Abstract
Continuous lifelong neurogenesis is typical of the vertebrate olfactory system. The regenerative ability of olfactory receptor neurons is dependent on the glial cell type specific to the olfactory pathway, designated ‘olfactory ensheathing cells'. Several studies to date have focused on mammalian olfactory ensheathing cells, owing to their potential roles in cell-based therapy for spinal cord injury repair. However, limited information is available regarding this glial cell type in non-mammalian vertebrates, particularly anamniotes. In the current immunocytochemical study, we analysed the features of olfactory ensheathing cells in the zebrafish, Danio rerio. Fish provide a good model for studying glial cells associated with the olfactory pathway of non-mammalian vertebrates. In particular, zebrafish has numerous valuable features that enable its use as a prime model organism for genetic, neurobiological and developmental studies, as well as toxicology and genomics research. Paraffin sections from decalcified heads of zebrafish were processed immunocytochemically to detect proteins used in the research on mammalian olfactory ensheathing cells, including glial fibrillary acid protein (GFAP), S100, neural cell adhesion molecule (NCAM), polysialylated NCAM (PSA-NCAM), vimentin (VIM), p75NTR and galactin (Gal)-1. Notably, GFAP, S100, NCAM and Gal-1 were clearly observed, whereas no vimentin staining was detected. Weak immunostaining for PSA-NCAM and p75NTR was evident. Moreover the degree of marker expression was not uniform in various tracts of the zebrafish olfactory pathway. The immunostaining patterns of the zebrafish olfactory system are distinct from those of other fish to some extent, suggesting interspecific differences. We also showed that the olfactory pathway of zebrafish expresses markers of mammalian olfactory ensheathing cells. The olfactory systems of vertebrates have similarities but there are also marked variations between them. The issue of whether regional and interspecific differences in immunostaining patterns of olfactory pathway markers have functional significance requires further investigation.
Keywords: ensheathing cells, fish, immunohistochemistry, olfactory system, zebrafish
Introduction
The olfactory system is sometimes considered a primitive system that has undergone little evolutionary modification over time, in terms of not only morphology but also molecular characteristics. Comparative studies in vertebrates show similarities but there are also marked variations between their olfactory systems (see Eisthen & Polese, 2006). In both terrestrial and aquatic vertebrates, the nasal cavities opening out directly to the external environment contain the olfactory epithelium. Different strategies of ventilation facilitate fluid change in these cavities. New neurons, mitotically produced by basal cells in the deeper region of the olfactory epithelium, replace dead olfactory receptor neurons (ORNs) throughout life (Graziadei & Monti-Graziadei, 1979; Mackay-Sim & Kittel, 1991; Franssen et al. 2007). During differentiation, newly formed ORNs direct their growing axons through the olfactory nerve towards the glomerular layer of the olfactory bulb (OB), in which they form synaptic connections with mitral cells (Graziadei & Monti-Graziadei, 1979).
In the olfactory pathway, from the basal lamina of the olfactory epithelium to the glomerular layer of the olfactory bulb, glial cells, designated olfactory ensheathing cells (OECs), envelop the olfactory axons and are involved in the regenerating capability of ORNs throughout life (see Pellitteri et al. 2010). Ensheathment of ORN axons stops just a short distance from the synapse with mitral cell dendrites in the glomeruli of the olfactory bulb. In this context, OECs represent the only glial population that continuously ensheathe axons from the peripheral nervous system to the CNS through the ethmoid bone. The OEC population exhibits characteristics of both astrocytes and Schwann cells (Raisman, 2001; Wewetzer et al. 2002; Mackay-Sim, 2005). An earlier prevailing viewpoint asserts that these two cell types do not share a common origin, as Schwann cells originate from the neural crest whereas OECs are derived from olfactory placodes (Chuah & Au, 1991). However, a recent study showed that OECs are derived during normal development from neural crest cells and not olfactory placodes (Barraud et al. 2010). During embryonic development, OEC ancestors are associated with primitive ORNs extending their un-myelinated axons from the olfactory epithelium to the developing olfactory bulb (Ramon-Cueto & Avila, 1998; Fairless & Barnett, 2005). Recently, OECs have been a focus of interest in neurobiology research, revealing a prominent role in promoting regeneration of the injured spinal cord (Raisman, 2001; Barnett & Riddell, 2004; Chung et al. 2004). In fact, OECs transplanted into injured spinal cord appear to promote remyelination of damaged axons, as well as regeneration to functional recovery (Lu et al. 2002; Mackay-Sim, 2005; Granger et al. 2012; Coutts et al. 2013). Pellitteri et al. (2010) suggested that this behaviour is related to the possibility that OECs operate as a source of growth factors and adhesion molecules (Ramon-Cueto & Avila, 1998; Boruch et al. 2001; Woodhall et al. 2001). Moreover, OECs express specific markers and are thus amenable to immunocytochemical characterisation (Franceschini & Barnett, 1996; Vincent et al. 2005; Pellitteri et al. 2010).
Although mammalian OECs, considered potential therapeutic candidates in spinal cord injury repair, have been extensively studied, limited information is available regarding the characteristics of OECs in non-mammalian vertebrates (Huang et al. 2005; Lazzari et al. 2013). Fish represent a good model for analysing the olfactory pathway in non-mammalian vertebrates. In fact, the olfactory system of fish is well characterised with regard to both anatomy and function (Franceschini et al. 1994, 1996, 1999, 2000; Lazzari et al. 2007; Bettini et al. 2009). Earlier studies indicate that its principal activity is related to feeding and intraspecific relationships, mainly sexual behaviour (Yamamoto, 1982; Byrd & Brunjes, 1995).
The olfactory system of teleost fish displays a heterogeneous anatomical structure. Accordingly, in a previous study (Lazzari et al. 2013), we selected models from species with different morphologies of the olfactory region. In particular, we focused on goldfish, Carassius auratus, and guppy, Poecilia reticulata, as representatives of macrosmatic and microsmatic fish, respectively. Macrosmatic fish have well developed multilamellar olfactory rosettes with a behaviourally predominant olfactory faculty. In contrast, microsmatic fish possess poorly developed, even unilamellar rosettes and rely more on a different sense perception, mainly vision, than on olfaction (Teichmann, 1954; Lazzari et al. 2007; Bettini et al. 2009). The macrosmatic condition prevails in teleosts and therefore the question arises whether OECs show the same immunocytochemical pattern throughout macrosmatic fish. In the present immunohistochemical investigation, we studied the expression of GFAP, S100, NCAM, PSA-NCAM, vimentin, p75NTR and galectin-1 (Gal-1) along the primary olfactory pathway of zebrafish, Danio rerio, a macrosmatic species. These proteins are commonly used in studies of OECs in mammals, but none of them is a specific marker for OECs (see Barnett & Chang, 2004; Vincent et al. 2005; Pellitteri et al. 2010; Higginson & Barnett, 2011). NCAM in mammals is an olfactory axon marker and has actually been used to distinguish axons from OECs (Au et al. 2002).
For more extensive information about expression of each of these proteins in OECs in other vertebrate species, suggested papers are listed in Table 1.
Table 1.
References to papers describing expression of each antigen, GFAP, S100, NCAM, PSA-NCAM, VIM, p75NTR, and GAL-1, in OECs in other vertebrate species.
| Antigen | Vertebrate Group | Species | Reference |
|---|---|---|---|
| GFAP | Chondrichthyes | Scyliorhinus canicula | Quintana-Urzainqui et al. (2012) |
| Actinopterygii | Carassius auratus | Lazzari et al. (2013) | |
| Poecilia reticulata | Lazzari et al. (2013) | ||
| Amphibia | Xenopus sp. | Huang et al. (2005) | |
| Mammalia | Cat, guinea, pig | Smithson & Kawaja, (2009) | |
| Dog | Krudewig et al. (2006) | ||
| Hamster, rabbit, monkey, mouse | Rawji et al. (2013) | ||
| Human | Liu et al. (2010a) | ||
| Mouse | Au & Roskams, (2003); Chehrehasa et al. (2010); Geller et al. (2013); Katoh et al. (2011); Nakajima et al. (2013) |
||
| Rat | Astic et al. (1998); Audisio et al. (2009); Babiarz et al. (2011);Franceschini & Barnett, (1996); Kumar et al. (2005); Li et al. (2012); Liu et al. (2010b) |
||
| S100 | Actinopterygii | Carassius auratus | Lazzari et al. (2013) |
| Poecilia reticulata | Lazzari et al. (2013) | ||
| Mammalia | Cat, Guinea pig | Smithson & Kawaja, (2009) | |
| Hamster rabbit monkey mouse | Rawji et al. (2013) | ||
| Human | Liu et al. (2010a) | ||
| Mouse | Au & Roskams, (2003); Chehrehasa et al. (2010); Geller et al. (2013); Katoh et al. (2011); Rela et al. (2010) |
||
| Rat | Astic et al. (1998); Audisio et al. (2009); Franceschini & Barnett, (1996); Li et al. (2012); Lipson et al. (2003) |
||
| NCAM | Actinopterygii | Carassius auratus | Lazzari et al. (2013) |
| Poecilia reticulata | Lazzari et al. (2013) | ||
| Mouse | Rela et al. (2010) | ||
| Rat | Franceschini & Barnett, (1996) | ||
| PSA-NCAM | Actinopterygii | Carassius auratus | Lazzari et al. (2013) |
| Poecilia reticulata | Lazzari et al. (2013) | ||
| Mammalia | Mouse | Geller et al. (2013) | |
| Rat | Franceschini & Barnett, (1996); Kumar et al. (2005) | ||
| VIM | Actinopterygii | Carassius auratus | Lazzari et al. (2013) |
| Poecilia reticulata | Lazzari et al. (2013) | ||
| Amphibia | Xenopus sp | Huang et al. (2005) | |
| Mammalia | Mouse | Au & Roskams, (2003); Geller et al. (2013) | |
| p75NTR | Actinopterygii | Carassius auratus | Lazzari et al. (2013) |
| Poecilia reticulata | Lazzari et al. (2013) | ||
| Aves | Chick | Barraud et al. (2010) | |
| Mammalia | Cat guinea pig | Smithson & Kawaja, (2009) | |
| Dog | Bock et al. (2007); Krudewig et al. (2006) | ||
| Human | Liu et al. (2010a) | ||
| Mouse | Au & Roskams, (2003); Chehrehasa et al. (2010); Geller et al. (2013); Katoh et al. (2011); Nakajima et al. (2013) |
||
| Rat | Audisio et al. (2009); Babiarz et al. (2011); Franceschini & Barnett, (1996); Kumar et al. (2005); Li et al. (2012); Lipson et al. (2003); Liu et al. (2010b) |
||
| GAL-1 | Actinopterygii | Carassius auratus | Lazzari et al. (2013) |
| Poecilia reticulata | Lazzari et al. (2013) | ||
| Mammalia | Rat | St John & Key, (1999) |
Zebrafish is a tropical freshwater fish belonging to the minnow family (Cyprinidae) of the order Cypriniformes, the same family as goldfish. Therefore, it is possible to determine whether uniform conditions are shared at the family level. Moreover, zebrafish has numerous valuable features that enable its use as a prime model organism for genetic, neurobiological and developmental studies, as well as toxicology and genomic research. The main aim of this study was to extend our knowledge of the biology of vertebrates that exhibit comparable organs and tissues to humans despite being genetically distant, with a view to using this model to obtain data for analysis, and ultimately, treatment of human biological problems.
Materials and methods
Animals
Ten adult zebrafish (Danio rerio, Hamilton, 1822) of both sexes (5–6 cm body length) were purchased locally from the Acquario Fossolo (Bologna, Italy). Fish were maintained in aquaria at 25 °C under a natural light-dark cycle, and fed once a day. All procedures conformed with the guidelines of European Communities Council Directive (86/609/CEE), the current Italian legislation regarding the use and care of animals, and the guidelines issued by the U.S. National Institutes of Health. The study was approved by the University of Bologna Scientific Ethics Committee.
Tissue preparation
Zebrafish were anaesthetised with 0.1% 3-aminobenzoic acid ethyl ester (MS-222, Sigma, St. Louis, MO) and killed via decapitation. After removal of the dorsal cranium, heads were promptly immersed in modified Bouin's fixative solution containing a saturated aqueous solution of picric acid and formalin (ratio 3 : 1), for 24 h at room temperature. Picric acid was removed by prolonged washing in 0.1 m sodium phosphate buffer (PB), pH 7.4, at room temperature. Heads were decalcified with 0.25 m EDTA in 0.1 m PB, pH 7.4, for 3–7 days at room temperature, depending on bone tissue size and degree of mineralisation. Specimens were dehydrated in a graded series of ethanol (70, 80, 95, 100%, for 40 min each at room temperature) and subsequently embedded in Paraplast plus (Sherwood Medical, St. Louis, MO; melting point 55–57 °C). Frontal 5-μm-thick sections were cut with a Leica RM2145 microtome, mounted on silane-coated slides (Sigma) and dried. Adjacent slides were used for immunohistochemical detection.
Immunohistochemistry
In immunohistochemical processing, all incubation and washing solutions, except those containing primary antibodies, were used at room temperature. After deparaffinisation with xylene and rehydration, endogenous peroxidase activity was quenched with 1% H2O2 in 0.01 m PB containing 0.15 m NaCl (PBS), pH 7.4, for 20 min. For antigen retrieval, tissue sections were immersed in 0.01 m citrate buffer, pH 6.0 (substituted with 0.01 m Tris 0.1 m EDTA buffer, pH 8.8, for PSA-NCAM), and heated in a microwave oven (750 W) for two cycles of 5 min each at 98 °C. Non-specific background staining was reduced by preincubation in PBS containing 10% normal goat serum (NGS; Vector Laboratories, Burlingame, CA), 1% bovine serum albumin (BSA; Sigma) and 0.1% Tween 20 (Merck, Darmstadt, Germany) for 30 min. Sections were incubated separately overnight with seven primary antibodies in a moist chamber on a floating plate at 4 °C: (i) rabbit polyclonal antibody against bovine glial fibrillary acidic protein (GFAP) (1 : 500, Z 0334, DAKO Cytomation, Glostrup, Denmark), (ii) rabbit polyclonal antibody against bovine S100 (1 : 1000, Z 0311, DAKO Cytomation) recognising both S100B and S100A1, (iii) rabbit polyclonal antibody against chicken neural cell adhesion molecule (NCAM) (1 : 2000, AB5032, Chemicon International, Temecula, CA), (iv) mouse monoclonal anti-meningococcus PSA-NCAM (1 : 50, MAB5324, Millipore, Billerica, MA), (v) rabbit polyclonal antibody against low-affinity nerve growth factor receptor, also known as p75 neurotrophin receptor (p75NTR) (1 : 250, 07-476, Millipore), (vi) rabbit polyclonal antibody against Gal-1 (LGALS1, 1 : 200, HPA000646, Sigma), and (vii) mouse monoclonal anti-human vimentin antibody (1 : 50, CBL202, Millipore). Antibodies were diluted in PBS containing 3% NGS, 1% BSA and 0.1% Tween 20. After rinsing in PBS with 0.1% Tween 20, sections were incubated for 1 h with secondary antibody, including HRP-conjugated goat anti-mouse IgG (1 : 200, A4416, Sigma) for vimentin, HRP-conjugated goat anti-mouse Ig μm (1 : 100, 12–489, Millipore) for PSA-NCAM, and HRP-conjugated goat anti-rabbit IgG (1 : 200, PI-1000, Vector Laboratories) for the other markers, in PBS containing 1% BSA and 0.1% Tween 20. After rinsing in PBS, immunoreaction was visualised using the intensified diaminobenzidine method (Adams, 1981). Sections were dehydrated in ethanol, cleared in xylene, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA). Negative controls for specificity of immunostaining were obtained by omission of primary antibodies, replaced with 3% NGS. Sections of olfactory epithelium and bulb and hippocampus from mouse and rat were used as positive controls. As the antibodies used in this study had not been reported previously to recognise the relevant antigens in zebrafish, positive control tests were carried out on zebrafish nervous system cells known to express these proteins. A different monoclonal anti-vimentin antibody (clone V9, Boehringer, also available from Millipore, MAB3400) was employed by Cerdà et al. (1998) to characterise zebrafish vimentin. Bernardos & Raymond (2006) utilised an anti-zebrafish GFAP monoclonal antibody, zrf-1, available from the Zebrafish International Resource Centre, ZIRC, Oregon (http://zfin.org/ZDB-ATB-081002-46). In their study of radial glia in the spinal cord of zebrafish embryos, Kim et al. (2008) made use of both zrf-1 monoclonal antibody (University of Oregon Monoclonal Antibody Facility) and mouse monoclonal anti-GFAP antibody, clone GA5 (Sigma). It is also worth noting that zrf-1 antibody recognises glia in both the central and peripheral nervous system (see citations in http://zfin.org/ZDB-ATB-081002-46).
Results
In the zebrafish olfactory system, the degree of immunostaining intensity differed in relation to the antigen and anatomical regions examined (Table 2).
Table 2.
Semiquantitative distribution of the immunostainings in the olfactory system of Danio rerio.
| GFAP | S100 | NCAM | PSA-NCAM | VIM | p75NTR | GAL-1 | |
|---|---|---|---|---|---|---|---|
| Olfactory neuroepithelium | + | +/++ | +/++ | − | − | +/++ | − |
| Epithelial lamina propria | + | + | ++ | −/+ | − | −/+ | − |
| Extracranial olfactory nerve | ++ | +/++ | +++ | − | − | + | − |
| Intracranial olfactory nerve | +/++ | +++ | ++/+++ | − | − | + | ++/+++ |
| Olfactory nerve layer | ++ | +++ | ++/+++ | −/+ | − | +/++ | ++/+++ |
| Glomerular layer | −/+ | +/++ | ++ | − | − | −/+ | ++/+++ |
−, absent; +, slight; ++, moderate; +++, intense.
The olfactory neuroepithelium exhibited slight GFAP immunopositivity. Slight GFAP staining was additionally localised in the neuroepithelial lamina propria (Fig. 1A,B: arrowheads, lamina propria; open arrowheads, immunolabelled elements). GFAP was moderately expressed in the extracranial olfactory nerve, whereas expression was somewhat reduced in the intracranial tract of the olfactory nerve (Fig. 1A–C: open arrowheads, immunolabelled elements). In the olfactory bulb, moderate GFAP staining was detected in the olfactory nerve layer, whereas both the glomerular layer and inner bulbar zone showed faint GFAP positivity (Fig. 1A,D: arrows, olfactory nerve layer; open arrowheads, immunolabelled elements).
Figure 1.
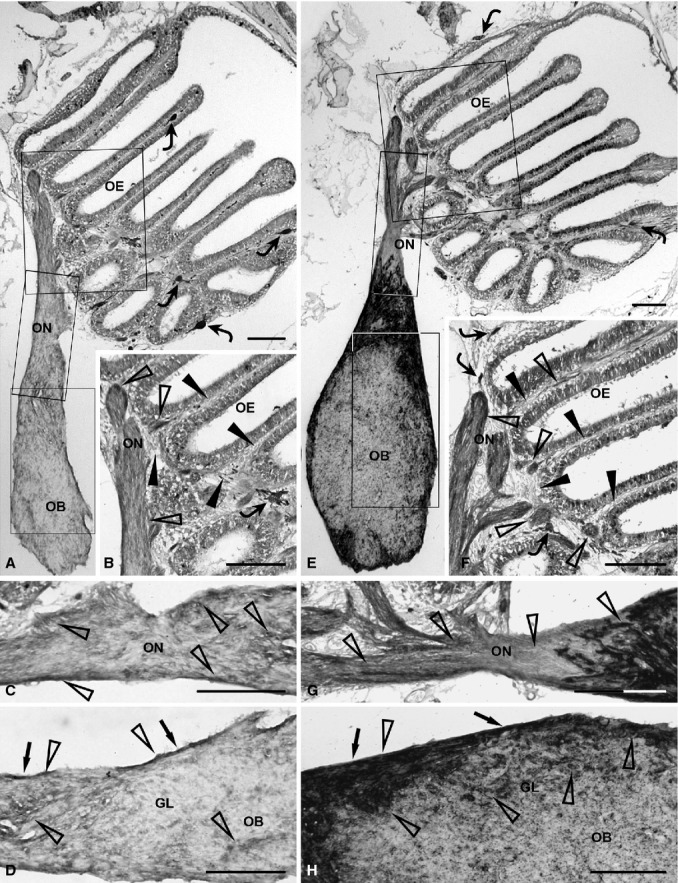
Immunohistochemical detection of olfactory ensheathing cell markers, GFAP (A–D) and S100 (E–H), in the olfactory system of zebrafish. (A) Distribution of GFAP immunopositivity in the complete olfactory system. Rectangular areas marked with OE, ON and OB are shown in (B), (C) and (D), respectively, at higher magnification. Some chromatophores (curved arrows) are present in the epithelial lamina propria. (B) GFAP immunopositivity (open arrowheads) appears slight in the epithelial lamina propria (arrowheads). A chromatophore (curved arrow) is visible. (C) The olfactory nerve shows clear GFAP immunopositivity (open arrowheads). (D) In the olfactory bulb, GFAP immunostaining (open arrowheads) is more evident in the olfactory nerve layer (arrows) than in the glomerular layer. (E) Distribution of S100 immunostaining in the whole olfactory system. Rectangular areas marked with OE, ON and OB are shown in (F), (G) and (H), respectively, at higher magnification. Some chromatophores (curved arrows) are evident in the epithelial lamina propria. (F) The epithelial lamina propria (arrowheads) shows clear immunostaining (open arrowheads) and some chromatophores (curved arrows). (G) In the olfactory nerve, the intracranial tract is heavily stained, compared with the extracranial portion (open arrowheads). (H) In the olfactory bulb, the olfactory nerve layer (arrows) exhibits intense staining (open arrowheads). Evident immunostaining (open arrowheads) appears in the glomerular layer. GL, glomerular layer; OB, olfactory bulb; OE, olfactory epithelium; ON, olfactory nerve. Scale bars: 100 μm.
The entire olfactory pathway, from the olfactory mucosa to glomerular layer of the olfactory bulb, showed clear S100 immunopositivity (Fig. 1E). Slight to moderate immunopositivity was observed in the olfactory neuroepithelium, whereas staining appeared reduced in the epithelial lamina propria (Fig. 1E,F: arrowheads, lamina propria; open arrowheads, immunolabelled elements). S100 immunopositivity was intense in the intracranial olfactory nerve as well as the olfactory nerve layer, but was slightly reduced in the extracranial olfactory nerve (Fig. 1E–H: arrows, olfactory nerve layer; open arrowheads, immunolabelled elements). The glomerular layer of the olfactory bulb showed slight to moderate S100 staining (Fig. 1E,H: open arrowheads, immunolabelled elements).
Additionally, the entire olfactory pathway was immunopositive for NCAM (Fig. 2A). Notably, expression of this antigen was slight to moderate in the olfactory neuroepithelium, moderate in the epithelial lamina propria, and intense in the extracranial olfactory nerve (Fig. 2A–C: arrowheads, lamina propria; open arrowheads, immunolabelled elements). NCAM staining appeared moderate to intense in both the intracranial olfactory nerve and olfactory nerve layer of the olfactory bulb (Fig. 2A,C,D: arrows, olfactory nerve layer; open arrowheads, immunolabelled elements). The bulbar glomerular layer showed moderate positivity (Fig. 2A,D: open arrowheads, immunolabelled elements).
Figure 2.
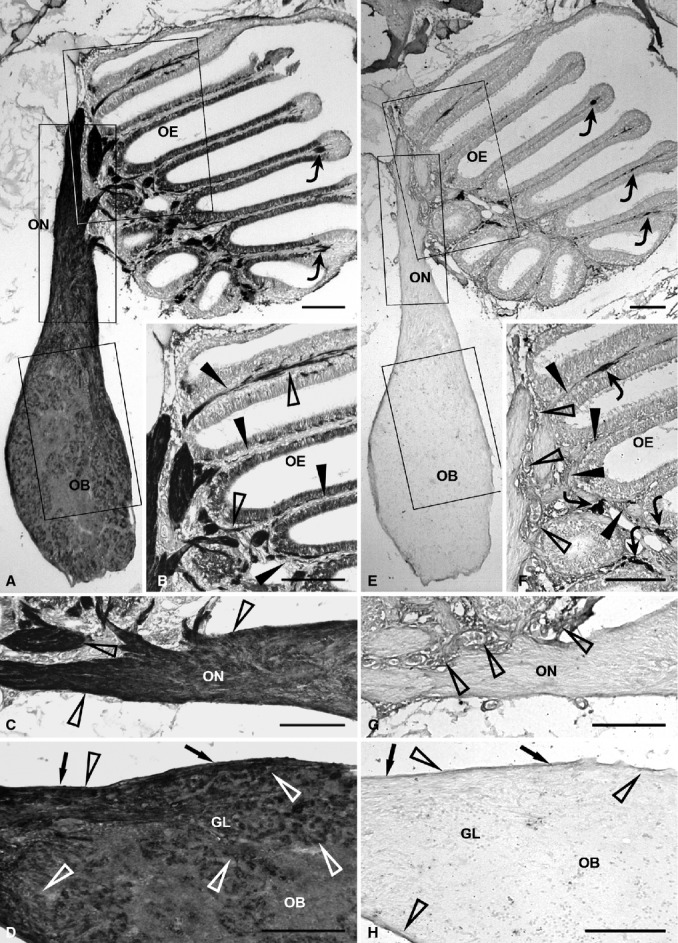
Immunohistochemical detection of olfactory ensheathing cell markers, NCAM (A-D) and PSA-NCAM (E-H), in the olfactory system of zebrafish. (A) Evident NCAM staining in the whole olfactory pathway. Rectangular areas marked with OE, ON and OB are shown in (B), (C) and (D), respectively, at higher magnification. The epithelial lamina propria contains some chromatophores (curved arrows) (B) The epithelial lamina propria (arrowheads) shows moderate immunostaining (open arrowheads). (C) The extracranial olfactory nerve (open arrowheads) shows stronger expression compared with the intracranial olfactory nerve. (D) In the olfactory bulb, the olfactory nerve layer (arrows) and glomerular layer are clearly immunostained (open arrowheads). (E) In the olfactory pathway, PSA-NCAM immunostaining appears weak. Rectangular areas marked with OE, ON and OB are shown in (F), (G) and (H), respectively, at higher magnification. Some chromatophores (curved arrows) are present in the epithelial lamina propria. (F) Weak PSA-NCAM immunoreactivity (open arrowheads) in the lamina propria (arrowheads) of the olfactory mucosa. Curved arrows point out some chromatophores. (G) The olfactory nerve is unstained. Immunostaining (open arrowheads) appears in the epithelial lamina propria. (H) In the olfactory bulb, weak immunopositivity (open arrowheads) is observed in the olfactory nerve layer (arrows), while the glomerular layer is unstained. GL, glomerular layer; OB, olfactory bulb; OE, olfactory epithelium; ON, olfactory nerve. Scale bars: 100 μm.
PSA-NCAM immunopositivity was absent in the olfactory neuroepithelium, extracranial and intracranial tracts of the olfactory nerve, and the glomerular layer of the olfactory bulb (Fig. 2E–H). PSA-NCAM staining was weak in the neuroepithelial lamina propria and the olfactory nerve layer of the olfactory bulb (Fig. 2F–H: arrows, olfactory nerve layer; arrowheads, lamina propria; open arrowheads, immunolabelled elements).
No vimentin expression was evident in the olfactory system of zebrafish, from the olfactory neuroepithelium to the glomerular layer of the olfactory bulb (Fig. 3A–D: arrows, olfactory nerve layer; arrowheads, lamina propria).
Figure 3.
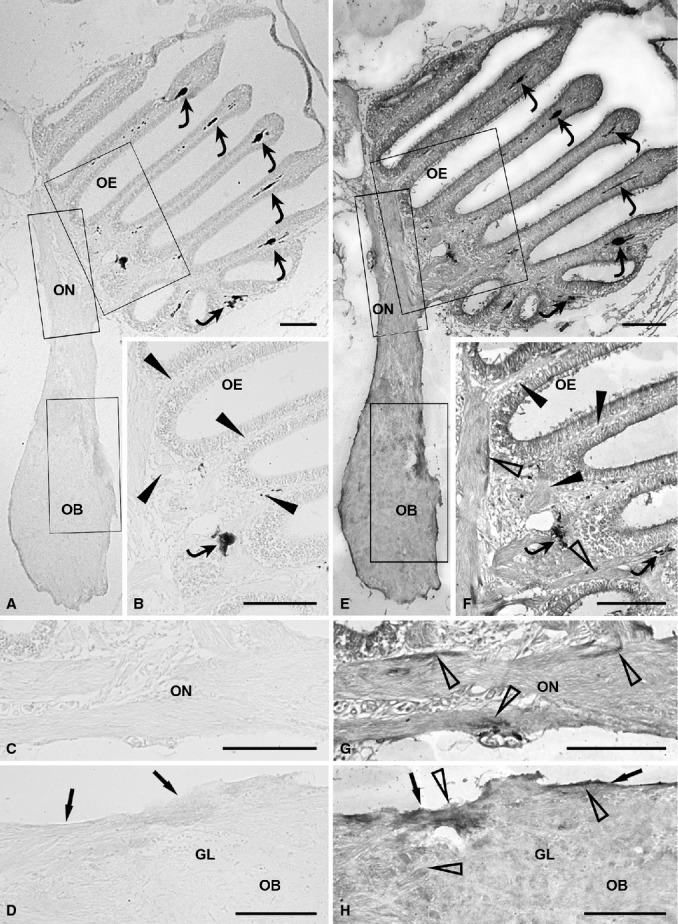
Immunohistochemical detection of olfactory ensheathing cell markers, Vim (A-D) and p75NTR (E-H), in the olfactory system of zebrafish. (A) Lack of vimentin expression in the olfactory pathway at low magnification. Rectangular areas marked with OE, ON and OB are shown in (B), (C) and (D), respectively, at higher magnification. Chromatophores are indicated by curved arrows. (B) The olfactory mucosa (arrowheads) is unstained. A chromatophore (curved arrow) is visible. (C) The olfactory nerve displays immmunonegativity for vimentin. (D) In the olfactory bulb, the olfactory nerve layer (arrows) and glomerular layer lack immunoreactivity for vimentin. (E) Distribution of p75NTR immunostaining in the whole olfactory system. Rectangular areas marked with OE, ON and OB are shown in (F), (G) and (H), respectively, at higher magnification. Some chromatophores (curved arrows) are present in the epithelial lamina propria. (F) Slight p75NTR immunoreactivity (open arrowheads) in the lamina propria of the olfactory mucosa (arrowheads). Two chromatophores (curved arrows) are visible. (G) The olfactory nerve shows slight immunopositivity (open arrowheads). (H) In the olfactory bulb, p75NTR immunopositivity (open arrowheads) is slight to moderate in the olfactory nerve layer (arrows) and reduced in the glomerular layer. GL, glomerular layer; OB, olfactory bulb; OE, olfactory epithelium; ON, olfactory nerve. Scale bars: 100 μm.
The dendritic and apical processes of the sensory cells in the olfactory neuroepithelium appeared slightly to moderately immunopositive for p75NTR. Expression of this antigen was faint in the neuroepithelial lamina propria (Fig. 3E,F: arrowheads, lamina propria; open arrowheads, immunolabelled elements). The olfactory nerve was weakly stained (Fig. 3E,G: open arrowheads, immunolabelled elements). In the olfactory bulb, the olfactory nerve layer showed slight to moderate p75NTR immunopositivity, whereas the glomerular layer was weakly stained (Fig. 3E,H: arrows, olfactory nerve layer; open arrowhead, immunolabelled elements).
The olfactory neuroepithelium, epithelial lamina propria and extracranial olfactory nerve appeared immunonegative for Gal-1 (Fig. 4A–C: arrowheads, lamina propria). In contrast, the intracranial olfactory nerve, olfactory nerve layer and glomerular layer of the olfactory bulb displayed strong Gal-1 immunopositivity (Fig. 4A,C,D: arrows, olfactory nerve layer; open arrowheads, immunolabelled elements).
Figure 4.
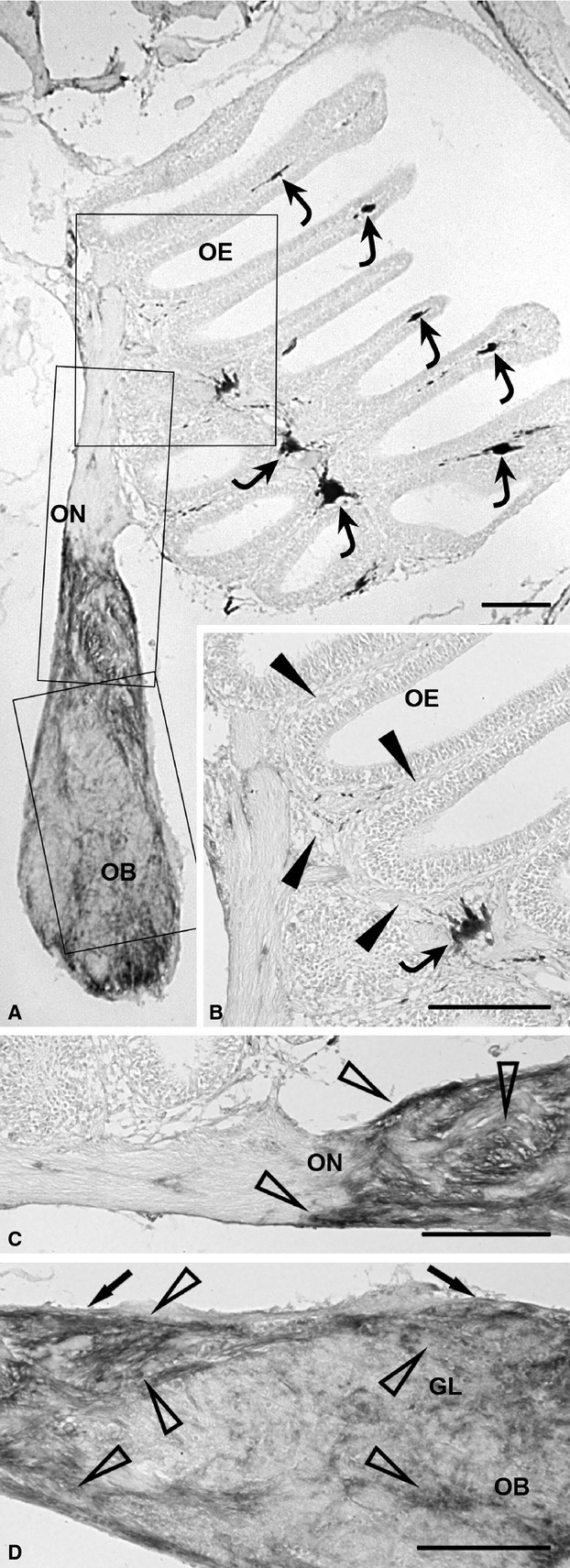
Immunohistochemical detection of the olfactory ensheathing cell marker, Gal-1 (A-D), in the olfactory system of zebrafish. (A) Distribution of Gal-1 immunostaining in the whole olfactory system at low magnification. Rectangular areas marked with OE, ON and OB are shown in (B), (C) and (D), respectively, at higher magnification. Some chromatophores (curved arrows) are present in the epithelial lamina propria. (B) The olfactory mucosa (arrowheads), which contains a chromatophore (curved arrow), is not immunostained. (C) In the olfactory nerve, the extracranial segment is unstained, whereas the intracranial segment shows strong immunoreaction (open arrowheads). (D) In the olfactory bulb, the olfactory nerve layer (arrows) and glomerular layer exhibit strong immunostaining (open arrowheads). GL, glomerular layer; OB, olfactory bulb; OE, olfactory epithelium; ON, olfactory nerve. Scale bars: 100 μm.
Chromatophores were present in the epithelial lamina propria (Figs 1–4: curved arrows, chromatophores).
Omission of primary antisera completely prevented immunostaining in the olfactory mucosa, olfactory nerve and olfactory bulb (Fig. 5A–H; negative controls presented only for GFAP, PSA-NCAM, vimentin and p75NTR immunostaining: arrows, olfactory nerve layer; curved arrows, chromatophores).
Figure 5.
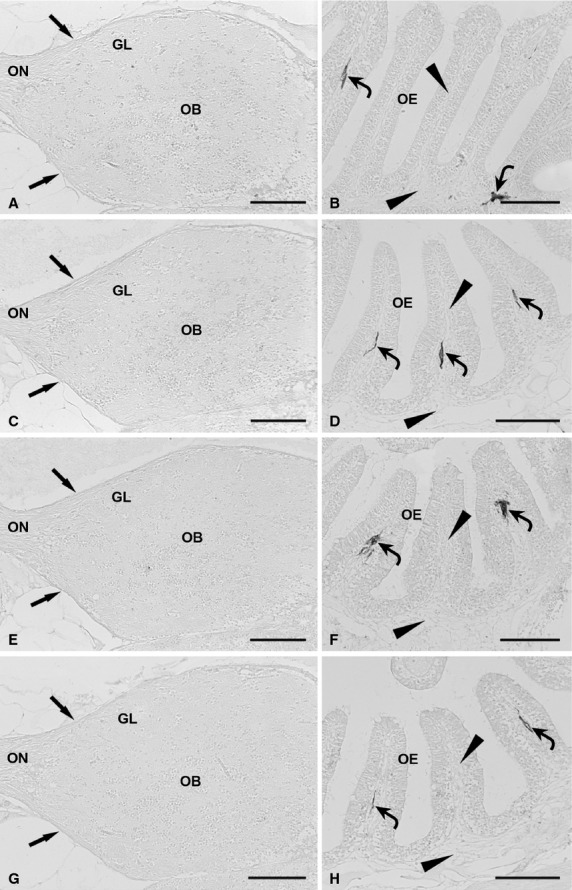
Negative controls for immunostaining specificity in the olfactory system of zebrafish, obtained by omission of the primary antibodies. (A) Omission of anti-GFAP antibody results in lack of staining in the olfactory nerve, olfactory nerve layer (arrows) and glomerular layer. (B) The epithelial lamina propria (arrowheads), which contains chromatophores (curved arrows), shows no immunoreaction as a result of anti-GFAP antibody omission. (C) Omission of anti-PSA-NCAM antibody causes immunostaining deficiency in the olfactory nerve, olfactory nerve layer (arrows) and glomerular layer. (D) Omission of anti-PSA-NCAM antibody results in immunonegativity of the olfactory mucosa (arrowheads). Some chromatophores (curved arrows) are displayed. (E) The olfactory nerve, olfactory nerve layer (arrows) and glomerular layer show lack of immunostaining due to anti-vimentin antibody omission. (F) In the olfactory mucosa (arrowheads), immunostaining deficiency results from anti-vimentin antibody omission. Chromatophores (curved arrows) are shown. (G). Omission of the anti-p75NTR antibody causes immunonegativity in the olfactory nerve, olfactory nerve layer (arrows) and glomerular layer. (H) Immunonegativity of the olfactory mucosa (arrowheads) ensues from omission of anti-p75NTR antibody. Curved arrows point out chromatophores. GL, glomerular layer; OB, olfactory bulb; OE, olfactory epithelium; ON, olfactory nerve. Scale bars: 100 μm.
Immunohistochemical reactions with positive controls resulted in clear immunopositive staining of the sections. In zebrafish spinal cord, strong GFAP immunopositivity was found in radial glial processes extending from the periependymal zone outwards to the pial surface, particularly in the dorsal and ventral horns (Fig. 6A: arrowheads, immunolabelled elements). Zebrafish spinal cord showed vimentin immunopositivity in radial glial processes and projecting spinal nerve roots (Fig. 6B: arrowheads, immunolabelled elements; curved arrows, chromatophores).
Figure 6.
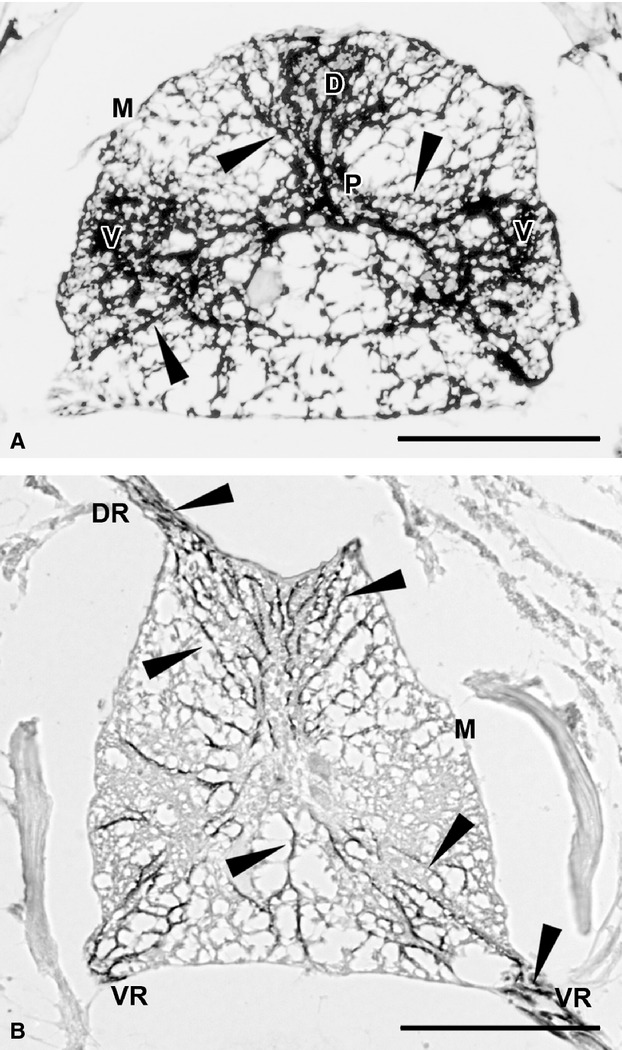
Positive controls showing expression in known antigen-positive cells in the nervous system of zebrafish (A,B). (A) In a transverse section through the spinal cord, GFAP-positive radial glia processes (arrowheads) extend from the periependymal zone to the meningeal surface. They are thicker in the dorsal and ventral horns. (B) Transverse sectioned spinal cord showing vimentin immunopositive radial glia processes (arrowheads). Immunopositivity is also found in the projecting roots of a spinal nerve. Curved arrows point out chromatophores in the dorso-lateral meningeal layer. D, dorsal horn; DR, dorsal root; M, meningeal surface; P, periependymal zone; V, ventral horn; VR, ventral root. Scale bars: 100 μm.
Table 3 gives references to previously published use in zebrafish and other actinopterygians of the antibodies here employed and different antibodies against the same antigens.
Table 3.
Previously published use in zebrafish and other actinopterygians of the antibodies employed in this study and different antibodies against the same proteins.
| Antigen | Host | Type, clone | Source | Code | Fish studied | Reference |
|---|---|---|---|---|---|---|
| GFAP | Mouse | Monoclonal | ZIRC | zrf-1 | Danio rerio | Bernardos & Raymond (2006) |
| GFAP | Rabbit | Polyclonal | Dako | Z0334 | Oryzias latipes | Alunni et al. (2010) |
| GFAP | Rabbit | Polyclonal | Dako | Z0334 | Danio rerio | Baumgart et al. (2012) |
| GFAP | Rabbit | Polyclonal | Dako | Z0334 | Nothobranchius guentheri | Genade & Lang (2013); |
| GFAP | Rabbit | Polyclonal | Dako | Z0334 | Poecilia reticulata Carassius auratus | Lazzari et al. (2013); |
| GFAP | Rabbit | Polyclonal | Dako | Z0334 | Astatotilapia burtoni | Mack & Tiedemann (2013) |
| GFAP | Mouse | Monoclonal GA5 | Sigma | G3893 | Danio rerio | Kim et al. (2008) |
| GFAP | Rabbit | Polyclonal | Sigma | G9269 | Danio rerio | Hagström & Olsson (2010) |
| S100 | Rabbit | Polyclonal | Dako | Z0311 | Danio rerio | Braubach et al. (2012) |
| S100 | Rabbit | Polyclonal | Dako | Z0311 | Poecilia reticulata Carassius auratus | Lazzari et al. (2013) |
| S100 | Rabbit | Polyclonal | NeoMarkers | RB9018 | Oreochromis sp | Wen et al. (2008) |
| S100 | Rabbit | Polyclonal | Swant | Danio rerio | Zupanc et al. (2005) | |
| S100 | Rabbit | Polyclonal | Swant | Apteronotus leptorhynchus | Sirbulescu et al. (2009) | |
| NCAM | Mouse | Monoclonal | Dev Studies Hybridoma Bank | Carassius auratus | Harman et al. (2003) | |
| NCAM | Rabbit | Polyclonal | Martin Bastmeyer, Univ. of Karlsruhe (Marx et al. 2001) | Zebrafish | Kustermann et al. (2010) | |
| NCAM | Rabbit | Polyclonal | Chemicon | AB5032 | Poecilia reticulata Carassius auratus | Lazzari et al. (2013) |
| PSA-NCAM | Mouse | Monoclonal | Millipore | MAB 5324 | Zebrafish | Lindsey et al. (2012) |
| PSA-NCAM | Mouse | Monoclonal | Millipore | MAB 5324 | Poecilia reticulata Carassius auratus | Lazzari et al. (2013) |
| PSA | Mouse | Monoclonal | Frosch et al. (1985) | Danio rerio | Kustermann et al. (2010) | |
| Vim | Mouse | Monoclonal V9 | Boehringer | Danio rerio | Cerdà et al. (1998) | |
| Vim | Mouse | Monoclonal V9 | Sigma | Danio rerio | Costa et al. (2003) | |
| Vim | Mouse | Monoclonal V9 | NeoMarkers | Oreochromis sp | Wen et al. (2008) | |
| Vim | Mouse | Monoclonal V9 | Dako | M0725 | Symphysodon aequifasciatus | Magi et al. (2013) |
| Vim | Mouse | Monoclonal VIM 3B4 | Millipore | CBL202 | Poecilia reticulata Carassius auratus | Lazzari et al. (2013) |
| Vim | Mouse | Monoclonal | Developmental Studies Hybridoma Bank | Danio rerio | Chablais et al. (2011) | |
| p75NTR | Rabbit | Polyclonal | Millipore | 07-476 | Poecilia reticulata Carassius auratus | Lazzari et al. (2013) |
| Zebrafish galectin-1-like lectin 2 Drgal1-L2 | Rabbit | Polyclonal | Ahmed et al. (2004) | Danio rerio | Craig et al. (2010) | |
| Galectin-1 LGALS1 | Rabbit | Polyclonal | Sigma | Poecilia reticulata Carassius auratus | Lazzari et al. (2013) |
Discussion
In the present study, we examined the immunostaining patterns of GFAP, S100, NCAM, PSA-NCAM, vimentin, p75NTR and Gal-1 in the olfactory system of zebrafish. Different degrees of staining in the olfactory structures were observed for all biomarkers. Moreover, the degree of expression of the same marker was not uniform in different tracts of the zebrafish olfactory pathway. Interestingly, immunostaining patterns of these markers differed among macrosmatic fish, even within the same family. The results indicate that biomarkers conventionally used in research on mammalian OECs (Boyd et al. 2006; Jahed et al. 2007; Kawaja et al. 2009; Smithson & Kawaja, 2009) are also applicable to the fish model.
Although fish species show intense labelling for the cytoskeleton-associated calcium binding protein, S100 (present study; Germanà et al. 2008; Lazzari et al. 2013), upon comparison of zebrafish with guppy, only the intracranial olfactory nerve and olfactory nerve layer displayed similar immunostaining intensities in the two species, whereas other parts of the olfactory system were less stained in zebrafish. The olfactory nerve, olfactory nerve layer and glomerular layer appeared similarly immunostained in zebrafish and goldfish. Moreover, analogous to goldfish, the extracranial olfactory nerve of zebrafish displayed evident reduction in immunostaining relative to the intracranial region. S100 immunostaining patterns of zebrafish were more similar to those of goldfish than guppy. In the olfactory pathways of fish, both intra-and interspecific regional differences may be associated with different degrees of S100-related activities. This calcium-binding protein may function in the cytoskeletal dynamics of OECs and regulation of numerous intracellular processes, including proliferation, differentiation, protein phosphorylation and cell survival (Garbuglia et al. 1999; Santamaria-Kisiel et al. 2006; Smithson & Kawaja, 2009).
Braubach et al. (2012) state that inconsistencies in S100 staining results for zebrafish olfactory epithelia by different authors may arise from different fixation methods used to prepare zebrafish tissues for immunohistochemical analysis.
GFAP, associated with intermediate filaments and an accepted marker of astrocytes, is another reliable marker for OECs in zebrafish (present study), as well as guppy and goldfish (Lazzari et al. 2013). The expression patterns in the extracranial olfactory nerve, olfactory nerve layer and glomerular layer of zebrafish resemble those in goldfish. In zebrafish, GFAP expression in the glomerular layer matches the immunopositivity pattern in guppy, whereas staining intensity is lower in the intracranial olfactory nerve and higher in the epithelial lamina propria, extracranial olfactory nerve and olfactory nerve layer. In accordance with the data obtained by Huang et al. (2005) in Xenopus, our results demonstrate that GFAP-positive cells are present in the olfactory nerve and olfactory nerve layer of the olfactory bulb but are almost absent in deeper layers of the olfactory bulb, particularly the glomerular layer in fish.
Immunolabelling for vimentin, which represents a major constituent of OEC intermediate filaments in mammals during both development and adulthood (Franceschini & Barnett, 1996), differs among the fish species (present study; Lazzari et al. 2013). Analogous to guppy, the olfactory system of zebrafish displays no vimentin staining, whereas faint vimentin immunopositivity is observed in the olfactory bulb in goldfish. This condition of goldfish is reminiscent of the vimentin staining pattern in rat, whereby vimentin immunolabelling is decreased towards the centre of the olfactory bulb (Franceschini & Barnett, 1996). GFAP is considered a reliable marker of mature glial cells, whereas vimentin is typical of immature elements (Kalman, 1998; Lazzari & Franceschini, 2005, 2006). Therefore, on the basis of the vimentin immunolabelling of intermediate filaments in fish, we hypothesise that the olfactory pathways of zebrafish and guppy contain only fully differentiated OECs. In contrast, goldfish retain differentiating elements in the olfactory bulb. The presence of differentiating OECs is related to tissue-repairing activities or turnover of glial conduits for ORN axons, which may be more intense in goldfish, as they are macrosmatic fish with a very extended olfactory system.
In the zebrafish olfactory system, p75NTR immunopositivity is higher than that in goldfish and lower than that in guppy. In agreement with the findings of Smithson & Kawaja (2009), we suggest that the data require validation with more sensitive methods (such as RT-PCR) to ascertain whether OECs express p75NTR or other markers at levels below that detectable with immunohistochemical techniques. Our studies on fish show that the degree of p75NTR expression differs among various species and shows regional differences within a given species. The receptor may recruit distinct intracellular binding proteins to mediate specific functions in distinct cell types. In addition, p75NTR is involved in various functions requiring the response of other co-receptors to different ligands, and may facilitate or inhibit axonal growth or promote cell survival or death, determined by its receptor partners (see Cragnolini & Friedman, 2008). The differences in p75NTR expression patterns in fish OECs may thus be indicative of its involvement in distinct functions.
In the olfactory system of zebrafish (present study), Gal-1 immunopositivity appears moderate to intense in the intracranial olfactory nerve, olfactory nerve layer and glomerular layer. Both goldfish and guppy differ considerably from zebrafish, as their olfactory systems are entirely immunonegative for Gal-1 (Lazzari et al. 2013). Staining of some tracts of the olfactory pathway in zebrafish would rule out lack of specificity for the anti Gal-1 antibody used in our studies on fish OECs. During the development of the olfactory pathway, cytoplasmic processes of OECs form conduits expressing cell adhesion and extracellular molecules, including Gal-1 (Puche et al. 1996; St John & Key, 1999; Vincent et al. 2005). The growth-promoting pathways created by OECs are thought to facilitate fasciculation and elongation of new axons towards their bulbar targets (Key & St John, 2002; Li et al. 2005a,b2005b; Vincent et al. 2005). Cell surface carbohydrates on primary olfactory axons act as ligands for bivalent carbohydrate-binding proteins, such as Gal-1 (Puche & Key, 1996; Puche et al. 1996; St John & Key, 1999). However, the exact mechanism underlying Gal-1 activity in targeting axons to glomeruli remains to be established.
In adult mammals, OECs express developmentally regulated proteins normally found in progenitor or immature cells, such as nestin, vimentin and PSA-NCAM (Barnett et al. 1993; Franceschini & Barnett, 1996; Pixley, 1996). In particular, both NCAM and PSA-NCAM are expressed in the olfactory pathway at all developmental stages (Miragall et al. 1989; Key & Akeson, 1990; Franceschini & Barnett, 1996). NCAM belongs to the immunoglobulin superfamily that mediates cell–cell interactions or cell–extracellular matrix adhesion and recognition functions (see Berezin, 2009). In the goldfish olfactory pathway, PSA-NCAM and vimentin are expressed at higher levels than those detected in guppy, indicative of the presence of differentiating cells (Lazzari et al. 2013). In contrast, in zebrafish, PSA-NCAM and vimentin expressions are reduced and absent, respectively, resembling the patterns found in guppy (Lazzari et al. 2013). The early assumption that macrosmatic fish have a faster turnover of olfactory axons and their ensheathing structures than microsmatic fish needs reconsideration in view of the present findings that immunopatterns of the macrosmatic zebrafish resemble those of the microsmatic guppy. The differences between the two macrosmatic fish may be attributed to the fact that the number of simultaneously differentiating cells is greater in a bigger species than in a smaller species. The higher number of simultaneously differentiating cells may therefore generate a more intense signal in immunohistochemical detection. Further studies using more sensitive detection methods are required to verify the presence of differentiating OECs in zebrafish.
Conclusions
The current study has focused on preliminary immunocytochemical characterisation of OECs in zebrafish, a species extensively used in genetic, developmental and neurobiological studies. Our findings emphasise the differences in OEC marker expression between macrosmatic and microsmatic fish, and similarities in the immunostaining patterns of glial cells in the olfactory pathways of fish (anamniotes) and mammals (amniotes). We assume that the glial cells associated with the fish olfactory pathway at least partially correspond to the well-known OECs of mammals. Similar to mammals, in the fish olfactory system, neurogenesis occurs in the basal layer of the olfactory epithelium (Bettini et al. 2006, 2009), possibly conditioned by components of the local microenvironment. These basal cells participate in regenerative processes of the olfactory receptors throughout life. OECs in the fish system could promote axon elongation to specific synaptic connections onto the dendritic processes of bulbar mitral cells. Moreover, in the fish olfactory pathway, conduits expressing cell adhesion and growth-promoting molecules may be produced by OECs, indicating that the mammalian and fish pathways share some basic structural and functional features. Clearly, the olfactory system is a primitive system that has undergone some evolutionary changes over time, in terms of not only morphology but also molecular characteristics associated with neurite outgrowth and elongation. However, the issue of whether regional and interspecific differences in immunostaining patterns of olfactory pathway markers are of functional significance requires further investigation. New immunohistochemical studies with double-labelling techniques and different section planes of the specimens will improve our knowledge of OECs in fish and, more generally, in non-mammalian vertebrates.
Acknowledgments
This study was supported by grants from the Italian Ministry of University and Research: FFO2009, FFO2010.
Author contributions
All authors contributed equally to this work.
References
- Adams JC. Heavy-metal intensification of DAB-based reaction product. J Histochem Cytochem. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Ahmed H, Du SJ, O'Leary N, et al. Biochemical and molecular characterization of galectins from zebrafish (Danio rerio): notochord-specific expression of a prototype galectin during early embryogenesis. Glycobiology. 2004;14:219–232. doi: 10.1093/glycob/cwh032. [DOI] [PubMed] [Google Scholar]
- Alunni A, Hermel JM, Heuzé A, et al. Evidence for neural stem cells in the medaka optic tectum proliferation zones. Dev Neurobiol. 2010;70:693–713. doi: 10.1002/dneu.20799. [DOI] [PubMed] [Google Scholar]
- Astic L, Pellier-Monnin V, Godinot F. Spatio-temporal patterns of ensheathing cell differentiation in the rat olfactory system during development. Neuroscience. 1998;84:295–307. doi: 10.1016/s0306-4522(97)00496-x. [DOI] [PubMed] [Google Scholar]
- Au E, Roskams J. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia. 2003;41:224–236. doi: 10.1002/glia.10160. [DOI] [PubMed] [Google Scholar]
- Au WW, Treloar HB, Greer CA. Sublaminar organization of the mouse olfactory bulb nerve layer. J Comp Neurol. 2002;446:68–80. doi: 10.1002/cne.10182. [DOI] [PubMed] [Google Scholar]
- Audisio C, Raimondo S, Nicolino S, et al. Morphological and biomolecular characterization of the neonatal olfactory bulb ensheathing cell line. J Neurosci Methods. 2009;185:89–98. doi: 10.1016/j.jneumeth.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Babiarz J, Kane-Goldsmith N, Basak S, et al. Juvenile and adult olfactory ensheathing cells bundle and myelinate dorsal root ganglion axons in culture. Exp Neurol. 2011;229:72–79. doi: 10.1016/j.expneurol.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Barnett SC, Chang L. Olfactory ensheathing cells and CNS repair: Going solo or in need of a friend? Trends Neurosci. 2004;27:54–60. doi: 10.1016/j.tins.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Barnett SC, Riddell JS. Olfactory ensheathing cells (OECs) and the treatment of CNS injury: Advantages and possible caveats. J Anat. 2004;204:57–67. doi: 10.1111/j.1469-7580.2004.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SC, Hutchins AM, Noble M. Purification of olfactory nerve ensheathing cells from the olfactory bulb. Dev Biol. 1993;155:337–350. doi: 10.1006/dbio.1993.1033. [DOI] [PubMed] [Google Scholar]
- Barraud P, Seferiadis AA, Tyson LD, et al. Neural crest origin of olfactory ensheathing glia. Proc Natl Acad Sci U S A. 2010;107:21040–21045. doi: 10.1073/pnas.1012248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart EV, Barbosa JS, Bally-Cuif L, et al. Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia. 2012;60:343–357. doi: 10.1002/glia.22269. [DOI] [PubMed] [Google Scholar]
- Berezin V. Advances in Experimental Medicine and Biology. New York: Springer; 2009. Structure and function of the neural cell adhesion molecule NCAM. Vol. 663, [Google Scholar]
- Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–1013. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Bettini S, Ciani F, Franceschini V. Recovery of the olfactory receptor neurons in the African Tilapia mariae following exposure to low copper level. Aquat Toxicol. 2006;76:321–328. doi: 10.1016/j.aquatox.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Bettini S, Lazzari M, Ciani F, et al. Immunohistochemical and histochemical characteristics of the olfactory system of the guppy, Poecilia reticulata (Teleostei, Poecilidae) Anat Rec. 2009;292:1569–1576. doi: 10.1002/ar.20944. [DOI] [PubMed] [Google Scholar]
- Bock P, Beineke A, Techangamsuwan S, et al. Differential expression of HNK-1 and p75NTR in adult canine Schwann cells and olfactory ensheathing cells in situ but not in vitro. J Comp Neurol. 2007;505:572–585. doi: 10.1002/cne.21519. [DOI] [PubMed] [Google Scholar]
- Boruch AV, Conners JJ, Pipitone M, et al. Neurotrophic and migratory properties of an olfactory ensheathing cell line. Glia. 2001;33:225–229. [PubMed] [Google Scholar]
- Boyd JG, Jahed A, McDonald TG, et al. Proteomic evaluation reveals that olfactory ensheathing cells but not Schwann cells express calponin. Glia. 2006;53:434–440. doi: 10.1002/glia.20299. [DOI] [PubMed] [Google Scholar]
- Braubach OR, Fine A, Croll RP. Distribution and functional organization of glomeruli in the olfactory bulbs of zebrafish (Danio rerio. J Comp Neurol. 2012;520:2317–2339. doi: 10.1002/cne.23075. [DOI] [PubMed] [Google Scholar]
- Byrd CA, Brunjes PC. Organization of the olfactory system in the adult zebrafish: Histological, immunohistochemical, and quantitative analysis. J Comp Neurol. 1995;358:247–259. doi: 10.1002/cne.903580207. [DOI] [PubMed] [Google Scholar]
- Cerdà J, Conrad M, Markl J, et al. Zebrafish vimentin: molecular characterization, assembly properties and developmental expression. Eur J Cell Biol. 1998;77:175–187. doi: 10.1016/S0171-9335(98)80105-2. [DOI] [PubMed] [Google Scholar]
- Chablais F, Veit J, Rainer G, et al. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev Biol. 2011;11:21. doi: 10.1186/1471-213X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehrehasa F, Windus LC, Ekberg JA, et al. Olfactory glia enhance neonatal axon regeneration. Mol Cell Neurosci. 2010;45:277–288. doi: 10.1016/j.mcn.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Chuah MI, Au C. Olfactory Schwann cells are derived from precursor cells in the olfactory epithelium. J Neurosci Res. 1991;29:72–180. doi: 10.1002/jnr.490290206. [DOI] [PubMed] [Google Scholar]
- Chung RS, Woodhouse A, Fung S, et al. Olfactory ensheathing cells promote neurite sprouting of injured axons in vitro by direct cellular contact and secretion of soluble factors. Cell Mol Life Sci. 2004;61:1238–1245. doi: 10.1007/s00018-004-4026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa ML, Escaleira R, Manasfi M, et al. Cytoskeletal and cellular adhesion proteins in zebrafish (Danio rerio) myogenesis. Braz J Med Biol Res. 2003;36:1117–1120. doi: 10.1590/s0100-879x2003000800019. [DOI] [PubMed] [Google Scholar]
- Coutts DJC, Humphries CE, Zhao C, et al. Embryonic-derived olfactory ensheathing cells remyelinate focal areas of spinal cord demyelination more efficiently than neonatal or adult-derived cells. Cell Transplant. 2013;22:1249–1261. doi: 10.3727/096368912X656153. [DOI] [PubMed] [Google Scholar]
- Cragnolini AB, Friedman WJ. The function of p75NTR in glia. Trends Neurosci. 2008;31:99–104. doi: 10.1016/j.tins.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Craig SE, Thummel R, Ahmed H, et al. The zebrafish galectin Drgal1-L2 is expressed by proliferating Müller glia and photoreceptor progenitors and regulates the regeneration of rod photoreceptors. Invest Ophthalmol Vis Sci. 2010;51:3244–3252. doi: 10.1167/iovs.09-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisthen HL, Polese G. Evolution of vertebrate olfactory subsystems. In: Kaas JH, editor. Evolution of Nervous Systems. 2. Non-Mammalian Vertebrates. Oxford: Academic Press; 2006. pp. 355–406. [Google Scholar]
- Fairless R, Barnett S. Olfactory ensheathing cells: their role in central nervous system repair. Int J Biochem Cell Biol. 2005;37:693–699. doi: 10.1016/j.biocel.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Franceschini IA, Barnett SC. Low-affinity NGF receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Dev Biol. 1996;173:327–343. doi: 10.1006/dbio.1996.0027. [DOI] [PubMed] [Google Scholar]
- Franceschini V, Ciani F, Lazzari M. Histochemical study of glycoconjugates in the actinopterygian olfactory system. In: Van Driessche E, Fischer J, Beeckmans S, Bøg-Hansen TC, editors. Lectins: Biology, Biochemistry, Clinical Biochemistry. Vol. 10. Hellerup, DK: Textop; 1994. pp. 24–30. [Google Scholar]
- Franceschini V, Lazzari M, Ciani F. Cell surface glycoconjugates in the vertebrate olfactory system. In: Fabbri E, Biondi C, editors. Olfaction: From Pre-Receptorial to Behavioural Aspects. Trivandrum, India: Research Signpost; 1996. pp. 29–40. [Google Scholar]
- Franceschini V, Lazzari M, Ciani F. Lectin characterization of the olfactory system in brachiopterygian fish. Int J Dev Neurosci. 1999;17:31–36. doi: 10.1016/s0736-5748(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Franceschini V, Lazzari M, Ciani F. Cell surface glycoconjugates in the olfactory system of lungfish Protopterus annectens Owen. Acta Zool. 2000;81:131–137. [Google Scholar]
- Franssen EH, de Bree FM, Verhaagen J. Olfactory ensheathing glia: their contribution to primary olfactory nervous system regeneration and their regenerative potential following transplantation into the injured spinal cord. Brain Res Rev. 2007;56:236–258. doi: 10.1016/j.brainresrev.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Frosch M, Gorgen I, Boulnois GJ, et al. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci USA. 1985;82:1194–1198. doi: 10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuglia M, Verzini M, Sorci G, et al. The calcium-modulated proteins, S100A1 and S100B, as potential regulators of the dynamics of type III intermediate filaments. Braz J Med Biol Res. 1999;32:1177–1185. doi: 10.1590/s0100-879x1999001000001. [DOI] [PubMed] [Google Scholar]
- Geller S, Kolasa E, Tillet Y, et al. Olfactory ensheathing cells form the microenvironment of migrating GnRH-1 neurons during mouse development. Glia. 2013;61:550–566. doi: 10.1002/glia.22455. [DOI] [PubMed] [Google Scholar]
- Genade T, Lang DM. Resveratrol extends lifespan and preserves glia but not neurons of the Nothobranchius guentheri optic tectum. Exp Gerontol. 2013;48:202–212. doi: 10.1016/j.exger.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Germanà A, Marino F, Guerrera MC, et al. Expression and distribution of S100 protein in the nervous system of the adult zebrafish (Danio rerio. Microsc Res Tech. 2008;71:248–255. doi: 10.1002/jemt.20544. [DOI] [PubMed] [Google Scholar]
- Granger N, Blamires H, Franklin RJM, et al. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain. 2012;135:3227–3237. doi: 10.1093/brain/aws268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei PPC, Monti-Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Hagström C, Olsson C. Glial cells revealed by GFAP immunoreactivity in fish gut. Cell Tissue Res. 2010;341:73–81. doi: 10.1007/s00441-010-0979-3. [DOI] [PubMed] [Google Scholar]
- Harman AM, Rodger J, Ahmat A, et al. PSA-NCAM is up-regulated during optic nerve regeneration in lizard but not in goldfish. Exp Neurol. 2003;182:180–185. doi: 10.1016/s0014-4886(03)00081-5. [DOI] [PubMed] [Google Scholar]
- Higginson JR, Barnett SC. The culture of olfactory ensheathing cells (OECs) – a distinct glial cell type. Exp Neurol. 2011;229:2–9. doi: 10.1016/j.expneurol.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Zhao S, Gaudin A, et al. Glial fibrillary acidic protein and vimentin expression in the frog olfactory system during metamorphosis. NeuroReport. 2005;16:1439–1442. doi: 10.1097/01.wnr.0000177009.06485.89. [DOI] [PubMed] [Google Scholar]
- Jahed A, Rowland JW, McDonald T, et al. Olfactory ensheathing cells express smooth muscle alpha-actin in vitro and in vivo. J Comp Neurol. 2007;503:209–223. doi: 10.1002/cne.21385. [DOI] [PubMed] [Google Scholar]
- Kalman M. Astroglial architecture of the carp (Cyprinus carpio) brain as revealed by immunocytochemical staining against glial fibrillary acidic protein (GFAP) Anat Embryol. 1998;198:409–433. doi: 10.1007/s004290050193. [DOI] [PubMed] [Google Scholar]
- Katoh H, Shibata S, Fukuda K, et al. The dual origin of the peripheral olfactory system: Placode and neural crest. Mol Brain. 2011;4:34. doi: 10.1186/1756-6606-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaja MD, Boyd JG, Smithson LJ, et al. Technical strategies to isolate olfactory ensheathing cells for intraspinal implantation. J Neurotrauma. 2009;26:155–177. doi: 10.1089/neu.2008.0709. [DOI] [PubMed] [Google Scholar]
- Key B, Akeson RA. Olfactory neurons express a unique glycosylated form of neural cell adhesion molecule (N-CAM) J Cell Biol. 1990;110:1729–1743. doi: 10.1083/jcb.110.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key B, St John J. Axon navigation in the mammalian primary olfactory pathway: where to next? Chem Senses. 2002;27:245–260. doi: 10.1093/chemse/27.3.245. [DOI] [PubMed] [Google Scholar]
- Kim H, Shin J, Kim S, et al. Notch-regulated oligodendrocyte specification from radial glia in the spinal cord of zebrafish embryos. Dev Dyn. 2008;237:2081–2089. doi: 10.1002/dvdy.21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krudewig C, Deschl U, Wewetzer K. Purification and in vitro characterization of adult canine olfactory ensheathing cells. Cell Tissue Res. 2006;326:687–696. doi: 10.1007/s00441-006-0238-9. [DOI] [PubMed] [Google Scholar]
- Kumar R, Hayat S, Felts P, et al. Functional differences and interactions between phenotypic subpopulations of olfactory ensheathing cells in promoting CNS axonal regeneration. Glia. 2005;50:12–20. doi: 10.1002/glia.20154. [DOI] [PubMed] [Google Scholar]
- Kustermann S, Hildebrandt H, Bolz S, et al. Genesis of rods in the zebrafish retina occurs in a microenvironment provided by polysialic acid-expressing Müller glia. J Comp Neurol. 2010;518:636–646. doi: 10.1002/cne.22232. [DOI] [PubMed] [Google Scholar]
- Lazzari M, Franceschini V. Intermediate filament immunohistochemistry of astroglial cells in the leopard gecko, Eublepharis macularius. Anat Embryol. 2005;210:275–286. doi: 10.1007/s00429-005-0049-x. [DOI] [PubMed] [Google Scholar]
- Lazzari M, Franceschini V. Glial cytoarchitecture in the central nervous system of the soft-shell turtle, Trionyx sinensis, revealed by intermediate filament immunohistochemistry. Anat Embryol. 2006;211:497–506. doi: 10.1007/s00429-006-0101-5. [DOI] [PubMed] [Google Scholar]
- Lazzari M, Bettini S, Ciani F, et al. Light and transmission electron microscopy study of the peripheral olfactory organ of the guppy, Poecilia reticulata (Teleostei, Poecilidae) Microsc Res Tech. 2007;70:782–789. doi: 10.1002/jemt.20487. [DOI] [PubMed] [Google Scholar]
- Lazzari M, Bettini S, Franceschini V. Immunocytochemical characterization of olfactory ensheathing cells in fish. Brain Struct Funct. 2013;218:539–549. doi: 10.1007/s00429-012-0414-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Li D, Raisman G. Interaction of olfactory ensheathing cells with astrocytes may be the key to repair of tract injuries in the spinal cord: the ‘pathway hypothesis'. J Neurocytol. 2005a;34:343–351. doi: 10.1007/s11068-005-8361-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Olfactory ensheathing cells and olfactory nerve fibroblasts maintain continuous open channels for regrowth of olfactory nerve fibres. Glia. 2005b;52:245–251. doi: 10.1002/glia.20241. [DOI] [PubMed] [Google Scholar]
- Li BC, Xu C, Zhang JY, et al. Differing Schwann cells and olfactory ensheathing cells behaviors, from interacting with astrocyte, produce similar improvements in contused rat spinal cord's motor function. J Mol Neurosci. 2012;48:35–44. doi: 10.1007/s12031-012-9740-6. [DOI] [PubMed] [Google Scholar]
- Lindsey BW, Darabie A, Tropepe V. The cellular composition of neurogenic periventricular zones in the adult zebrafish forebrain. J Comp Neurol. 2012;520:2275–2316. doi: 10.1002/cne.23065. [DOI] [PubMed] [Google Scholar]
- Lipson AC, Widenfalk J, Lindqvist E, et al. Neurotrophic properties of olfactory ensheathing glia. Exp Neurol. 2003;180:167–171. doi: 10.1016/s0014-4886(02)00058-4. [DOI] [PubMed] [Google Scholar]
- Liu K, Li Y, Wang H, et al. The immunohistochemical characterization of human fetal olfactory bulb and olfactory ensheathing cells in culture as a source for clinical CNS restoration. Anat Rec. 2010a;293:359–369. doi: 10.1002/ar.21030. [DOI] [PubMed] [Google Scholar]
- Liu Q, Ye J, Yu H, et al. Survival-enhancing of spiral ganglion cells under influence of olfactory ensheathing cells by direct cellular contact. Neurosci Lett. 2010b;478:37–41. doi: 10.1016/j.neulet.2010.04.065. [DOI] [PubMed] [Google Scholar]
- Lu J, Féron F, Mackay-Sim A, et al. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transacted spinal cord. Brain. 2002;125:14–21. doi: 10.1093/brain/awf014. [DOI] [PubMed] [Google Scholar]
- Mack AF, Tiedemann K. Cultures of astroglial cells derived from brain of adult cichlid fish. J Neurosci Methods. 2013;212:269–275. doi: 10.1016/j.jneumeth.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A. Olfactory ensheathing cells and spinal cord repair. Keio J Med. 2005;54:8–14. doi: 10.2302/kjm.54.8. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Kittel PW. On the life span of olfactory receptor neurons. Eur J Neurosci. 1991;3:209–215. doi: 10.1111/j.1460-9568.1991.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Magi GE, Renzoni G, Piccionello AP, et al. Primary ocular chondrosarcoma in a discus (Symphisodon aequifasciatus. J Zoo Wildl Med. 2013;44:225–231. doi: 10.1638/1042-7260-44.1.225. [DOI] [PubMed] [Google Scholar]
- Marx M, Rutishauser U, Bastmeyer M. Dual function of polysialic acid during zebrafish central nervous system development. Development. 2001;128:4949–4958. doi: 10.1242/dev.128.24.4949. [DOI] [PubMed] [Google Scholar]
- Miragall F, Kadmon G, Schachner M. Expression of L1 and N-CAM cell adhesion molecules during development of the mouse olfactory system. Dev Biol. 1989;135:272–286. doi: 10.1016/0012-1606(89)90179-6. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Tsuruta M, Mori H, et al. A comparative study of axon-surrounding cells in the two nasal nerve tracts from mouse olfactory epithelium and vomeronasal organ. Brain Res. 2013;1503:16–23. doi: 10.1016/j.brainres.2013.01.053. [DOI] [PubMed] [Google Scholar]
- Pellitteri R, Spatuzza M, Stanzani S, et al. Biomarkers expression in rat olfactory ensheathing cells. Front Biosci. 2010;S2:289–298. doi: 10.2741/s64. [DOI] [PubMed] [Google Scholar]
- Pixley SK. Characterization of olfactory receptor neurons and other cell types in dissociated rat olfactory cell cultures. Int J Dev Neurosci. 1996;14:823–839. doi: 10.1016/s0736-5748(96)00057-3. [DOI] [PubMed] [Google Scholar]
- Puche AC, Key B. N-acetyl-lactosamine in the rat olfactory system: Expression and potential role in neurite growth. J Comp Neurol. 1996;364:267–278. doi: 10.1002/(SICI)1096-9861(19960108)364:2<267::AID-CNE6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Puche AC, Poirier F, Hair M, et al. Role of galectin-1 in the developing mouse olfactory system. Dev Biol. 1996;179:274–287. doi: 10.1006/dbio.1996.0257. [DOI] [PubMed] [Google Scholar]
- Quintana-Urzainqui I, Rodriguez-Moldes I, Candal E. Developmental, tract-tracing and immunohistochemical study of the peripheral olfactory system in a basal vertebrate: insights on Pax6 neurons migrating along the olfactory nerve. Brain Struct Funct. 2012 doi: 10.1007/s00429-012-0486-2. doi: 10.1007/s00429-012-0486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G. Olfactory ensheathing cells: Another miracle cure for spinal cord injury. Nat Rev Neurosci. 2001;2:369–375. doi: 10.1038/35072576. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Avila J. Olfactory ensheathing cells: Properties and function. Brain Res Bull. 1998;46:175–187. doi: 10.1016/s0361-9230(97)00463-2. [DOI] [PubMed] [Google Scholar]
- Rawji KS, Zhang SX, Tsai YY, et al. Olfactory ensheathing cells of hamsters, rabbits, monkeys, and mice express α-smooth muscle actin. Brain Res. 2013;1521:31–50. doi: 10.1016/j.brainres.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Rela L, Bordey A, Greer CA. Olfactory ensheathing cell membrane properties are shaped by connectivity. Glia. 2010;58:665–678. doi: 10.1002/glia.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and-independent interactions of the S100 protein family. Biochem J. 2006;396:201–214. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbulescu RF, Ilies I, Zupanc GK. Structural and functional regeneration after spinal cord injury in the weakly electric teleost fish, Apteronotus leptorhynchus. J Comp Physiol A. 2009;195:699–714. doi: 10.1007/s00359-009-0445-4. [DOI] [PubMed] [Google Scholar]
- Smithson LJ, Kawaja MD. A comparative examination of biomarkers for olfactory ensheathing cells in cats and guinea pigs. Brain Res. 2009;1284:41–53. doi: 10.1016/j.brainres.2009.06.011. [DOI] [PubMed] [Google Scholar]
- St John JA, Key B. Expression of galectin-1 in the olfactory nerve pathway of rat. Dev Brain Res. 1999;117:171–178. doi: 10.1016/s0165-3806(99)00118-2. [DOI] [PubMed] [Google Scholar]
- Teichmann H. Vergleichende Untersuchungen an der Nase der Fische. Z Morphol Ökol Tiere. 1954;43:171–212. [Google Scholar]
- Vincent AJ, West AK, Chuah MI. Morphological and functional plasticity of olfactory ensheathing cells. J Neurocytol. 2005;34:65–80. doi: 10.1007/s11068-005-5048-6. [DOI] [PubMed] [Google Scholar]
- Wen CM, Cheng YH, Huang YF, et al. Isolation and characterization of a neural progenitor cell line from tilapia brain. Comp Biochem Physiol A. 2008;149:167–180. doi: 10.1016/j.cbpa.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Wewetzer K, Verdù E, Angelov DN, et al. Olfactory ensheathing glia and Schwann cells: two of a kind? Cell Tissue Res. 2002;309:337–345. doi: 10.1007/s00441-002-0607-y. [DOI] [PubMed] [Google Scholar]
- Woodhall E, West AK, Chuah MI. Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Mol Brain Res. 2001;88:203–213. doi: 10.1016/s0169-328x(01)00044-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto M. Comparative morphology of the peripheral olfactory organ in teleosts. In: Hara TJ, editor. Chemoreception in Fishes. Amsterdam: Elsevier; 1982. pp. 39–59. [Google Scholar]
- Zupanc GK, Hinsch K, Gage FH. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J Comp Neurol. 2005;488:290–319. doi: 10.1002/cne.20571. [DOI] [PubMed] [Google Scholar]


