Abstract
Due to lack of access in healthy patients, the structural properties underlying the inherent regenerative power and advanced material properties of the human periosteum are not well understood. Periosteum comprises a cellular cambium layer directly apposing the outer surface of bone and an outer fibrous layer encompassed by the surrounding soft tissues. As a first step to elucidating the structural and cellular characteristics of periosteum in human bone, the current study aims to measure cambium and fibrous layer thickness as well as cambium cellularity in human femora and tibiae of aged donors. The major and minor centroidal axes (CA) serve as automated reference points in cross-sections of cadaveric mid-diaphyseal femora and tibiae. Based on the results of this study, within a given individual, the cambium layer of the major CA of the tibia is significantly thicker and more cellular than the respective layer of the femur. These significant intraindividual differences do not translate to significant interindividual differences. Further, mid-diaphyseal periosteal measures including cambium and fibrous layer thickness and cellularity do not correlate significantly with age or body mass. Finally, qualitative observations of periosteum in amputated and contralateral or proximal long bones of the lower extremity show stark changes in layer organization, thickness, and cellularity. In a translational context, these novel data, though inherently limited by availability and accessibility of human mid-diaphyseal periosteum tissue, provide important reference values for the use of periosteum in the context of facilitated healing and regeneration of tissue.
Keywords: amputee, loading history, mechanobiology, periosteal cells, periosteum
Introduction
Periosteum is the thin, bounding membrane enveloping all outer bone surfaces not covered by cartilage. The periosteum comprises a cellular, or cambium layer, which directly apposes the outer surface of bone, and an outer fibrous layer, which lies adjacent to the surrounding soft fibrous and muscular tissue. The cambium layer is host to osteochondroprogenitor cells with unique tissue-building capacity (Chang & Knothe Tate, 2012). Recently, there has been renewed scientific interest in the periosteum due to its inherent regenerative power and stimuli-responsive (‘smart') material properties (Colnot et al., 2012; Evans et al., 2012b).
A number of studies describe mechanical, permeability, and regenerative properties of periosteal tissue and periosteum-derived cells in a variety of animal models (McBride et al., 2011c; Evans et al., 2012a). Yet, little is known regarding the structural and cellular characteristics of human periosteum because the tissue is not routinely accessible in living patients, except for example as metaphyseal tissue resected during the normal course of joint replacement (Chang et al., 2013). Further, though mid-diaphyseal periosteum tissue may exhibit even higher regenerative capacity than that derived from metaphyseal bone (Chang & Knothe Tate, 2012), accessibility and hence understanding of mid-diaphyseal tissue is even more limited than that of metaphyseal tissue. Finally, during surgical treatment of critical-sized bone defects or non-unions, adjacent, healthy diaphyseal periosteum might be accessible (Knothe & Springfield, 2005; Sauser, 2010), but further study is warranted for effective harnessing of its regenerative potential and smart material properties. As a first step, measurements of thickness of the respective fibrous and cambium layers as well as cellularity of the cambium layer are crucial for the translation and development of regenerative medicine therapies (Knothe Tate et al., 2011; Chang et al., 2013).
Minor and major centroidal axes (CAs) serve as cross-sectional, anatomical reference points that can be obtained in an automated fashion, thus obviating observer bias. Further, CAs can also serve as a proxy for relative loading history in matched experimental cohorts, allowing for testing of mechanobiological hypotheses (McBride et al., 2011b), e.g. that the structure of periosteum (thickness, cellularity) relates to the prevailing mechanical loads to which it is subjected. Finally, recent studies of aged human femora indicate great variability in femur cross-sectional shape attributable to the presence and prominence of the linea aspera, a characteristic ridge along the length of the bone that is unique to humans and some non-human primates. These studies underscore that CAs may be appropriate references for mechanical loading history within but not between patients or human cadaveric donors (Moore et al., 2013).
Hence, as a first step to understanding the structural and cellular characteristics of periosteum in human bone, the current study aims to measure cambium and fibrous layer thickness as well as cambium cellularity of human femoral and tibial periosteum, using the major and minor centroidal axes as reference points, in cadaveric mid-diaphyseal femora and tibiae of aged donors. Further, to determine whether loading history relates to periosteal properties including thickness and cellularity, outcome measures are tested for correlation between respective major and minor centroidal axes of each pair of long bone cross-sections. Finally, a limited subset of specimens obtained from cadaveric bone of amputees provides pilot data to initiate study of periosteal structure and cellularity in both amputated as well as associated non-amputated long bones of the lower extremity.
Materials and methods
Sample collection and preparation
Segments of the mid-diaphysis 5 cm long were harvested from the left and right tibiae and femora of formalin-fixed cadavers donated to the Department of Anatomy at the Ludwig Maximilians University of Munich. Overlying skin and musculature were preserved during embedding to avoid disruption of periosteal tissue. A total of 29 mid-diaphyseal samples from tibiae and femora were collected from eight donors, aged between 68 and 99 (Table1). An additional nine mid-diaphyseal samples were obtained from three donors exhibiting amputations, including one donor with double transfemoral amputations and two donors with single transtibial amputations (Table2). Cross-sections were excluded from study if there was damage during embedding, or accidental stripping of muscle and subsequent removal of periosteum during harvesting.
Table 1.
Donor and sample details for non-amputees. Specimens were obtained from the mid-diaphysis of the tibia as well as the femur, excluding cross-sections that were damaged during harvesting or histological processing.
| Donor number | Age | Gender | Body mass (kg) | Cause of death | Bone samples collected |
|---|---|---|---|---|---|
| 1 | 68 | M | 80 | Heart failure | RT, LT, RF, LF |
| 2 | 69 | F | 50 | Heart failure | RT, LT |
| 3 | 82 | F | 80 | Multiple organ failure | RT, LT, RF, LF |
| 4 | 86 | M | 77 | Unknown | RT, LT, RF, LF |
| 5 | 88 | F | 69 | Unknown | RT, LT, RF, LF |
| 6 | 93 | F | 68 | Unknown | RT, LT, RF, LF |
| 7 | 93 | F | 37 | Multi-causal related to dementia and nutrition status | RT, LT, RF |
| 8 | 99 | M | 70 | Heart failure | RT, LT, RF, LF |
M, male; F, female; R, right; L, left; T, tibia; F, femur.
Table 2.
Donor and sample details for amputees. Specimens from amputated bones were obtained just proximal to the site of amputation (indicated as bold in the bones collected column). Associated specimens were obtained from the mid-diaphysis of from non-amputated bones of the amputees.
| Donor number | Age | Gender | Weight (kg) | Cause of death | Amputated bone(s) | Bones collected |
|---|---|---|---|---|---|---|
| 9 | 83 | M | 65 | Unknown | RT | RT, LT, RF, LF |
| 10 | 87 | M | 52 | Unknown | LT | RT, LT, RF |
| 11 | 92 | F | 43 | Heart Failure | RF, LF | RF, LF |
Due to the tendency of the periosteum to degrade during paraffin histology processing, as well as to maintain the spatial dimensions of the native tissue, we proceeded with undecalcified histology and embedded the specimens in poly(methyl methacrylate) (PMMA). Following embedding, the blocks were sectioned perpendicular to the longitudinal axis of the bone and polished to a thickness of 100–140 μm using a saw grinding device (Patho-service GmbH, Oststeinbek, Germany). Specimens were then mounted onto opaque Perspex slides. For each sample, one mid-diaphyseal section was stained using Giemsa-Eosin stain to visualize mineralized tissue (pink) and cell nuclei (blue).
Determination of regions of interest
Stained and mounted sections were scanned with an HP flatbed scanner at 1200 dpi. The digitized images were then processed in photoshop (CS5) to segment them into binary images (black and white) after thresholding. A modified version of Ruff's momentmacro was used to trace the major and minor centroidal axes onto the cross-sections (Warfel et al., 2005). The centroidal axes were then transferred onto the slides to indicate periosteal regions along the major and minor centroidal axes.
Image acquisition, processing and analysis
A region of interest comprising a distance of 500 μm from the CA in each direction along the bone/periosteum interface was imaged using transmitted light at 200× magnification on a Leica DMIRE2 inverted microscope (Fig. 1). Approximately 20 fields of view were captured for each region of interest to enable analysis of the entire region. Individual images were collaged using photoshop's Photomerge feature. Cambium layer thickness, fibrous thickness, and cambium cell number were measured at regular 100-μm intervals from the centroidal axis along the outer bone surface (imagej 1.42q).
Figure 1.
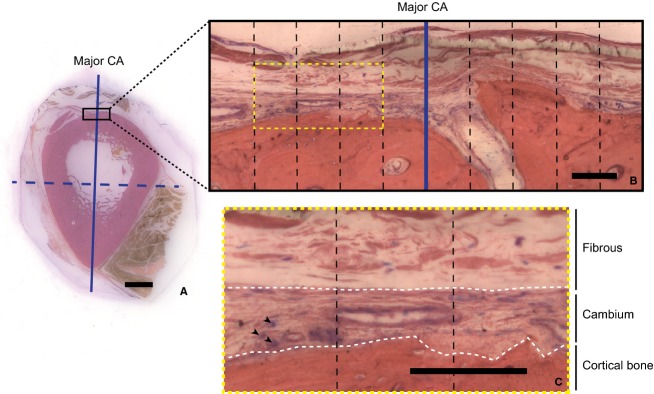
(A) Tibial cross-section from Donor Number 4 showing major (solid) and minor (dashed) CAs. Scale bar: 5 mm. (B) Region of interest along bone surface and 100-μm divisions (black dashed line) from axis. Scale bar: 100 μm (C) White dashed lines delineate the cambium layer. Cells (blue-staining nuclei, indicated by black arrow heads) are counted within the cambium layer, and thickness of both cambium and fibrous layers are measured in the center of each 100-μm region. Scale bar: 100 μm.
Cambium cell counts were carried out in contiguous regions containing clearly focused cells, in a single focal plane, and directly adjacent to bone (Fig. 1). All cells were counted on randomized samples by one observer to minimize bias. The Cell Counter feature of imagej was used to mark cells in each region, preventing double-counting or missed counts, and providing a read-out of cell number in each region. The number of cells in the cambium layer represents the number of cell nuclei counted in a 100-μm region along the surface of the bone (Fig 1). The region of counted and marked cambium cells defined the cambium layer, where the thickness was measured as the perpendicular distance from the bone surface to furthest border of the counted cells. The fibrous layer is defined as the measured distance from the edge of the cambium layer not adjacent to bone to the interface of periosteum with muscle.
Statistical methods
The thickness and number of cells in each sample was calculated as the mean of the 20 measurements along each CA for total thickness and cell number comparisons. As data are normally distributed, parametric methods were employed. A manova was used to assess differences in pooled parameters from femora and tibiae. Paired t-tests were used to compare bones from the same donor, and two-sample t-tests were used to compare non-matched groups. Correlations were calculated as the Pearson correlation coefficient. The level for significance was set at P < 0.05, and all statistical calculations comparisons were performed using minitab 16.
Results
Total periosteal thickness is approximately 100 μm for both tibiae and femora (Fig. 2A), with respective mean cambium layer thicknesses of 29 ± 3.1 and 23 ± 2.5 μm, and mean fibrous layer thicknesses of 72 ± 5.1 and 77 ± 8.8 μm. The fibrous layer along the centroidal axes of both tibiae and femora is significantly thicker than the respective cambium layer (paired t-tests, P < 0.05). In general, both thickness and cellularity measures show high interindividual variability (Fig. 2A,B). Furthermore, manova tests comparing outcome measures between the femora and tibiae of the group as a whole (n = 7 or 8, Table1, Fig. 2) show no significant differences.
Figure 2.
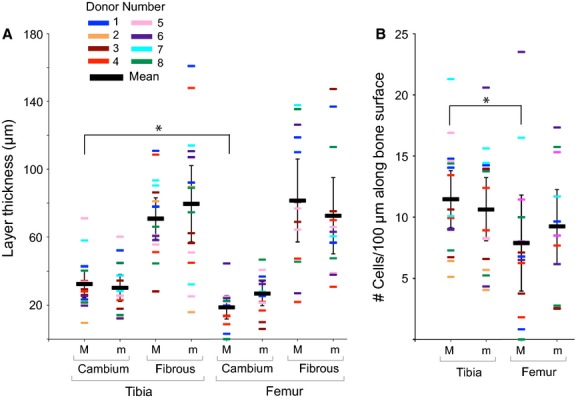
Donor-specific mean measurements major (M) and minor (m) centroidal axes (donors summarized in Table1). Error bars indicate 95% confidence intervals of mean measurement for all samples, where each value is a mean of 20 measurements. (A) Measurements of layer thickness along the major centroidal axes indicated a significantly (*P < 0.05) thicker cambium layer covering the tibia than femur. (B) Significantly more cells (*P < 0.05) are located in the cambium layer along the major axis in the tibia than the femur.
Paired t-tests comparing tibia and femur measures within individual donors indicate a significantly thicker cambium layer covering the major CA of the tibia than on the same location on the femur (Fig. 2A, P = 0.021). In addition, the number of cells along the major CA of the tibia is significantly higher than along the femur (Fig. 2B, P = 0.047). Finally, for both the tibia and the femur of individual donors, no significant differences are observed between cell number along the major and minor CA.
Additionally, for both tibiae and femora, each respective variable (cambium thickness, fibrous thickness, and cambium cell number) was plotted against age as well as weight and a linear regression was performed. Neither correlation with age nor donor weight showed statistical significance (data not shown).
In general, periosteal parameters from amputees exhibit high variability (Supporting Information Fig. S1). Although the number of specimens from amputees precluded statistical analysis, qualitative comparison of an amputated tibia with its donor-matched contralateral side revealed a visibly less cellular cambium layer, with a looser fibrous organization (Fig. 3). Comparison of the periosteum of a non-amputee femur with that of the double femoral amputee also indicated a much looser fibrous layer organization in the amputee.
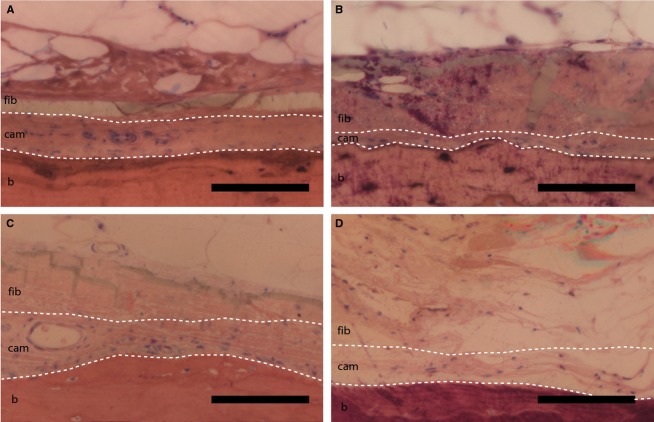
Figure 3.
Comparisons of periosteum from amputated and non-amputated bone (b: cortical bone, cam: cambium layer of periosteum, fib: fibrous layer of periosteum). (A,B) Matched comparison of the contralateral, non-amputated tibia (A) compared to the amputated tibia (B), from Donor 10. (C) Normal femur (Donor 7), and (D) periosteum from an amputated femur (Donor 11). Of note, cambium cellularity is lower directly adjacent to amputated bone, and layers are more difficult to distinguish (B,D). Scale bar: 100 μm.
Discussion
Based on the results of this study, within a given individual, the cambium layer of the major axis of the tibia is significantly thicker and more cellular than the respective layer of the femur. These significant differences between the periosteum of the tibia and femur of a given individual do not translate to significant differences between the periosteum of the tibia and femur of the cohort. Specifically, accounting for interindividual variability and limited sample size, no significant differences are observed between measures of the group as a whole. Further, mid-diaphyseal periosteal measures including cambium and fibrous layer thickness and cellularity do not appear to correlate significantly with age or body mass. Finally, qualitative observations of periosteum in amputated and associated (contralateral or proximal) long bones of the lower extremity exhibit stark changes in layer organization, thickness, and cellularity. In a translational context, these novel data, though inherently limited by availability and accessibility of human mid-diaphyseal periosteum tissue, provide important reference values for use of periosteum in the healing and regeneration of tissue.
Of particular clinical interest, the cambium layer of periosteum covering the major axis of the mid-diaphyseal tibia is significantly thicker and more cellular than that of the layer covering the femur. Hence, the tibia may represent a preferred site for harvesting a maximal number of periosteal cells or for resecting tissue for transplantation. Additionally, due to the relative ease of access of the tibia compared with the femur, collection of periosteum from this site may minimize soft tissue injury due to harvesting. Finally, although the results of the current study relate to cell numbers, the specific proliferative and differentiation potentials of periosteal cells from these sites should be investigated in future studies (Chang et al., 2013).
Furthermore, the age of donors reported in the current study spans approximately 30 years. Previous studies using a rat model have noted a decrease in periosteal thickness throughout life as the membrane becomes denser, more tightly organized, and less cellular (Ellender et al., 1988; Fan et al., 2008). Rat periosteum also shows a continual decrease in mid-diaphyseal cambium layer cell number and cambium thickness with increasing age, including juvenile, adult, and aged rat femora. In the present study of an elderly human group (68–99 years of age), however, correlations between age and layer thicknesses and age and cellularity did not indicate that the trend observed in rat periosteum persists in humans during advanced age. Further, the observed variation in cross-sectional geometry and associated calculation of CA for the femoral mid-diaphysis has been noted by our group previously and may underscore the appropriateness of CAs as anatomical reference points within but not between patients or human cadaveric donors; hence, repeat measures within individuals (over time) may be necessary to capture potential changes in periosteal structure attributable to age. Finally, in a study of periosteal cells derived from metaphyseal periosteum tissue resected during hip replacement surgery, cell proliferation and differentiation capacities do not correlate significantly with patient age (Chang et al., 2013).
Comparing all outcome measures taken along the major vs. minor CA, no significant differences were observed either within or between individuals, indicating lack of a significant relationship between mechanical loading function and the thickness and cellularity of the periosteum. Interestingly, in an ovine model study of periosteally mediated tissue regeneration in a critical-sized defect, early periosteum-mediated bone formation was significantly correlated with large net changes in strain environment, including a net change from its baseline pre-stressed state, rather than the maximum magnitudes of periosteal strain (McBride et al., 2011a). However, neither mid-diaphyseal periosteum in normal ovine femora nor regenerated periosteum from denuded transport segments exhibit significant differences in thickness along the minor and major CA (Knothe Tate et al., 2012). Yet, in culture, periosteal cells are sensitive to mechanical strains and respond by altering their shape, proliferating, and upregulating chondrogenic and osteogenic differentiation factors (Saris et al., 1999; Kanno et al., 2005). Periosteal cells have also been observed to respond to applied loads in vivo through reorganization of their cytoskeleton and bone formation (LaMothe et al., 2005; Kock et al., 2010; Sakai et al., 2011). In addition, cultured periosteal cells increase cell division, collagen synthesis, and collagenase activity in response to physiological strains (Jones et al., 1991). At a tissue level, the membrane's collagen alignment and anisotropic material properties have been implicated in guiding long bone growth (Bertram et al., 1998; Foolen et al., 2008; McBride et al., 2011c).
Centroidal axes, which are calculated as the second moments of area, represent planes about which a cross-section offers the most and least resistance to bending. In this sense, CAs could in theory provide references for mechanical function due to predominant bending loads. Lieberman et al. (2004) have assessed this relationship in an ovine model, comparing calculated CA to experimentally measured strain data from the bone surface. They found that although the absolute magnitudes of the experimental vs. calculated axes are notably different, the patterns, or relative values, of calculated axes are highly correlated and useful in comparing bones from the same or similar species. While major and minor CAs can serve as a substitute for relative loading history in matched experimental cohorts (McBride et al., 2011b), a recent study in our lab group indicates that they may not be an appropriate reference for mechanical loading history in human femora, which exhibit great anatomical variation with increasing age. Further, it would not be feasible or ethical to use the current methodology to determine whether these axes correlate in situ with actual patterns of mechanical loading in humans (as this would require strain gauging or strain mapping of fresh tissues). Hence, for the purpose of the current study, we use the CAs as landmarks for comparing regions of periosteum overlying bone that are likely to have experienced different mechanical loading histories.
In addition, the extent to which muscular forces affect bone cross-sectional geometry and thus CAs is currently not well known. The muscular environment influences the cross-sectional geometry of long bones (Carpenter & Carter, 2008), and we have assessed this recently in the context of the emergence of the linea aspera along the posterior aspect of the human femur using finite element modeling and simulations of adaptation with advancing age. Muscular strength, size, and geometry are very difficult to measure experimentally, but offer an interesting area for future study in the context of the periosteum and bone functional adaptation.
Taken together, periosteum tissue per se may not be as mechanosensitive as cells derived from the periosteum. Rather, our working hypothesis is that the periosteum provides a quiescent niche for stem cells to reside in at steady state; however, gross perturbation of periosteum baseline mechanical state, e.g. through surgical procedures such as periosteal lifting, or through trauma, may induce proliferation, egression, and differentiation of the multipotent cells within the cambium and fibrous layers. Interestingly, recent studies of periosteum-derived multipotent cells indicate intrinsic differences between the cells of the fibrous and cambium layers of periosteum from the femoral neck, as cells isolated via enzymatic digestion of the periosteum show subtle, although not significant, differences in expression of surface markers as well as differentiation capacity compared with cells isolated using a migration protocol. Follow-on studies will determine whether the isolation method per se more efficiently isolates cells from the fibrous and/or cambium layers or directly influences differentiation capacity (Chang et al., 2013).
Ongoing studies will seek to describe further the microstructural parameters of the periosteum in relation to nutrient transport, as it is known that the membrane exhibits direction-as well as load-dependent permeability properties (Evans et al., 2012b). Periosteal microstructural parameters are of particular interest to engineers looking to replicate the mechanoactive and barrier functions of the periosteum. Guided bone regeneration offers one such example of a successful tissue engineering technique to mimic the selective permeability properties of the periosteum (Knothe Tate et al., 2011; Gamal & Iacono, 2012), where surgical membranes are used to strategically exclude rapidly proliferating fibrous tissue from the bone defect concomitant with facilitating de novo direct intramembranous and indirect endochondral bone formation within the defect (Knothe Tate et al., 2008). Customizing the replacement membrane permeability based on physical and mechanical parameters of native periosteum, for example the permeability of the fibrous layer based on local collagen alignment and thickness, may help enhance tissue regeneration outcomes (Taguchi et al., 2005; Colnot et al., 2012).
Recognizing inherent limitations of small sample sizes due to paucity of appropriate human cadaveric specimens, the results of this study nonetheless indicate a significant difference in thickness and cellularity of the cambium layer from mid-diaphyseal periosteum of the femur and tibia of aged patients. The growing population of aged individuals as well as contradictory reports regarding the regenerative potential of periosteum with advanced age (O'Driscoll et al., 2001; Fan et al., 2008; Chang et al., 2013) further underscore the clinical relevance of the current study of periosteum from aged donors between 68 and 99. Follow-on studies should include not only more samples but also samples from a greater range of ages; given the lack of accessibility of tissue from healthy donors, follow-on studies will likely necessitate collection of tissue from the Organ Procurement and Transplantation Network (OPTN) as well as close collaboration with groups that have significant experience in undecalcified histology to minimize preparation artifacts.
Despite growing clinical interest in harnessing the regenerative potential of periosteum, relatively little is known about human periosteal parameters in situ (Allen et al., 2004). In contrast to the growing body of work on periosteal cell proliferation and differentiation characteristics (Chang & Knothe Tate, 2012), other functional parameters of the biological, mechanical, barrier, nervous and vascular dynamics of periosteum are not yet as well described. Accordingly, thickness and cellularity of human periosteum are important parameters for engineering replacements as well as for surgeons looking to minimize tissue damage while harvesting the most viable periosteum possible for autologous regenerative therapies. In particular, the current study provides a new context for understanding the basic structural features of mid-diaphyseal periosteum from femora and tibiae of aged donors.
Acknowledgments
Alexander von Humboldt Foundation, AO ASIF Research Foundation, Ludwig-Maximilians-University, Christopher Columbus Foundation and the Whitaker International Program.
Authors' contributions
Study design: S.R.M., S.M. and M.L.K.T. Data collection: S.R.M. Data analysis: S.R.M. and M.L.K.T. Data interpretation: S.R.M., S.M. and M.L.K.T. Drafting manuscript: S.R.M. and M.L.K.T. Approving final version of manuscript: S.R.M., S.M. and M.L.K.T. Responsibility for the integrity of the data analysis: S.R.M.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Periosteal outcome measures from amputees, comparing amputated bones and non-amputated bones (donors are summarized in Table 2).
References
- Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–1012. doi: 10.1016/j.bone.2004.07.014. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Bertram JE, Polevoy Y, Cullinane DM. Mechanics of avian fibrous periosteum: tensile and adhesion properties during growth. Bone. 1998;22:669–675. doi: 10.1016/s8756-3282(98)00035-0. [DOI] [PubMed] [Google Scholar]
- Carpenter RD, Carter DR. The mechanobiological effects of periosteal surface loads. Biomech Model Mechanobiol. 2008;7:227–242. doi: 10.1007/s10237-007-0087-9. [DOI] [PubMed] [Google Scholar]
- Chang H, Knothe Tate ML. Concise review: the periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl Med. 2012;1:480–491. doi: 10.5966/sctm.2011-0056. doi: 10.5966/sctm.2011-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Docheva D, Knothe UR, et al. 2013. Arthritic periosteal tissue from joint replacement surgery as an autologous source of stem cells In Review.
- Colnot C, Zhang X, Knothe Tate ML. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J Orthop Res. 2012;30:1869–1878. doi: 10.1002/jor.22181. doi: 10.1002/jor.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellender G, Feik SA, Carach BJ. Periosteal structure and development in a rat caudal vertebra. J Anat. 1988;158:173–187. [PMC free article] [PubMed] [Google Scholar]
- Evans S, Chang H, Knothe Tate ML. Elucidating multiscale periosteal mechanobiology: a key to unlocking the smart properties and regenerative capacity of the periosteum? Tissue Eng Part B Rev. 2012a;19:1–47. doi: 10.1089/ten.teb.2012.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SF, Parent JB, Lasko CE, et al. Periosteum, bone's ‘smart' bounding membrane, exhibits direction dependent permeability. J Bone Miner Res. 2012b;28:608–617. doi: 10.1002/jbmr.1777. [DOI] [PubMed] [Google Scholar]
- Fan W, Crawford R, Xiao Y. Structural and cellular differences between metaphyseal and diaphyseal periosteum in different aged rats. Bone. 2008;42:81–89. doi: 10.1016/j.bone.2007.08.048. [DOI] [PubMed] [Google Scholar]
- Foolen J, Van Donkelaar C, Nowlan N, et al. Collagen orientation in periosteum and perichondrium is aligned with preferential directions of tissue growth. J Orthop Res. 2008;26:1263–1268. doi: 10.1002/jor.20586. [DOI] [PubMed] [Google Scholar]
- Gamal AY, Iacono VJ. Enhancing guided tissue regeneration of periodontal defects by using a novel perforated barrier membrane. J Periodontol. 2012;1:14. doi: 10.1902/jop.2012.120301. [DOI] [PubMed] [Google Scholar]
- Jones DB, Nolte H, Scholübbers JG, et al. Biochemical signal transduction of mechanical strain in osteoblast-like cells. Biomaterials. 1991;12:101–110. doi: 10.1016/0142-9612(91)90186-e. [DOI] [PubMed] [Google Scholar]
- Kanno T, Takahashi T, Ariyoshi W, et al. Tensile mechanical strain up-regulates Runx2 and osteogenic factor expression in human periosteal cells: implications for distraction osteogenesis. J Oral Maxillofac Surg. 2005;63:499–504. doi: 10.1016/j.joms.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Knothe UR, Springfield DS. A novel surgical procedure for bridging of massive bone defects. World J Surg Oncol. 2005;3:7. doi: 10.1186/1477-7819-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe Tate ML, Falls TD. Mechanical modulation of osteochondroprogenitor cell fate. Int J Biochem Cell Biol. 2008;40:2720–2738. doi: 10.1016/j.biocel.2008.05.011. McBride SH, et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe Tate ML, Chang H, Moore SR, et al. Surgical membranes as directional delivery devices to generate tissue: testing in an ovine critical sized defect model. PLoS ONE. 2011;6:e28702. doi: 10.1371/journal.pone.0028702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe Tate ML, Merritt FC, Erinc A, et al. Periosteum regenerates on periosteum-denuded, transported bone segment. Trans Orthopaedic Res Soc. 2012;37:1570. [Google Scholar]
- Kock LM, Ravetto A, Van Donkelaar CC, et al. Tuning the differentiation of periosteum-derived cartilage using biochemical and mechanical stimulations. Osteoarthritis Cartilage. 2010;18:1528–1535. doi: 10.1016/j.joca.2010.09.001. [DOI] [PubMed] [Google Scholar]
- LaMothe JM, Hamilton NH, Zernicke RF. Strain rate influences periosteal adaptation in mature bone. Med Eng Phys. 2005;27:277–284. doi: 10.1016/j.medengphy.2004.04.012. doi: 10.1016/j.medengphy.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Polk JD, Demes B. Predicting long bone loading from cross-sectional geometry. Am J Phys Anthropol. 2004;123:156–171. doi: 10.1002/ajpa.10316. [DOI] [PubMed] [Google Scholar]
- McBride SH, Dolejs S, Brianza S, et al. Net change in periosteal strain during stance shift loading after surgery correlates to rapid de novo bone generation in critically sized defects. Ann Biomed Eng. 2011a;39:1570–1581. doi: 10.1007/s10439-010-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SH, Dolejs S, Knothe U, et al. Major and minor centroidal axes serve as objective, automatable reference points to test mechanobiological hypotheses using histomorphometry. J Biomech. 2011b;44:1205–1208. doi: 10.1016/j.jbiomech.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SH, Evans SF, Knothe Tate ML. Anisotropic mechanical properties of ovine femoral periosteum and the effects of cryopreservation. J Biomech. 2011c;44:1954–1959. doi: 10.1016/j.jbiomech.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Milz S, Knothe TM. The emergence of the linea aspera: a virtual case study testing emergence of form and function. The Anat Record. 2013 doi: 10.1002/ar.22840. in press. [DOI] [PubMed] [Google Scholar]
- O'Driscoll SW, Saris DB, Ito Y, et al. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001;19:95–103. doi: 10.1016/S0736-0266(00)00014-0. [DOI] [PubMed] [Google Scholar]
- Sakai D, Kii I, Nakagawa K, et al. Remodeling of actin cytoskeleton in mouse periosteal cells under mechanical loading induces periosteal cell proliferation during bone formation. PLoS ONE. 2011;6:e24847. doi: 10.1371/journal.pone.0024847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saris DB, Sanyal A, An KN, et al. Periosteum responds to dynamic fluid pressure by proliferating in vitro. J Orthop Res. 1999;17:668–677. doi: 10.1002/jor.1100170508. [DOI] [PubMed] [Google Scholar]
- Sauser B. 2010. Technology review: faster healing for severe fractures MIT Tech Review. Available: http://www.technologyreview.com/news/417946/faster-healing-for-severe-fractures/?a=f (accessed 5 April 2013)
- Taguchi Y, Amizuka N, Nakadate M, et al. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials. 2005;26:6158–6166. doi: 10.1016/j.biomaterials.2005.03.023. doi: 10.1016/j.biomaterials.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Warfel M, Serafin S, DeLeon VB. 2005. MomentMacroJ. Version 1.3 Available: http://www.hopkinsmedicine.org/fae/mmacro.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Periosteal outcome measures from amputees, comparing amputated bones and non-amputated bones (donors are summarized in Table 2).