Abstract
The caudal zona incerta is the target of a recent modification of established procedures for deep brain stimulation (DBS) for Parkinson's disease and tremor. The caudal zona incerta contains a number of neuronal populations that are distinct in terms of their cytoarchitecture, connections, and pattern of immunomarkers and is located at a position where a number of major tracts converge before turning toward their final destination in the forebrain. However, it is not clear which of the anatomical features of the region are related to its value as a target for DBS. This paper has tried to identify features that distinguish the caudal zona incerta of rodents (mouse and rat) and primates (marmoset, rhesus monkey, and human) from the remainder of the zona incerta. We studied cytoarchitecture, anatomical relationships, the pattern of immunomarkers, and gene expression in both of these areas. We found that the caudal zona incerta has a number of histological and gene expression characteristics that distinguish it from the other subdivisions of the zona incerta. Of particular note are the sparse population of GABA neurons and the small but distinctive population of calbindin neurons. We hope that a clearer appreciation of the anatomy of the region will in the end assist the interpretation of cases in which DBS is used in human patients.
Keywords: caudal zona incerta, deep brain stimulation, Parkinson's disease, subthalamic nucleus, zona incerta
Introduction
The caudal zona incerta can be an effective site for deep brain stimulation (DBS) in cases of Parkinson's disease (Plaha et al. 2006; Blomstedt et al. 2012) and essential tremor (Plaha et al. 2008, 2011; Blomstedt et al. 2009b; Fytagoridis et al. 2012), although it should be noted that randomised controlled trials comparing this target with others have not been performed. DBS in this region of the brain for Parkinson's disease has previously focused on the subthalamic nucleus, anterior to the caudal zona incerta (Deuschl et al. 2006; Weaver et al. 2009; Follett et al. 2010; Schuepbach et al. 2013). In addition, DBS in the ventral posterior lateral thalamic nucleus has been shown to be effective in cases of essential tremor (Deuschl et al. 2011). The putative advantages of the caudal zona incerta as a target are improved clinical reliability, improvement in proximal tremor, and better control of the cardinal symptoms of Parkinson's disease, with less potential for clinical depression as a side effect (Plaha et al. 2006, 2008). This paper explores the anatomical features of the caudal zona incerta to see if they might provide an explanation for the effects of DBS in this area.
The zona incerta is a complex region of the brain. First of all, it contains a number of neuronal populations that are distinct in terms of their cytoarchitecture, connections, and pattern of immunomarkers (Kawana & Watanabe, 1981; Kolmac & Mitrofanis, 1999; Mitrofanis, 2005). Secondly, the zona incerta is located at a position where a number of major tracts converge before turning toward their final destination in the forebrain. These tracts include the superior cerebellar peduncle (the h field of Forel), the basis pedunculi, the nigrostriatal tract, the medial lemniscus, the thalamic fasciculus (the h1 field), and the lenticular fasciculus (the h2 field). In fact, the zona incerta was originally defined according to the anatomy of some of these prominent tracts, leading Burton & Jones (1976) to suggest that the neuronal population of the zona incerta would be better named ‘the nucleus of the zona incerta' to distinguish it from the nearby fibre sheets.
In developmental terms, the zona incerta is a part of prosomere 3 of the true diencephalon. By true diencephalon, we refer to the modern concept of the diencephalon, which excludes the hypothalamus. In developmental and gene expression terms, the hypothalamus, diencephalon, and midbrain form a rostrocaudal sequence (Puelles et al. 2012a,b2012b, 2013). Furthermore, gene expression studies show that the diencephalon is divided into three segments from caudal to rostral – prosomere 1 (the pretectum), prosomere 2 (the thalamus), and prosomere 3 (the prethalamus) (Puelles et al. 2012b). Prosomere 3 consists of the reticular nucleus of the thalamus, the zona incerta, and the pregeniculate nucleus (formerly the ventral lateral geniculate). Prosomere 3 is dominated by GABAergic neurons, whereas prosomere 2 is filled mainly with glutamatergic neurons. Note that GABAergic neurons are scattered through the primate thalamus, but in rodents they are restricted to a small population in the dorsal lateral geniculate nucleus (Jones, 2007; Puelles et al. 2012b).
The rodent zona incerta receives afferents from a number of different regions of the central nervous system (Mitrofanis, 2005) and it seems likely these same connections are present in the primate brain. Within the neuronal population of the central part of the zona incerta, there is a distinct subdivision into dorsal and ventral sheets, and a number of areas are characterised by the expression of particular immunomarkers (Mitrofanis et al. 2004; Paxinos et al. 2009a; Watson & Paxinos, 2010). Up until now the pattern of markers in the caudal part of the zona incerta has not received specific attention.
The zona incerta in rats has been more extensively studied than in other species. The rat zona incerta can be divided into four major regions on the basis of cytoarchitecture (Kawana & Watanabe, 1981; Kolmac & Mitrofanis, 1999; Mitrofanis, 2005). The rat brain atlas of Paxinos & Watson (2007) identifies these four regions as a rostral part, a ventral sheet (ZIV), a dorsal sheet (ZID), and a caudal part. At the medial border of the ZIV and ZID is a small group of dopaminergic cells (A13), which belongs to prosomere 3. We believe that the A13 group should not be included in the zona incerta because the A13 group has a distinctive dopaminergic population, which is absent from the zona incerta. It should be noted that the boundaries between the different areas of the rat zona incerta in many places are not distinct in Nissl-stained sections.
The zona incerta in rodents appears to be relatively much larger than that seen in primates. In the mouse, the zona incerta occupies a space about as large as the substantia nigra (Paxinos & Franklin, 2013), whereas in primate brains its size is much less striking (Mai et al. 2008; Paxinos et al. 2009a, 2012).
This paper aims to compare the caudal zona incerta of rodents (mouse and rat) with that found in primates (marmoset, rhesus monkey, and human) in terms of published data on cytoarchitecture, anatomical relationships, the pattern of immunomarkers, and gene expression. We hope that a clearer appreciation of the anatomy of the region will in the end assist in understanding the role DBS may play in human patients with parkinsonism and tremor.
Methods
Our sources of data were the serial sections presented in major histological atlases of the mouse, rat, pygmy marmoset, rhesus, and human brains (Paxinos & Watson, 2007; Mai et al. 2008; Paxinos et al. 2009a,b2009b, 2012, 2013; Paxinos & Franklin, 2013). In the case of the rat, mouse, and the pygmy marmoset, an extensive series of sections stained with histochemical markers and immunomarkers was also available (Paxinos et al. 2009b, 2012; Watson & Paxinos, 2010).
Mouse brain
We studied Nissl and acetylcholinesterase patterns in images of coronal, sagittal, and horizontal sections from the atlas of Paxinos & Franklin (2013) (three brains). We studied images of immunohistochemistry coronal and sagittal sections showing the presence of calbindin, parvalbumin, and SMI-32 (neurofilament H non-phosphorylated protein) in the chemoarchitectonic atlas of Watson & Paxinos (2010) (two brains). We recorded staining intensity in the different parts of the zona incerta, on a four-point scale (weak, light, medium, and strong – shown in Table 1 as + to ++++). Where it was obvious, we recorded whether the staining was present in neuronal cell bodies, fibres, or the neuropil.
Table 1.
Immunohistochemical marker distribution in rat zona incerta.
| Marker | ZID | ZIV | Caudal ZI |
|---|---|---|---|
| Calbindin | Negative | Negative | Positive cells ++ |
| Calretinin | Positive cells ++ | Negative | Positive cells + |
| NADPH-d | Positive cells +++ | Negative | Positive cells + |
| Parvalbumin | Medial part positive +++ | Positive cells and neuropil ++++ | Negative or weak neuropil + |
| SMI-32 | Positive cells and fibres ++ | Positive cells and fibres ++ | Negative |
| Tyrosine hydroxylase | Negative | Negative | Negative |
| Acetylcholinesterase | Light neuropil staining ++ | Light neuropil staining ++ | Medium intensity patch – caudal pole +++ |
The intensity of immunohistochemical staining was categorised as weak (+), light (++), medium (+++) or strong (++++). Where it was obvious, we recorded whether the staining was present in neuronal cell bodies, fibres, or the neuropil.
Rat brain
We studied Nissl and acetylcholinesterase patterns in images of coronal, sagittal, and horizontal sections from the atlas of Paxinos & Watson (2007) (three brains). We studied images of immunohistochemical stains in a continuous series of coronal sections from a single brain showing the presence of calbindin, calretinin, parvalbumin, tyrosine hydroxylase, NADPH diaphorase, and SMI-32 in the chemoarchitectonic atlas of Paxinos et al. (2009b). We recorded staining intensity in the different parts of the zona incerta, on a four-point scale (weak, light, medium, and strong – shown in Table 1 as + to ++++). Where it was obvious, we recorded whether the staining was present in neuronal cell bodies, fibres, or the neuropil.
Marmoset brain
We studied Nissl, acetylcholinesterase, calbindin, and SMI-32 patterns in images of coronal sections from the brain of a single common marmoset (Callithrix jacchus) in the atlas of Paxinos et al. (2012). We also studied the pattern of immunohistochemical stains in coronal sections showing the presence of calretinin, parvalbumin, tyrosine hydroxylase, and NADPH diaphorase, in images of sections of two brains shown on the website of Professor Hironobu Tokuno of the Tokyo Metropolitan Institute of Medical Science (http://www.marmoset-brain.org). We recorded staining intensity in the different parts of the zona incerta on a four-point scale (weak, light, medium, and strong). Where it was obvious, we recorded whether the staining was present in neuronal cell bodies, fibres, or the neuropil.
Rhesus monkey brain
We studied Nissl and acetylcholinesterase patterns in images of coronal sections from the brain of a single rhesus monkey (Macaca mulatta) shown in the atlas of Paxinos et al. (2009a). We recorded staining intensity in the different parts of the zona incerta on a four-point scale (weak, light, medium, and strong). Where it was obvious, we recorded whether the staining was present in neuronal cell bodies, fibres, or the neuropil.
Human brain
We studied images of Nissl and myelin stained coronal sections from a single brain in the atlas of Mai et al. (2008). We also studied acetlycholinesterase patterns in the most rostral sections of a single brain in the human brain atlas of Paxinos et al. (2013).
Gene expression in the mouse zona incerta
To establish patterns of gene expression in different parts of the zona incerta and neighbouring structures, we searched the mouse gene expression data on the website Allen Institute for Brain Science [Website: ©2012 Allen Institute for Brain Science. Allen Mouse Brain Atlas (Internet). Available from: http://mouse.brain-map.org/]. To identify patterns of gene expression, we used the agea tool (Ng et al. 2009). The agea tool allows a specific small region of the brain (such as the caudal zona incerta) to be selected in a three-dimensional display. Once an area is selected with the agea tool, a list appears showing the patterns of expression of genes that are more or less specific to the area selected. In most cases, over 100 candidates are offered, among which five to 10 are directly relevant to the area of interest. The selection of genes is based on a search of expression of over 20 000 genes that are represented in the mouse brain atlas on the Allen Institute website. We recorded the intensity of gene expression on a four-point scale, as shown in Table 2.
Table 2.
Gene expression of 15 different genes in different parts of the mouse zona incerta and adjacent subthalamic nucleus and substantia nigra.
| Gene | Caudal ZI | ZIV | ZID | STh | SNR | SNC |
|---|---|---|---|---|---|---|
| GAD67 (GAD1) | + | ++++ | ++++ | -ve | +++ | -ve |
| Slc32a1 (GABA transporter) | + | ++++ | ++++ | -ve | +++ | -ve |
| SEBOX homeobox | ||||||
| (Og9x, OG9) | -ve | ++ | -ve | -ve | -ve | -ve |
| Pax6 | + | +++ | ++ | -ve | -ve | -ve |
| C13008E23Rik | -ve | +++ | + | +++ | -ve | |
| Rreb1 | -ve | ++ | -ve | -ve | + | + |
| Nos1ap | -ve | +++ | -ve | -ve | -ve | +++ |
| Press12 | -ve | +++ | -ve | -ve | -ve | -ve |
| St8sia6 | -ve | ++ | -ve | -ve | -ve | -ve |
| Ubash3b | ||||||
| (2810457I06Rik) | -ve | ++++ | ||||
| + | ++ | -ve | -ve | |||
| Gfra1 | -ve | ++++ | -ve | -ve | -ve | + |
| Hap1 | -ve | +++ | +++ | -ve | -ve | -ve |
| Coch | -ve | +++ | -ve | -ve | -ve | -ve |
| Pou6f2 | -ve | ++ | -ve | -ve | -ve | -ve |
| Cdh23 | + | +++ | -ve | -ve | -ve | ++ |
The intensity of immunohistochemical staining was categorised as either weak (+), light (++), medium (+++) or strong (++++).
Results
The anatomical relationships of the zona incerta in rodents and primates
The central and caudal regions of the zona incerta in the mouse
The zona incerta in the mouse extends from the rostral pole of the thalamus, level with the rostrocaudal centre of the hypothalamic paraventricular nucleus (interaural 2.98 mm) to the rostral pole of the red nucleus (interaural 0.64 mm) (for explanation of the coordinates please refer to the atlas of Paxinos & Franklin, 2013). The central portion of the zona incerta (interaural 1.86 mm) lies between the subthalamic nucleus and the nigrostriatal tract (ventrolaterally), and the ventroposterior and ventromedial thalamic nuclei (dorsally) (Fig. 1A). At this level the superior cerebellar peduncle is enclosed by the ventromedial thalamic nucleus. The caudal zona incerta (interaural 0.88 mm) is sandwiched between the basis pedunculi and the substantia nigra ventrolaterally, and parts of the posterior thalamic group and medial lemniscus dorsally (Fig. 1B). At this level, the dorsal part of the caudal zona incerta is only 0.1 mm away from the dorsalmost fibres of the basis pedunculi.
Figure 1.
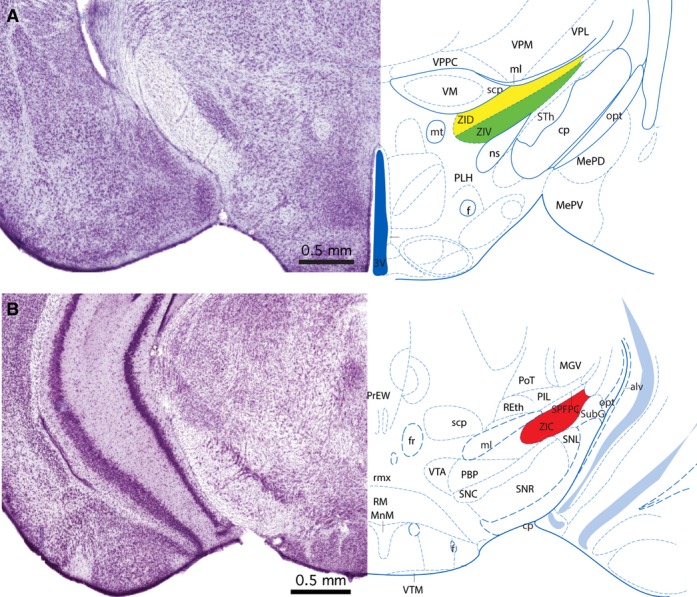
Mouse zona incerta. (A) Nissl-stained coronal section through the central part of the zona incerta in a mouse matched with a labelled diagram of the same section. The section is 1.86 mm rostral to the interaural plane. The dorsal lamina of the zona incerta (ZID) is coloured yellow, and the ventral lamina of the zona incerta (ZIV) is coloured green. (B) Nissl-stained coronal section through the caudal part of the zona incerta in a mouse matched with a labelled diagram of the same section. The section is 0.88 mm rostral to the interaural plane. The caudal zona incerta is coloured red. The image and diagram are taken from the atlas of Paxinos & Franklin (2013).
The central and caudal regions of the zona incerta in the rat
The zona incerta in the rat extends from the rostral pole of the thalamus, level with the rostrocaudal centre of the hypothalamic paraventricular nucleus (interaural 2.98 mm) to the rostral pole of the red nucleus (interaural 3.80 mm) (coordinates refer to the atlas of Paxinos & Watson, 2007). The central portion of the zona incerta (interaural 5.52 mm) lies between the basis pedunculi, subthalamic nucleus, and the nigrostriatal tract (ventrolaterally). The ventroposterior and ventromedial thalamic nuclei and the superior cerebellar peduncle are dorsal to the central zona incerta (Fig. 2A). At this level the superior cerebellar peduncle lies at the lateral edge of the ventromedial thalamic nucleus. The caudal zona incerta (interaural 4.20 mm) is sandwiched between the basis pedunculi and the substantia nigra (SNL) ventrolaterally, and parts of the posterior thalamic group, the subparafascicular parvicellular thalamic nucleus, and the medial lemniscus dorsally (Fig. 2B). The dorsolateral edge of the caudal zona incerta is continuous with the pregeniculate nucleus, which is a companion part of prosomere 3. The most caudal part of the zona incerta is strongly stained in an AChE section at interaural 3.96 mm, but this patch of staining is very localised and is not seen in interaural 4.20 mm.
Figure 2.
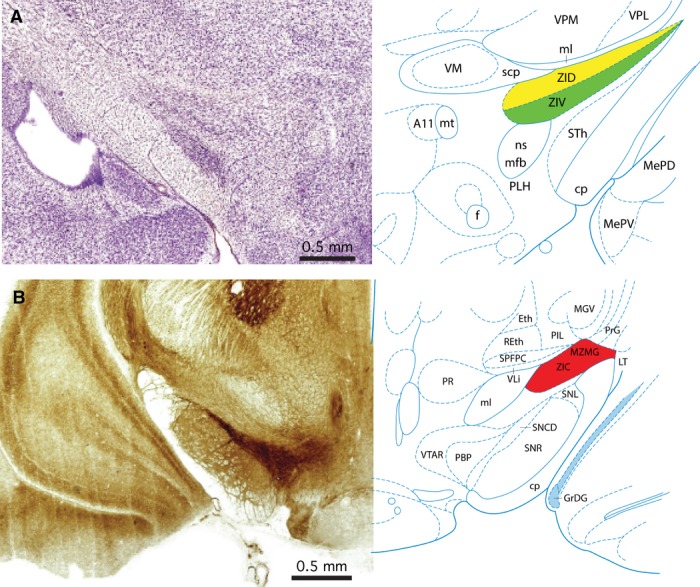
Rat zona incerta. (A) Nissl-stained coronal section through the central part of the zona incerta in a rat matched with a labelled diagram of the same section. The section is 5.52 mm rostral to the interaural plane. The dorsal lamina of the zona incerta (ZID) is coloured yellow, and the ventral lamina of the zona incerta (ZIV) is coloured green. (B) Acetylcholinesterase-stained coronal section through the caudal part of the zona incerta in a rat matched with a labelled diagram of the same section. The section is 4.20 mm rostral to the interaural plane. The caudal zona incerta is coloured red. The image and diagram are taken from the atlas of Paxinos & Watson (2007).
The central and caudal regions of the zona incerta in the marmoset
The zona incerta in the marmoset extends from the rostral pole of the thalamus, level with the rostrocaudal centre of the hypothalamic paraventricular nucleus (interaural 8.05 mm), to the caudal pole of the subgeniculate nucleus (interaural 5.00 mm) (coordinates refer to the image series on the website http://www.marmoset-brain.org and the atlas of Paxinos et al. 2012). The central portion of the zona incerta (interaural 6.50 mm) lies between the subthalamic nucleus (ventrolaterally) and the ventral anterior thalamic nucleus (dorsally). At this level the superior cerebellar peduncle lies at the medial edge of the zona incerta, and the nigrostriatal tract is ventromedial to the zona incerta. In this central region, the ventral nucleus of the zona incerta contains prominent calbindin-positive cells (interaural 6.30 mm) but there are few of these cells in the dorsal part. On the other hand, the dorsal part is more intensely stained with antibodies to SMI-32 than the ventral tier (interaural 6.05 mm). The caudal zona incerta (interaural 5.15 mm) is sandwiched between the substantia nigra ventrolaterally and the ventroposterior thalamic nucleus and the medial lemniscus dorsally. The caudal part of the caudal zona incerta is strongly AChE-positive, and is separated from the substantia nigra by the AChE-negative peripeduncular area (interaural 5.15 mm).
The central and caudal regions of the zona incerta in the rhesus monkey
The zona incerta in the rhesus monkey extends from the rostral pole of the thalamus, just caudal to the hypothalamic paraventricular nucleus (interaural 13.35 mm), to the rostral pole of the subgeniculate nucleus (interaural 7.05 mm) (coordinates refer to the atlas of Paxinos et al. 2009a). The central portion of the zona incerta (interaural 10.20 mm) lies between the subthalamic nucleus (ventrolaterally) and the ventral lateral thalamic nucleus (dorsally) (Fig. 3A). At this level, the superior cerebellar peduncle lies at the medial edge of the zona incerta. The caudal zona incerta (interaural 7.95 mm) is sandwiched between the substantia nigra and the basis peduncli ventrolaterally and the ventroposterior thalamic nucleus and the medial lemniscus dorsally (Fig. 3B). As in the marmoset, the caudal part of the caudal zona incerta is strongly AChE-positive (7.95 mm).
Figure 3.
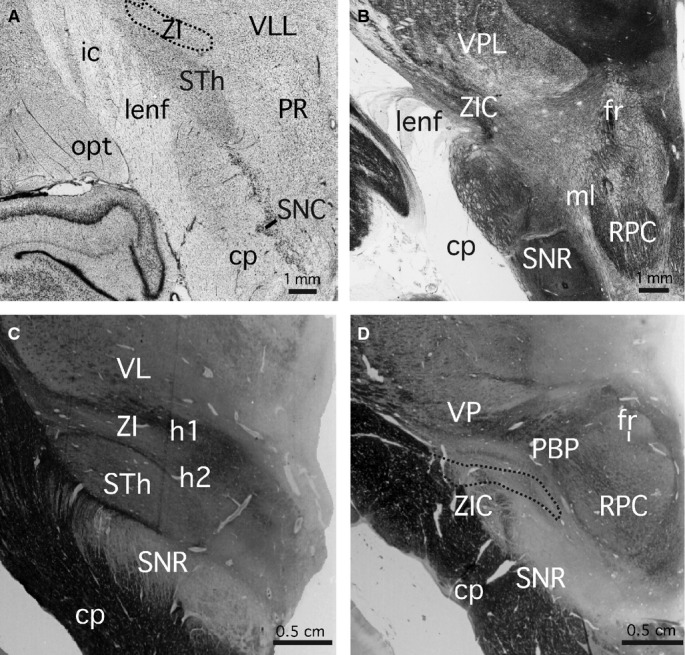
Rhesus monkey and human zona incerta. (A) Nissl-stained coronal section through the central part of the zona incerta in a rhesus monkey. The section is 10.20 mm rostral to the interaural plane. The central part of the zona incerta (ZI) is outlined. (B) Acetylcholinesterase-stained coronal section through the caudal part of the zona incerta in a rhesus monkey. The section is 7.95 mm rostral to the interaural plane. The images in (A) and (B) are taken from the atlas of Paxinos et al. (2009b). (C) Myelin-stained coronal section through the central part of the zona incerta in a human. The section is 16.0 mm rostral to the interaural plane. (D) Myelin-stained coronal section through the caudal part of the zona incerta in a human. The section is 26.5 mm rostral to the interaural plane. The caudal zona incerta (ZIC) is outlined. The images in (C) and (D) are taken from the atlas of Mai et al. (2008).
The central and caudal regions of the zona incerta in the human
The zona incerta in the human extends from the rostral pole of the thalamus, level with the caudal pole of the hypothalamic paraventricular nucleus (interaural 5.4 mm), to the rostral pole of the medial geniculate nucleus (interaural 26.5 mm) (Mai et al. 2008; Paxinos et al. 2013) (coordinates refer to the atlas of Mai et al. 2008). The central portion of the zona incerta (interaural 16.0 mm) (which neurosurgeons term the dorsal zona incerta (Plaha et al. 2006), lies between the subthalamic nucleus (ventrolaterally) and the ventrolateral and ventromedial thalamic nuclei (dorsally) (Fig. 3C). At this level the superior cerebellar peduncle lies at the medial edge of the zona incerta. The caudal zona incerta (interaural 23.9 mm) is sandwiched between the basis pedunculi and the substantia nigra (SNL) ventrolaterally, the h1 fibre tract and ventroposterior thalamic nucleus dorsally, the subthalamic nucleus anteriorly, and the lateral and medial lemnisci posteriorly (Fig. 3D). The caudal zona incerta region targeted by neurosurgeons ranges from interaural 19.9 mm and 23.9 mm with a recent trend to the more anterior part of this range (S. Gill, personal communication 2007). The most caudal part of the caudal zona incerta (26.5 mm), which is not targeted by neurosurgeons, is separated from the substantia nigra by the peripeducular area.
The chemoarchitecture of the caudal zona incerta in the rat
Our data on the details of marker expression in the caudal zona incerta are drawn from the chemoarchitectonic atlas of a single rat brain (Paxinos et al. 2009b). The section series studied contained serial rotations of the following sequence of markers – calbindin, calretinin, parvalbumin, NADPH diaphorase, SMI-32, tyrosine hydroxylase, acetlycholinesterase, and Nissl. The acetylcholinesterase sections from this series are not shown in this atlas, but this disadvantage can be overcome partly by referring to the one in four acetylcholinesterase series in the rat brain atlas of Paxinos & Watson (2007).
An examination of the marker-stained sections in the rat shows a clear difference between the caudal zona incerta and the more rostral regions, where the zona incerta is divided into dorsal and ventral layers (ZID and ZIV) (Fig. 4). The caudal zona incerta contains a prominent population of calbindin-positive cells (Plate #237; Fig. 4D in this paper), but such cells are rare in ZIV and ZID (Plates #195, 202, 223; Fig. 4C in this paper), in sharp contrast to the major calbindin population in the nearby VM. All parts of the caudal zona incerta are sprinkled with callretinin-positive (#238) and NADPH diaphorase-positive cells (#235), whereas such cells are restricted to the dorsal tier of the central ZI (#214, 217). The caudal zona incerta shows negligible parvalbumin staining (#236) compared with the darkly stained ZIV (#222, 229; Fig. 4B), which is filled with parvalbumin-stained cells and neuropil (as is the adjacent reticular nucleus of the thalamus). The medial part of the ZID is also marked by parvalbumin-stained cells and neuropil, but the lateral part is not stained (#222). ZID and ZIV are both prominently stained with SMI-32, both cells and fibres (#219), but the caudal zona incerta is largely negative (#233). The ZID in the central region of the zona incerta contains a prominent group of neurons positive for NADPH diaphorase (Fig. 4A). The ZID, ZIV, and caudal zona incerta are all negative for tyrosine hydroxylase (#213, 234), except where ZID and ZIV are traversed by TH fibres that appear to arise in the adjacent nigrostriatal tract (#206). The dopamine A13 group (DA13), which borders the medial edge of ZIV, is of course strongly positive for TH but, as noted above, we do not classify this group as part of the zona incerta.
Figure 4.
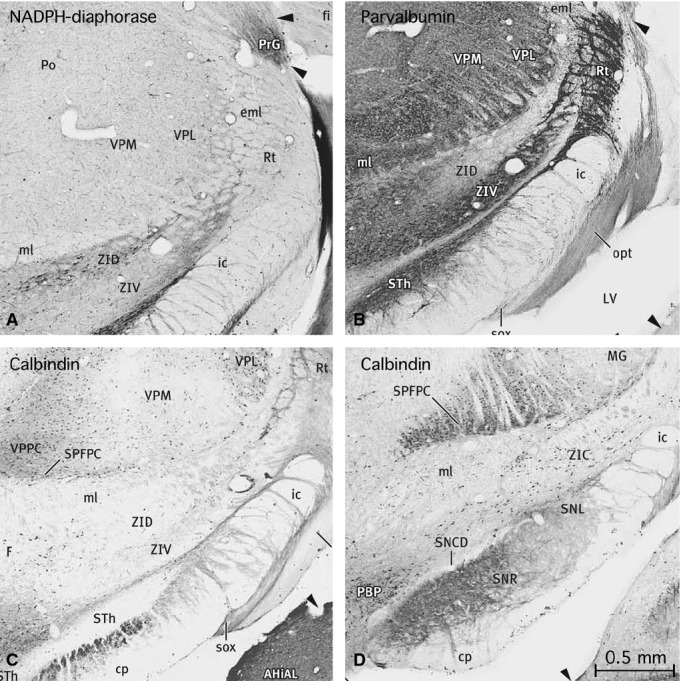
Four images taken from a chemoarchitectonic atlas of the rat brain (Paxinos et al. 2009b). These four images show the distribution of selected markers in the central and caudal parts of the zona incerta. (A) An image taken from Figure 221 of the atlas showing the distribution of NADPH diaphorase in the central zona incerta. Note the population of the dorsal part (ZID). (B) Image taken from Figure 222 of the atlas showing the distribution of parvalbumin in the central zona incerta. Note the heavy labelling in the ventral tier (ZIV), and in the medial part of the dorsal tier (ZID). (C) Image taken from Figure 223 of the atlas showing the absence of calbindin in the dorsal and ventral tiers of the zona incerta at this level. (D) Image taken from Figure 237 of the atlas showing the distribution of calbindin in the caudal zona incerta. Note the distinctive population of calbindin-labelled cells in this area.
We do not have access to a similar comprehensive series of markers for the mouse, but examination of the mouse chemoarchitectonic atlas of Watson & Paxinos (2010) shows that the pattern of distribution of calbindin (#177, 182, 187) and parvalbumin (#181, 186) is essentially the same as in the rat. In acetylcholinesterase sections in the mouse brain altas of Paxinos & Franklin (2013), a small patch of positivity at the caudal pole can be distinguished, although it is not as prominent as in the rat.
In summary, the caudal zona incerta in rodents displays a characteristic pattern of histochemical and immunohistochemical markers, and this pattern distinguishes it from the central parts of the zona incerta (Table 1). These features are:
a sparse but distinct population of calbindin-positive cells
negative staining in SMI-32 and parvalbumin preparations
the presence of a small patch of medium intensity AChE neuropil staining.
Chemoarchitecture of the caudal zona incerta in the marmoset
In the marmoset atlas of Paxinos et al. (2012) the caudal zona incerta shows a prominent patch of AChE neuropil staining (#99a) and contains a sparse population of calbindin-positive cells (#98a), which are absent from more rostral sections (#90a). This pattern is broadly consistent with that observed in the mouse and rat.
Selective gene expression in the caudal zona incerta in the mouse
Gene expression in the caudal zona incerta in the mouse was studied with reference to the mouse brain gene expression data on the website of the Allen Brain Institute. Using the agea tool, we searched for genes that were expressed in the zona incerta and not in neighbouring structures such as the thalamus and subthalamic nucleus. Our search was by no means exhaustive but we did examine sections showing the expression of over 200 genes. Table 2 summarises the expression of 15 different genes in different parts of the zona incerta and adjacent subthalamic nucleus and substantia nigra. Overall, we found many genes to be strongly expressed in ZIV, whereas only a few were expressed in ZID. The most significant result seen in relation to caudal zona incerta was that this area shows only sparse expression of GABA genes (GAD67 and Slc32a1) (Fig. 5) and light expression of Pax6. Considering that the GABA genes are intensely expressed in all of the other major components of prosomere 3 (ZID, ZIV, reticular nucleus of thalamus, pregeniculate nucleus), the relative lack of GABA neurons in caudal ZI may be significant.
Figure 5.
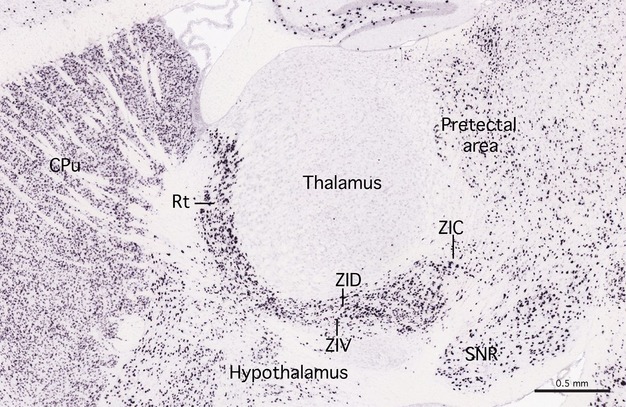
Expression of the GABA gene in mouse brain. A sagittal section of the brain of a C57BL mouse showing the expression of the GABA gene GAD67. The sagittal section is approximately 1.5 mm from the midline. The GABA gene is heavily expressed in the reticular nucleus of the prethalamus (Rt) and the dorsal and ventral laminae of the zona incerta (ZID and ZIV). There is only light expression in the caudal zona incerta. Note that GABA gene expression is virtually absent in the thalamus but that there is moderate expression in the pretectal area. The image is courtesy of the Allen Brain Institute [Website: ©2012 Allen Institute for Brain Science. Allen Mouse Brain Atlas (Internet). Available from: http://mouse.brain-map.org/].
Discussion
General characteristics of the zona incerta
The zona incerta contains a wide variety of neurotransmitters. Apart from the adjacent dopaminergic neurons in A13, the zona incerta contains neurons with GABA, somatostatin, angiotensin II, and melanocyte-stimulating hormone (Mitrofanis et al. 2004), glutamate (Heise & Mitrofanis, 2004), and melanocyte-concentrating hormone (Bittencourt et al. 1992). Among these transmitters, GABA is by far the best represented. All of the components of prosomere 3 (reticular nucleus, pregeniculate nucleus, subgeniculate nucleus, and zona incerta) are packed with cells that express the GABA gene GAD67 (GAD1) and the GABA solute carrier gene Slc32a1.
The zona incerta is closely associated with major tracts involved in motor control (such as the lenticular fasciculus, the superior cerebellar peduncle) and is also located very close to a number of nuclear areas known to play an important role in the control of automatic or stereotyped movement. The central part of the zona incerta lies dorsal to the subthalamic nucleus, and its caudal region is close to the rostral substantia nigra. Neurosurgeons consider the caudal zona incerta to comprise all parts of the nucleus posterior to the subthalamic nucleus. And they consider the dorsal zona incerta to comprise all the nucleus located between the subthalamic nucleus and the thalamus. In primates, the caudal tip of the zona incerta lies close to the lateral margin of the red nucleus. Dorsomedial to the zona incerta is the ventral medial nucleus of the thalamus; the ventral lateral nucleus of the thalamus lies dorsolaterally. The latter nucleus is relevant for neurosurgeons using trajectories which attempt to avoid the lateral ventricles, The zona incerta therefore lies at the crossroads of centres that are important in motor control. These centres belong to the hypothalamus (the subthalamic nucleus), the diencephalon (the ventromedial nucleus of the thalamus), and the midbrain (substantia nigra). The zona incerta also lies at the centre of other brain structures at which DBS is known to modify tremor, akinesia, bradykinesia, and rigidity in movement disorders to various extents; these include the prelemniscal radiation medially (Murata et al. 2003; Carrillo-Ruiz et al. 2007), the subthalamic nucleus anteriorly, and the ventral intermediate part of the ventral posterior lateral thalamic nucleus.
In some publications dealing with human DBS, the term ‘posterior subthalamic area‘ (PSA) has been used to describe an area that contains the zona incerta. The term is used to acknowledge the potential current spread into adjacent structures that may partially or totally explain therapeutic effects (see Blomstedt et al. 2009a). Defined in this way, the PSA is characterised as an area lying inferior to the thalamus, lateral to the red nucleus, and posteromedial to the subthalamic nucleus (Blomstedt et al. 2009b). This, in fact, corresponds with Steven Gill's description of the human caudal zona incerta, but PSA is a more inclusive and anatomically accurate term for this purpose. The published PSA DBS target of Gill (Plaha et al. 2006) is superiorly located in the caudal zona incerta. Since 2007, however, that group has shifted their electrode about 1 to 2 mm more anteriorly, closer to the junction of the posteromedial superior border of the subthalamic nucleus and the caudal zona incerta because of late side effects from stimulating at the original target (S. Gill pers. commun., 2007–2013; Fig. 6). Detailed target analysis in the PSA is made possible by using the MRI-directed guide tube technique that enables accurate intraoperative imaging of eventual electrode location (Patel et al. 2007; Thani et al. 2011, 2012). Other variations of PSA targeting are difficult to verify anatomically with precision because of technical disadvantages in standard computed tomographic and magnetic resonance imaging of actual brain electrodes as compared with guide tube stylettes (Thani et al. 2011).
Figure 6.
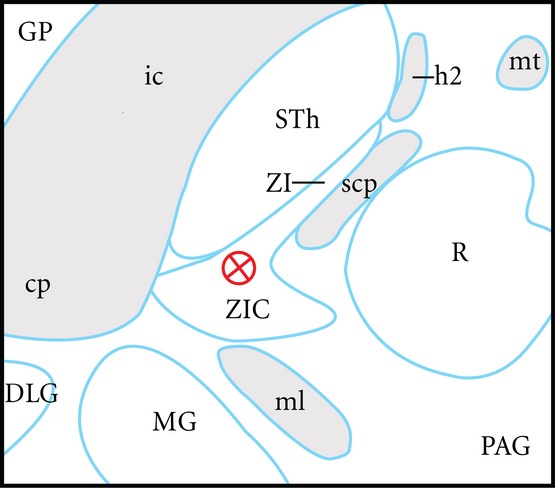
Scale diagram of an example of a modern clinical posterior subthalamic area deep brain stimulation (DBS) target 3.5 mm ventral to the modified axial commissural plane of Schaltenbrand & Wahren (1977). This plane is favoured by neurosurgeons for DBS planning. The target shown (a red circle and cross) represents an attempt to optimise neuromodulation of the caudal zona incerta (ZIC) and posterodorsal subthalamic nucleus (STh). In this position, the effects of proximity to the internal capsule (ic), medial lemniscus (ML) and the ventromedial subthalamic nucleus are minimised. The result is a reduction in clinical side effects such as skeletal muscle contraction, dysaesthesiae, and limbic effects, respectively. The combination of surgical targeting error and the relatively symmetrical current spread from the electrode is a source of side effects. Moreover, these factors create uncertainty as to which brain structures underlie positive clinical effects in parkinsonism and tremor. This composite diagram has been adapted from drawings of human brain horizontal sections as shown in diagram LXVIII (−3.5) of Schaltenbrand & Wahren (1977) and horizontal sections V2.7 and V3.6 of Morel (2007). In this diagram, the midline is to the left, and rostral is at the top. Other structures shown in this diagram are the central part of the zona incerta (ZI), red nucleus (R), medial geniculate (MG), cerebral peduncle, dorsal lateral geniculate nucleus (DLG), superior cerebellar peduncle (scp), lenticular fasciculus (h2), mammilothalamic tract (mt), globus pallidus (GP), and periaqueductal grey (PAG).
Although the term PSA ignores clearly defined features of the prosomeric organisation of the forebrain (Puelles et al. 2013), its clinical utility is the recognition that DBS is limited to non-specific volumetric modulation of the brain that must include all structures that lie within any several millimetre radius of an electrode despite the lower excitability threshold of myelinated axons (Butson & McIntyre, 2005; Maks et al. 2009). Our clinical endorsement of the term PSA should not be taken to imply that there is some kind of organisational continuity between the zona incerta and the subthalamic nucleus, when in fact the two are developmentally distinct, being derived from prosomere 3 and the hypothalamus, respectively. In particular, it should be noted that the subthalamic nucleus was formerly considered to be a separate part of the human diencephalon, at the same level of status as the hypothalamus and the thalamus, but developmental gene expression shows this to be incorrect (Puelles et al. 2012a). Now that the hypothalamus is recognised as rostral to and distinct from the true diencephalon, it is clear that the subthalamic nucleus is part of the hypothalamus, and not part of the diencephalon (Puelles et al. 2012b). Thus although PSA is a more accurate term to describe the DBS intervention centred on the caudal zona incerta, or even its border with the subthalamic nucleus, precise specification of the caudal zona incerta is important for mechanistic advances which may in the future be exploited by new technologies such as the use of stereotactically targeted neurotransmitter-specific infusions (Lam et al. 2011).
Characteristics of the caudal zona incerta
We found that the caudal zona incerta has a number of histological properties that distinguish it from the main body of the zona incerta; it has immunohistochemical and gene expression patterns that distinguish it from the main dorsal and ventral subdivisions of the main body of the zona incerta. Although this may be consistent with the preferential selection of this small area of the zona incerta for DBS, it unfortunately does not tell us why the caudal zona incerta is so responsive to DBS. It is possible that the responsiveness of the caudal zona incerta to DBS is due to stimulation of neuronal populations in the caudal zona incerta or close to the caudal zona incerta, but it is also possible that the effect depends on stimulation of major fibre bundles that traverse this area (Fig. 6). The two large fibre bundles within a 2–3 mm radius of neuromodulation are the h1 field (thalamic fasciculus, which is continuous with the medial lemniscus) and the h2 field (lenticular fasciculus). This volume corresponds to common clinical DBS settings in the vicinity of the caudal zona incerta, and the h1 and h2 fields may subserve positive clinical effects. The other major tracts that are demonstrably sources of side effects during intra-and peri-operative testing include the corticospinal component of the basis pedunculi/internal capsule, and the medial and lateral lemnisci. Because of the somewhat dorsal location of the PSA target in the caudal zona incerta, the area that separates the zona incerta from the substantia nigra, but which is not labelled in the atlases of the mouse (Paxinos & Franklin, 2013) and rat (Paxinos & Watson, 2007), is unlikely to be of clinical significance. In the human brain atlas of Mai et al. (2008) this area is labelled the peripeduncular area. Interestingly, part of this structure may have been targeted in error by neurosurgeons attempting to implant the pedunculopontine nucleus for l-dopa-resistant gait freezing in Parkinson's disease, with some apparent success in this condition (Mazzone et al. 2005, 2007; Zrinzo et al. 2007). It seems likely that this cell-sparse area is occupied by fibre bundles of some type, but we cannot at this stage identify them.
In summary, the potential significance of the caudal zona incerta in relation to DBS may relate to one or more of the following factors:
it is close to the posterodorsal pole (possibly the sensorimotor area noted by Mallet et al. 2007) of the subthalamic nucleus
it is adjacent to the cerebello-rubro-thalamic (prelemniscal) pathway, which is targeted by some neurosurgeons (Velasco et al. 2001; Murata et al. 2003)
it is adjacent to the pallidothalamic fibre tracts, which have themselves been suggested as targets (Gallay et al. 2008)
it is adjacent to the ventral posterior lateral thalamic nuclei
it does not contain a significant population of GABA neurons, whereas GABA neurons are plentiful in the main body of the zona incerta (based on rodent data)
it contains a distinct patch of acetylcholinesterase activity in the caudal pole
it contains a distinct population of calbindin neurons (based on rodent data).
In a study of neurosecretory neurons of the hypothalamic supraoptic nucleus, Li et al. (1995) reported that calbindin acts as an endogenous Ca2+ buffer, and this allows it to play a role in the regulation of intrinsic firing patterns. Supraoptic neurons that release vasopressin fire phasically, whereas those that release calbindin fire continuously. Those authors suggest that calbindin may determine the selection of one firing pattern over another in particular neurons. If the calbindin population in the caudal zona incerta has a similar role, it may be involved in firing patterns that control specific motor outputs.
Gene expression in the caudal zona incerta
The most notable finding in our studies of gene expression is the very low level of the GABA gene GAD67 in the caudal zona incerta. The remainder of the zona incerta is packed with GAD67-expressing neurons, consistent with the GABAergic functions associated with most of the remainder of the prethalamus. It seems reasonable to postulate that the caudal pole of the zona incerta is sharply different in function to the remainder of the zona incerta. The GABA population of the main part of the zona incerta (along with the rest of prosomere 3) has been found to exert a powerful inhibitory control over the thalamus during sleep (Llinas & Jahnsen, 1982).
Of the remaining genes in which we identified relatively specific expression in the zona incerta, most were concentrated in the ventral tier of the zona incerta. Apart from a low level of expression of GABA genes in caudal zona incerta, the genes that we found to be prominently expressed in the ventral tier of the zona incerta were not significantly expressed in the caudal zona incerta. It seems that the caudal zona incerta does represent an unusual part of the prethalamus, having little in common with the neuron groups in the main part of the zona incerta.
The beta oscillation theory of Parkinson's disease and the caudal zona incerta
Bradykinesia in Parkinson's disease is associated with enhanced beta oscillations in the forebrain, and a decrease in the beta frequency oscillations in the subthalamic nucleus and cortex has been shown to correlate with a reduction in bradykinesia (Brown, 2007). However, McCarthy et al. (2011) argue that beta oscillations of parkinsonism may primarily arise in the striatum, and not in the cortex or subthalamic systems. Their study was based on mathematical modelling of the activity of striatal medium spiny neurons, supported by striatal carbachol injection experiments. Previous to this study, the circuits considered most likely were the subthalamic nucleus/external globus pallidus circuit (Plenz & Kital, 1999; Bevan et al. 2002) and the cortex/subthalamic ‘patterning' circuit (Magill et al. 2001). In support of the ‘striatal' hypothesis of McCarthy et al. (2011) is the demonstration by Costa et al. (2006) and Kreitzer (2009) that removal of the dopamine input to the striatum increases the excitability of dopamine receptors. This is consistent with the idea that beta oscillations arise from abnormalities of activity in corticostriatal circuits rather than primary variations in cortical activity. The ‘striatal' hypothesis fits well with the core finding of loss of striatal dopamine input from the substantia nigra in Parkinson's disease. These studies also support the increasingly popular idea that suppression of beta oscillation may be the mechanism of DBS in Parkinson's disease.
The importance of beta oscillations in DBS in the subthalamic region has recently been challenged. The first and obvious argument against it is that beta oscillations are not evident in essential tremor, despite the fact that the same PSA, thalamic and subthalamic nucleus targets that can improve Parkinson's disease also improve essential tremor. In the context of parkinsonism, Li et al. (2012) showed that beta wave activity can be suppressed by antidromic modification of the corticofugal projection to the subthalamic nucleus. This does not directly dismiss the possibility of an underlying striatal origin for the beta oscillations but it does argue against a primary role for the neurons of the subthalamic nucleus. An even more fundamental challenge to the beta-oscillation theory of Parkinson's disease was provided by interventions using optogenetics. In these experiments, laser-induced beta oscillations in subthalamic neurons were not sufficient to produce Parkinsonian signs in a transgenic mouse model and the mechanism of DBS-like high-frequency electrical stimulation of the subthalamic nucleus was retrograde modulation of subthalamic neuron afferents (Gradinaru et al. 2009). Optogenetic studies have not yet been published for the caudal zona incerta neurons or their afferents and this technology cannot be used to study primates at this stage. Like the subthalamic nucleus, the zona incerta has extensive connections throughout the brain, leading to the hypothesis that it may have a fundamental regulatory function (Power & Mitrofanis, 1999). Although beta oscillations are present in Parkinson's disease, they appear unlikely to be the underlying cause of symptoms. The extensive connectivity of both the subthalamic nucleus and possibly the zona incerta may yet underpin the comprehensive motor therapeutic effects on rigidity, akinesia, bradykinesia, and tremor when DBS is used in these targets.
In terms of the anatomy of the caudal zona incerta in relation to DBS, current concepts and recent studies have not ruled out the possibility that therapeutic effects may be due to one or more of (i) orthodromic efferent nuclear neuron axonal, (ii) antidromic afferent nuclear neuron axonal, or (iii) ortho-or anti-dromic adjacent tract axonal electrical modulation. In this respect, the usefulness of the caudal zona incerta as a target for DBS may indeed be related to either its connectivity with many brain areas or its proximity to major fibre pathways, rather than the histological characteristics we have demonstrated in this paper.
Concluding remarks
The caudal zona incerta has clinical promise as an effective site for DBS in Parkinson's disease. We have searched for characteristics which distinguish this region from the remainder of the zona incerta. We found that the caudal zona incerta contains only a sparse population of GABA neurons, whereas GABA neurons fill the main body of the zona incerta. We found that the caudal zona incerta contains a distinct patch of acetylcholinesterase activity and a small but distinct population of calbindin neurons. The caudal pole therefore appears to constitute a distinct part of the zona incerta. However, it is by no means clear whether these cytological characteristics are specifically related to the responsiveness of this region to DBS, or whether DBS acts by modulating the adjacent pathways.
Acknowledgments
M.G.T. was funded by Edith Cowan University's Office of Research and Innovation, the Faculty of Computing, Health and Science, and the Vario Health Institution. This work was undertaken with support from the Parkinson's Association WA and the Young Onset Group. We thank Miss Tiza Chipungu for her efforts in exploratory work that led to the formulation of the present project.
References
- Bevan MD, Magill PJ, Terman D, et al. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, et al. The melanin-concentrating hormone system of the rat brain: an immuno-and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Blomstedt P, Sandvik U, Fytagoridis A, et al. The posterior subthalamic area in the treatment of movement disorders: past, present, and future. Neurosurgery. 2009a;64:1029–1038. doi: 10.1227/01.NEU.0000345643.69486.BC. [DOI] [PubMed] [Google Scholar]
- Blomstedt P, Fytagoridis A, Tisch S. Deep brain stimulation of the posterior subthalamic area in the treatment of tremor. Acta Neurochir. 2009b;151:31–36. doi: 10.1007/s00701-008-0163-7. [DOI] [PubMed] [Google Scholar]
- Blomstedt P, Fytagoridis A, Astrom M. Unilateral caudal zona incerta deep brain stimulation for Parkinsonian tremor. Parkinsonism Relat Disord. 2012;18:1062–1066. doi: 10.1016/j.parkreldis.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol. 2007;17:656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Burton H, Jones EG. The posterior thalamic region and its cortical projection in new world and old world monkeys. J Comp Neurol. 1976;168:249–302. doi: 10.1002/cne.901680204. [DOI] [PubMed] [Google Scholar]
- Butson CR, McIntyre CC. Tissue and electrode capacitance reduce neural activation volumes during deep brain stimulation. Clin Neurophysiol. 2005;116:2490–2500. doi: 10.1016/j.clinph.2005.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Ruiz JD, Velasco F, Jimenez F, et al. Neuromodulation of prelemniscal radiations in the treatment of Parkinson's disease. Acta Neurochirurg. 2007;97:185–190. doi: 10.1007/978-3-211-33081-4_20. Supp. [DOI] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, et al. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [Erratum appears in N Engl J Med. 2006: 355, 1289] [DOI] [PubMed] [Google Scholar]
- Deuschl G, Raethjen J, Hellriegel H, et al. Treatment of patients with essential tremor. Lancet Neurol. 2011;10:148–161. doi: 10.1016/S1474-4422(10)70322-7. [DOI] [PubMed] [Google Scholar]
- Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. 2010;362:2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- Fytagoridis A, Sandvik U, Astrom M, et al. Long term follow-up of deep brain stimulation of the caudal zona incerta for essential tremor. J Neurol Neurosurg Psychiatry. 2012;83:258–262. doi: 10.1136/jnnp-2011-300765. [DOI] [PubMed] [Google Scholar]
- Gallay MN, Jeanmonod D, Liu J, et al. Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct. 2008;212:443–463. doi: 10.1007/s00429-007-0170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, et al. Optical deconstruction of Parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise CE, Mitrofanis J. Evidence for a glutamatergic projection from the zona incerta to the basal ganglia of rats. J Comp Neurol. 2004;468:482–495. doi: 10.1002/cne.10971. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. 2nd edn. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Kawana E, Watanabe K. A cytoarchitectonic study of zona incerta in the rat. J Hirnforsch. 1981;22:535–541. [PubMed] [Google Scholar]
- Kolmac C, Mitrofanis J. Distribution of various neurochemicals within the zona incerta: an immunocytochemical and histochemical study. Anat Embryol. 1999;199:265–280. doi: 10.1007/s004290050227. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Lam MF, Thomas MG, Lind CR. Neurosurgical convection-enhanced delivery of treatments for Parkinson's disease. J Clin Neurosci. 2011;18:1163–1167. doi: 10.1016/j.jocn.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Li Z, Decavel C, Hatton GI. Calbindin-D28k: role in determining intrinsically generated firing patterns in rat supraoptic neurons. J Physiol. 1995;488:601–608. doi: 10.1113/jphysiol.1995.sp020993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ke Y, Chan DC, et al. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron. 2012;76:1030–1041. doi: 10.1016/j.neuron.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurons in-vitro. Nature. 1982;297:406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. 2001;106:313–330. doi: 10.1016/s0306-4522(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Mai J, Paxinos G, Voss T. Atlas of the Human Brain. 3rd edn. Amsterdam: Elsevier; 2008. [Google Scholar]
- Maks CB, Butson CR, Walter BL, et al. Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. J Neurol Neurosurg Psychiatry. 2009;80:659–666. doi: 10.1136/jnnp.2007.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L, Schupbach M, N'Diaye K, et al. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci U S A. 2007;104:10661–10666. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone P, Lozano A, Stanzione P, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. NeuroReport. 2005;16:1877–1881. doi: 10.1097/01.wnr.0000187629.38010.12. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Insola A, Lozano A, et al. Peripeduncular and pedunculopontine nuclei: a dispute on a clinically relevant target. NeuroReport. 2007;18:1407–1408. doi: 10.1097/WNR.0b013e3282638614. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Moore-Kochlacs C, Gub X, et al. Striatal origin of the pathologic beta oscillations in Parkinson's disease. Proc Natl Acad Sci U S A. 2011;108:11620–11625. doi: 10.1073/pnas.1107748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrofanis J. Some certainty for the ‘zone of uncertainty'? Exploring the function of the zona incerta. Neuroscience. 2005;130:1–15. doi: 10.1016/j.neuroscience.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J, Ashkan K, Wallace A, et al. Chemoarchitectonic heterogeneities in the primate zona incerta: clinical and functional implications. J Neurocytol. 2004;33:429–440. doi: 10.1023/B:NEUR.0000046573.28081.dd. [DOI] [PubMed] [Google Scholar]
- Morel A. Stereotactic Atlas of the Human Thalamus and Basal Ganglia. New York: Informa Health Care; 2007. [Google Scholar]
- Murata J, Kitagawa M, Uesugi H, et al. Electrical stimulation of the posterior subthalamic area for the treatment of intractable proximal tremor. J Neurosurg. 2003;99:708–715. doi: 10.3171/jns.2003.99.4.0708. [DOI] [PubMed] [Google Scholar]
- Ng L, Bernard A, Lau C, et al. An anatomic gene expression atlas of the adult mouse brain. Nat Neurosci. 2009;12:356–362. doi: 10.1038/nn.2281. [DOI] [PubMed] [Google Scholar]
- Patel NK, Plaha P, Gill SS. Magnetic resonance imaging-directed method for functional neurosurgery using implantable guide tubes. Neurosurgery. 2007;61(Suppl):358–366. doi: 10.1227/01.neu.0000303994.89773.01. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 4th edn. San Diego: Elsevier Academic Press; 2013. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th edn. San Diego: Elsevier Academic Press; 2007. [Google Scholar]
- Paxinos G, Huang X-F, Petrides M, et al. The Rhesus Monkey Brain in Stereotaxic Coordinates. 2nd edn. San Diego: Elsevier Academic Press; 2009a. [Google Scholar]
- Paxinos G, Watson C, Carrive P, et al. Chemoarchitectonic Atlas of the Rat Brain. 2nd edn. San Diego: Elsevier Academic Press; 2009b. [Google Scholar]
- Paxinos G, Watson C, Petrides M, et al. The Marmoset Brain in Stereotaxic Coordinates. San Diego: Elsevier Academic Press; 2012. [Google Scholar]
- Paxinos G, Watson C, Sengul G. Organization of brainstem nuclei. In: Mai JK, Paxinos G, editors. The Human Nervous System. 3rd edn. San Diego: Elsevier Academic Press; 2013. pp. 260–327. [Google Scholar]
- Plaha P, Ben-Shlomo Y, Patel NK, et al. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral Parkinsonism. Brain. 2006;129:1732–1747. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- Plaha P, Khan S, Gill SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. 2008;79:504–513. doi: 10.1136/jnnp.2006.112334. [DOI] [PubMed] [Google Scholar]
- Plaha P, Javed S, Agombar D, et al. Bilateral caudal zona incerta nucleus stimulation for essential tremor: outcome and quality of life. J Neurol Neurosurg Psychiatry. 2011;82:899–904. doi: 10.1136/jnnp.2010.222992. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kital ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- Power BD, Mitrofanis J. Evidence for extensive inter-connections within the zona incerta in rats. Neurosci Lett. 1999;267:9–12. doi: 10.1016/s0304-3940(99)00313-4. [DOI] [PubMed] [Google Scholar]
- Puelles L, Martinez-de-la-Torre M, Bardet S. Hypothalamus. In: Watson C, Paxinos G, Puelles L, et al., editors. The Mouse Nervous System. San Diego: Elsevier Academic Press; 2012a. pp. 221–312. (Chapter 10) [Google Scholar]
- Puelles L, Martinez-de-la-Torre M, Ferran JL. Diencephalon. In: Watson C, Paxinos G, Puelles L, et al., editors. The Mouse Nervous System. San Diego: Elsevier Academic Press; 2012b. pp. 313–336. (Chapter 9) [Google Scholar]
- Puelles L, Paxinos G, Harrison M, et al. A developmental ontology for the mammalian brain using the prosomere model. Trends Neurosci. 2013;36:570–578. doi: 10.1016/j.tins.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Schaltenbrand G, Wahren W. Atlas for Stereotaxy of the Human Brain. 2nd edn. Stuttgart: Thieme; 1977. [Google Scholar]
- Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013;368:610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- Thani NB, Bala A, Swann GB, et al. Accuracy of postoperative computed tomography and magnetic resonance image fusion for assessing deep brain stimulation electrodes. Neurosurgery. 2011;69:207–214. doi: 10.1227/NEU.0b013e318218c7ae. [DOI] [PubMed] [Google Scholar]
- Thani NB, Bala A, Lind CR. Accuracy of magnetic resonance imaging-directed frame-based stereotaxis. Neurosurgery. 2012;70:114–123. doi: 10.1227/NEU.0b013e3182320bd6. [DOI] [PubMed] [Google Scholar]
- Velasco F, Jimenez F, Perez ML, et al. Electrical stimulation of the prelemniscal radiation in the treatment of Parkinson's disease: an old target revised with new techniques. Neurosurgery. 2001;49:293–306. doi: 10.1097/00006123-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Watson C, Paxinos G. Chemoarchitectonic Atlas of the Mouse Brain. San Diego: Elsevier Academic Press; 2010. [Google Scholar]
- Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. J Am Med Assoc. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrinzo L, Zrinzo LV, Hariz M. The pedunculopontine and peripeduncular nuclei: a tale of two structures. Brain. 2007;130:e73. doi: 10.1093/brain/awm079. [DOI] [PubMed] [Google Scholar]


