Abstract
The axial musculoskeletal system is important for the static and dynamic control of the body during both locomotor and non-locomotor behaviour. As a consequence, major evolutionary changes in the positional habits of a species are reflected by morpho-functional adaptations of the axial system. Because of the remarkable phenotypic plasticity of muscle tissue, a close relationship exists between muscle morphology and function. One way to explore major evolutionary transitions in muscle function is therefore by comparative analysis of fibre type composition. In this study, the three-dimensional distribution of slow and fast muscle fibres was analysed in the lumbar perivertebral muscles of two lemuriform (mouse lemur, brown lemur) and four hominoid primate species (white-handed gibbon, orangutan, bonobo, chimpanzee) in order to develop a plausible scenario for the evolution of the contractile properties of the axial muscles in hominoids and to discern possible changes in muscle physiology that were associated with the evolution of orthogrady. Similar to all previously studied quadrupedal mammals, the lemuriform primates in this study exhibited a morpho-functional dichotomy between deep slow contracting local stabilizer muscles and superficial fast contracting global mobilizers and stabilizers and thus retained the fibre distribution pattern typical for quadrupedal non-primates. In contrast, the hominoid primates showed no regionalization of the fibre types, similar to previous observations in Homo. We suggest that this homogeneous fibre composition is associated with the high functional versatility of the axial musculature that was brought about by the evolution of orthograde behaviours and reflects the broad range of mechanical demands acting on the trunk in orthograde hominoids. Because orthogrady is a derived character of euhominoids, the uniform fibre type distribution is hypothesized to coincide with the evolution of orthograde behaviours.
Keywords: autochthonous, epaxial, great ape, hypaxial, lesser ape, skeletal musculature
Introduction
The axial musculoskeletal system plays a central role in both locomotor and non-locomotor behaviour and the musculature surrounding the vertebral column therefore reflects the functional demands of the static and dynamic control of the body's posture and the integration of the action of the limbs and the trunk (Schilling, 2011). As a consequence, major evolutionary changes in the postural and/or locomotor habits of a species are accompanied by morpho-functional adaptations of the axial system. For example, locomotor deviations from typical mammalian quadrupedalism, such as bipedal saltation in jerboas and kangaroo rats, are associated with changes in their axial musculoskeletal system that reflect adaptation to hindlimb-dominated locomotion (Lull, 1904; Howell, 1932). Similarly, primates such as atelines and hominoids that regularly display orthograde positional behaviours show a suite of convergent adaptations to orthogrady in their truncal skeleton and musculature (e.g. Benton, 1967; Johnson & Shapiro, 1998).
Muscle tissue has a remarkable phenotypic plasticity that allows it to adapt to changing functional demands, both ontogenetically and phylogenetically (Schaeffer & Lindtstedt, 2013). A key element of this plasticity is variability in the density of the three main components that determine the contractile properties of muscle fibres – myofibrils, mitochondria and sarcoplasmic reticulum (Lindstedt et al. 1998). Although classifications of fibre types are somewhat artificial because their contractile properties represent a continuum and muscle fibres are dynamic structures (Staron, 1997), different muscle fibre types vary in the relative volume occupied in each by the myofibrils, mitochondria and the sarcoplasmic reticulum (Lindstedt et al. 1998). Because the volume that can be occupied by each of these components is limited in a muscle fibre, the different fibre types are optimized for different motor tasks by containing more of one of the components but less of others (Rome et al. 1988; Schaeffer & Lindtstedt, 2013). The result of the selection for specific functional properties is a striking diversity in the structural characteristics of muscle tissue among extant animals and a close relationship between muscle morphology and function (e.g. Chanaud et al. 1991; Jouffroy et al. 1999; Scholle et al. 2001). In general, muscles or muscle regions that fulfil a single dominant function will comprise the fibre type best suited for that particular function, whereas those with multiple functions (e.g. regarding force, speed or frequency of contraction) will possess a mixture of fibre types.
Because of the close relationship between muscle function and fibre properties, one way to explore diversity in muscle function among animals is the comparative analysis of fibre type composition (e.g. limb muscles in primates: Ariano et al. 1973; Sickles & Pinkstaff, 1981; Roy et al. 1984; McIntosh et al. 1985; Anapol & Jungers, 1986; Acosta & Roy, 1987; Suzuki, 1996; Jouffroy et al. 1999; Myatt et al. 2011). A recurrent finding has been that muscles or muscle groups are regionalized; that is, they show regional accumulations of the type of fibre best suited for the function of that particular muscle or muscle region (Kernell, 1998). In general, greater proportions of slow fibres tend to be found deep in the muscle and greater proportions of fast fibres tend to be located superficially. The deeper, fatigue-resistant regions are involved in repetitive and/or long-lasting activities such as postural functions, whereas the more superficial muscle areas produce or restrict fast motion (e.g. Jungers et al. 1980; Anapol & Jungers, 1987; Jouffroy & Stern, 1990; Jouffroy et al. 1999).
This morpho-functional dichotomy has also been broadly observed in the lumbar perivertebral musculature of various quadrupedal mammals (Schilling, 2009). The deeper stabilizer muscles consistently possessed high proportions of slow, fatigue-resistant fibres well suited to providing postural stability over extended periods of time (e.g. to ensure the structural linking of the vertebrae). In contrast, the more superficial muscles that dynamically stabilize the trunk against rapid loading or produce motion consisted primarily of fast fibres. Although the absolute fibre type proportions showed some species-specific variability (e.g. Moritz et al. 2007) and depended for example on body size (e.g. Hesse et al. 2010), the occurrence of greater proportions of slow fibres in the deeper areas of the perivertebral musculature (i.e. near the vertebrae, intramuscular tendons, fascia) compared with the more superficial regions is a common trait shared by all quadrupedal mammals investigated so far (Schilling, 2009). The fibre type composition of these muscles has been studied in only a few non-hominoid primates to date (i.e. crab-eating macaque (Macaca fascicularis) and pig-tailed macaque (Macaca nemestrina): Yokoyama, 1982; rhesus macaque (Macaca mulatta): Bagnall et al. 1983; Ford et al. 1986; Japanese macaque (Macaca fuscata): Kojima & Okada, 1996) and suggests a greater proportion of slow fibres in deeper muscle areas similar to other quadrupedal mammals. However, only a few samples of some epaxial muscles and/or some vertebral levels of these primates were studied (except Kojima & Okada, 1996).
In contrast, the human perivertebral musculature differs from that of other mammals in lacking distinct regionalizations and showing a homogeneous fibre composition throughout the lumbar region (Hesse et al. 2013). All human lumbar muscles, deep and superficial, are composed of approximately half slow and half fast fibres (e.g. Bagnall et al. 1983; Hesse et al. 2013). This homogeneous fibre type distribution has been suggested to be associated with the high functional versatility of the human back musculature and to reflect the broad range of mechanical demands acting on the human trunk during upright movement (Hesse et al. 2013).
The evolutionary context of this difference, however, is not well understood. A detailed interpretation of the fibre type distribution patterns throughout cross-sections and along the trunk in primates is hampered by the small amount of data available to date. In addition, only one study has investigated the fibre composition in a perivertebral muscle in another hominoid (orangutan: Kimura, 2002). Nevertheless, such data are important because it remains unclear whether the condition observed in humans is indicative of generalized orthogrady, which is increasingly thought to be the primary adaptation that characterizes crown hominoids (i.e. euhominoids) and thus is characteristic of all extant hominoids (e.g. Crompton et al. 2008), or whether it is a derived character of Homo reflecting specific adaptations to habitual terrestrial bipedalism.
The aim of this study was to increase our understanding of the functional morphology of the lumbar perivertebral musculature in primates in general and in hominoids in particular in order to develop a plausible scenario for the evolution of the contractile properties of this musculature in hominoid primates. To this end, we first reconstruct the fibre type distribution pattern that was likely present in the most recent common ancestor of primates by investigating the three-dimensional fibre type distribution in two prosimian species that resemble early primates in body size and locomotor style. Secondly, we integrate our findings with previously published observations from selected muscles and vertebral levels of other non-hominoid primates (i.e. cercopithecines) to discern traits shared by the quadrupedal non-hominoid primates investigated so far. Thirdly, we study the contractile properties of the lumbar perivertebral muscles in several non-human hominoids (i.e. white-handed gibbon, orangutan, bonobo and chimpanzee) to test whether the homogeneous fibre composition of humans is a derived character of Homo, hominids or hominoids.
Because of the great similarities in the use, function and loading regime of their axial system, we hypothesize that the fibre type distribution pattern in quadrupedal non-hominoid primates will be very similar to that observed in other quadrupedal mammals. Furthermore, we expect all hominoid primates to show no regionalizations in the fibre type distribution of their lumbar perivertebral musculature, based on the hypothesis that the evolution of orthograde trunk behaviours was associated with a need for great muscular versatility and thus the loss of muscular specialization (i.e. the evolution of a homogeneous fibre composition).
Material and methods
Specimens
The distribution patterns of slow (type I) and fast (type II) muscle fibres were studied in one female mouse lemur (Microcebus murinus, M), one male brown lemur (Eulemur fulvus, E), one female white-handed gibbon (Hylobates lar, G), one female orangutan (Pongo abelii, O), one female bonobo (Pan paniscus, B) and one female and two male common chimpanzees (Pan troglodytes, C). The specimens were all adults and obtained after death from European zoos or research institutes (for subject details, see Table 1). The animals had no known musculoskeletal diseases and were considered healthy, active individuals prior to death. The sample size is low because of the considerable rarity of endangered primates, and particularly non-human hominoids, and cadavers from musculoskeletally healthy, adult (but not elderly) individuals that are in addition accessible within days after death to ensure proper fixation. Nevertheless, publication of the data, even from single individuals, is important because these can reveal broad patterns in morphology and over time will contribute to the building of a robust dataset to facilitate more detailed inter-and intraspecific comparisons. Furthermore, the profound differences in the fibre distribution patterns that we observed between non-hominoid and hominoid primates in this study prevail over interindividual variations.
Table 1.
Subject information.
| Subject | Individual number | Sex | Age, years | Mass, kg | Cause of death | Name of institution |
|---|---|---|---|---|---|---|
| Mouse lemur | M | Female | 8 | 92 × 10−3 | Euthanized | Biology & Health Department, University of Montpellier II, France |
| Brown lemur | E | Male | > 20 | 3.2 | Euthanized | Inst. f. Spezielle Zoologie, Friedrich-Schiller-Universität Jena, Germany |
| White-handed gibbon | G | Female | 22 | 6.5 | Intestinal congestion | Royal Zoological Society Antwerp, Belgium |
| Orangutan | O | Female | 12 | 42 | Drowning | Tierpark Hagenbeck, Hamburg, Germany |
| Bonobo | B | Female | 18 | 38 | Drowning | Zoo Antwerpen, Belgium |
| Common chimpanzee | C1 | Female | 23 | 56 | ? | Belfast Zoological Gardens, Nothern Ireland |
| Common chimpanzee | C2 | Male | 34 | 62 | Pulmonary embolism | Zoologischer Garten, Halle, Germany |
| Common chimpanzee | C3 | Male | 56 | 45 | Respiratory arrest | Zoologischer Garten, Landau, Germany |
Sample preparation
The lemur cadavers were embalmed in buffered 4% formalin prior to dissection. In preparation for the histological processing, they were eviscerated and skin, fat and limbs removed. Then, the sternum was removed by severing all ribs in their middle and the obtained specimens, comprising the vertebral column and its surrounding musculature, were divided into two (for M) or four (for E) pieces after the specimens had been frozen overnight (−18 °C). To preserve the topographical relationships (i.e. the association with the vertebral levels), sutures were placed at the levels of the intervertebral joints. After decalcification of the specimens with ethylenediaminetetraacetic acid (EDTA), serial histological cross-sections of the complete backs including the vertebral column were prepared and immunohistochemical labelling was used to identify slow-and fast-twitch fibres as described below.
The hominoids had been eviscerated during the standard post mortem examinations and were frozen for transport to the Friedrich-Schiller-University Jena, Germany. Upon arrival, they were thawed, skinned and embalmed in 4% formalin. The gibbon was already embalmed (4% formalin) and had been partially dissected for other purposes. As with the lemuriforms, sutures were placed at the intervertebral joint levels in all cadavers to preserve the vertebral affiliation (Fig. 1a). The musculature was then removed in a stepwise procedure by severing the origins and insertions of the muscles and carefully removing the dorsovertebral and ventrovertebral musculature as a whole. Removing the complete musculature instead of a muscle-wise dissection preserved the topographical relationships within and among muscles and prevented distortion after the muscles were detached from their attachment sites. After freezing overnight (−18 °C), the musculature was cut into histologically manageable muscle blocks using a band saw (Fig. 1b). The number of blocks varied depending on the overall size of musculature, as they had to be no larger than 3.5 × 3.5 cm for the histological processing.
Figure 1.
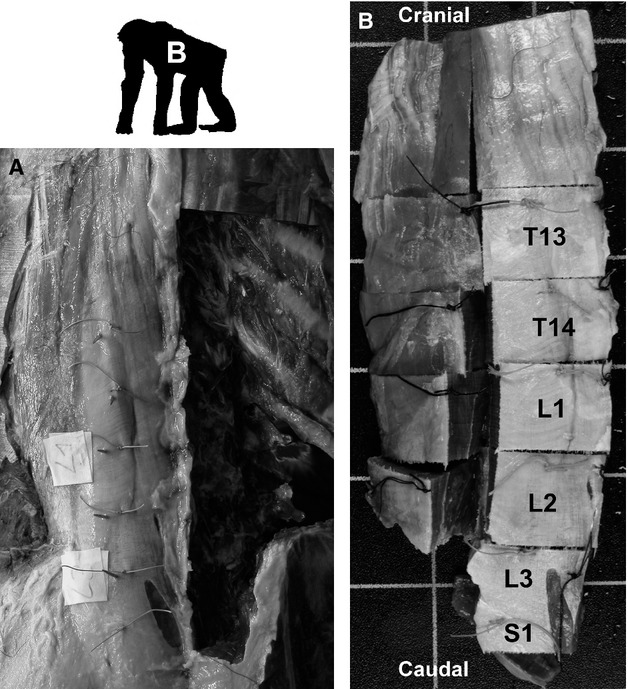
Preparation and tissue sampling in example of the epaxial musculature from the female bonobo. (a) Dorsal perspective of the embalmed cadaver to illustrate the preparation for sampling by marking the mid-vertebral levels. The epaxial musculature on the right had already been removed. (b) Division of the left musculature into histologically manageable tissue blocks. The light sutures served as indicators for the mid-vertebral levels, the dark ones indicated the medial and dorsal edges of the block. Grid size: 30 mm.
Immunohistochemistry
We used a previously developed immunohistochemical protocol for the hominoids which uses a primary antibody to slow myosin (MHC I, Clone NoQ7.5.4D) and a primary antibody to fast myosin (MHC II, Clone MY-32; both Sigma-Aldrich, Germany) (for details, see Myatt et al. 2011 as well as Schmidt & Schilling, 2007). The anti-fast antibody labels all fast myosin isoforms (Havenith et al. 1990), therefore no subtypes (e.g. 2A, 2X or 2B) were identified. We tested this protocol for the lemur species and found that it produced complementary results and allowed unequivocal identification of slow and fast fibres (Fig. 2). Therefore, the same immunohistochemical protocol was used for all species studied here.
Figure 2.
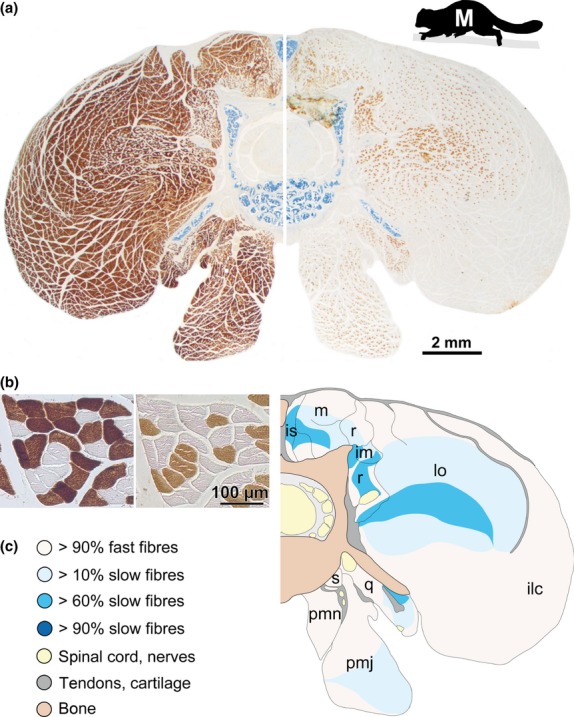
Results of the immunohistochemical protocol in example (a) of the cross-section at the vertebral level L4/5 for the mouse lemur. (b) Magnification of the complementary staining results. (a,b) Left: labelling with the primary anti-fast antibody (i.e. fast fibres are brown), right: labelling with the primary anti-slow antibody (i.e. slow fibres are brown). (c) Schematic illustration of the segregation of slow and fast fibres (colour-coded as indicated in the legend) to allow for the interspecific comparison illustrated in Fig. 5. Muscle abbreviations: ilc, m. iliocostalis; im, mm. intermammillares (et mammilloaccessorii); is, mm. interspinales; lo, m. longissimus lumborum; m, m. multifidus; pmj, m. psoas major; pmn, m. psoas minor; q, m. quadratus lumborum; r, mm. rotatores; s, m. sacrocaudalis.
The tissue blocks were washed in distilled water and dehydrated with a graded series of ethanol and then propanol, before being embedded in paraffin. Serial cross-sections were prepared (10 μm; microtome HM360, Microm International GmbH, Walldorf, Germany). Several sections were sampled from the mid-vertebral levels of all lumbar vertebrae. Using the above-mentioned commercially available mouse monoclonal antibodies, raised against rabbit skeletal muscle, slow-twitch (type I) and fast-twitch (type II) fibres were identified. Because the primary antibody to fast myosin produced both the greatest staining intensity and ease of distinction between the fibre types, it was used for all samples. For this, the immunoreactivity of the muscle tissue was first reinstated using trypsin (0.1%) in phosphate-buffered saline (PBS, 0.01 m, pH 7.4). Peroxidase activity was then blocked using 3% H2O2 in methanol before treating the cross-sections with normal goat serum (1.5% in PBS). The primary antibody was added and visualized using a Mouse ExtrAvidin®-Peroxidase staining kit (Sigma-Aldrich, Germany), consisting of biotinylated purified goat antibody to mouse IgG and ExtrAvidin®-Peroxidase. By covering the muscle samples with a diaminobenzidine-H2O2 substrate, the fibres containing the type II MHC were stained brown. A counterstaining with methylene blue was used to contrast slow fibres.
Data analysis
The proportion and distribution of slow and fast fibres were determined using one of the serial histological cross-sections collected from each of the mid-vertebral lumbar levels in the hominoid and from four selected lumbar levels in the lemuriform primates (i.e. L1, L3, L5, L7). These four levels were selected to facilitate the comparison of the fibre type patterns in the upper, middle and lower lumbar regions between the species, irrespective of differences in vertebral count. The sections were photographed using a digital camera mounted to an Axiolab microscope (Carl Zeiss Jena, Germany). Fibre composition was then analysed using a grid that covered the complete cross-section and contained a manageable amount of fibres to be counted per square while providing appropriate coverage of the fibre composition. Therefore, grid size varied somewhat depending on body size (i.e. cross-sectional area of the musculature). In the mouse lemur, sequential images (1280 × 1024 pixels, 0.71 × 0.56 mm) were taken in a grid-like manner covering the complete cross-section using the Axiolab microscope, which was also equipped with a motor-driven object table controlled by the image analysis software AnalySIS® 3.2 (Olympus Soft Imaging Solutions GmbH, Muenster, Germany; following Schilling, 2009). Every other image was then analysed. In the brown lemur and the gibbon, a grid (0.5 × 0.5 mm) was placed over the cross-sections and images were taken from every fourth square in every second row and every fourth square in every fourth row, respectively. In the hominid samples, every fourth square in every fourth row was analysed using a larger grid (1.0 × 1.0 mm).
The total number of muscle fibres per image differed among the species due to grid-size and the location the image was taken from. Thus, for each image, the percentage of slow and fast fibres was assessed by counting the fibres of each type using AnalySIS® 3.2. The images contained a maximum of 275 muscle fibres for the mouse lemur, 145 for the brown lemur, 92 for the gibbon, 340 fibres for the orangutan, and 280 for the bonobo and the common chimpanzees. To prevent double counting, the software marked the fibres already counted. Descriptive statistics were studied using Microsoft® Office Excel 2007.
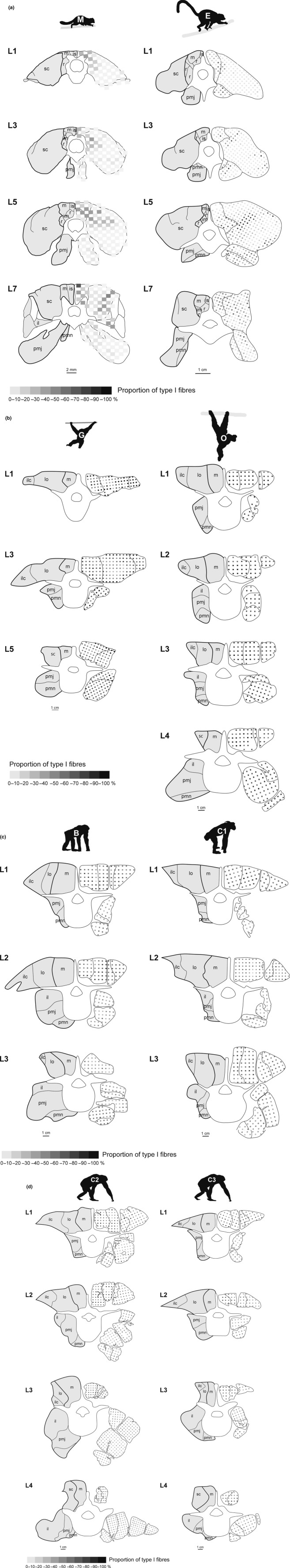
Drawings of the cross-sections were prepared using Adobe® Illustrator® CS3 and the percentages of slow fibres were encoded using a grey scale (higher percentage, darker colour) to illustrate the overall fibre type distribution pattern throughout the cross-sections (e.g. Fig. 3a). To facilitate the correct reassembling of the cross-sections from the various tissue blocks of the hominoids, computerized tomographic scans were taken of the cadavers before the dissection. For the gibbon, a CT scan from a siamang (Symphalangus syndactylus) was used because no scan was obtained from the individual used for fibre typing. These cross-sectional CT images served to prepare the outlines of the bones and the perivertebral muscles and provided anatomical landmarks for rearranging of the sections.
Figure 3.
Distribution pattern of muscle fibre types in the lumbar regions of (a) mouse lemur (M) and the brown lemur (E), (b) the gibbon (G) and the orangutan (O), (c) the bonobo (B) and the female chimpanzee (C1) and (d) the two male chimpanzees (C2, C3). The percentage of slow fibres is illustrated by a grey scale (see colour code on the bottom). Note that the percentage of fast fibres is complementary to that of slow fibres. The muscles grouped together as the dorsomedial, dorsolateral and the ventral tracts, respectively, are surrounded by thicker lines. Because the right side was reconstructed from the tissue blocks, the lines separating the muscles do not perfectly match the lines on the left, which were drawn after the CT scans. Muscle abbreviations: il, m. iliacus; ilc, m. iliocostalis; im, mm. intermammillares (et mammilloaccessorii); is, mm. interspinales; lo, m. longissimus lumborum; m, m. multifidus; pmj, m. psoas major; pmn, m. psoas minor; r, mm. rotatores; sc, m. sacrospinalis.
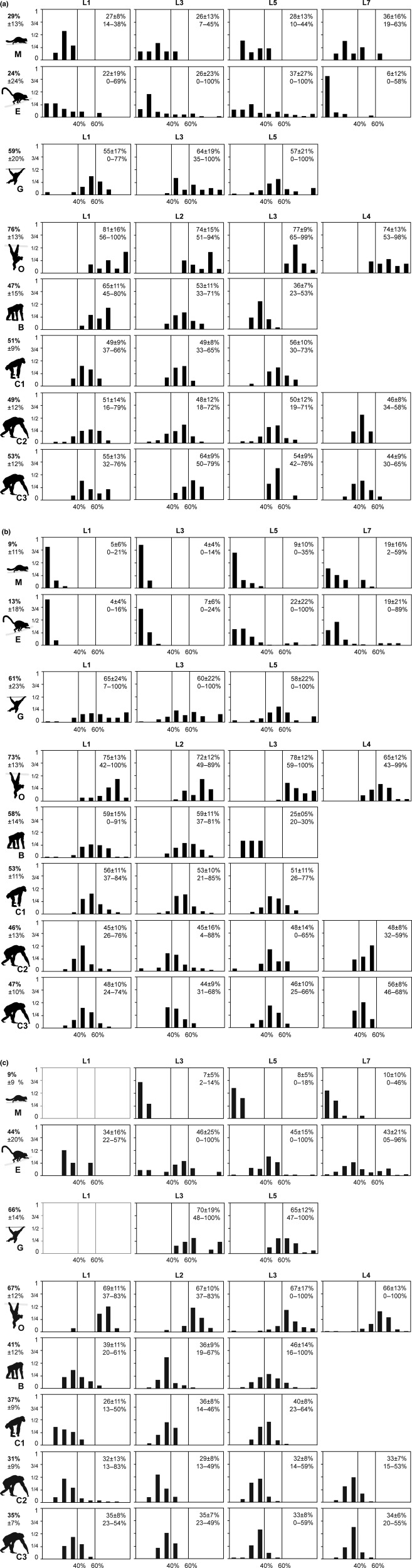
The muscles were grouped in three tracts to facilitate meaningful comparison of the fibre type composition between species and because the individual muscles were sometimes indistinguishable in the cross-sections, whereas the large muscles tracts were always clearly recognizable. The lumbar epaxial muscles were grouped in a dorsomedial tract, comprising interspinales and transversospinales muscles (i.e. mm. rotatores, intermammillares et multifidi, if applicable), and a dorsolateral tract, comprising the sacrospinalis muscle (i.e. mm. longissimus et iliocostalis). The ventral tract comprised the mm. iliopsoas et psoas minor. For each muscle tract, the abundance of a given fibre type ratio was expressed in classes of 10% slow fibres and these results were illustrated as frequency plots (e.g. Fig. 4a). Additionally, mean ± standard deviation (SD) of the slow fibre proportion as well as minimum and maximum percentage of slow fibres were determined per muscle tract and each vertebral level of a given tract (e.g. Fig. 4a, top right corner of each plot). Note that we follow the standard description of fibre type distributions by referring to the proportions of slow fibres (e.g. Kernell, 1998). However, the interpretation of the results should not be biased towards the slow fibres, as slow and fast fibres are inversely related.
Figure 4.
Frequency plots of the proportion of slow fibres in the three lumbar muscle tracts studied: (a) dorsomedial, (b) dorsolateral, (c) ventral of the mouse lemur (M), brown lemur (E), gibbon (G), orangutan (O), bonobo (B) and the three chimpanzees (C1, C2, C3) (from top to bottom). The proportions of slow fibres were divided into classes of 10% for each lumbar level and the frequency of their occurrence in a given tract and level was plotted. Numbers in the top right corner of each graph represent means ± SD (first line) as well as minimum and maximum (second line) of the slow fibre proportions observed at the respective vertebral level. Numbers to the left of each row indicate mean ± SD of the percentage of slow fibres for all lumbar levels investigated.
Results
Mouse and brown lemur
In the dorsomedial tracts of both lemur species, the deeper muscle areas near the vertebrae consisted of greater percentages of slow fibres compared with the more superficial areas (e.g. M & E: L1, Fig. 3a). For example, at least one-third of the fibres adjacent to the bone were slow in both species, compared with the more superficial areas, which sometimes comprised only fast fibres (e.g. E: L5, Fig. 3a). Along the cranio-caudal axis, the proportion of slow fibres increased slightly in the dorsomedial tract of the mouse lemur, whereas it decreased in the brown lemur (Fig. 4a, compare means in the top right corners). However, at all lumbar levels, the deep-to-superficial difference in slow vs. fast fibre proportion persisted in both species. When averaged across the lumbar levels, both lemuriforms contained similar proportions of slow fibres (means M: 29%, E: 24%; Fig. 4a), although, overall, more fibre type classes were present in the brown lemur, particularly in the mid-lumbar region. The most abundant classes in both lemur species were those that contained < 40% slow fibres (Fig. 4a).
The dorsolateral muscle tract had the lowest proportion of slow fibres of all muscles investigated (means M: 9%, E: 14%). Large areas with no slow fibres at all occurred particularly towards the lateral superficial aspect (i.e. the m. iliocostalis; Fig. 3a). The proportion of slow fibres increased caudally (Fig. 4b) but this was mainly due to the accumulation of slow fibres in the middle of the tract (i.e. on the medial aspect of the lateral longissimus muscle). In this slow region, about half the fibres were slow (e.g. M: L7 or E: L5, Fig. 3a). At all lumbar levels and in both lemur species, the fibre class 1–10% was clearly the most abundant in the dorsolateral tract (except E: L5 due to the extensive region of slow fibres in the lateral longissimus muscle), corroborating the bias towards high proportions of fast fibres in this dorsolateral tract.
Similar to the dorsolateral tract, the ventral tract was dominated by fast fibres in the mouse lemur, which were homogeneously distributed throughout the cross-sections as well as cranio-caudally (Fig. 3a). In the brown lemur, a greater proportion of slow fibres occurred without clear accumulation in a particular muscle region. The most abundant fibre classes were between 40 and 60% slow fibres in the mid-lumbar region. However, in the most caudal vertebral level, fewer fast fibres occurred (Fig. 4c).
White-handed gibbon
Overall, the percentage of slow fibres was greater in the perivertebral muscles of the white-handed gibbon than in the two lemuriforms (means: dorsomedial tract 59%, dorsolateral 61%, ventral 66%; Fig. 4a-c). The slow fibres were more or less equally distributed over the cross-sectional area in all three tracts, with no clear deep-to-superficial difference in fibre proportions. The only exception to this was a somewhat greater proportion of slow fibres (> 80% class) in close proximity to the thoracolumbar fascia and the transverse processes in the dorsolateral tract (Fig. 3b). No substantial changes in the percentages of slow fibre were observed in either of the longitudinal tracts (Fig. 4a-c). The fibre type classes of 40–70% slow fibres were most frequent in the gibbon, although all classes between 10–20% and 90–100% slow fibres were present (Fig. 4a–c).
Orangutan
Among the hominoids studied here, the overall proportion of slow fibres was greatest in the orangutan. On average, at least two-thirds of all fibres were slow in all muscle tracts (means for all lumbar levels: dorsomedial 76%, dorsolateral 73%, ventral 67%). Therefore, the fibre type composition was very homogeneous across the cross-sections (Fig. 3b). Only a minor decrease in the proportion of slow fibres was observed in the cranio-caudal direction in the epaxial musculature (Fig. 4a,b). All classes between 30–40% and 90–100% slow fibres were present in the orangutan, but the most abundant ones were 60–70% and 70–80% slow fibres in all three muscle tracts (Fig. 4a-c).
Bonobo
The bonobo was similar to the gibbon and the orangutan in that the proportion of slow fibres was relatively homogeneously distributed across the cross-sections, respective muscle tracts and among the different lumbar levels. However, in contrast to the gibbon and particularly the orangutan, the bonobo possessed on average less than two-thirds of the slow fibres (means for all lumbar levels: dorsomedial 47%, dorsolateral 58%, ventral 41%; Figs 3 and 4). Slow fibres were somewhat more abundant in the middle of the dorsolateral tract at the more cranial lumbar levels (e.g. L1&2, Fig. 3c). Along the cranio-caudal axis, the proportion of slow fibres decreased substantially in the dorsovertebral musculature but increased in the ventral muscle tract (Fig. 3a-c). The classes between 10–20% and 90–100% slow fibres were all present in the bonobo, although not in all tracts or at all lumbar levels. Interestingly, the slow fibre classes in the bonobo show a nearly bell-shaped curve with the highest frequency of the classes of 30–70% slow fibres in all muscle tracts compared with the skewed frequency plots of the muscle tracts of the two prosimians, indicating a high proportion of either slow or fast fibres (e.g. Fig. 4a,b).
Common chimpanzee
The dorsovertebral musculature of the three common chimpanzees contained almost equal proportions of slow and fast fibres (means for all levels dorsomedial vs. dorsolateral tracts: C1: 51 vs. 53%; C2: 49 vs. 46%; C3: 53 vs. 47%; Figs 3c,d and 4a,b). In contrast, only one-third of the fibres were slow in the ventral tract (means for all lumbar levels: C1: 37%; C2: 31%; C3: 35%; Fig. 4c). The distribution pattern of slow and fast fibres was homogeneous across the cross-sections in all individuals. There were no clear differences from deep to superficial muscle regions or from cranial to caudal lumbar levels. The only exception was a central accumulation of slow fibres in the dorsolateral tract at the first two lumbar levels in all three specimens (Fig. 3c,d). All classes between 10–20% and 80–90% slow fibres were present, but the classes of 40–50% and 50–60% slow fibres were clearly the most frequent and this pattern was found at all lumbar levels (Fig. 4). Therefore, the frequency plots generally show a symmetrical bell-shape for the slow fibre proportion, which is more pronounced in the dorsovertebral tracts than in the ventral tract. Among the hominoids, the fibre type proportions of the chimpanzees and the bonobo most closely resembled each other.
In summary, of the six primate species studied here, the orangutan and the white-handed gibbon possessed on average the greatest proportions of slow fibres, the chimpanzee species were in the middle, and the smallest species – the mouse lemur and the brown lemur – contained the least slow fibres. The two prosimians clearly showed regionalizations of the two fibre types, that is, slow fibres were accumulated near the vertebrae (e.g. dorsomedial tract) and fast fibres were primarily superficial (e.g. dorsolateral tract). In contrast, all hominoids were characterized by a homogeneous fibre type composition throughout the cross-sections. This resulted in a more skewed fibre class distribution in the dorsovertebral musculature of the lemuriforms compared with the overall more bell-shaped frequency plots in the hominoids. In all primates, however, an accumulation of slow fibres was consistently found in the middle of the dorsolateral tract.
Discussion
Fibre type composition in quadrupedal, pronograde primates
The fibre type composition of the perivertebral musculature has been studied in only very few non-hominoid primates, but these results uniformly indicate a greater proportion of slow fibres in deeper muscles or muscle areas (crab-eating and pig-tailed macaques: Yokoyama, 1982; rhesus macaque: Bagnall et al. 1983; Ford et al. 1986; Japanese macaque: Kojima & Okada, 1996). In the studies that sampled several muscles or locations, and thus were able to compare deep and superficial compositions, in particular greater proportions of slow fibres in deeper locations were observed compared with more superficial locations. For example, Bagnall and colleagues analysed biopsies from the multifidus and the longissimus muscles in the rhesus macaque and found twice as many slow fibres in the multifidus than in the longissimus muscle (Bagnall et al. 1983). Later, the same group confirmed this deep-to-superficial difference in fibre proportion for several vertebral levels (e.g. L3: 46% slow fibres in the multifidus vs. 21% in the longissimus; Ford et al. 1986). The deep-to-superficial difference was similar in the brown lemur studied here, with twice as many slow fibres deep compared with superficial, and was even stronger in the mouse lemur, with three times more slow fibres. Comparing the fibre composition in four non-primate mammals (i.e. mouse, rat, cat, dog) with that of the crab-eating and the pig-tailed macaques, Yokoyama observed in both primate and non-primate species the same clear deep-to-superficial difference in the slow fibre percentage within as well as among muscles (Yokoyama, 1982). Similarly, in the Japanese macaque, the multifidus muscle contained about two-thirds slow fibres, whereas the longissimus muscle possessed less than a fifth at the mid-lumbar level (Kojima & Okada, 1996). In summary, the muscle fibre type distribution in the perivertebral musculature of quadrupedal, pronograde primates is strikingly similar among the species studied so far. Uniformly, muscle areas close to the vertebrae contain much greater proportions of slow, fatigue-resistant fibres compared with more superficial regions, which possess higher proportions of fast fibres.
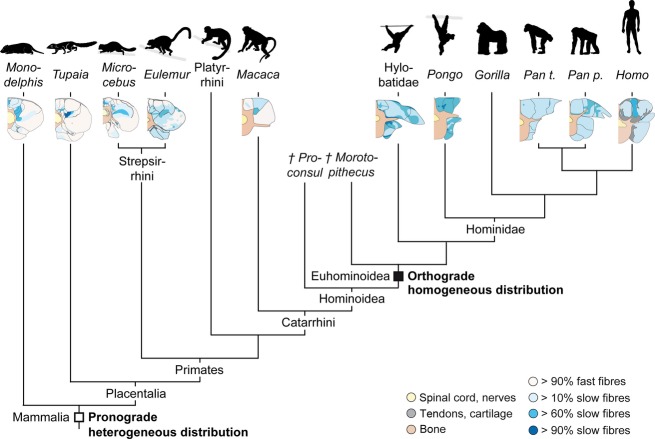
The fibre composition of the perivertebral muscles that was most likely present in the last common ancestor (LCA) of primates can be deduced by studying extant species that resemble the hypothetical LCA in their locomotor habits and the decisive postcranial characters. The fossil record indicates that early primate representatives were arboreal, quadrupedal and relatively small, i.e. comparable with two lemur species studied here (e.g. Cartmill, 1972; Jenkins, 1974; Dagosto, 1988; Martin, 1990; Bloch & Boyer, 2002; Gebo, 2004). Therefore, we suggest that the fibre pattern observed in their perivertebral musculature most likely represents the ancestral pattern for primates (Fig. 5).
Figure 5.
Hypothesized evolutionary transformation of the fibre distribution pattern in the perivertebral muscles in primates. Data were assembled from various studies (Kojima & Okada, 1996; Schilling, 2009; Hesse et al. 2013; this study) and mapped onto a simplified phylogenetic hypothesis (based on Crompton et al. 2008). The regionalized fibre distribution symplesiomorphic for non-primate mammals and primates is indicated by an open square. The homogeneous pattern suggested as a derived character for euhominoids and hypothesized to be associated with the evolution of orthograde behaviours is indicated by a filled square. Illustrated cross-sections from the following mid-lumbar levels: L2: Pan; L3: Hylobates, Pongo; L4: Monodelphis, Tupaia, Macaca, Homo; L5: Microcebus, Eulemur.
The fibre type distribution observed in the mouse lemur and the brown lemur also strikingly resembles the pattern described previously for various quadrupedal non-primate mammals (reviewed in Schilling, 2009). For example, the longissimus and iliocostalis muscles of the mouse and brown lemurs consist of 91 and 87% fast fibres, respectively, compared with clearly more than two-thirds to up to 100% of fast fibres in these muscles of the rabbit (McFadden et al. 1984), cat (Carlson, 1978; Yokoyama, 1982), rat (Schwartz-Giblin et al. 1983; Schilling et al. 2005), mouse (Hesse et al. 2010), pika, (Schilling, 2005), spiny mouse, cui, and tree-shrew (Schilling, 2009). As in the lemuriform species studied here, deeper muscle regions contained between 40% and up to 100% slow fibres in the non-primate mammals (Schilling, 2009). Thus, the fibre distribution in the perivertebral musculature of quadrupedal non-hominoid primates closely resembles the regionalized pattern observed in quadrupedal non-primate mammals. Based on the great similarity in body size, proportions and locomotor patterns as well as axial muscle function between quadrupedal non-primate and primate mammals (Shapiro & Jungers, 1994; Schmidt, 2005, 2008), we conclude that early primates retained the typical mammalian fibre distribution pattern in their perivertebral musculature. This suggests that the lumbar perivertebral musculature of the LCA of primates generally had to meet the same functional requirements as in its quadrupedal non-primate ancestor (Fig. 5).
Fibre type composition in hominoids
The general fibre type distribution was very similar among the hominoids studied here and is consistent with the previous observations in Homo (Hesse et al. 2013). In humans, the lumbar perivertebral muscles were composed of about 50% slow and 50% fast fibres, homogeneously distributed throughout the musculature (Hesse et al. 2013). Thus, in all hominoids studied so far, the fibre distribution was characterized by a (remarkably) homogeneous fibre composition, although the specific proportion of slow vs. fast fibres varied between the species. Among the non-human hominoids, the chimpanzee and bonobo fibre type proportions were most comparable with those of humans. These three hominids showed a fibre ratio for slow-to-fast fibres of about 50 : 50 [human: dorsomedial: 47%, dorsolateral: 56%, ventral: 46% slow fibres (Hesse et al. 2013) vs. 47, 58, 41% in the bonobo and 51, 49, 34% mean of the three chimpanzees]. In contrast to the regionalizations observed in the perivertebral musculature of pronograde non-hominoid primates, this 50 : 50 ratio together with the homogeneous distribution throughout the cross-sections of the hominoid primates indicates that all perivertebral muscles are equally well equipped to stabilize and mobilize the spine over varying periods of time.
A somewhat greater mean proportion of slow fibres was observed in the gibbon than in the hominids. Between half and two-thirds of the fibres on average were slow in its perivertebral muscles, compared with the 50% average for the hominids. Although gibbons are adept at terrestrial locomotion (including bipedalism, tripedalism and quadrupedalism; Vereecke et al. 2006), the majority of their locomotor active time is spent arboreally using bipedalism, leaping (Channon et al. 2009), and below branch suspensory behaviours (Fleagle, 1976; Gittins, 1983; Sati & Alfred, 2002). Among the latter, brachiation is by far the most important locomotor mode in gibbons and siamangs, accounting for 50–80% of their locomotor time compared with about 15% in hominids (Thorpe & Crompton, 2006; Michilsens et al. 2009). Gibbons and siamangs cover distances mostly using brachiation (Fleagle, 1976), which requires relatively high endurance, consistent with high overall proportions of slow, fatigue-resistant fibres. However, similar to the hominids, the fibres were evenly distributed throughout the musculature and no local accumulations of either fibre type were observed.
By far the highest proportion of slow fibres was observed in the orangutan, with on average two-thirds to three quarters of the fibres being slow contracting. This confirms previous observations from hindlimb muscles (Myatt et al. 2011) and agrees well with the orangutan's cautious and deliberate locomotor habits (MacKinnon, 1974; Thorpe & Crompton, 2005, 2006). Similarly, a larger proportion of slow fibres have been observed in slow-moving species such as the slow loris (Ariano et al. 1973; Sickles & Pinkstaff, 1981; Kimura et al. 1987; Suzuki, 1996) and the sloth (Barany et al. 1967) when compared with similar-sized species that engage in swifter and more powerful movements. However, our results are in contrast to previous findings for the psoas muscle in orangutans (Kimura, 2002), which reported less than a third slow fibres compared with about the two-thirds observed in this study. Unfortunately, no details on the sample location were provided by Kimura (2002). Because local variability in fibre proportions may be high, local samples are often not representative of the whole muscle (Hesse et al. 2013). The low proportion of slow fibres reported by Kimura (2002) may therefore reflect, among other things, the result of his local sampling technique.
In contrast to the gibbon, orangutan (this study) and human (Hesse et al. 2013), which all possessed comparable fibre percentages in their dorso-and ventrovertebral tracts (mean difference < 10% slow fibres), a slightly greater proportion of fast fibres was observed in the ventral muscles of the bonobo and particularly the chimpanzees (mean difference > 10%). The ventrovertebral musculature consisted primarily of the m. iliopsoas, which is the main hip flexor and a ventral flexor of the spine. A greater proportion of fast fibres in the hip flexor potentially points to an increased need for fast, powerful hip flexion in Pan and is consistent with the fast vertical climbing and the quadrupedal, high speed running bursts they engage in (Hunt, 1992; K. D'Aout and J. Neufuss, pers. obs.). Additionally, the greater proportion of fast fibres in the spinal flexor compared with the extensors is consistent with the comparable spinal flexion but reduced spinal extension that has been reported for galloping chimpanzees compared with other mammals (i.e. lack of hyperextension; Preuschoft, 2004; J. Neufuss, pers. obs.).
A consistent finding among all species investigated in this and previous studies (reviewed in Schilling, 2009; Hesse et al. 2013) is that the dorsolateral tract contains a distinct central accumulation of slow fibres. Because of its greater proportion of muscles spindles, Schilling (2011) proposed that this dorsolateral area acts as a proprioceptive system that monitors the position of the pelvis relative to the spine in mammals. The same may apply to hominoids, but this hypothesis remains to be tested by evaluating the receptor density in this central region vs. other regions of the epaxial musculature.
Implications for the evolution of axial muscle properties in hominoids
The hominoids investigated in this and a previous study (i.e. Homo; Hesse et al. 2013) shared a homogeneous fibre composition in their lumbar perivertebral musculature that is well suited to function in varying ways regarding contractile speed and duration. We hypothesize that this functional versatility reflects the broad range of mechanical requirements that their trunk has to meet.
During the evolution of hominoids, the positional repertoire of crown hominoids (i.e. ‘euhominoids', Fig. 5) was substantially expanded by a whole suite of new behaviours connected with the evolution of orthogrady and above-head arm motions. As a consequence, the loading regime on the trunk in hominoids broadened because orthograde postures and locomotor behaviours placed new mechanical demands on the trunk, such as tension and compression, in addition to the bending or twisting demands characteristic of ancestral primates (Johnson & Shapiro, 1998). Furthermore, in hominoids, trunk loading depends substantially on how many and which limbs provide support and where the substrate is relative to the centre of body mass. This, combined with frequent changes between behaviours due to the discontinuity of the habitat, necessitated a uniquely broad range of functional roles for the perivertebral muscles. Therefore, we suggest that the evolution of a homogeneous fibre composition that equips all axial muscles to act equally well as mobilizers and stabilizers of the vertebral column was related to the extension of the positional repertoire to orthograde behaviours that characterize the living hominoids (e.g. vertical climbing, orthograde clambering, brachiation, bipedalism, forelimb suspension; Hunt, 1992; Thorpe & Crompton, 2006). Clearly, this hypothesis needs further testing. Fortunately, a natural control group exists because atelines are non-hominoid primates that display large amounts of orthograde behaviours and as such form a key group for understanding the evolution of the upright postures (Johnson & Shapiro, 1998).
The fossil record indicates clear skeletal adaptations to habitual orthogrady in early representatives of the crown hominoids such as Morotopithecus, Pierolapithecus and Hispanopithecus/Dryopithecus, but not in stem hominoids such as Proconsul (Ward, 1993, 2007; Moyà-Solà & Köhler, 1996; Crompton et al. 2008; Alba, 2012). If the loss of fibre type regionalizations were associated with the evolution of orthograde behaviours, as we propose, then the evolutionary transition from a regionalized to a uniform fibre distribution would have coincided with the appearance of crown hominoids (‘euhominoids', Fig. 5). During hominin evolution, facultative bipedalism became an increasing part of the locomotor repertoire (Crompton et al. 2008). The transition from an arboreal ancestor that displayed a range of quadrupedal, tripedal or bipedal behaviours to a striding biped was most likely gradual and not associated with fundamental anatomical changes (D'Aout et al. 2004; Vereecke et al. 2006), because several postcranial adaptations facilitated a permanent orthograde trunk posture and bipedalism (e.g. Häusler et al. 2002; Schmitt, 2003; Crompton et al. 2008). The soft tissue results from this study support this evolutionary scenario because humans do not differ from other hominoids in their fibre type distribution, suggesting the contractile properties of the lumbar perivertebral musculature of the human predecessors were well suited for terrestrial bipedalism.
Acknowledgments
We thank D. R. Carrier for stimulating discussions. The cadavers were kindly provided by J.-M. Verdier (University of Montpellier II, France), M. Schmidt (Friedrich-Schiller-University Jena, Germany), T. Kaiser (Zoological Museum, University Hamburg, Germany) and the Tierpark Hagenbeck (Hamburg, Germany), F. Vercammen (Royal Zoological Society Antwerp, Belgium) and the Zoo Antwerp (Belgium), A. Kitchener (National Museums Scotland, UK), J. Heuer (Zoologischer Garten Halle, Germany) and J.-O. Heckel (Zoo Landau, Germany). This study would not have been possible without the technical assistance and skills of I. Weiß and M. Krüger. J. Schmidt helped with parts of the data analysis. The CT scans were prepared in cooperation with the Institute of Diagnostic and Interventional Radiology of the Jena University Hospital (T. Schulz, E. Lopatta, A. Kubin) and the Small Animal Teaching Hospital of the University of Liverpool (M. Baker, A. Channon). Grant sponsor: Centre of Interdisciplinary Prevention of Diseases related to Professional Activities founded and funded by the Friedrich-Schiller-University (Jena, Germany) and the Berufsgenossenschaft Nahrungsmittel und Gastgewerbe (Erfurt, Germany), Grant number: 1.1.9 (to N.S.); The Royal Society, UK, International Joint project Scheme (to S.K.S.T. and N.S.).
Author contributions
N.S. and S.K.S.T. designed the study. J.N., B.H. and N.S. collected and analysed the data and drafted the manuscript. S.K.S.T., E.E.V., K.A. and M.S.F. provided essential insights and commented on the manuscript. All authors finalized and approved the manuscript.
References
- Acosta L, Roy RR. Fiber-type composition of selected hindlimb muscles of a primate (Cynomolgus monkey. Anat Rec. 1987;218:136–141. doi: 10.1002/ar.1092180207. [DOI] [PubMed] [Google Scholar]
- Alba DM. Fossil apes from the Vallès-Penedès Basin. Evol Anthropol. 2012;21:254–269. doi: 10.1002/evan.21312. [DOI] [PubMed] [Google Scholar]
- Anapol FC, Jungers WL. Architectural and histochemical diversity within the quadriceps femoris of the brown lemur (Lemur fulvus. Am J Phys Anthropol. 1986;69:355–375. doi: 10.1002/ajpa.1330690308. [DOI] [PubMed] [Google Scholar]
- Anapol FC, Jungers WL. Telemetered electromyography of the fast and slow extensors of the leg of the brown lemur (Lemur fulvus. J Exp Biol. 1987;130:341–358. doi: 10.1242/jeb.130.1.341. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Armstrong RB, Edgerton VR. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973;21:51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Bagnall KM, Ford DM, McFadden KD, et al. A comparison of vertebral muscle fiber characteristics between human and monkey tissue. Acta Anat. 1983;117:51–57. doi: 10.1159/000145770. [DOI] [PubMed] [Google Scholar]
- Barany M, Conover TE, Schliselfeld LH, et al. Relation of properties of isolated myosin to those of intact muscles of the cat and sloth. Eur J Biochem. 1967;2:156–164. doi: 10.1111/j.1432-1033.1967.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Benton R. Morphological evidence for adaptations within the epaxial region of the primates. In: Vagtborg H, editor. The Baboon in Medical Research. Austin: University of Texas Press; 1967. pp. 201–216. [Google Scholar]
- Bloch J, Boyer DM. Grasping primate origins. Science. 2002;298:1606–1610. doi: 10.1126/science.1078249. [DOI] [PubMed] [Google Scholar]
- Carlson H. Morphology and contraction properties of cat lumbar back muscles. Acta Physiol Scand. 1978;103:180–197. doi: 10.1111/j.1748-1716.1978.tb06206.x. [DOI] [PubMed] [Google Scholar]
- Cartmill M. Arboreal adaptations and the origin of the order primates. In: Tuttle RH, editor. Functional and Evolutionary Biology of Primates. Chicago: Aldine-Atherton; 1972. pp. 97–122. [Google Scholar]
- Chanaud CM, Pratt CA, Loeb GE. Functionally complex muscles of the cat hindlimb. V. The roles of histochemical fiber-type regionalization and mechanical heterogeneity in differential muscle activation. Exp Brain Res. 1991;85:300–313. doi: 10.1007/BF00229408. [DOI] [PubMed] [Google Scholar]
- Channon AJ, Günther MM, Crompton RH, et al. Mechanical constraints on the functional morphology of the gibbon hind limb. J Anat. 2009;215:383–400. doi: 10.1111/j.1469-7580.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton RH, Vereecke EE, Thorpe SKS. Locomotion and posture from the common hominoid ancestor to fully modern hominins, with special reference to the last common panin/hominin ancestor. J Anat. 2008;212:501–543. doi: 10.1111/j.1469-7580.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagosto M. Implications of postcranial evidence for the origin of euprimates. J Hum Evol. 1988;17:35–56. [Google Scholar]
- D'Aout K, Vereecke E, Shoonaert K, et al. Locomotion in bonobos (Pan paniscus): differences and similarities between bipedal and quadrupedal terrestrial walking, and a comparison with other locomotor modes. J Anat. 2004;204:353–361. doi: 10.1111/j.0021-8782.2004.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleagle JG. Locomotion and posture of the Malayan Siamang and implications for hominoid evolution. Folia Primatol. 1976;26:245–269. doi: 10.1159/000155756. [DOI] [PubMed] [Google Scholar]
- Ford DM, Bagnall KM, McFadden KD, et al. A comparison of muscle fiber characteristics at different levels of the vertebral column in the rhesus monkey. Acta Anat. 1986;126:163–166. doi: 10.1159/000146208. [DOI] [PubMed] [Google Scholar]
- Gebo DL. A shrew-sized origin for primates. Am J Phys Anthropol. 2004;125:40–62. doi: 10.1002/ajpa.20154. [DOI] [PubMed] [Google Scholar]
- Gittins SP. Use of the forest canopy by the agile gibbon. Folia Primatol. 1983;40:134–144. doi: 10.1159/000156095. [DOI] [PubMed] [Google Scholar]
- Häusler M, Martelli SA, Boeni T. Vertebrae numbers of the early hominid lumbar spine. J Hum Evol. 2002;43:621–643. doi: 10.1006/jhev.2002.0595. [DOI] [PubMed] [Google Scholar]
- Havenith MG, Visser R, Schrijvers-van Schendel JMS, et al. Muscle fiber typing in routinely processed skeletal muscle with monoclonal antibodies. Histochemistry. 1990;93:497–499. doi: 10.1007/BF00266407. [DOI] [PubMed] [Google Scholar]
- Hesse B, Fischer MS, Schilling N. Distribution pattern of muscle fibre types in the perivertebral musculature of two different sized species of mice. Anat Rec. 2010;293:446–463. doi: 10.1002/ar.21090. [DOI] [PubMed] [Google Scholar]
- Hesse B, Fröber R, Fischer MS, et al. Functional differentiation of the human lumbar perivertebral musculature revisited by means of muscle fiber type composition. Ann Anat. 2013 doi: 10.1016/j.aanat.2013.07.003. doi: 10.1016/j.aanat.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Howell AB. The saltatorial rodent Dipodomys. The functional and comparative anatomy of its muscular and osseus system. Proc Am Acad Arts Sci. 1932;67:375–536. [Google Scholar]
- Hunt KD. Positional behavior of Pan troglodytes in the Mahale Mountains and Gombe Stream National Parks, Tanzania. Am J Phys Anthropol. 1992;87:83–105. doi: 10.1002/ajpa.1330870108. [DOI] [PubMed] [Google Scholar]
- Jenkins FAJ. Primate Locomotion. New York: Academic Press; 1974. pp. 1–390. [Google Scholar]
- Johnson SE, Shapiro L. Positional behavior and vertebral morphology in Atelines and Cebines. Am J Phys Anthropol. 1998;105:333–354. doi: 10.1002/(SICI)1096-8644(199803)105:3<333::AID-AJPA4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Jouffroy FK, Stern JTJ. Telemetered EMG study of the antigravity versus propulsive actions of knee and elbow muscles in the slow loris (Nycticebus coucang. In: Jouffroy FK, Stack MH, Niemitz D, editors. Gravity, Posture and Locomotion in Primates. Florence: Editrice II Sedicesimo; 1990. pp. 221–236. [Google Scholar]
- Jouffroy FK, Stern JTJ, Medina MF, et al. Function and cytochemical characteristics of postural limb muscles of the Rhesus monkey: a telemetered EMG and immunofluorescence study. Folia Primatol. 1999;70:235–253. doi: 10.1159/000021703. [DOI] [PubMed] [Google Scholar]
- Jungers WL, Jouffroy FK, Stern JTJ. Gross structure and function of the quadriceps femoris in Lemur fulvus: an analysis based on telemetered electromyography. J Morph. 1980;164:287–299. doi: 10.1002/jmor.1051640305. [DOI] [PubMed] [Google Scholar]
- Kernell D. Muscle regionalization. Can J Appl Physiol. 1998;23:1–22. doi: 10.1139/h98-001. [DOI] [PubMed] [Google Scholar]
- Kimura T. Composition of psoas major muscle fibers compared among humans, orangutans, and monkeys. Z Morph Anthropol. 2002;83:305–314. [PubMed] [Google Scholar]
- Kimura T, Kumakura H, Inokuchi S, et al. Composition of muscle fibers in the slow loris, using the m. biceps brachii as an example. Primates. 1987;28:525–532. [Google Scholar]
- Kojima R, Okada M. Distribution of muscle fibre types in thoracic and lumbar epaxial muscles of Japanese macaques (Macaca fuscata. Folia Primatol. 1996;66:38–43. doi: 10.1159/000157183. [DOI] [PubMed] [Google Scholar]
- Lindstedt SL, McGothlin T, Percy E, et al. Task-specific design of skeletal muscle: balancing muscle structural composition. Comp Biochem Physiol B Biochem Mol Biol. 1998;120:35–40. doi: 10.1016/s0305-0491(98)00021-2. [DOI] [PubMed] [Google Scholar]
- Lull RS. Adaptations to aquatic, arboreal, fossorial, and cursorial habits in mammals. IV. Cursorial adaptations. Am Nat. 1904;38:1–11. [Google Scholar]
- MacKinnon J. The behaviour and ecology of wild Orang-Utans (Pongo pygmaeus. Anim Behav. 1974;22:3–74. [Google Scholar]
- Martin RD. Primate Origin and Evolution: A Phylogenetic Reconstruction. London: Chapman Hall; 1990. pp. 1–840. [Google Scholar]
- McFadden KD, Bagnall KM, Mahon M, et al. Histochemical fiber composition of lumbar back muscles in the rabbit. Acta Anat. 1984;120:146–150. doi: 10.1159/000145909. [DOI] [PubMed] [Google Scholar]
- McIntosh JS, Rinquist M, Schmidt EM. Fiber type composition of monkeys forearm muscle. Anat Rec. 1985;211:403–409. doi: 10.1002/ar.1092110405. [DOI] [PubMed] [Google Scholar]
- Michilsens F, Vereecke EE, D'Août K, et al. Functional anatomy of the gibbon forelimb: adaptations to a brachiating lifestyle. J Anat. 2009;215:335–354. doi: 10.1111/j.1469-7580.2009.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz S, Fischer MS, Schilling N. 3D-Fibre type distribution in the paravertebral muscles of the domestic ferret (Mustela putorius f. furo) related to trunk movements during locomotion. Zoology. 2007;110:197–211. doi: 10.1016/j.zool.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Moyà-Solà S, Köhler M. A Dryopithecus skeleton and the origins of great-ape locomotion. Nature. 1996;379:156–159. doi: 10.1038/379156a0. [DOI] [PubMed] [Google Scholar]
- Myatt J, Schilling N, Thorpe SKS. Distribution patterns of fibre types in the triceps surae muscle group of chimpanzees and orangutans. J Anat. 2011;218:402–412. doi: 10.1111/j.1469-7580.2010.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoft H. Mechanisms for the acquisition of habitual bipedality: are there biomechanical reasons for the acquisition of upright bipedal posture? J Anat. 2004;204:363–384. doi: 10.1111/j.0021-8782.2004.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome LC, Funke RP, Alexander RM, et al. Why animals have different muscle fibre types. Nature. 1988;335:824–827. doi: 10.1038/335824a0. [DOI] [PubMed] [Google Scholar]
- Roy RR, Bello MA, Powell PL, et al. Architectural design and fiber-type distribution of the major elbow flexors and extensors of the monkey (Cynomolgus. Am J Anat. 1984;171:285–293. doi: 10.1002/aja.1001710305. [DOI] [PubMed] [Google Scholar]
- Sati J, Alfred J. Locomotion and posture in the hoolock gibbon. Ann Forestry. 2002;10:298–306. [Google Scholar]
- Schaeffer PJ, Lindtstedt SL. How animals move: comparative lessons on animal locomotion. Compr Physiol. 2013;3:289–314. doi: 10.1002/cphy.c110059. [DOI] [PubMed] [Google Scholar]
- Schilling N. Characteristics of paravertebral muscles – fibre type distribution pattern in Ochotona rufescens (Mammalia: Lagomorpha) J Zool Syst Evol Res. 2005;43:38–48. [Google Scholar]
- Schilling N. Metabolic profile of the perivertebral muscles of small therian mammals: implications for the evolution of the mammalian trunk musculature. Zoology. 2009;112:279–304. doi: 10.1016/j.zool.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Schilling N. Evolution of the axial system in craniates: morphology and function of the perivertebral musculature. Front Zool. 2011;8:4. doi: 10.1186/1742-9994-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling N, Arnold D, Wagner H, et al. Evolutionary aspects and muscular properties of the trunk – Implications for human low back pain. Pathophysiology. 2005;12:233–242. doi: 10.1016/j.pathophys.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Hind limb proportions and kinematics: are small primates different from other small mammals? J Exp Biol. 2005;208:3367–3383. doi: 10.1242/jeb.01781. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Forelimb proportions and kinematics: how are small primates different from other small mammals? J Exp Biol. 2008;211:3775–3789. doi: 10.1242/jeb.019802. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Schilling N. Fiber type distribution in the shoulder muscles of the tree shrew, the cotton-top tamarin, and the squirrel monkey related to shoulder movements and forelimb loading. J Hum Evol. 2007;52:401–419. doi: 10.1016/j.jhevol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Schmitt D. Insights into the evolution of human bipedalism from experimental studies of humans and other primates. J Exp Biol. 2003;206:1437–1448. doi: 10.1242/jeb.00279. [DOI] [PubMed] [Google Scholar]
- Scholle HC, Schumann NP, Biedermann FHW, et al. Spatiotemporal surface EMG characteristics from rat triceps brachii muscle during treadmill locomotion indicate selective recruitment of functionally distinct muscle regions. Exp Brain Res. 2001;138:26–36. doi: 10.1007/s002210100685. [DOI] [PubMed] [Google Scholar]
- Schwartz-Giblin S, Rosello L, Pfaff DW. A histochemical study of lateral longissimus muscle in rat. Exp Neurol. 1983;79:497–518. doi: 10.1016/0014-4886(83)90229-7. [DOI] [PubMed] [Google Scholar]
- Shapiro LJ, Jungers WL. Electromyography of back muscles during quadrupedal and bipedal walking in primates. Am J Phys Anthropol. 1994;93:491–504. doi: 10.1002/ajpa.1330930408. [DOI] [PubMed] [Google Scholar]
- Sickles DW, Pinkstaff CA. Comparative histochemical study of prosimian primate hindlimb muscles. II. Populations of fiber types. Am J Anat. 1981;160:187–194. doi: 10.1002/aja.1001600205. [DOI] [PubMed] [Google Scholar]
- Staron RS. Human skeletal muscle fiber types: delineation, development, and distribution. Can J Appl Physiol. 1997;22:307–327. doi: 10.1139/h97-020. [DOI] [PubMed] [Google Scholar]
- Suzuki A. Adaptation of skeletal myofiber types to arboreality. Primate Res. 1996;12:133–146. [Google Scholar]
- Thorpe SKS, Crompton RH. Locomotor ecology of wild orangutans (Pongo pygmaeus abelii) in the Gunung Leuser ecosystem, Sumatra, Indonesia: a multivariate analysis using log-linear modelling. Am J Phys Anthropol. 2005;127:58–78. doi: 10.1002/ajpa.20151. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH. Orangutan positional behavior and the nature of arboral locomotion in Hominoidea. Am J Phys Anthropol. 2006;131:384–401. doi: 10.1002/ajpa.20422. [DOI] [PubMed] [Google Scholar]
- Vereecke E, D'Aout K, Aerts P. Locomotor versatility in the white-handed gibbon (Hylobates lar): a spatiotemporal analysis of the bipedal, tripedal, and quadrupedal gaits. J Hum Evol. 2006;50:552–567. doi: 10.1016/j.jhevol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Ward CV. Torso morphology and locomotion in Proconsul nyanzae. Am J Phys Anthropol. 1993;92:291–328. doi: 10.1002/ajpa.1330920306. [DOI] [PubMed] [Google Scholar]
- Ward CV. Postcranial and locomotor adaptations of hominoids. In: Henke W, Tattersall I, editors. Handbook of Paleoanthropology. Berlin: Springer-Verlag; 2007. pp. 1011–1030. [Google Scholar]
- Yokoyama I. Analyses of the fibre composition of the lumbar back muscles in mammals. Nihon Seikeigeka Gakkai Zasshi. 1982;56:579–594. [PubMed] [Google Scholar]