Abstract
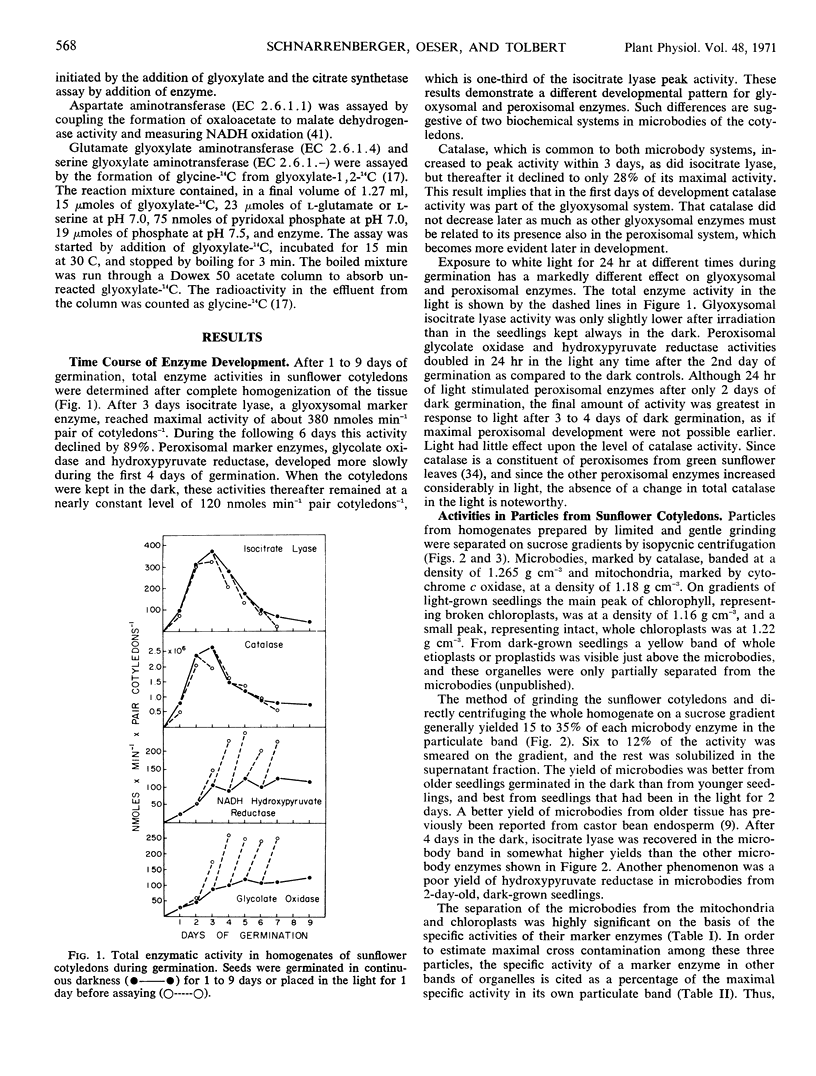
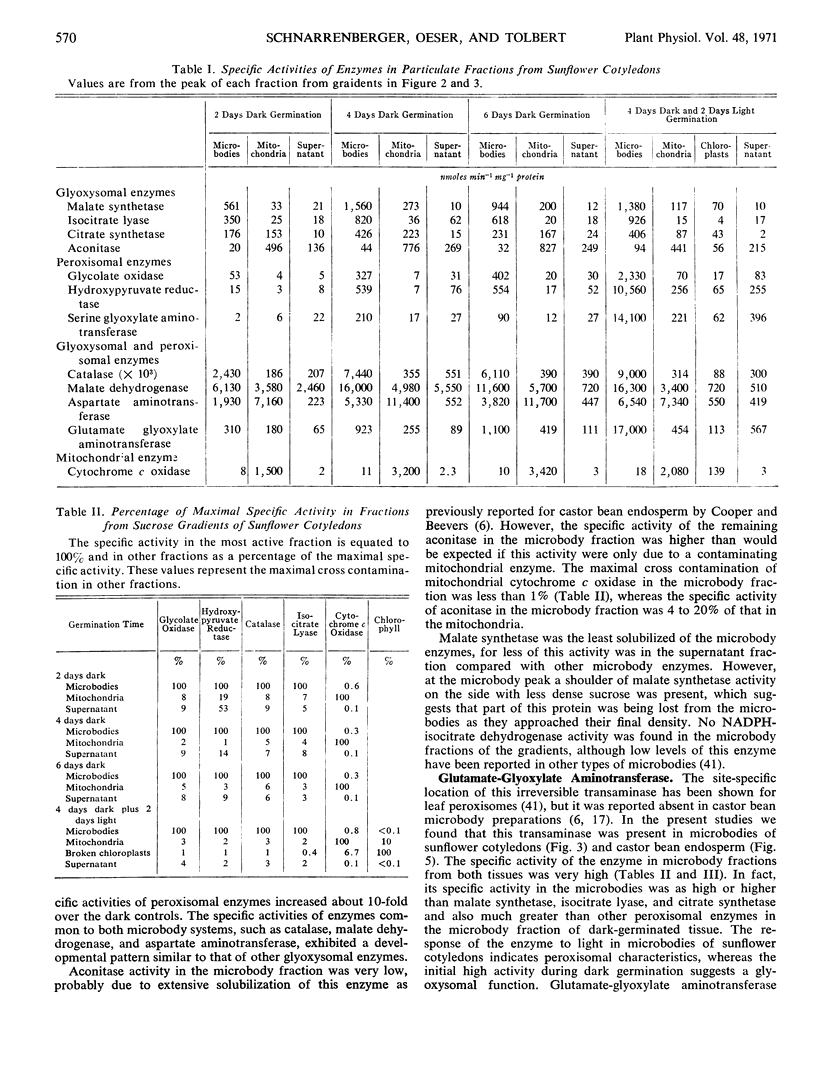
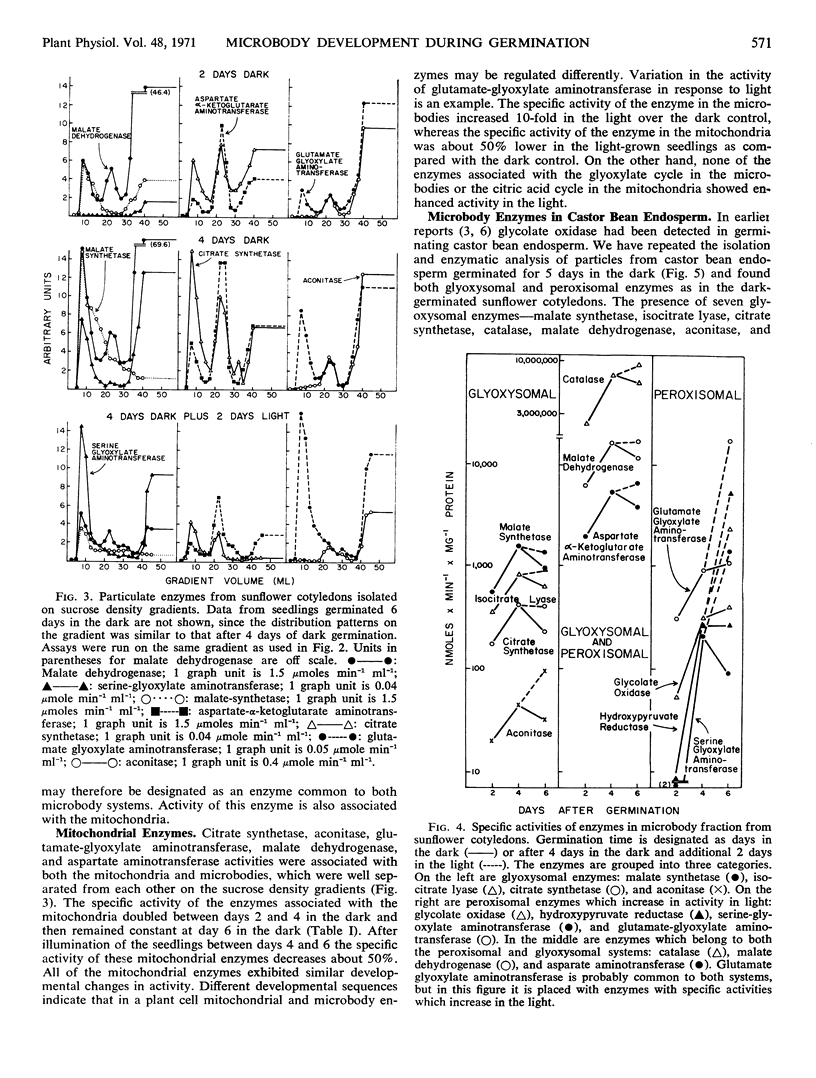
In cotyledons of sunflower seedlings glyoxysomal and peroxisomal enzymes exhibit different rates of development during germination. The total activity of isocitrate lyase, a glyoxysomal marker enzyme, rapidly increased during the first 3 days, and then decreased 89% by day 9. Exposure to light accelerated this decrease only slightly. The specific activity of glyoxysomal enzymes (malate synthetase, isocitrate lyase, citrate synthetase, and aconitase) in the microbody fraction from sucrose density gradients increased between days 2 and 4 about 2- to 3-fold, and thereafter it remained about constant in light or darkness.
Total activity of the peroxisomal enzymes increased slowly in the dark during the first 4 days of germination and thereafter remained at a constant level of activity in the dark or increased 2-fold in 24 hours of light. The specific activties of glycolate oxidase, hydroxypyruvate reductase, and serine-glyoxylate aminotransferase in the isolated microbody fraction increased about 10-fold between days 2 and 4 in the dark and then remained constant or increased again 10-fold after an additional 48 hours in the light.
The total activity of the common microbody marker, catalase, developed similarly to isocitrate lyase, but decreased only 72% by day 9. The specific activities of enzymes (catalase, malate dehydrogenase, and aspartate aminotransferase) common to both microbody systems were 10- to 1000-fold greater than those of other enzymes. It is proposed that malate and aspartate may be involved in hydrogen transport between microbodies and other cellular sites.
Glutamate-glyoxylate aminotransferase was very active in microbodies from castor bean endosperm and sunflower cotyledons. The specific activity of this aminotransferase developed similarly to glyoxysomal enzymes in the dark but further increased in the light, as did peroxisomal enzymes.
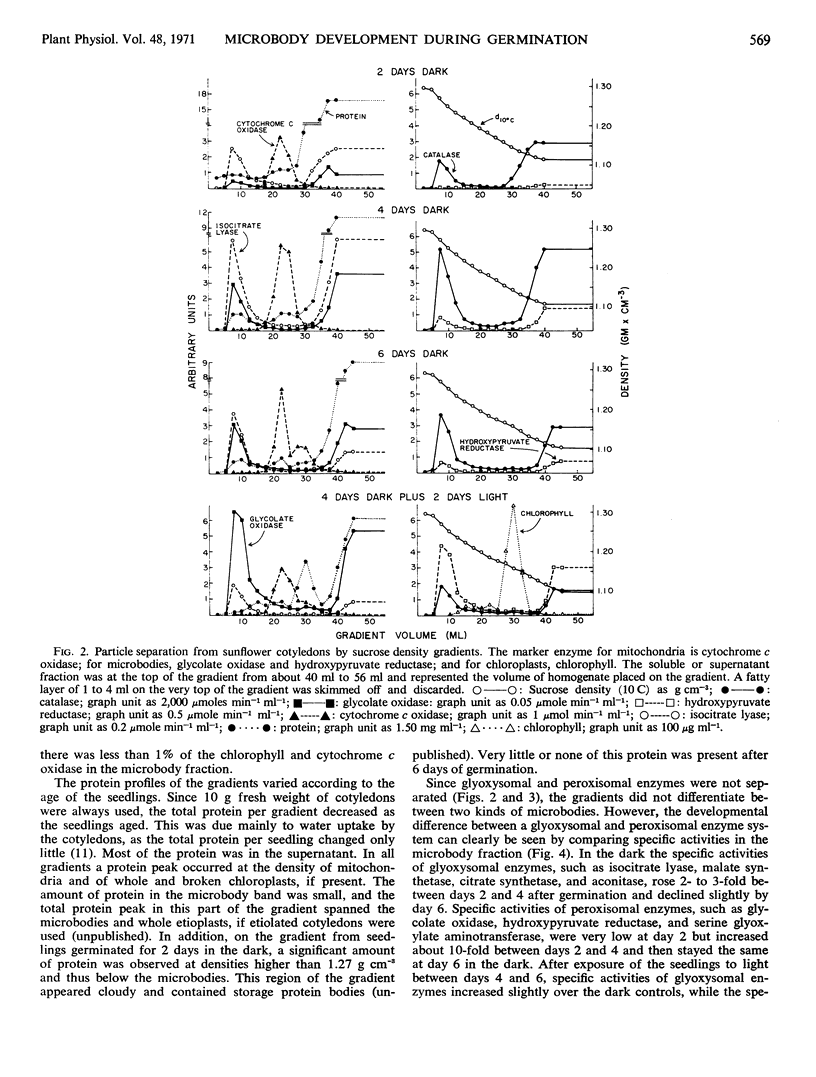
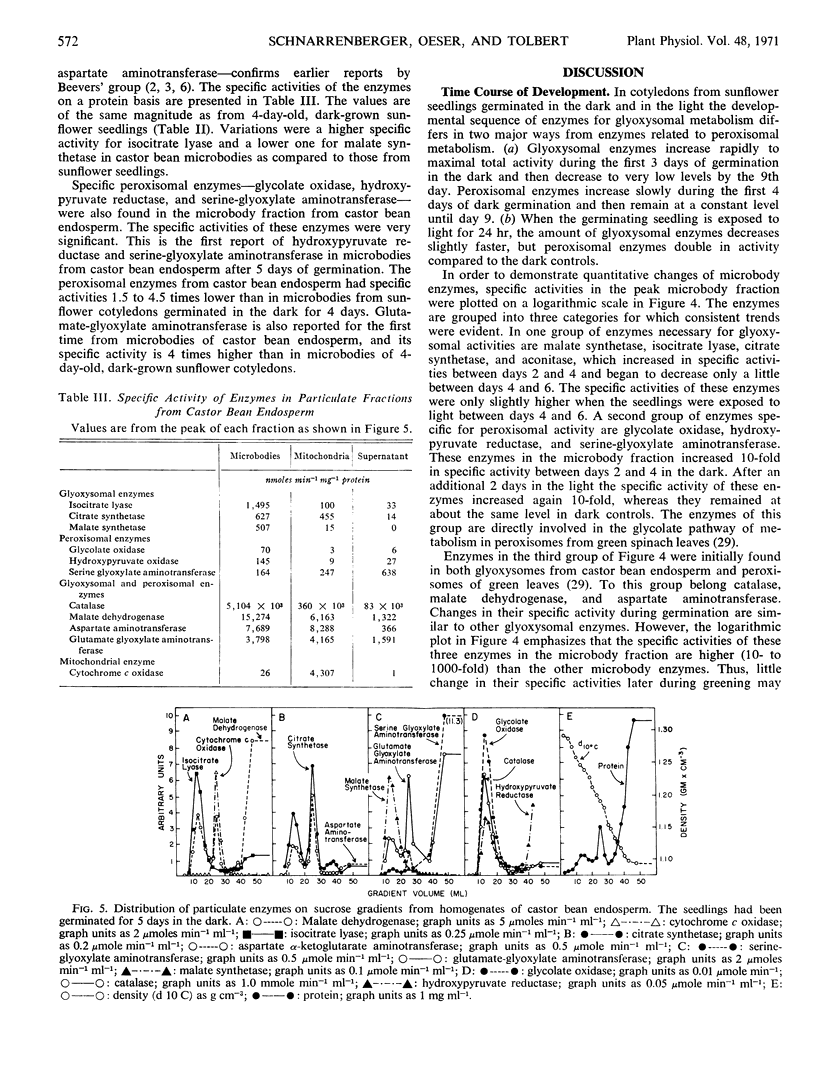
The microbody fraction of castor bean endosperm germinated in the dark for 5 days contained both glyoxysomal and peroxisomal enzymes of similar specific activity.
Adjacent to the microbody fraction on sucrose gradients from sunflower cotyledons were etioplasts at slightly lower densities and protein bodies at similar and higher densities. Their presence in the microbody fractions resulted in artificially low specific activities.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter W. D., Beevers H. Distribution and Properties of Isocitritase in Plants. Plant Physiol. 1959 Jul;34(4):403–409. doi: 10.1104/pp.34.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung G. P., Rosenblum I. Y., Sallach H. J. Comparative studies of enzymes related to serine metabolism in higher plants. Plant Physiol. 1968 Nov;43(11):1813–1820. doi: 10.1104/pp.43.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Filner B., Klein A. O. Changes in enzymatic activities in etiolated bean seedling leaves after a brief illumination. Plant Physiol. 1968 Oct;43(10):1587–1596. doi: 10.1104/pp.43.10.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gientka-Rychter A., Cherry J. H. De Novo Synthesis of Isocitritase in Peanut (Arachis hypogaea L.) Cotyledons. Plant Physiol. 1968 Apr;43(4):653–659. doi: 10.1104/pp.43.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisaki T., Tolbert N. E. Glycolate and glyoxylate metabolism by isolated peroxisomes or chloroplasts. Plant Physiol. 1969 Feb;44(2):242–250. doi: 10.1104/pp.44.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. O. Persistent photoreversibility of leaf development. Plant Physiol. 1969 Jun;44(6):897–902. doi: 10.1104/pp.44.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmak M., Tolbert N. E. Glycolic acid oxidase formation in greening leaves. Plant Physiol. 1962 Nov;37(6):729–734. doi: 10.1104/pp.37.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Longo C. P. Evidence for de novo synthesis of isocitratase and malate synthesis in germinating peanut cotyledons. Plant Physiol. 1968 Apr;43(4):660–664. doi: 10.1104/pp.43.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Rocha V., Ting I. P. Tissue distribution of microbody, mitochondrial, and soluble malate dehydrogenase isoenzymes. Plant Physiol. 1970 Nov;46(5):754–756. doi: 10.1104/pp.46.5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford H. A., Magaldi A. A Developmental Study of D-Glyceric Acid Dehydrogenase. Plant Physiol. 1954 Nov;29(6):504–508. doi: 10.1104/pp.29.6.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLBERT N. E., BURRIS R. H. Light activation of the plant enzyme which oxidizes glycolic acid. J Biol Chem. 1950 Oct;186(2):791–804. [PubMed] [Google Scholar]
- TOLBERT N. E., COHAN M. S. Activation of glycolic acid oxidase in plants. J Biol Chem. 1953 Oct;204(2):639–648. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Yamamoto Y., Beevers H. Malate Synthetase in Higher Plants. Plant Physiol. 1960 Jan;35(1):102–108. doi: 10.1104/pp.35.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Enzymic characterization of leaf peroxisomes. J Biol Chem. 1970 Oct 10;245(19):5137–5144. [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Malate dehydrogenase in leaf peroxisomes. Biochim Biophys Acta. 1969 Mar 18;178(1):11–20. doi: 10.1016/0005-2744(69)90127-2. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]



