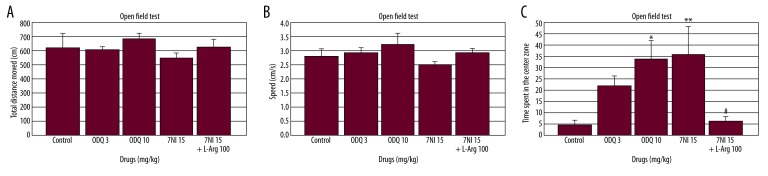
Effects of 7-NI, ODQ and 7-NI ± L-Arginine on locomotion and anxiety in the open field test
The individual drugs [F(3.29)=0.88, p=0.46; Figure 5A; F(3.29)=1.18, p=0.33; Figure 5B] and the combination [F(1.29)=1.27, p=0.26; Figure 5A; F(1.29)=0.78, p=0.38; Figure 5B] did not significantly affect the total distance moved or speed of the animals in the open field test. The individual drugs [F(3.29)=4.84, p=0.009; Figure 5C] and the combination [F(1.29)=10.29, p=0.004; Figure 5C] significantly affected the center zone duration in the open field test. ODQ (10 mg/kg) and 7-NI (15 mg/kg) significantly increased center zone duration compared to the control group in the open field test (p<0.05 and p<0.01; respectively; Figure 5C), whereas L-arginine (100 mg/kg) combined with 7-NI (15 mg/kg) significantly decreased center zone duration compared to the 7-NI (15 mg/kg) alone group (p<0.01; Figure 5C).
Figure 5.
Effect of ODQ (3 and 10 mg/kg), 7-NI (15 mg/kg) and L-arginine (100 mg/kg) (n=6) administration on locomotion and anxiety in the open field test. The drugs were injected 30, 30, and 60 min prior to testing, respectively. The data are expressed as the mean ±SEM values. (A) Total distance moved, (B) speed, and (C) center zone duration in the open field test. * p<0.05, ** p<0.01 compared to the control group. # p<0.01 compared to the 7-NI alone group.
Discussion
This study revealed that both the GC inhibitor, ODQ (10 mg/kg), and the NOS inhibitor, 7-NI (15 mg/kg), decreased retention latency in the PA test, ratio index in the NOR test, and percent cued food/non-cued food eaten in the STFP test. ODQ (3 and 10 mg/kg) and 7-NI also decreased total food consumption in the STFP test. Neither ODQ nor 7-NI altered total distance moved and speed of the animals in the open field test, but both increased center zone duration. In the Hughes box test, neither drug affected the time spent in the new area. However, ODQ (10 mg/kg) increased the number of entries into the new area, whereas 7-NI had no effect. 7-NI (15 mg/kg)-induced effects in the PA, NOR, STFP, and open field tests were reversed by a NO precursor, L-arginine (100 mg/kg).
Our literature search revealed no studies investigating the effect of ODQ on memory in the NOR and STFP tests, and both ODQ and 7-NI had not been studied in the Hughes box. The NOR test for rodents, in which the spontaneous exploratory activity toward a novel object and a familiar object is measured, was formulated by Ennaceur and Delacour [30]. This test does not involve rule learning or reinforcement, and it evaluates working and visual memory. In the NOR test, the failure to discriminate between familiar and novel objects can be related to either impaired memory of the object or the inability to use spatial information. Occasionally, the effect might be unrelated to an amnesic action, as mice displayed an inhibition of locomotion. However, this hypothesis does not apply to ODQ and 7-NI at the doses used because they did not affect locomotion in the open field test in our study. Therefore, the performance observed in the NOR test can be attributed to memory-disturbing effects of ODQ and 7-NI.
Hall [38] originally described the open field test for the study of rat emotion. The procedure consists of placing an animal in an unknown environment from which it cannot escape due to the surrounding walls [39]. The open field test is a very common procedure used in animal psychology [33]. In this situation, rodents naturally prefer the periphery of the apparatus to the middle area of the open field. Indeed, mice and rats walk close to the walls, a behavior called thigmotaxis. Treatments that increase the time spent in the central area without impairment of locomotion and vertical exploration are deemed anxiolytic-like, while treatments that decrease these variables produce anxiogenic effects. In the free exploratory test (Hughes box), mice that spend more time in the novel side are considered less anxious. In our study, the observed behavior in the Hughes box test cannot be linked to any difference in the level of locomotion, as the drugs did not alter the total distance moved and speed of the animals in the open field test. The effect of NO on anxiety is controversial. In some studies, NOS inhibitors displayed anxiolytic effects [3], whereas NO donors had anxiogenic effects [40]. In our study, both ODQ and 7-NI exerted anxiolytic effects in the open field test, while only ODQ increased exploratory activity in the Hughes box test, a result that can be attributed to its anxiolytic effect. Doses of 7-NI above 15 mg/kg have been reported to exert anxiolytic effects in the Hughes box test, which may explain why we did not observe any effect of 7-NI in the Hughes box test.
Social transmission of food preference is a hippocampal-dependent olfactory memory test [31]. Both ODQ (10 mg/kg) and 7-NI disturbed olfactory memory in the STFP test, but ODQ (3 and 10 mg/kg) and 7-NI also diminished total food consumption, suggesting that these effects are related to nonspecific processes. These results are compatible with previous studies [37]. Several studies indicate that NO release is involved in the acquisition phase of an olfactory recognition test, but it does not affect post-acquisition recall [41]. This can explain why ODQ and 7-NI did not have a clear effect on olfactory memory retrieval in our study.
Passive avoidance is an adaptive response to a stressful experience, which serves as a measure of learning and memory [42]. In our study, drugs were injected just before giving an electrical shock so that we could study the effect of the drugs on memory acquisition and retrieval. Administering the drug just before the electrical shock can cause some nonspecific effects (e.g., analgesic effect, pain perception, and motility). Both ODQ (10 mg/kg) and 7-NI (15 mg/kg) treatment led to memory deterioration in the passive avoidance test in our study, in accordance with previous studies [28,37,43].
Recent studies have postulated that both eNOS and nNOS activities are necessary for memory formation. Additionally, in a passive avoidance test in chicks, nNOS cannot substitute for the role of eNOS on LTP [44]. Son et al. [45] demonstrated that LTP decreased only when both NO synthase isoforms were absent in the hippocampal CA1 area. Taken together, these results support the claim that both isoforms have a role in LTP and can substitute for each other, but the mechanism underlying their action and to what degree eNOS can compensate nNOS remains unknown. These findings can explain why the nonselective nNOS and eNOS inhibitor, 7-NI, leads to deterioration in learning and memory and also the discrepancy among memory studies using NOS inhibitors. The difference between the effects of NOS inhibitors on cognitive functions could be related to the selectivity differences between the drugs, but also to the dosage, administration route, and pharmacokinetic and pharmacodynamic properties of the compounds.
One of the major problems with NO inhibitors or donors is the effect of these drugs on blood pressure [46]. It is difficult to dissociate the neural effects of these drugs from their cardiovascular effects. Prickaerts et al. [14] reported that disturbing effects of 7-NI were not correlated with its effects on blood pressure. In another study, Hölscher et al. [26] demonstrated that 30 mg/kg 7-NI disturbed spatial learning without changing blood pressure in both the water maze and 8-arm radial maze tests. Taken together, these data indicate that one can exclude the blood pressure effects of 7-NI from the effects observed in our study.
NO is an intercellular messenger that plays a critical role in learning and memory processes. NO modulates the release and reuptake of various neurotransmitters [47], resulting in long-term potentiation of synaptic transmission. This phenomenon is believed to be the neuronal basis of memory formation. NOS inhibitors have been reported to impair memory in learning-memory tasks, but these findings are discrepant in this context. Some studies reported that NOS inhibitors do not change learning performance or memory in the passive avoidance [48,49] and Morris water maze tests, respectively [17,20], whereas other studies reported that they inhibit the retention trial of the passive avoidance test [50,51], spatial learning in the water maze test [52,53], or object recognition [14] in the radial arm maze [54]. Previous studies indicated that 7-NI impaired learning and memory in several tests [26,28,37], and those findings are similar to the results observed in our study. Our results are in accordance with the studies proposing the disturbing effect of NOS inhibitors on learning and memory.
Here, the fact that L-arginine reversed the impairment effect of 7-NI supports the theory that this effect is specific to NOS. Despite considerable evidence for the involvement of NO in at least some forms of memory processing [14,53,54], its function remains unknown. However, a majority of NO-mediated physiological processes result from the activation of GC and, in turn, cGMP-mediated activation of protein kinase G (PKG). Edwards et al. [55] reported that inhibition of GC and PKG impairs retention for the passive avoidance task, suggesting that GC mediates 2 memory retrieval processes. Interestingly, in a study using the GC inhibitor, ODQ, and ADP-ribosyltransferase inhibitor nicotinamide, ODQ failed to affect LTP but effectively suppressed cGMP production in the hippocampus. Similarly, Kleppsich et al. [56] reported that PKGs are not involved in LTP in mice, but NO induces LTP through an alternative cGMP-independent pathway, possibly ADP-ribosylation. However, studies on the modulation of synaptic function by cGMP indicate that it regulates synaptic efficacy via multiple mechanisms, and its action may include regulating synaptic plasticity [6]. Our results support the role of cGMP in cognition and disturbing effect of ODQ in learning and memory.
Conclusions
The present study demonstrates that both the GS inhibitor, ODQ, and the NOS inhibitor, 7-NI, disturbed emotional memory in the PA test, visual memory in the NOR test, and olfactory memory in the STFP test. Neither drug had any effect on locomotion, but they exerted an anxiolytic effect in the open field test. ODQ also increased exploratory activity in the Hughes box test, whereas 7-NI had no effect. L-arginine, the NO precursor, reversed 7-NI-induced changes, confirming that the effects of 7-NI were NO-dependent. Our findings suggest that the nNOS-sGC-cGMP pathway is involved in the pathophysiology of memory functions. Future studies using different NOS inhibitors with different cognition methods are needed to support our findings.
Footnotes
Source of support: Departmental sources
References
- 1.Prast H, Philippu A. Nitric oxide as a modulator of neuronal function. Prog Neurobiol. 2001;64:51–56. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 2.Yildiz F, Erden F, Ulak G, et al. Antidepressant-like effect of 7-nitroindazole in the forced swimming test in rats. Psychopharmacology. 2000;149:41–44. doi: 10.1007/s002139900316. [DOI] [PubMed] [Google Scholar]
- 3.Yildiz F, Ulak G, Erden F, Gacar N. Anxiolytic- like effects of 7-nitroindazole in the rat plus-maze test. Pharmacol Biochem Behav. 2000;65:199–202. doi: 10.1016/s0091-3057(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 4.Trainor BC, Workman JL, Jessen R, Nelson RJ. Impaired nitric oxide synthase signaling dissociates social investigation and aggression. Behav Neurosci. 2007;121:362–69. doi: 10.1037/0735-7044.121.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Susswein AJ, Katzoff A, Miller N, Hurwitz I. Nitric oxide and memory. Neuroscientist. 2004;10:153–62. doi: 10.1177/1073858403261226. [DOI] [PubMed] [Google Scholar]
- 6.Barnstable CJ, Wei JY, Han MH. Modulation of synaptic function by cGMP and GMP-gated cation channels. Neurochem Int. 2004;45:875–84. doi: 10.1016/j.neuint.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Chien WL, Liang KC, Teng CM, et al. Enhancement of learning behaviour by a potent nitric oxide-guanylate cyclase activator YC-1. Eur J Neurosci. 2005;21:1679–88. doi: 10.1111/j.1460-9568.2005.03993.x. [DOI] [PubMed] [Google Scholar]
- 8.Chien WL, Liang KC, Fu WM. Enhancement of active shuttle avoidance response by the NO-cGMP-PKG activator YC-1. Eur J Pharmacol. 2008;590:233–40. doi: 10.1016/j.ejphar.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 9.Zhuo M, Hu Y, Schultz C, et al. Role of guanylyl cyclase and cGMPdependent protein kinases in long-term potentiation. Nature. 1994;368:635–39. doi: 10.1038/368635a0. [DOI] [PubMed] [Google Scholar]
- 10.Garthwaite J. Glutamate, nitric oxide and cell–cell signalling in the nervous system. Trends Neurosci. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- 11.Southam E, Garthwaite J. The nitric oxide-cyclic GMP signalling pathway in rat brain. Neuropharmacology. 1993;32:1267–77. doi: 10.1016/0028-3908(93)90021-t. [DOI] [PubMed] [Google Scholar]
- 12.Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3X, 5Xcyclic-GMP in cultured hippocampal neurons. Nature. 1995;376:74–80. doi: 10.1038/376074a0. [DOI] [PubMed] [Google Scholar]
- 13.Boulton CL, Southam E, Garthwaite J. Nitric oxide-dependent long-term potentiation is blocked by a specific inhibitor of soluble guanylyl cyclase. Neuroscience. 1995;69:699–703. doi: 10.1016/0306-4522(95)00349-n. [DOI] [PubMed] [Google Scholar]
- 14.Prickaerts J, Steinbusch HW, Smits JF, Vente J. Possible role of nitric oxide-cyclic GMP pathway in object recognition memory: effects of 7-nitroindazole and zaprinast. Eur J Pharmacol. 1997;33:125–36. doi: 10.1016/s0014-2999(97)01301-0. [DOI] [PubMed] [Google Scholar]
- 15.Meyer CR, Spangler EL, Patel N, et al. Impaired learning in rats in a 14-unit T-maze by 7-nitroindazole, a neuronal nitric oxide synthase inhibitor, is attenuated by the nitric oxide donor, molsidomine. Eur J Pharmacol. 1998;341:17–22. doi: 10.1016/s0014-2999(97)01428-3. [DOI] [PubMed] [Google Scholar]
- 16.Knepper BR, Kurylo DD. Effects of nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on spatial and cued learning. Neuroscience. 1998;83:837–41. doi: 10.1016/s0306-4522(97)00457-0. [DOI] [PubMed] [Google Scholar]
- 17.Blokland A, De Vente J, Prickaerts J, et al. Local inhibition of hippocampal nitric oxide synthase does not impair place learning in the Morris water escape task in the rat. Eur J Neurosci. 1999;11:223–32. doi: 10.1046/j.1460-9568.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- 18.Estall LB, Grant SJ, Cicala GA. Inhibition of nitric oxide (NO) production selectively impairs learning and memory in the rat. Pharmacol Biochem Behav. 1993;46:959–62. doi: 10.1016/0091-3057(93)90228-l. [DOI] [PubMed] [Google Scholar]
- 19.Yamada K, Noda Y, Nakayama S, et al. Role of nitric oxide in learning and memory and in monoamine metabolism in the rat brain. Br J Pharmacol. 1995;115:852–58. doi: 10.1111/j.1476-5381.1995.tb15011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bannerman DM, Chapman PF, Kelly PA, et al. Inhibition of nitric oxide synthase does not impair spatial learning. J Neurosci. 1994;14:7404–14. doi: 10.1523/JNEUROSCI.14-12-07404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bon C, Böhme GA, Doble A, et al. A role for nitric oxide in long-term potentiation. Eur J Pharmacol. 1992;4:420–24. doi: 10.1111/j.1460-9568.1992.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 22.Doyle C, Hölscher C, Rowan MJ, Anwyl R. The selective neuronal NO synthase inhibitor 7-nitro-indazole blocks both long-term potentiation and depotentiation of field EPSPs in rat hippocampal CA1 In Vivo. J Neurosci. 1996;16:418–26. doi: 10.1523/JNEUROSCI.16-01-00418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Southam E, Charles SL, Garthwaite J. The nitric oxide-cyclic GMP pathway and synaptic plasticity in the rat superior cervical ganglion. Br J Pharmacol. 1996;119:527–32. doi: 10.1111/j.1476-5381.1996.tb15703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings JA, Nicola SM, Malenka RC. Induction in the rat hippocampus of long-term potentiation (LTP) and long-term depression (LTD) in the presence of a nitric oxide synthase inhibitor. Neurosci Lett. 1994;176:110–14. doi: 10.1016/0304-3940(94)90883-4. [DOI] [PubMed] [Google Scholar]
- 25.Murphy KPSJ, Williams JH, Bettache N, Bliss TVP. Photolytic release of nitric oxide modulates NMDA receptor-mediated transmission but does not induce long-term potentiation at hippocampal synapses. Neuropharmacol. 1994;33:1375–85. doi: 10.1016/0028-3908(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 26.Hölscher C, McGlinchey L, Anwyl R, Rowan MJ. 7-Nitroindazole, a selective neuronal nitric oxide synthase inhibitor in vivo, impairs spatial learning in the rat. Learn Mem. 1996;2:267–78. doi: 10.1101/lm.2.6.267. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno M, Yamada K, Olariu A, et al. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–21. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yildiz-Akar F, Ulak G, et al. 7-Nitroindazole, a neuronal nitric oxide synthase inhibitor, impairs passive-avoidance and elevated plus-maze memory performance in rats. Pharmacol Biochem Behav. 2007;87:434–43. doi: 10.1016/j.pbb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Ogren SO, Johansson C, Magnusson O. Forebrain serotonergic involvement in avoidance learning. Neurosci Lett. 1985;58:305–9. doi: 10.1016/0304-3940(85)90071-0. [DOI] [PubMed] [Google Scholar]
- 30.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1. Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 31.Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus. 1995;5:546–56. doi: 10.1002/hipo.450050606. [DOI] [PubMed] [Google Scholar]
- 32.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 33.Belzung C. Measuring exploratory behavior. In: Crusio WE, Gerlai RT, editors. Handbook of Molecular Genetic Techniques for Brain and Behavior Research (Techniques in the Behavioral and Neural Sciences) Amsterdam: Elsevier; 1999. pp. 739–49. [Google Scholar]
- 34.Hughes RN. Food deprivation and locomotor exploration in the white rat. Anim Behav. 1965;13:30–32. [Google Scholar]
- 35.Ergün Y, Ergün UGÖ. Prevention of pro-depressant effect of L-arginine in the forced swim test by NG-nitro-L-arginine and [1H-[1,2,4]Oxadiazole[4,3-a]quinoxalin-1-one] Eur J Pharmacol. 2007;554:150–54. doi: 10.1016/j.ejphar.2006.09.067. [DOI] [PubMed] [Google Scholar]
- 36.Yıldız Akar F, Komsuoğlu FI, Ulak G, Mutlu O. Effects of L -Arginine on 7-Nitroindazole- Induced Reference and Working Memory Performance of Rats. Pharmacology. 2009;84:211–18. doi: 10.1159/000235997. [DOI] [PubMed] [Google Scholar]
- 37.Mutlu O, Ulak G, Belzung C. Effects of nitric oxide synthase inhibitors 1-(2 trifluoromethylphenyl) – imidazole (TRIM) and 7-nitroindazole (7-NI) on learning and memory in mice. Fundam Clin Pharmacol. 2011;25:368–77. doi: 10.1111/j.1472-8206.2010.00851.x. [DOI] [PubMed] [Google Scholar]
- 38.Hall CS. Emotional behavior in the rat: I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol. 1934;18:385–403. [Google Scholar]
- 39.Walsh RN, Cummins RA. The open field test: a critical review. Psychol Bull. 1976;83:481–504. [PubMed] [Google Scholar]
- 40.Caton PW, Tousman SA, Quock RM. Involvement of nitric oxide in nitrous oxide anxiolysis in the elevated plus-maze. Pharmacol Biochem Behav. 1994;48:689–92. doi: 10.1016/0091-3057(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Andrade G, James BM, Kendrick KM. Neural encoding of olfactory recognition memory-review. J Reprod Dev. 2005;51:547–58. doi: 10.1262/jrd.17031. [DOI] [PubMed] [Google Scholar]
- 42.Tsuji M, Takeda H, Matsumiya T. Modulation of passive avoidance in mice by the 5-HT1A receptor agonist flesinoxan: comparison with the benzodiazepine receptor agonist diazepam. Neuropsychopharmacol. 2003;28:664–74. doi: 10.1038/sj.npp.1300080. [DOI] [PubMed] [Google Scholar]
- 43.Komsuoglu-Celikyurt I, Gocmez SS, Mutlu O, et al. Evidence for the involvement of neuronal nitric oxide synthase and soluble guanylate cyclase on cognitive functions in rats. Life Sci. 2011;89(23–24):905–10. doi: 10.1016/j.lfs.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Rickard NS, Gibbs ME, Ng KT. Inhibition of the endothelial isoform of nitric oxide synthase impairs long-term memory formation in the chick. Learn Mem. 1999;6(5):6458–66. doi: 10.1101/lm.6.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Son H, Hawkins RD, Martin K, et al. Long-term potentiation is reduced in mice that are doubly mutant in endothelial and neuronal nitric oxide synthase. Cell. 1996;87:1015–23. doi: 10.1016/s0092-8674(00)81796-1. [DOI] [PubMed] [Google Scholar]
- 46.Pitsikas N, Rigamonti AE, Cella SG, et al. Effects of molsidomine on scopolamine-induced amnesia and hypermotility in the rat. Eur J Pharmacol. 2001;426:193–200. doi: 10.1016/s0014-2999(01)01164-5. [DOI] [PubMed] [Google Scholar]
- 47.Guevara-Guzman R, Emson PC, Kendrick KM. Modulation of in vivo striatal transmitter release by nitric oxide and cyclic GMP. J Neurochem. 1994;62:807–10. doi: 10.1046/j.1471-4159.1994.62020807.x. [DOI] [PubMed] [Google Scholar]
- 48.Böhme GA, Bon C, Lemaire M, et al. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc Natl Acad Sci USA. 1993;90:9191–94. doi: 10.1073/pnas.90.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Telegdy G, Kokavszky R. The role of nitric oxide in passive avoidance learning. Neuropharmacology. 1997;36:1583–87. doi: 10.1016/s0028-3908(97)00154-8. [DOI] [PubMed] [Google Scholar]
- 50.Finn C, da Cunha C, Bromberg E, et al. Experiments suggesting a role for nitric oxide in the hippocampus in memory processes. Neurobiol Learn Mem. 1995;63:113–15. doi: 10.1006/nlme.1995.1011. [DOI] [PubMed] [Google Scholar]
- 51.Kopf SR, Benton SR, Kanfin R, et al. NO synthesis inhibition decreases cortical Ach release and impairs retention of a conditioned response. Brain Res. 2001;894:141–44. doi: 10.1016/s0006-8993(00)03148-6. [DOI] [PubMed] [Google Scholar]
- 52.Toyoda M, Saito H, Matsuki N. Nitric oxide but not carbonmonoxide is involved in spatial learning of mice. Jpn J Pharmacol. 1996;71:205–11. doi: 10.1254/jjp.71.205. [DOI] [PubMed] [Google Scholar]
- 53.Prendergast MA, Buccafusco JJ, Terry AV., Jr Nitric oxide inhibition impairs spatial navigation learning and induces contitioned taste aversion. Pharmacol Biochem Behav. 1998;57:347–52. doi: 10.1016/s0091-3057(96)00313-9. [DOI] [PubMed] [Google Scholar]
- 54.Zou LB, Yamada K, Tanaka T, et al. Nitric oxide synthase inhibitors impair reference memory formation in a radial arm maze. Neuropharmacology. 1998;37:323–30. doi: 10.1016/s0028-3908(98)00042-2. [DOI] [PubMed] [Google Scholar]
- 55.Edwards TM, Rickard NS, Ng KT. Inhibition of guanylate cyclase and protein kinase G impairs retention for the passive avoidance in the day old chick. Neurobiol Learn Mem. 2002;77:313–26. doi: 10.1006/nlme.2001.4021. [DOI] [PubMed] [Google Scholar]
- 56.Kleppsich T, Pfeifer A, Klatt P, et al. Long-term potentiation in the hippocampal CA1 region ofmice lacking cGMP-dependent kinases is normal and susceptible to inhibition of nitric oxide synthase. J Neurosci. 1999;19:48–55. doi: 10.1523/JNEUROSCI.19-01-00048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]