Abstract
The inhibitory effects of gamma-aminobutyric acid (GABA) in the central and peripheral nervous systems and the endocrine system are mediated by two different GABA receptors: GABAA-receptor (GABAA-R) and GABAB-receptor (GABAB-R). GABAA-R, but not GABAB-R, has been observed in the rat adrenal gland, where GABA is known to be released. This study sought to determine whether both GABA and GABAB-R are present in the endocrine and neuronal elements of the rat adrenal gland, and to investigate whether GABAB-R may play a role in mediating the effects of GABA in secretory activity of these cells. GABA-immunoreactive nerve fibers were observed in the superficial cortex. Some GABA-immunoreactive nerve fibers were found to be associated with blood vessels. Double-immunostaining revealed GABA-immunoreactive nerve fibers in the cortex were choline acetyltransferase (ChAT)-immunonegative. Some GABA-immunoreactive nerve fibers ran through the cortex toward the medulla. In the medulla, GABA-immunoreactivity was seen in some large ganglion cells, but not in the chromaffin cells. Double-immunostaining also showed GABA-immunoreactive ganglion cells were nitric oxide synthase (NOS)-immunopositive. However, neither immunohistochemistry combined with fluorescent microscopy nor double-immunostaining revealed GABA-immunoreactivity in the noradrenaline cells with blue-white fluorescence or in the adrenaline cells with phenylethanolamine N-methyltransferase (PNMT)-immunoreactivity. Furthermore, GABA-immunoreactive nerve fibers were observed in close contact with ganglion cells, but not chromaffin cells. Double-immunostaining also showed that the GABA-immunoreactive nerve fibers were in close contact with NOS-or neuropeptide tyrosine (NPY)-immunoreactive ganglion cells. A few of the GABA-immunoreactive nerve fibers were ChAT-immunopositive, while most of the GABA-immunoreactive nerve fibers were ChAT-immunonegative. Numerous ChAT-immunoreactive nerve fibers were observed in close contact with the ganglion cells and chromaffin cells in the medulla. The GABAB-R-immunoreactivity was found only in ganglion cells in the medulla and not at all in the cortex. Immunohistochemistry combined with fluorescent microscopy and double-immunostaining showed no GABAB-R-immunoreactivity in noradrenaline cells with blue-white fluorescence or in adrenaline cells with PNMT-immunoreactivity. These immunoreactive ganglion cells were NOS-or NPY-immunopositive on double-immunostaining. These findings suggest that GABA from the intra-adrenal nerve fibers may have an inhibitory effect on the secretory activity of ganglion cells and cortical cells, and on the motility of blood vessels in the rat adrenal gland, mediated by GABA-Rs.
Keywords: adrenal gland, GABA, GABAB-receptor, ganglion cells, rat
Introduction
Gamma-aminobutyric acid (GABA) exerts its inhibitory actions through two distinct types of receptors. The GABAA-receptor (GABAA-R) is an ionotropic receptor, permeable to chloride ions, at which the action of GABA is antagonized by bicuculline (Macdonald & Olsen, 1994). The GABAB-receptor (GABAB-R) is a metabotropic receptor, blocked by baclofen, which mediates neuronal responses via the second messenger systems regulating calcium and potassium channels (Bowery, 1989; Bettler et al. 1998).
The adrenal medulla comprises two types of chromaffin cells, adrenaline and noradrenaline cells, and these cells secrete large amounts of catecholamines containing adrenaline and noradrenaline (Ungar & Phillips, 1983). Furthermore, a few large ganglion cells are also present in the medulla (Oomori et al. 1994; Holgert et al. 1996a,b1996b).
Previous immunohistochemical studies have demonstrated GABA or glutamate decarboxylase (GAD)-immunoreactivity in the chromaffin cells and nerve fibers of the adrenal gland (Kataoka et al. 1984; Alho et al. 1986; Ahonen et al. 1989; Oomori et al. 1993; Iwasa et al. 1998, 1999).
Pharmacological, physiological and molecular data all point to the presence of GABAA-R and/or GABAB-R in the adrenal chromaffin cells (Kataoka et al. 1986; Castro et al. 1989, 2003; Ymer et al. 1989). However, although previous physiological studies have reported the presence of GABAA-R in rat adrenal chromaffin cells (Busik et al. 1996; Matsuoka et al. 2008), it remains unclear whether the GABAB-R exists in the cells of the rat adrenal gland and, if so, what functional significance it plays.
To clarify these issues, we examined the cellular and neuronal GABA-and GABAB-R-immunoreactive elements and the co-localization of other bioactive substances and enzymes, such as neuropeptide tyrosine (NPY), nitric oxide synthase (NOS) and choline acetyltransferase (ChAT) in the immunoreactive cells of the rat adrenal gland by light microscopy.
Materials and methods
Five male Wistar rats (Japan SLC, Shizuoka, Japan; 8 weeks old; body weight 180–200 g) were used in this study. The animals received commercial food pellets and water ad libitum. They were kept under constant conditions (temperature 22 °C, relative humidity 45%, LD 14 h light from 05:00 to 19:00 hours). All experimental procedures were performed according to the Guidelines for Animal Care by the Japanese Red Cross Hokkaido College of Nursing.
The animals were anesthetized with ether, and perfused through the heart with 200 mL of physiological saline and 200 mL of 4% paraformaldehyde or 0.1% glutaraldehyde plus 4% paraformaldehyde in 0.1 m phosphate buffer (PB) pH 7.3. The adrenal gland was then removed and immersed in the same fixative for 2 h at 4 °C. After rinsing in PB, the adrenal gland was left overnight in phosphate-buffered saline (PBS) containing 30% sucrose at 4 °C. The adrenal gland was cut at a thickness of 12 μm using a cryostat, and mounted on glass slides coated with poly-l-lysine (Sigma; St Louis, MO, USA).
For immunohistochemistry, the sections were incubated with primary antibodies (Table 1) overnight at 4 °C, followed by incubation for 2 h with a secondary antibody conjugated with indocarbocyanine (Cy3) or cyanine (Cy2; Table 1). To identify noradrenaline cells in the medulla, the cryostat sections were examined and photographed using a Zeiss fluorescent microscope equipped with a filter for noradrenaline fluorescence. Fixation containing 4% paraformaldehyde is suitable for demonstrating noradrenaline fluorescence in these tissues (Falck & Torp, 1961). In order to confirm the distribution of noradrenaline cells or adrenaline cells, we used both the formaldehyde-induced fluorescence (FIF) method for noradrenaline cells and phenylethanolamine N-methyltransferase (PNMT) immunohistochemistry for adrenaline cells in the same sections of the rat adrenal medulla. For immunohistochemistry and FIF, the cryostat sections were photographed by fluorescence microscope and then immunostained by the primary antibodies.
Table 1.
List of primary antisera and secondary fluorescence conjugated antisera used for immunohistochemistry in the present study.
| Host animals | Dilution | Catalogue no. | Source | |
|---|---|---|---|---|
| Primary antisera | ||||
| ChAT | Goat | 1 : 250 | AB144p | Chemicon International, Temecula, CA, USA |
| GABA | Rabbit | 1 : 5000 | A-2052 | SIGMA BIO SCIENCES, MO, USA |
| GABAB-R | Guinea pig | 1 : 4000 | AB1531 | Chemicon International |
| NOS | Rabbit | 1 : 4000 | B220-1 | EURO-DIAGNOSTICA, Beijerinckweg, the Netherlands |
| NPY | Rabbit | 1 : 5000 | 6730-0204 | Biogenesis, England, UK |
| PNMT | Sheep | 1 : 5000 | AB146 | Chemicon |
| Secondary antisera | ||||
| Anti-goat IgG | Cy2 | 1 : 100 | 705-225-147 | Jackson ImmunoResearch, West Grove, PA, USA |
| Anti-guinea pig IgG | Cy3 | 1 : 250 | 706-165-148 | Jackson ImmunoResearch |
| Anti-rabbit IgG | Cy2 | 1 : 100 | 711-225-152 | Jackson ImmunoResearch |
| Anti-rabbit IgG | Cy3 | 1 : 250 | 711-165-152 | Jackson ImmunoResearch |
| Anti-sheep IgG | Cy3 | 1 : 250 | 713-165-147 | Jackson ImmunoResearch |
ChAT, choline acetyltransferase; Cy2, cyanine; Cy3, indocarbocyanine; GABA, gamma-aminobutyric acid; GABAB-R, GABAB-receptor; NOS, nitric oxide synthase; NPY, neuropeptide tyrosine; PNMT, phenylethanolamine N-methyltransferase.
For double-immunostaining, the sections were incubated with a mixture of two primary antisera (GABA/ChAT, GABAB-R/NOS, GABAB-R/NPY)-raised different species for 12 h at 4 °C. The immunoreacted sections were rinsed in PBS and then incubated with a mixture of secondary antibodies conjugated with Cy3 or Cy2. In order to show double-staining of GABA/NPY, GABA/NOS, the elution technique of Nakane (1968) was used. The sections were first incubated with GABA antiserum and photographed; the antibody was then eluted and finally incubated with NPY or NOS antiserum, respectively.
The specificity of the immunohistochemical staining was confirmed by replacing the primary antibodies with normal rabbit serum, and by using diluted antiserum pretreated with adequate antigen (5–5.7 μg mL−1) for 24 h at 4 °C. No immunostaining was observed under this condition.
Results
In the cortex, no GABA-immunoreactivity was seen in the cortical cells. GABA-immunoreactive nerve fibers were seen in the blood vessels under the capsule and in high numbers in the superficial cortex (zona glomerulosa) compared with other cortex areas. GABA-immunoreactive nerve fibers were both associated and not associated with the blood vessels (Fig. 1). In addition, some GABA-immunoreactive nerve fibers ran through the cortical cells. Double-immunostaining with GABA and ChAT antibodies revealed the GABA-immunoreactive nerve fibers in the cortex were ChAT-immunonegative (Fig. 2A,B). Some GABA-immunoreactive nerve fibers and bundles ran through the cortex and divided into thinner nerve fibers in the medulla.
Figure 1.
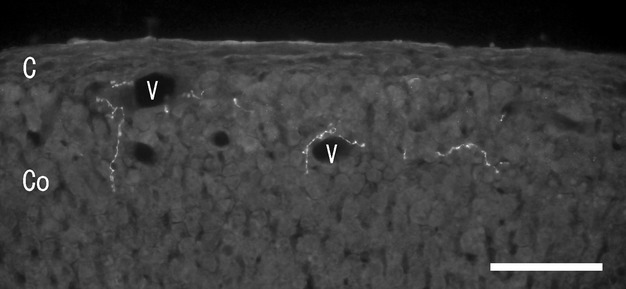
Fluorescent micrograph of gamma-aminobutylic acid (GABA) immunoreactivity in the rat adrenal cortex. GABA-immunoreactive nerve fibers are seen along the blood vessels (V) and among the cortical cells. C, capsule; Co, cortex; V, blood vessel. Scale bar: 30 μm.
Figure 2.
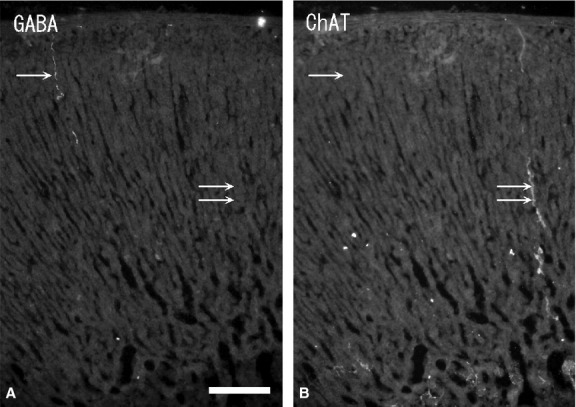
Fluorescent micrographs of double-immunostaining with gamma-aminobutyric acid (GABA) (A) and choline acetyltransferase (ChAT) (B) antibodies in the same section of the rat adrenal cortex. GABA-immunoreactive nerve fiber (single arrow) is ChAT-immunonegative, while ChAT-immunoreactive nerve fiber (double arrows) is GABA-immunonegative. Scale bar: 60 μm.
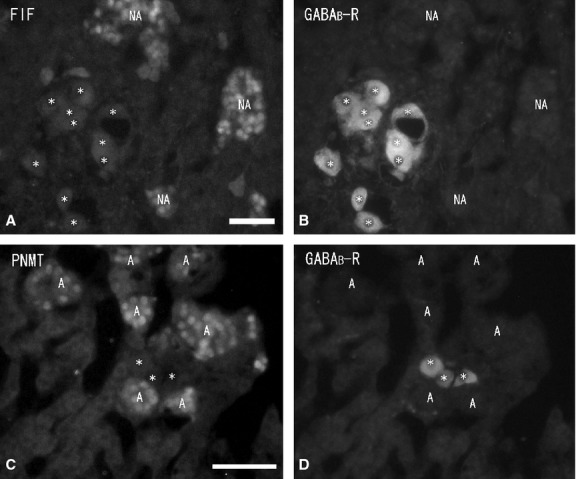
Numerous ganglion cells in the medulla were GABA-immunonegative and had long cytoplasmic processes and a large nucleus in the cytoplasm. Ganglion cells were located mainly in the periphery or in the center of the medulla, and sometimes in the juxtamedullary cortex (zona reticularis). Double-immunostaining with GABA and NOS antibodies showed some of the GABA-immunoreactive ganglion cells to be NOS-immunopositive (Fig. 3A,B), but no GABA-immunoreactivity was found in the chromaffin cells. Immunohistochemistry combined with FIF showed no GABA-immunoreactivity in noradrenaline cells with blue-white fluorescence (Fig. 4A,B). Double-immunostaining revealed no GABA-immunoreactivity in adrenaline cells with PNMT-immunoreactivity (Fig. 4C,D). In some cases, a few GABA-immunoreactive nerve fibers were seen running into the medulla. However, these GABA-immunoreactive nerve fibers did not closely appose the chromaffin cells and finally reached the ganglion cells in the medulla. GABA-immunoreactive nerve bundles and nerve fibers were found in clusters of the large ganglion cells or in single ganglion cells in the medulla, and were in close contact with the ganglion cells. Double-immunostaining with GABA, NOS and NPY antibodies revealed the GABA-immunoreactive nerve fibers in the medulla were in close contact with the NPY-immunoreactive ganglion cells, and in even closer contact with NOS-immunoreactive ganglion cells (Fig. 5A,B). In the medulla, NPY-immunoreactive ganglion cells (40–60 μm in diameter) were present in large clusters, while NOS-immunoreactive ganglion cells (30–40 μm in diameter) were present as single cells or in small clusters. Furthermore, a few GABA-immunoreactive nerve fibers were ChAT-immunopositive, while most of the GABA-immunoreactive nerve fibers were ChAT-immunonegative (Fig. 6A,B). Numerous ChAT-immunoreactive nerve fibers without GABA-immunoreactivity were in close contact with the ganglion cells and chromaffin cells in the medulla.
Figure 3.
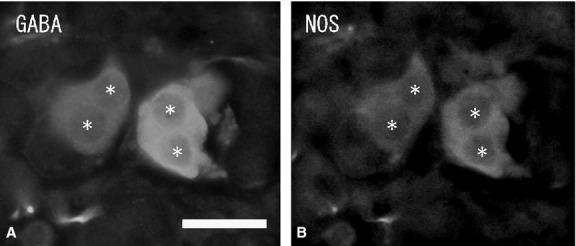
Fluorescent micrographs of double-immunostaining of gamma-aminobutyric acid (GABA) (A) and nitric oxide synthase (NOS) (B) antibodies in the same section of the rat adrenal juxtamedullary cortex. GABA-immunoreactive ganglion cells (asterisks) are NOS-immunopositive. Scale bar: 30 μm.
Figure 4.
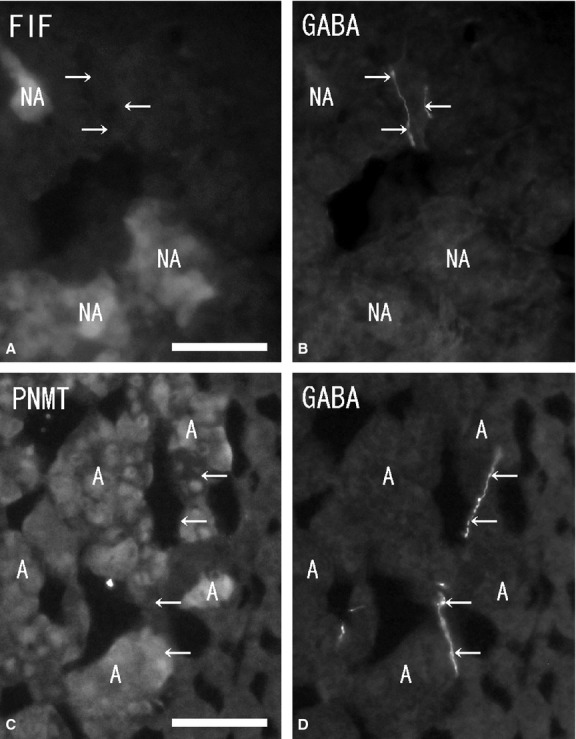
Fluorescent micrographs of formaldehyde-induced fluorescence (FIF) (A) and immunostaining of gamma-aminobutyric acid (GABA) (B) antibody, double-immunostaining of phenylethanolamine N-methyltransferase (PNMT) (C) and GABA (D) antibodies in the same section (A and B, C and D) of the rat adrenal medulla. A few GABA-immunoreactive nerve fibers (arrows) are found in the medulla (B, D). However, no GABA-immunoreactivity is seen in noradrenaline cells (NA) showing blue-white fluorescence and in adrenaline cells (A) demonstrating PNMT-immunoreactivity (A and B, C and D). Scale bar: 40 μm.
Figure 5.
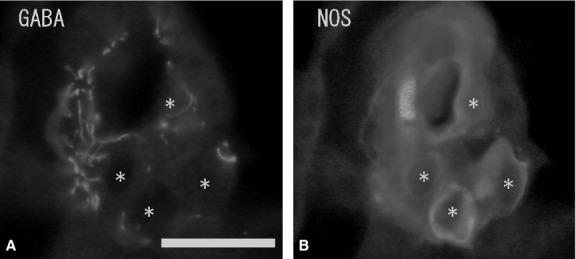
Fluorescent micrographs of double-immunostaining of gamma-aminobutyric acid (GABA) (A) and nitric oxide synthase (NOS) (B) antibodies in the same section of the rat adrenal medulla. Numerous GABA-immunoreactive nerve fibers are in close contact with GABA-immunonegative ganglion cells (A) (asterisks). NOS-immunoreactivity is seen in these ganglion cells (B) (asterisks). Scale bar: 30 μm.
Figure 6.
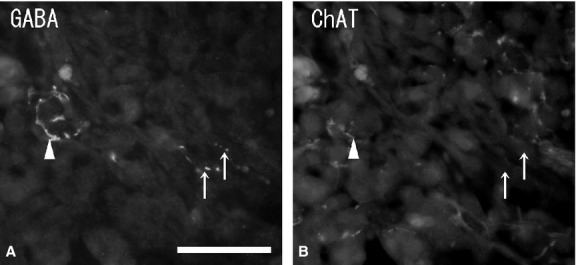
Fluorescent micrographs of double-immunostaining of gamma-aminobutyric acid (GABA) (A) and choline acetyltransferase (ChAT) (B) antibodies in the same section of the rat adrenal medulla. Few GABA-immunoreactive nerve fibers (arrowhead) are ChAT-immunopositive, while some GABA-immunoreactive nerve fibers (arrows) are ChAT-immunonegative. Numerous ChAT-immunoreactive nerve fibers are GABA-immunonegative. Scale bar: 50 μm.
In the cortex, no GABAB-R-immunoreactivity was seen in the cortical cells, whereas in the medulla, GABAB-R-immunoreactivity was observed in both large and small ganglion cells, but not in chromaffin cells or nerve fibers (Fig. 7). In the medulla, immunohistochemistry combined with FIF showed no GABAB-R-immunoreactivity in the noradrenaline cells with blue-white fluorescence (Fig. 8A,B). Double-immunostaining showed no GABAB-R-immunoreactivity in the adrenaline cells with PNMT-immunoreactivity (Fig. 8C,D). Immunoreactivity was observed as fine dots on the membrane of the ganglion cells. Double-immunostaining with GABAB-R and NPY antibodies showed the GABAB-R-immunoreactive ganglion cells were NPY-immunopositive (∼50% of ganglion cells; Fig. 9A–D). Large NPY-immunoreactive ganglion cells were present as clusters in the medulla. Similarly, some GABAB-R-immunoreactive ganglion cells were also NOS-immunopositive, while others were NOS-immunonegative (Fig. 9E,F). In the medulla, the NOS-immunopositive ganglion cells were smaller than the NPY-immunoreactive ganglion cells.
Figure 7.
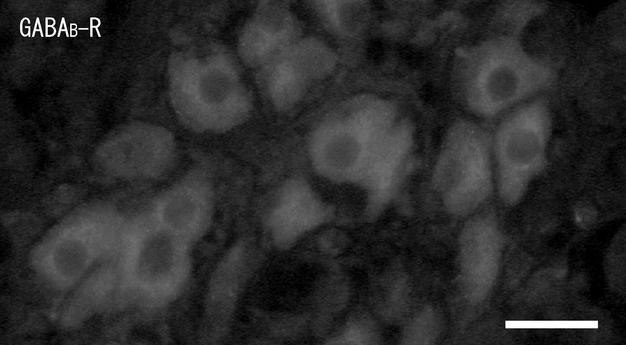
Fluorescent micrograph of gamma-aminobutyric acidB-receptor (GABAB-R)-immunoreactivity in the rat adrenal medulla. A cluster of GABAB-R-immunoreactive ganglion cells is found in the medulla. Scale bar: 50 μm.
Figure 8.
Fluorescent micrographs of formaldehyde-induced fluorescence (FIF) (A) and immunostaining of gamma-aminobutyric acidB-receptor (GABAB-R) (B) antibody, double-immunostaining of phenylethanolamine N-methyltransferase (PNMT) (C) and GABAB-R (D) antibodies in the same section (A and B, C and D) of the rat adrenal medulla. GABAB-R-immunoreactivity is seen in the ganglion cells (asterisks) in the medulla (B, D). No GABAB-R-immunoreactivity is observed in noradrenaline cells (NA) demonstrating blue-white fluorescence and in adrenaline cells (A) demonstrating PNMT-immunoreactivity (A and B, C and D). Scale bar: 40 μm.
Figure 9.
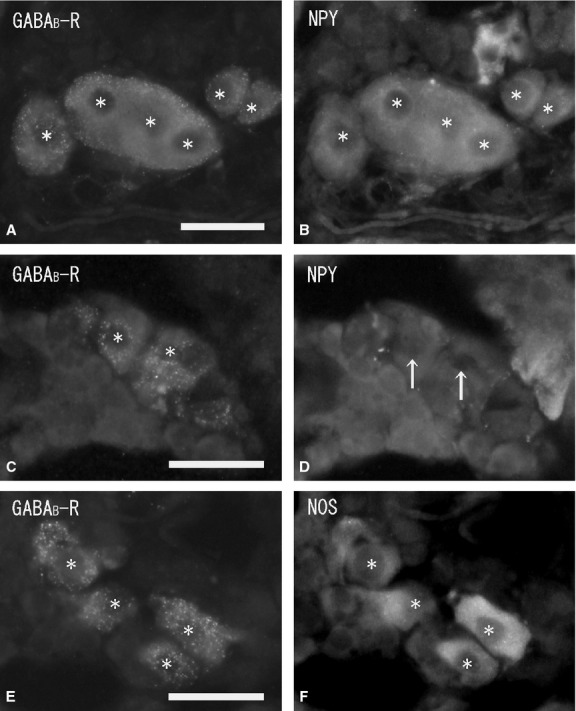
Fluorescent micrographs of double-immunostaining of gamma-aminobutyric acidB-receptor (GABAB-R) (A, C, E), and neuropeptide tyrosine (NPY) (B, D) and nitric oxide synthase (NOS) (F) antibodies in the same section (A and B, C and D, E and F) of the rat adrenal medulla. GABAB-R-immunoreactive ganglion cells (A) (asterisks) are all NPY-immunopositive (B) (asterisks). GABAB-R-immunoreactive ganglion cells (C) (asterisks) are all NPY-immunonegative (D) (arrows). GABAB-R-immunoreactive ganglion cells (asterisks) are all NOS-immunopositive (F) (asterisks). Scale bar: 50 μm.
In the control, immunohistochemical staining was confirmed by replacing the primary antibody with normal rabbit serum. No immunostaining was observed in the control sections of the rat cortex and medulla (Fig. 10A,B).
Figure 10.
Fluorescent micrographs showing replacement of the primary antibody with normal rabbit serum in the sections of the rat adrenal medulla (A) and cortex (B). No immunoreactivity is seen in the cortex (A) and in the medulla (B). C, capsule; Co, cortex; M, medulla; V, blood vessel. Scale bar: 60 μm.
Discussion
In the present study, no GABA-or GABAB-R-immunoreactivity was observed in the adrenaline cells or noradrenaline cells, and no GABA-immunoreactive nerve fibers were observed in close contact with the chromaffin cells in the rat adrenal medulla. It is therefore likely that neither GABA nor GABAB-R is expressed in the chromaffin cells of the rat adrenal medulla. In contrast, previous studies have shown the presence of GABA-or GAD-immunoreactive chromaffin cells in various mammals (Alho et al. 1986), including mice (Oomori et al. 1993; Iwasa et al. 1998, 1999), and close apposition of GABA-immunoreactive nerve fibers to chromaffin cells in the adrenal medulla of both dogs and mice (Alho et al. 1986; Oomori et al. 1993; Iwasa et al. 1998, 1999). Furthermore, GABA-R agonists bound to the plasma membranes of bovine chromaffin cells have also been detected (Alho et al. 1986). A GABA-R agonist has been shown to inhibit the release of catecholamines from canine adrenal chromaffin cells elicited by nicotinic receptor stimulation (Kataoka et al. 1986). These results suggest that GABA exists in adrenal chromaffin cells and that intra-adrenal nerve fibers may mediate the secretory activity of the chromaffin cells via the GABA-R. On the other hand, previous physiological studies have indicated that GABAA-R but not GABAB-R exists in the chromaffin cells of the rat adrenal medulla (Busik et al. 1996). Analysis of physiological, molecular and immunohistochemical data has revealed the expression of GAD, vesicular GABA transporter, and GABAA-R mRNA and proteins in rat adrenal chromaffin cells, but no GAD-immunoreactive nerve fibers in the medulla (Matsuoka et al. 2008). Adrenal chromaffin cells are generally regarded as homologs of sympathetic ganglion cells and are innervated by preganglionic neurons (Ungar & Phillips, 1983). In fact, our previous studies have demonstrated the presence of dense GABA-immunoreactive nerve fibers in adrenaline cells, but not noradrenaline cells, and in ganglion cells of the mouse adrenal medulla (Oomori et al. 1993; Iwasa et al. 1998, 1999). Thus, although chromaffin cells secretion is regulated by neuronal elements, it is enigmatic that chromaffin cell secretion in the rat is controlled via the GABAA-R alone, with no GABA innervation.
The present study revealed that GABA-immunoreactive nerve fibers were in close contact with GABAB-R-immunoreactive ganglion cells in the rat adrenal medulla. These results suggest that GABA from intra-adrenal nerve fibers may have an inhibitory effect on the ganglion cells via the GABAB-R. Our previous electron microscopic study revealed that GABA-immunoreactive nerve fibers were in close apposition to the intra-adrenal ganglion cells and the postsynaptic membrane specialization of the ganglion cells in the mouse adrenal gland (Oomori et al. 1993). Other physiological studies have shown that GABA-produced depolarization depends on the resting membrane potential, and that GABA-induced depolarization inhibited synaptic transmission in the ganglion cells of the autonomic ganglia (DeGroat, 1970; Adams & Brown, 1975). Furthermore, inhibition of the GABAB-R in neurons was achieved mainly via modulating the release of neurotransmitters from presynaptic terminals and hyperpolarizing the postsynaptic membranes (Bowery et al. 2002). It is probable, therefore, that GABA from the intra-adrenal nerve fibers exerts an inhibitory effect on the secretory activity of the ganglion cells via pre-and postsynaptic GABAB-R.
In the present study, numerous GABA-immunoreactive nerve fibers without ChAT-immunoreactivity were in close contact with the ganglion cells in the medulla, while numerous ChAT-immunoreactive nerve fibers were in close contact with the ganglion cells in general. In contrast, a previous study revealed the co-localization of GABA and acetylcholinesterase in the intra-adrenal nerve fibers of the mouse, suggesting that GABA and acetylcholine are co-localized in the same nerve fibers (Iwasa et al. 1999). Another previous study reported differences in the frequency and distribution of GAD-immunoreactive nerve fibers in the adrenal gland of various mammals (Alho et al. 1986). Thus, this discrepancy in the co-localization of GABA and acetylcholine in the intra-adrenal nerve fibers might be due to interspecies differences. Previous studies have also described acetylcholinesterase activity, ChAT and vesicular acetylcholine transporter immunoreactivity in the intra-adrenal nerve fibers around the ganglion cells and chromaffin cells in the adrenal gland of both rats and mice (Oomori et al. 1994; Holgert et al. 1996b; Iwasa et al. 1999; Murabayashi et al. 2009). GABA from the intra-adrenal nerve fibers may have an inhibitory effect on cholinergic transmission via postsynaptic GABAB-R, and on GABA release from the nerve fibers via the presynaptic GABAB-R as an autoreceptor. In the present study, some GABAB-R-immunoreactive ganglion cells were NOS-immunopositive, while others were NPY-immunopositive. Studies involving the rat adrenal gland have reported two types of ganglion cell: large ganglion cells showing NPY-, tyrosine hydroxylase-and dopamine β-hydroxylase-immunoreactivity; and small ganglion cells exhibiting vasoactive intestinal polypeptide-and NOS-immunoreactivity (Oomori et al. 1994; Holgert et al. 1996a,b1996b). These findings imply that GABA from the nerve fibers may also inhibit the release of nitric oxide and vasoactive intestinal polypeptide or of NPY and catecholamines from the terminals of the ganglion cells via the GABAB-R.
In this study, GABA-immunoreactive nerve fibers were both associated and not associated with blood vessels in the rat adrenal cortex, while no GABAB-R-immunoreactivity was found in the vessels or in the cortical cells. These findings suggest that GABA from the intra-adrenal nerve fibers may mediate both the vasodilation and the secretion of cortical hormones from cells in the rat adrenal cortex via GABA-Rs other than GABAB-R. However, the direct effect of GABA on smooth muscles is not known. In fact, previous studies have reported that GABA exerts an inhibitory effect through the GABA-R located on the adrenergic nerve terminals innervating peripheral blood vessels (Starke & Weitzell, 1980) and that GABA nerve fibers innervated blood vessels (Imai et al. 1991). Furthermore, the GABAA-R in the glomerulosa cells of the adrenal cortex has been shown to inhibit aldosterone secretion (Kenyon et al. 1999).
Because the present study revealed the GABA-immunoreactive ganglion cells in the rat adrenal gland, GABA-immunoreactive nerve fibers in the rat adrenal gland may be of both intrinsic and extrinsic origin. This study also showed that intra-adrenal GABA-immunoreactive nerve fibers were mainly ChAT-immunonegative. It is well known that the neurons that innervate the adrenal medulla are located in the intermediolateral horn of the spinal cord (Kesse et al. 1988). Thus, GABA nerve fibers in the rat adrenal gland may originate mainly from all other neurons except those in the intermediolateral horn of the spinal cord. However, the precise origin of the extrinsic immunoreactive nerve fibers in the rat adrenal gland has not yet been established. There have been reports of GAD-or GABA-immunoreactive neurons in the spinal cord (McLaughlin et al. 1975; Hunt et al. 1981; Barber et al. 1982; Fuji et al. 1985; Ito et al. 2007) and the intestine (Jessen et al. 1986; Saito & Tanaka, 1986; Davanger et al. 1987; Hills et al. 1987; Furness et al. 1989; Sang & Young, 1998). Taken together, GABA-immunoreactive nerve fibers in the rat adrenal gland may originate from the extra-adrenal neurons running along blood vessels and partly from intrinsic ganglion cells.
Acknowledgments
The authors would like to thank Dr Sharon J.B. Hanley (Department of Reproductive Endocrinology and Oncology, Hokkaido University Graduate School of Medicine) for helpful suggestions and manuscript corrections.
References
- Adams PR, Brown DA. Actions of γ-amino butyric acid on sympathetic ganglion cells. J Physiol. 1975;250:85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen M, Joh TH, Wu J-Y, et al. Immunocytochemical localization of L-glutamate decarboxylase and catecholamine-synthesizing enzymes in the retroperitoneal sympathetic tissue of the newborn rat. J Auton Nerv Syst. 1989;26:89–96. doi: 10.1016/0165-1838(89)90156-2. [DOI] [PubMed] [Google Scholar]
- Alho H, Fujimoto M, Guidotti A. γ-Aminobutyric acid (GABA) in the adrenal medulla: location, pharmacology, and function. In: Panula P, Soinila S, et al., editors. Neurochemistry: Modern Methods and Applications. New York: Liss; 1986. pp. 453–464. [Google Scholar]
- Barber RP, Vaughn JE, Roberts E. The cytoarchitecture of GABAergic neurons in the rat spinal cord. Brain Res. 1982;238:305–328. doi: 10.1016/0006-8993(82)90107-x. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Bowery N. GABAB receptors: drugs meet clones. Curr Opin Neurol. 1998;8:345–350. doi: 10.1016/s0959-4388(98)80059-7. [DOI] [PubMed] [Google Scholar]
- Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci. 1989;10:401–407. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, et al. International union of pharmacology. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. XXXIII. Mammalian γ-aminobutyric acidB receptors: structure and function. [DOI] [PubMed] [Google Scholar]
- Busik J, Nakamura M, Shibuya I, et al. Effects of GABA on spontaneous [Ca2+]c dynamics and electrical properties of rat adrenal chromaffin cells. Brain Res. 1996;739:97–103. doi: 10.1016/s0006-8993(96)00814-1. [DOI] [PubMed] [Google Scholar]
- Castro E, Oset-Gasque MJ, Canadas S, et al. GABAA and GABAB sites in bovine adrenal medulla membranes. J Neurosci Res. 1989;20:241–245. doi: 10.1002/jnr.490200213. [DOI] [PubMed] [Google Scholar]
- Castro E, Gonzalez MP, Oset-Gasque MJ. Distribution of gamma-aminobutyric acid receptors in cultured adrenergic and noradrenergic bovine chromaffin cells. J Neurosci Res. 2003;71:375–382. doi: 10.1002/jnr.10488. [DOI] [PubMed] [Google Scholar]
- Davanger S, Ottersen OP, Strom-Mathisen J. Immunocytochemical localization of GABA in cat myenteric plexus. Neurosci Lett. 1987;73:27–32. doi: 10.1016/0304-3940(87)90025-5. [DOI] [PubMed] [Google Scholar]
- DeGroat WC. The action of γ-amino butyric acid related amino acids on mammalian autonomic ganglia. J Pharmacol Exp Ther. 1970;172:221–226. [PubMed] [Google Scholar]
- Falck B, Torp A. A fluorescence method for histochemical demonstration of noradrenaline in the adrenal medulla. Int J Exp Med. 1961;5:429–432. [PubMed] [Google Scholar]
- Fuji K, Senba E, Fuji S, et al. Distribution, ontogeny, and projections of cholecystokinin-8, vasoactive intestinal polypeptide and γ-amino butyrate-containing neuron systems in the rat spinal cord: an immunohistochemical analysis. Neuroscience. 1985;14:881–894. doi: 10.1016/0306-4522(85)90151-4. [DOI] [PubMed] [Google Scholar]
- Furness JB, Trussell DC, Pompolo S, et al. Shapes and projections of neurons with immunoreactivity for gamma-aminobutyric acid in the guinea-pig small intestine. Cell Tissue Res. 1989;256:293–301. doi: 10.1007/BF00218886. [DOI] [PubMed] [Google Scholar]
- Hills JM, Jessen KR, Mirsky R. An immunohistochemistry study of the distribution of enteric GABA-containing neurons in the rat and guinea-pig intestine. Neuroscience. 1987;22:301–312. doi: 10.1016/0306-4522(87)90220-x. [DOI] [PubMed] [Google Scholar]
- Holgert H, Dagerlind A, Hökfelt T. Phenotype of intraadrenal ganglion neurons during postnatal development in rat. J Comp Neurol. 1996a;371:603–620. doi: 10.1002/(SICI)1096-9861(19960805)371:4<603::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Holgert H, Lagercrantz H, Dagerlind A, et al. Effects of immunological sympathectomy on postnatal peptide expression in the rat adrenal medulla. Brain Res Dev Brain Res. 1996b;97:88–95. doi: 10.1016/s0165-3806(96)00135-6. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Kelly JS, Emson PC, et al. An immunohistochemical study of neuronal populations containing neuropeptides or γ-aminobutyrate within the superficial layer of the rat dorsal horn. Neuroscience. 1981;6:1883–1889. doi: 10.1016/0306-4522(81)90029-4. [DOI] [PubMed] [Google Scholar]
- Imai H, Okuno T, Wu JY, et al. GABAergic innervation in cerebral blood vessels: an immunohistochemical demonstration of L-glutamic acid decarboxylase and GABA transaminase. J Cereb Blood Flow Metab. 1991;11:129–134. doi: 10.1038/jcbfm.1991.15. [DOI] [PubMed] [Google Scholar]
- Ito T, Hioki H, Nakamura K, et al. γ-Aminobutyric acid-containing sympathetic preganglionic neurons in rat thoracic spinal cord send their axons to the superior cervical ganglion. J Comp Neurol. 2007;502:113–125. doi: 10.1002/cne.21309. [DOI] [PubMed] [Google Scholar]
- Iwasa K, Oomori Y, Tanaka H. Gamma-aminobutyric acid immunoreactivity in the mouse adrenal gland during postnatal development. Arch Histol Cytol. 1998;61:373–382. doi: 10.1679/aohc.61.373. [DOI] [PubMed] [Google Scholar]
- Iwasa K, Oomori Y, Tanaka H. Colocalization of gamma-aminobutyric acid immunoreactivity and acetylcholinesterase activity in nerve fibers of the mouse adrenal gland. J Vet Med Sci. 1999;61:631–635. doi: 10.1292/jvms.61.631. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Hills JM, Saffrey MJ. Immunohistochemical demonstration of GABAergic neurons in the enteric nervous system. J Neurosci. 1986;6:1628–1634. doi: 10.1523/JNEUROSCI.06-06-01628.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Gutman Y, Guirotti A, et al. Intrinsic GABAergic system of adrenal chromaffin cells. Proc Natl Acad Sci USA. 1984;81:3218–3222. doi: 10.1073/pnas.81.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Gutman Y, Guidotti A, et al. Intrinsic gamma aminobutyric acid receptors modulate the release of catecholamine from canine adrenal gland in situ. J Pharmacol Exp Ther. 1986;239:584–600. [PubMed] [Google Scholar]
- Kenyon CJ, Thompson I, Fraser R. Stimulation of aldosterone secretion by benzodiazepines in bovine adrenocortical cells. Fundam Clin Pharmacol. 1999;13:213–219. doi: 10.1111/j.1472-8206.1999.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Kesse WK, Parker TL, Coupland RE. The innervation of the adrenal gland I. The source of pre-and postganglionic nerve fibers to the rat adrenal gland. J Anat. 1988;157:33–41. [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Harada K, Endo Y, et al. Molecular mechanisms supporting a paracrine role of GABA in rat adrenal medullary cells. J Physiol. 2008;586:4825–4842. doi: 10.1113/jphysiol.2008.158709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin BJ, Barber R, Saito K, et al. Immunocytochemical localization of glutamate decarboxylase in rat spinal cord. J Comp Neurol. 1975;164:305–322. doi: 10.1002/cne.901640304. [DOI] [PubMed] [Google Scholar]
- Murabayashi H, Kuramoto H, Ishikawa K, et al. Acetylcholinesterase activity, choline acetyltransferase and vesicular acetylcholine transporter immunoreactivities in the rat adrenal gland during postnatal development. Anat Rec. 2009;292:371–380. doi: 10.1002/ar.20856. [DOI] [PubMed] [Google Scholar]
- Nakane P. Simultaneous localization of multiple tissue antigens using the peroxidase-labelled antibody method: a study in pituitary gland of the rat. J Histochem Cytochem. 1968;16:557–560. doi: 10.1177/16.9.557. [DOI] [PubMed] [Google Scholar]
- Oomori Y, Iuchi H, Nakaya K, et al. Gamma-aminobutyric acid (GABA) immunoreactivity in the mouse adrenal gland. Histochemistry. 1993;100:203–213. doi: 10.1007/BF00269093. [DOI] [PubMed] [Google Scholar]
- Oomori Y, Okuno S, Fujisawa H, et al. Ganglion cells immunoreactive for catecholamine-synthesizing enzymes, neuropeptide Y and vasoactive intestinal polypeptide in the rat adrenal gland. Cell Tissue Res. 1994;275:201–213. doi: 10.1007/BF00319418. [DOI] [PubMed] [Google Scholar]
- Saito N, Tanaka C. Immunohistochemical demonstration of GABA containing neurons in the guinea-pig ileum using purified GABA antiserum. Brain Res. 1986;376:78–84. doi: 10.1016/0006-8993(86)90901-7. [DOI] [PubMed] [Google Scholar]
- Sang Q, Young HM. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat Rec. 1998;251:185–199. doi: 10.1002/(SICI)1097-0185(199806)251:2<185::AID-AR6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Starke K, Weitzell R. Gamma-aminobutyric acid and postganglionic sympathetic transmission in the pulmonary artery of the rabbit. J Auton Pharmacol. 1980;1:45–51. doi: 10.1111/j.1474-8673.1980.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Ungar A, Phillips JH. Regulation of the adrenal medulla. Physiol Rev. 1983;63:787–843. doi: 10.1152/physrev.1983.63.3.787. [DOI] [PubMed] [Google Scholar]
- Ymer S, Schofield PR, Draguhn A, et al. GABAA receptor β subunit heterogeneity: functional expression of cloned cDNAs. EMBO J. 1989;8:1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


