Abstract
Cardiovascular diseases are the leading causes of death in men and women in industrialized countries. While the effects of biological sex on cardiovascular pathophysiology have long been known, the sex-specific mechanisms mediating these processes have been further elucidated over recent years. This review aims at analysing the sex-based differences in cardiac structure and function in adult mammals, and the sex-based differences in the main molecular mechanisms involved in the response of the heart to pathological situations. It emerged from this review that the sex-based difference is a variable that should be dealt with, not only in basic science or clinical research, but also with regards to therapeutic approaches.
Keywords: heart, gender, hypertrophy, cardiac failure
Linked Articles This article is part of a themed section on Biological Sex and Cardiovascular Pharmacology. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-3
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death in men and women in industrialized countries. Over the last decade, while little change was noticed on the sex ratio of cohorts in the majority of CVD studies (Mosca et al., 2011), several clinical trials provided evidence that sex is an important determinant of cardiovascular events in patients with vascular diseases or high-risk diabetes mellitus. Indeed, female diabetic patients had a higher risk for acute myocardial infarction compared to male diabetics (Kappert et al., 2012). Women also exhibited a marked increase in the incidence of left ventricular (LV) hypertrophy after the menopause, when the prevalence of arterial hypertension increases (Lopez-Ruiz et al., 2008). Furthermore, women generally display better cardiac function and survival in the face of CVD than men, although this advantage is lost when comparing postmenopausal women with age-matched men (Fujimoto et al., 2013).
The effects of biological sex on cardiovascular physiology or pathology have long been known, but the biological mechanisms responsible for sex-related differences started to be unravelled over the last decades. Indeed, sex steroid hormones (oestrogens in female, testosterone in male) contribute significantly to the sex-based differences in the outcome of cardiac diseases, although the contribution of environmental oestrogen-like molecules, such as phytoestrogens, must not be neglected. Thus, hormonal therapy such as the hormone replacement therapy (HRT) after menopause using synthetic oestrogens and progesterone, while broadly debated, may help to understand the effects of oestrogen in cardiac pathophysiology. In addition, some elucidation of the interrelation between the sex steroid hormones and peptides and/or hormones directly involved in the cardiac physiopathology such as the components of the renin angiotensin-aldosterone system, and the natriuretic peptides (NPs) will be provided. At least, as adequate exercise and nutrition programmes were shown to improve the prevention and the treatment of CVD and metabolic disorders (Hagey and Warren, 2008), these lifestyle patterns are beginning to be taken into account in the treatment of postmenopausal women. The collection of such data will be of interest to analyse the relation between exercise, sex and CVD.
This review aimed at focusing on the effects of sexual hormones on the pathophysiology of the heart. The effect of adjuvant therapy such as hormonal replacement therapy and physical training on the cardiac adaptive response to pathological situations will also be discussed.
Sex-based differences in the adult heart in mammals
Sex-based differences in cardiomyocytes
With age, the number of cardiomyocytes significantly decreases in men through different processes including apoptosis and necrosis, whereas cardiomyocyte number and size are preserved in age-matched women (Swynghedauw, 1999; Kajstura et al., 2010). At the cardiomyocyte level, various biochemical characteristics such as telomerase activity vary differently in male and female across the lifespan. Telomerase is an enzyme that repairs the telomeric repeat DNA lost during the cell cycle, thus restoring telomere length. Telomere maintenance is one mechanism through which cell viability is preserved, and telomere shortening occurs at the end stage of heart failure in humans (Oh et al., 2003). Telomerase activity is detectable in the cardiomyocytes of young adults and decreases with aging in males, whereas it markedly increases in females. These data emphasize a gender difference in the cardiomyocyte viability and replication (Leri et al., 2000; Torella et al., 2004; Kajstura et al., 2010). The enhanced telomerase activity in female cardiomyocytes provides a molecular basis for the preservation of cardiomyocyte population in women throughout their lifespan. Besides, oestrogen signalling prevents cardiac muscle mass loss through autophagy, in a context of cancer, resulting in lower cardiac atrophy in females than in males (Cosper and Leinwand, 2011). Altogether, these data suggest that oestrogens may be responsible for such differences in cardiomyocyte replication capacity/viability and may explain the greater ability of the female heart to resist the deleterious consequences associated with the aging process and/or the development of heart failure.
At the subcellular level of the cardiomyocyte, sex differences in excitation-contraction (E-C) coupling have been reported in adult rats. Ca2+ transients are smaller and the gain of E-C coupling is lower in the female cardiomyocytes than in the males. In addition, the aging-induced alterations of cardiac E-C coupling are more prominent in male, than in female hearts (Howlett, 2010). The sex-based differences in intracellular calcium handling also involved the phosphorylation state of phospholamban, L-type Ca2+ channel density and the K+ currents. In mouse ventricle, Saito et al. (2009) proposed a gender-related differences in K+ currents during ventricular repolarization, when examining fast transient outward K+ current (Ito,f) and ultrarapid delayed rectifier K+ current (IK,slow). They observed that under conditions where oestrogen levels were high, the induced K+ current was reduced following a down-regulation of the Kv4.3 and Kv1.5 channels carrying the Ito,f and IK,slow respectively (channel and receptor nomenclature follows Alexander et al., 2013). These changes provided a molecular correlation for the prolongation of the action potential duration and the corrected QT interval in female mice under conditions of high oestrogens. These findings suggest that knowledge of the hormonal status is important to set the appropriate timing of the treatment in women prone to arrhythmias (Saito et al., 2009). In addition, female cardiomyocytes have a lower density of β-adrenoceptors and thus also a decreased inotropic response to stimulation of these receptors (Ostadal et al., 2009).
Other sex-based differences are found at the level of mitochondria in the cardiomyocytes. The rate of Ca2+uptake by cardiac mitochondria is lower in females than in males (see Ostadal et al., 2009). In addition, female rats exhibit lower cardiac mitochondria contents, their lower number being compensated by a higher efficiency (Colom et al., 2007). Therefore, the mitochondria from female hearts generate less free radicals, hence leading to lower cardiac oxidative damage in these animals. Such mitochondrial properties might be involved in the lower incidence of aging-related disorders and/or cardiac disease in women than in men.
Sex-based differences in the vascular cells of the coronary network
Another major cellular target for the sex-based differences in the CVDs is the endothelial cell, mainly through the modulation of the endogenous vasodilator NO by the endothelial NOS (NOS3). The oestrogens via the oestrogen receptors (ER)-α play a key role in the control of NOS3 activity (Mendelsohn and Karas, 1999; Chambliss and Shaul, 2002; Fleming and Busse, 2003). The microdomains, such as caveolae, are involved in the fine-tuned regulation of oestrogen-dependent NOS3 activity (Fleming and Busse, 2003; Loyer et al., 2007a).
Sex differences have been also observed at the level of the vascular smooth muscle cell (VSMC) and affect the cell death and growth in vivo and in vitro. A ‘gender memory’ can be conserved in VMSC in primary culture (Straface et al., 2009) and sex-based inhibition of VMSC proliferation by endothelin-1 (ET-1) was proposed to contribute, in part, to the cardioprotection noted in oestrogen-repleted states (Antoniucci et al., 2001). Female VSMCs exhibit a resistance to anoikis, showing a more adhering phenotype that is characterized by a well-organized actin microfilament cytoskeleton and an increased level of phosphorylated kinases involved in focal adhesion, and more importantly, a higher propensity to undergo survival by autophagy (Straface et al., 2009). The regulating effects of oestrogens on artery myogenic tone appear to involve regulation of calcium-activated potassium (BKCa) channels (Geary et al., 1998; Rosenfeld et al., 2000). BKCa channel expression and activity depend on a cohort of hormones and factors including those of the renin-angiotensin aldosterone system. Hence a down-regulation of BKCa channels in VSMC (Ambroisine et al., 2007), is present only in males in presence of a cardiac hyperaldosteronism (Garnier et al., 2004); the oestrogen levels in female counteracting the aldosterone effect on BKCa channel expression (C. Delcayre, pers. comm.).
Sex-based differences in the inflammatory cells and fibroblasts in cardiac pathophysiology
Inflammatory cells, mast cells and cardiac fibroblasts are known to have a detrimental role during cardiac disease and these cell types might also be modulated by sex hormones. For example, oestrogens appeared to protect against the significant increases in mast cell density, collagen degradation, ET-1 and TNF-α, induced by volume overload (Lu et al., 2012). The gender effects on the cardiac fibroblast, one of the key cells involved in the development of fibrosis, have been investigated recently (Montalvo et al., 2012). By combining, gender analysis together with the effect of castration, it was demonstrated that circulating sex hormones contributed to the male sex-related increase in fibrosis and subsequent LV dysfunction after thoracic aorta constriction (TAC) through a mechanism involving TGF-β (Montalvo et al., 2012). Based on these results, it was proposed that upon aging, the detrimental effects of the circulating androgens in males, rather than the protective actions of oestrogens in females, contributed to sex-related differences in myocardial remodelling.
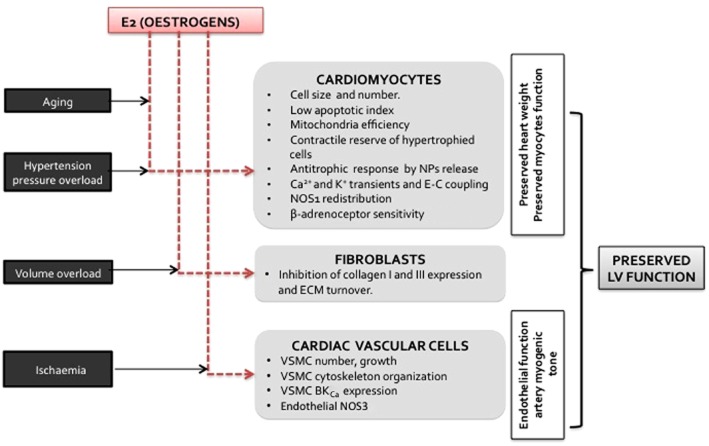
Thus, oestrogens may trigger some of the major sex-based differences observed in cardiac pathophysiology, through unique effects in the different cell types present in the heart (Figure 1).
Figure 1.
Summary of the effects of oestrogens, according to the cell types in the heart, which can be involved in cardioprotection induced by oestrogens.
Sex hormones and receptors, effect on target organs
The sex-specific effects in the CVDs are mediated by the oestrogen, progesterone and androgen receptors (ERs, PRs and ARs respectively). The two known ERs, ERα and ERβ (ESR1 and ESR2), have been described in the human and rodent hearts, (see Fielitz et al., 2007). All three receptor types, ERs, ARs and PRs, act by a number of genomic and non-genomic pathways. They act as transcription factors able to initiate the transcription of hormone-sensitive genes or to modulate the activity of other transcription factors. On binding the corresponding hormones, the ER, AR and PR can activate or interfere with many signalling pathways, including that of PI3K. Furthermore, an orphan GPCR, GPR30, has been proposed to mediate rapid actions of oestrogens (Revankar et al., 2005), and was recently suggested to be a candidate receptor for non-genomic action of aldosterone in VSMC (see Wendler et al., 2012). Signalling after activation of GPR30 may involve the stimulation of the adenyl cyclase and cAMP-dependent pathways.
Both ERs are expressed both in male and female myocardium. Apart from the production of oestrogens by the ovary, it has been suggested that both men and women synthesize oestrogens locally through the conversion of androgen to oestrogens by aromatase, particularly in adipose tissue (see Regitz-Zagrosek et al., 2013). The increased oestrogen levels in older or obese men have been proposed to increase the risk for the development or the progression of CVD (Kararigas et al., 2012). These elevated oestrogen levels in men are suggested to be responsible for the age-related changes in cardiac gene expression (see below). However, Banka (2012) pointed out that longitudinal studies revealed a significant age-dependent decrease in estradiol bioavailability in men, whereas there was a high increase in estradiol levels associated with obesity. This adipose tissue-dependent increase in estradiol synthesis in men may account in part, for the increased risk of CVD associated with obesity and with the increase in cardiovascular events observed in a study on men treated for prostate cancer with high dose of diethylstilbestrol (Ferrini and Barrett-Connor, 1998) or of polyestradiol phosphate (Hedlund et al., 2008).
Hence, the oestrogen actions differ between male and female with direct sex- and cardiomyocyte-specific effects in the heart (Kararigas et al., 2012). The relative importance of ERα versus ERβ has not been addressed in normotensive models. However, genetic models of ERβ-deleted mice (ERβ-/-) showed that ERβ has a cardioprotective role in females, while having minor effects on fibrotic remodelling in heart of the ERβ-/- male (Regitz-Zagrosek et al., 2013). As described above, the stimulation of the ERα and ERβ by oestrogens activate kinases involved in different signalling pathways such as Akt, PI3K, ERK 1/2, the p38 MAPK, and regulate calcineurin expression (see Sussman et al., 2011; Figure 2). Sussman et al. (2011) have clearly established sex differences in the basal levels of Akt, and demonstrated a nuclear accumulation of Akt in response to estradiol or a phytoestrogen treatment. The activation of Akt by oestrogen is known to influence events such as cell metabolism, cell cycle and cell survival. Studies from Sussman's group have also highlighted the role of the PI3K/Akt signalling cascade in the cardioprotective effects mediated by oestrogens and oestrogenic treatment. In addition, many cardioprotective genes such as the heat shock protein (Hsp) 72 or Hsp70 are up-regulated either directly or indirectly by oestrogens (Bhupathy et al., 2010).
Figure 2.
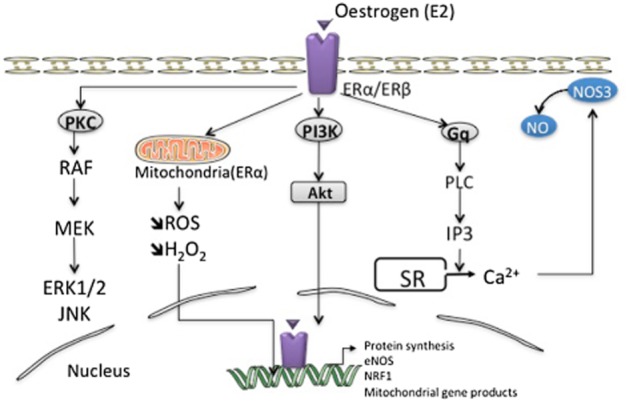
Molecular mechanisms of oestrogen action in cardiac cells, such as endothelial cells. Oestrogens can bind and activate the ERs, thereby inducing intracellular signalling cascades. Additionally, oestrogens influence other signalling pathways in the heart: oestrogens activate (i) the PI3K/Akt pathway, (ii) the Gq-coupled receptors, which results in the production of inositol trisphosphate (IP3) and the subsequent triggering of intracellular Ca2+ release and NO production, and (iii) the PKC/MAPK signal transduction pathways. All these signalling pathways are triggered at the level of the plasma membrane and activate intracellular cascades that converge to cytosolic targets and transcription factors and cofactors modulating gene expression. Finally, oestrogens can bind to mitochondria-specific receptors, through an increase in nuclear transcription of the nuclear respiratory factors (NRFs). MEK, mitogen-activated extracellular signal-regulated protein kinase; RAF, rapidly accelerated fibrosarcoma; ROS, reactive oxygen species.
Numerous results indicate that oestrogens have favourable metabolic effects. Oestrogen deficiency increased heart and muscle lipid content and the atherogenic index (Picard et al., 2000; Torto et al., 2006) and was associated with metabolic disorders such as insulin resistance and altered glucose metabolism (Champion et al., 2004; Bouwens and Rooman, 2005; Song et al., 2005; Sitnick et al., 2006).
Sex-based differences and neurohormonal disorders in CVD
An increasing body of evidence demonstrates sex differences in the renin-angiotensin-aldosterone system (RAAS), and their involvement in the development and progression of CVD and hypertension (see Bubb et al., 2012). The greater activation of the RAAS leading to greater BP levels in males may be attributed in part to androgens, as castration leads to a normalization of BP and a down-regulation of the intra-renal RAAS. In addition, oestrogens have been shown to down-regulate the expression and activity of various components of the RAAS, potentially explaining the lower BP levels observed in females compared to males (see Maric-Bilkan and Manigrasso, 2012). Oestrogens decreased renin levels, ACE activity, angiotensin AT1 receptor density and aldosterone production (Bubb et al., 2012). Besides, oestrogens increase the expression of NPs, AT2 receptor density and angiotensin-(1–7) (Baiardi et al., 2005; Bubb et al., 2012; Gupte et al., 2012; Maric-Bilkan and Manigrasso, 2012). The sex difference in the RAAS may also involve the GPR30 receptor, which has been proposed to trigger the non-genomic effects of oestrogens and aldosterone. Progesterone competes with aldosterone for the mineralocorticoid receptor. Little is known about androgens, but testosterone seems to increase renin levels and ACE activity (Komukai et al., 2010). These effects of sex hormones on the RAAS can explain some of the sex differences in CVDs.
We previously described that cardiac hyperaldosteronism induced coronary artery defects in a sex-specific fashion (Garnier et al., 2004). More recently, our data suggested that oestrogen may counteract the effect of hyperaldosteronism on the BKCa channel-mediated coronary relaxation in normotensive animals (Azibani et al., 2013). In male mice, cardiac hyperaldosteronism was shown to worsen hypertension-induced fibrosis through two mineralocorticoid receptor-dependent mechanisms: the activation of inflammation/galectin-3-induced fibrosis and the inhibition of anti-fibrotic factor expression (NPs and bone morphogenetic protein-4; Azibani et al., 2012). As proposed by Regitz-Zagrosek et al., 2013, these features of adverse remodelling strongly suggested a role for ERβ in males. This suggestion was supported by the fact that in deoxycorticosterone acetate (DOCA)-salt mice, a model for low-renin salt-sensitive hypertension similar to aldosterone, a higher increase in cardiomyocyte diameter, pro-inflammatory and pro-fibrosis transcripts was observed in male when compared to female. In addition, female DOCA mice did not exhibit any signs of heart failure (Regitz-Zagrosek et al., 2013). Interestingly, in diabetic animals, aldosterone plasma levels were shown to be increased in males, but not in females (Shimoni et al., 2008). The sex-dependent elevation of aldosterone in plasma and in cardiac cells was proposed to contribute to oxidative stress in this metabolic disorder (Shimoni et al., 2008).
Finally, the association of NPs, such as BNP and ANP, with gender has been examined in several studies. Despite disparity among results, higher BNP plasma levels were observed in females than in males. In basal conditions, NT-proBNP plasma levels, like those of BNP, tend to be higher in female patients and older individuals, through mechanisms involving either the clearance receptor for BNP or an increased NP expression (Redfield et al., 2002; Costello-Boerrigter et al., 2006). However, a population-based study indicated that in women, LV mass and NP concentrations increased to a lesser extent when compared to men and only upon severe LV dysfunction (Luchner et al., 2002). Regarding postmenopausal women, HRT has been associated with higher BNP levels (Redfield et al., 2002). In line with these findings, oestrogens exert anti-hypertrophic effects on cardiomyocytes in vitro, through the transactivation of the ANP gene (Horio et al., 2000; Babiker et al., 2004), hence preventing cardiomyocyte hypertrophy (Horio et al., 2000). Taken together, it seems that tight regulation of NP expression is of importance for the sex-based differences involved in the development of cardiac hypertrophy.
From a pharmacological standpoint, the differences observed between males and females affected by neurohormonal disorders suggest that the potency of a number of cardiovascular drugs may vary with sex. A greater benefit for women or female animals was suggested by some studies for the aldosterone antagonist eplerenone (Kanashiro-Takeuchi et al., 2009). Pharmacokinetic studies using eplerenone indicate that male rats metabolized the drug better than female rats due to an increased expression of cytochrome P-450 enzymes (Cook et al., 2003). In addition, enhanced adrenergic responses have been described in females, in which direct sex hormone-dependent mechanisms may be involved. Indeed, women appear to have fewer α-adrenoceptors, resulting in a lower α-adrenoceptor response to noradrenaline, and an increased β-adrenoceptor sensitivity. The oestrogen-enhanced, β-adrenoceptor-mediated response partially involved NO mechanisms (Grossini et al., 2008). Along these lines, the ability of the β-adrenoceptors to offset noradrenaline-mediated vasoconstriction that is seen in younger women disappears in postmenopausal women (Hart et al., 2012). Altogether, these data highlight that efforts are still needed to take into account the sex of the patient when prescribing cardiovascular medication; efforts that should be undertaken from the initial pharmacokinetic and pharmacodynamic studies.
Effects of sex-based differences in the cardiac adaptability to haemodynamic overloads
Sex-based differences in the cardiac adaptability to pressure overload
Pathological cardiac hypertrophy (PCH) per se is an independent risk factor for heart failure (see Swynghedauw, 1999) and is frequently secondary to a mechanical pressure overload, due to arterial hypertension or aortic stenosis. The sex difference in the myocardial ability to adapt to mechanical overload has long been described (Douglas et al., 1995; 1998,), but received recently an increased interest (Loyer et al., 2007a,b2007b; Regitz-Zagrosek et al., 2007; Luczak and Leinwand, 2009; Ostadal et al., 2009; Bhupathy et al., 2010; Petrov et al., 2010). More interestingly, there are significant differences in the way male and female hearts respond to various challenges. In rodents, pressure overload increases left ventricular mass to the same extent in males and females, but cardiac function is better preserved in females (Weinberg et al., 1999; 2003,; Loyer et al., 2007b). It is well known that premenopausal women have a better prognosis than men in response to hypertension and to aortic stenosis (Legget et al., 1996). Based on clinical trials, heart failure with normal ejection fraction is much more common in women than in men and was related to sex-based differences in ventricular diastolic distensibility, in vascular stiffness and ventricular/vascular coupling and in skeletal muscle adaptation to heart failure (Regitz-Zagrosek et al., 2007). When focusing on patients suffering from aortic stenosis (see Luczak and Leinwand, 2009), women, and specially the elderly, develop a more concentric form of hypertrophy than men. Interestingly, when analysing the regression of hypertrophy after aortic valve replacement, LV hypertrophy reversed more frequently in women than in men (Petrov et al., 2010). Furthermore, women with congestive heart failure survive better than men (Luczak and Leinwand, 2009), although recent epidemiological studies failed to demonstrate sex differences in death rate (Laribi et al., 2012).
Experimental studies exposed sex-based differences in the development of PCH. Recently, Bubb et al. (2012) have highlighted the role of sex hormones in a genetic model of essential hypertension, the SHR. In response to mechanical triggers such as at the onset of a pressure overload secondary to a TAC, female rats developed more cardiac hypertrophy than male (Douglas et al., 1998; Loyer et al., 2007b) and did not exhibit any signs of acute heart failure (Loyer et al., 2007b). In mice, similar sex-based differences were observed at later stages of cardiac hypertrophy (Witt et al., 2008). Such sex differences in the adaptation of hearts to pressure overload draw attention to the underlying mechanistic pathways and induced gene expression profiles. Indeed, the sex-based differences in remodelling of the whole heart are mirrored by the differences in signalling pathways or gene expression profiles. These sex-based differences in cardiac response to TAC included a higher β-myosin heavy chain expression, lower levels of mRNA for the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and lower expression of several genes controlling mitochondrial function, including the transcription factor PGC-1α, in males displaying hypertrophied hearts than in females. These transcriptional changes were associated with a preserved contractile reserve in females with hypertrophied hearts (Weinberg et al., 1999; Witt et al., 2008).
Oestrogens can prevent PCH development indirectly by counteracting hypertension, through the direct triggering of ANP release (van Eickels et al., 2001; Jankowski et al., 2001; Zhu et al., 2002), by blocking the p38 MAPK phosphorylation (van Eickels et al., 2001) and by preventing Ca2+ deregulation (Xin et al., 2002). Conversely, oestrogen deficiency enhanced adverse cardiac remodelling (capillary rarefaction, cardiomyocyte hypertrophy and loss) following TAC in rats (Marques et al., 2006; Loyer et al., 2007a). Recently, Kararigas et al. (2012) used elegant approaches including genome-wide expression profiling of oestrogen-treated human cardiac tissues and gene expression and functional analysis of mouse cardiomyocytes according to biological sex and oestrogen treatment in order to investigate the effects of oestrogens on gene regulation in the heart. Using this combined approach, they showed that the gene encoding the myosin regulatory light chain interacting protein was specifically induced in the cardiac tissues of men, in response to oestrogens. Conversely, the expression of the myosin regulatory light chain protein, a protein involved in cardiomyocyte contractility, was decreased only in male heart tissue. These changes in gene expression were related to impaired contractile function. All together, the data suggest a male-specific, oestrogen-regulated, effect resulting in an alteration of cardiac contractility.
Marked sex-based differences are also described in the development of cardiac fibrosis and include the expression of genes associated with the remodelling of the extracellular matrix (ECM), including those for collagen 3, MMP 2, TIMP2 and TGFβ2, which are lower in female heart after TAC in mice (Witt et al., 2008; Fliegner et al., 2010). Oestrogens reduced the turnover of the ECM, especially that of proteins involved in the collagen network (Xu et al., 2003; Mahmoodzadeh et al., 2010). Insights into the sex-specific regulation of fibrosis-related genes were provided by genetic heart failure models and in vitro approaches. In these models, β-estradiol significantly increased collagen-I and -III gene expressions in male fibroblasts, contrary to the effects observed in female cells (Petrov et al., 2010); these effects being mediated by ERβ (Fliegner et al., 2010). The sex-based differences observed in the regulation of genes encoding ECM proteins and MMPs may represent one of the major mechanisms slowing the progression of heart failure and the enhanced recovery of the heart in females.
Other lines of evidence of oestrogen-induced cardioprotection were provided by studies devoted to NO bioavailability or endothelial function. The reduction in the bioavailability of NO is a key feature of endothelial dysfunction during heart failure. In response to a severe TAC, sex differences changes in NOS3 activity were observed (Loyer et al., 2007b). In the hypertrophied female rat heart, the NOS3 activity remained constant before the onset of signs of heart failure (Loyer et al., 2007b) while after TAC, oestrogen deficiency blunted the increased NOS3 expression and exacerbated cardiac dysfunction (Loyer et al., 2007a). Besides the putative role of NOS3-derived NO, the involvement of NOS1-derived NO has been demonstrated during the development of PCH and heart failure (Bendall et al., 2004; Damy et al., 2004; Loyer et al., 2008). In male mice lacking both NOS isoforms, NOS1/3-/-, a twofold increase in mortality was observed when compared to NOS1/3-/- females (Barouch et al., 2003). The changes in NOS1 expression in hearts following TAC seemed to be triggered by mechanotransduction pathways, independently of oestrogen status (Loyer et al., 2007a). However, in the failing heart, sex-based differences were reported regarding the sub-cellular localization of NOS1, as NOS1/caveolin-3 association was significantly higher in female mice in response to cardiac injury than in males (Sun et al., 2006) or following TAC in rats (Loyer et al., 2007b). According to Murphy and Steenbergen (2007), the increase in NOS1 co-localization with caveolin-3 in females under stress conditions (ischaemia/reperfusion) associated with increased Ca2+ (which activates NOS) resulted in an increase in S-nitrosylation of the L-type Ca2+ channel, lower Ca2+ entry and therefore lower Ca2+ load, altogether constituting a cardioprotective mechanism (Chakrabarti et al., 2010).
Sex-based differences in the cardiac adaptability to volume overload
In rats, a volume overload secondary to aortocava fistula induces clear gender-specific differences in ventricular function, structural remodelling and mortality (Gardner et al., 2002). The eccentric dilated hypertrophy was only observed in male Sprague-Dawley rats in response to volume overload, not in females (Gardner et al., 2008). Using hormonal therapy in ovariectomized animal, it was proposed that oestrogens prevented adverse cardiac remodelling to a sustained volume overload through the direct or indirect inhibition of ET-1, the prevention of mast cell maturation and the inhibition of TNF-α synthesis by the mast cells (Gardner et al., 2008; Lu et al., 2012). Differences in the remodelling responses can also be seen after myocardial infarction (MI), as female rats developed less thickening of the non-infarcted regions and a less pronounced diastolic dysfunction than their male counterparts. Also, post-MI rupture of the left ventricle was less frequent in female than in male mice (Deschepper and Llamas, 2007).
Sex-based differences in adjuvant therapy of CVD
Besides the classical therapeutic approach that includes ACE inhibitors, diuretics, β-blockers and that is prescribed to patients regardless of their gender, new therapeutic approaches taking into account the sex-based difference may profoundly affect the prognosis of the patient.
Sex-based differences in cardiac benefit following exercise training; effects during pathological conditions
Physical training is recognized as beneficial in the context of cardiac diseases. Sex-based difference during experimental physical training revealed better exercise capacity of female than male animals, and sex-specific difference in cardiac hypertrophic signalling have been identified, such as a relative higher cardiac increase in Ca2+/calmodulin-dependent protein kinase in females than in males (Konhilas et al., 2004). In human, physical exercise reveals clearly sex-related differences in both healthy subjects and patients with asymptomatic aortic stenosis (see Higginbotham et al., 1984; Legget et al., 1996; Regitz-Zagrosek et al., 2007). Interaction between exercise training and female sex hormones on cardiac performance have been reported (Bupha-Intr and Wattanapermpool, 2004; Brown et al., 2005; Coimbra et al., 2008; Bupha-Intr et al., 2009). Regular exercise cardio-protective in terms of cardiac sarcoplasmic reticulum Ca2+ uptake in oestrogen-deprived status through the regulation of SERCA expression and phospholamban B phosphorylation (Bupha-Intr et al., 2009). In addition, exercising reduces inflammation and cell adhesion molecule expression in postmenopausal women (Wegge et al., 2004). Such results support the idea that exercise training exerts much more benefit on cardiac function after menopause, albeit clinical studies revealed conflicting results (Zarins et al., 2009; Swank et al., 2010; Ryan et al., 2012).
Exercise training in oestrogen-deficient rats improved resting haemodynamic status and arterial baroreflex sensitivity, most likely through the reduction of oxidative stress (Irigoyen et al., 2005). Indeed, exercise training in oestrogen-deficient animals can restore cardiac reserve function, the normal levels of antioxidant molecules (Patten et al., 2004; Rakpongsiri and Sawangkoon, 2008) and prevented pathology-related expression of β-myosin heavy chain (Bupha-Intr and Wattanapermpool, 2004). The metabolic syndrome and insulin resistance induced by oestrogen-deprivation were shown to be corrected by endurance exercise training alone or by oestrogen replacement alone (Saengsirisuwan et al., 2009). In addition, Pighon et al. (2010) found that exercise training acts as oestrogen supplementation surrogate, by decreasing several genes of lipogenesis in liver, as well as decreasing several biomarkers of inflammation (IL-6, NFkB, TNF-α) in oestrogen-deprived rats. Growing evidence suggest that most of these effects are dependent on the AMP-activated protein kinase pathway (Park et al., 2002; Lavoie and Gauthier, 2006).
During pathological situations, a prospective randomized controlled exercise trial indicated that exercise training had no major impact on the cardio-metabolic risk profile of overweight or obese postmenopausal women with moderate hypertension, despite considerable improvements in maximal oxygen consumption (Arsenault et al., 2009). These results contrast with data obtained in aging men indicating that progressive resistance training can be used as anti-hypertensive therapy as well as for the control of metabolic diseases such as obesity or type 2 diabetes (Ibanez et al., 2005). Furthermore, physical exercise was identified as a potent anti-senescent intervention to up-regulate telomere-stabilizing proteins and the telomerase activity in the diseased heart (Werner et al., 2008).
The marked prevalence of hypertension observed in postmenopausal sedentary women (Staessen et al., 1998) not only underlined the effects of oestrogen deficiency in the induction of vascular dysfunction, but also highlighted that exercise training may bring beneficial effects in this context. Postmenopausal women who engage in intermittent, moderate-intensity physical training experience demonstrated a significant reduction in systolic BP (Staffileno et al., 2001). In rats, exercise training dramatically reduces systolic BP of both normo- and hypertensive oestrogen-deficient animals and prevents adverse cardiac remodelling (Irigoyen et al., 2005; Marques et al., 2006). Also in SHR rats, exercise training reduced BP only in males (Coimbra et al., 2008). Among the molecular and biochemical mechanisms responsible for this cardioprotective effect of exercise, it has been postulated that these exercise-induced changes in the myocardium result from local increases in the oxidative stress detoxifying mechanisms or the levels of heat shock proteins (Powers et al., 1998; Demirel et al., 2001). Exercise training increased also antioxidant mechanisms through the expression of catalase and glutathione peroxidase (Adams et al., 2005), and NO bioactivity through an enhanced NOS3 expression (Knowles and Moncada, 1994; Hagg et al., 2004). Interestingly, Marques et al. (2006) demonstrated that a major effect of exercise training was the prevention of oestrogen deprivation-enhanced myocyte loss in the SHR. It is hypothesized that, as under normal conditions, regular exercise induces a protective effect on cardiac sarcoplasmic reticulum Ca2+ uptake in oestrogen-deficient animals (Bupha-Intr et al., 2009). Additional benefit arose through lowering BP by increasing the capillary density in the heart and the muscles and physiological activation of cardiac hypertrophy (Akt pathway). Taken together, these data clearly demonstrate the sex difference on the effects of exercise on cardiac structure and function. Exercise training has beneficial effects by diminishing the PCH induced by pressure overload, mainly by reducing interstitial myocardial fibrosis, improving myocardial vascularization and preventing reduction in the number of cardiomyocytes.
Hormonal replacement therapy (HRT) and CVD
The change in relative CVD risk and incidence in women aged 50 years and older as a result of aging, loss of oestrogen protection after menopause or the changing incidence of other cardiovascular risk factors is largely controversial (Valdiviezo et al., 2013). The safety of HRT in postmenopausal women using synthetic oestrogens and progesterone was extensively debated after the report of increased risk of heart disease, stroke and venous thromboembolism (Rossouw et al., 2002). Lam et al. indicated that the use of HRT in premenopausal women is associated with higher circulating NT-proBNP levels, compared with untreated age-matched women (Lam et al., 2011). However, a recent randomized study (Schierbeck et al., 2012) showed that women receiving HRT early after menopause had a significantly reduced risk of mortality, heart failure or MI, without any apparent increase in risk of cancer or stroke. In humans, HRT not only alleviated the metabolic consequences of menopause (Hassager and Christiansen, 1989; Arabi et al., 2003; Green et al., 2004) but maintained or improved cardiac performance (Alecrin et al., 2004). Thus, the cardiac hypertrophy frequently observed after menopause can be significantly prevented by HRT (Bhupathy et al., 2010). Consequently, the assessment of lifestyle patterns should be taken into account in the treatment of postmenopausal women, as adequate exercise and nutrition programmes were beneficial in the prevention and the treatment of obesity, diabetes and CVD in postmenopausal women (Hagey and Warren, 2008).
Hence, there is a need to encourage the implementation of well-proven interventions such as lifestyle changes of exercise, weight, BP and lipid control to prevent and reduce CVD risk, together with the inclusion of the sex-based differences in cardiac physiopathology in the therapeutic approaches adopted.
Conclusions
So far, the potentially important cardiovascular influences of endogenous oestrogens in men have received little attention. Recent evidence emphasizing the sexually dimorphic response of the heart to sex steroids according to pathophysiological status, suggests some novel therapeutic targets. For example, the negative responses to oestrogens in older men suggest the use of aromatase inhibitors as a potential pharmacological approach. In addition to the sex-based differences listed above, significant differences in the way the hearts of males and females respond to various challenges bring important insights into the mechanisms whereby female gender may influence favourably the remodelling and the adaptive response to myocardial insult. It is worth mentioning that, contrary to the apparently low improvement in treatment and outcome that has been suggested regarding women with MI over the past 25 years (Nauta et al., 2012), a recent study demonstrated that the temporal mortality reductions between 1985 and 2008 were at least as high in women as in men with MI, in terms of both 30 day mortality and long-term mortality hazard (Nauta et al., 2012). Recently, the epidemiological study of Laribi and co-workers also showed that over the last decade, the age-standardized death rate following heart failure was unrelated to sex differences, in seven European countries (Figure 3, Laribi et al., 2012). Such data must not divert the efforts necessary to improve or develop therapeutic approaches to treatment of cardiac diseases that take into account gender-related differences.
Figure 3.
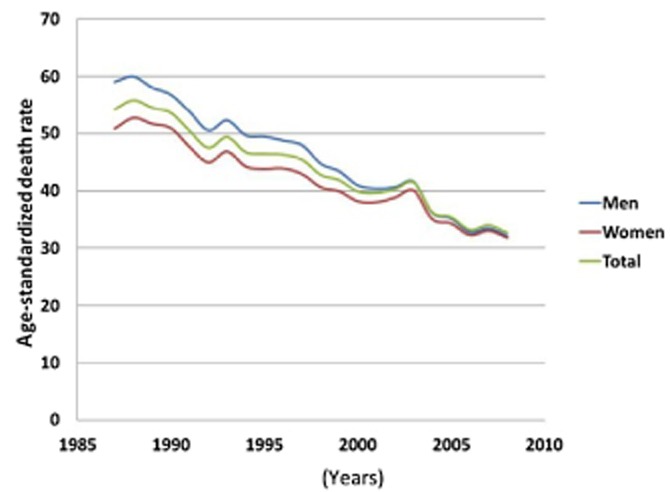
Heart failure as the underlying cause of death. The analysis clearly demonstrated that during the last decade, the age-standardized death rate per 100 000 inhabitants was unrelated to sex differences in seven European countries (reprinted from Laribi et al., 2012 with permission).
Acknowledgments
C. Delcayre was supported by CNRS. L. Fazal was supported by a PhD training grant from Univ-Paris Diderot and GRRC (Groupe de Réflexion sur la Recherche Cardiovasculaire). F. Azibani received grant from SFHTA. J. L. Samuel benefited from Contrat d'interface Inserm- Hôpital Lariboisière AP-HP.
Glossary
- AR
androgen receptor
- CVD
cardiovascular disease
- DOCA
deoxycorticosterone acetate
- E-C
excitation-contraction
- ET-1
endothelin-1
- ER
oestrogen receptor
- HRT
hormone replacement therapy
- LV
left ventricular
- MI
myocardial infarction
- NP
natriuretic peptide
- PCH
pathological cardiac hypertrophy
- PR
progesterone receptor
- RAAS
renin-angiotensin-aldosterone system
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- TAC
thoracic aorta constriction
- VSMC
vascular smooth muscle cell
Conflict of interest
The authors state that there is no conflict of interest.
References
- Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, et al. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- Alecrin IN, Aldrighi JM, Caldas MA, Gebara OC, Lopes NH, Ramires JA. Acute and chronic effects of oestradiol on left ventricular diastolic function in hypertensive postmenopausal women with left ventricular diastolic dysfunction. Heart. 2004;90:777–781. doi: 10.1136/hrt.2003.016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroisine ML, Favre J, Oliviero P, Rodriguez C, Gao J, Thuillez C, et al. Aldosterone-induced coronary dysfunction in transgenic mice involves the calcium-activated potassium (BKCa) channels of vascular smooth muscle cells. Circulation. 2007;116:2435–2443. doi: 10.1161/CIRCULATIONAHA.107.722009. [DOI] [PubMed] [Google Scholar]
- Antoniucci D, Miller VM, Sieck GC, Fitzpatrick LA. Gender-related differences in proliferative responses of vascular smooth muscle cells to endothelin-1. Endothelium. 2001;8:137–145. doi: 10.3109/10623320109165322. [DOI] [PubMed] [Google Scholar]
- Arabi A, Garnero P, Porcher R, Pelissier C, Benhamou CL, Roux C. Changes in body composition during post-menopausal hormone therapy: a 2 years prospective study. Hum Reprod. 2003;18:1747–1752. doi: 10.1093/humrep/deg331. [DOI] [PubMed] [Google Scholar]
- Arsenault BJ, Cote M, Cartier A, Lemieux I, Despres JP, Ross R, et al. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009;207:530–533. doi: 10.1016/j.atherosclerosis.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azibani F, Benard L, Schlossarek S, Merval R, Tournoux F, Fazal L, et al. Aldosterone inhibits antifibrotic factors in mouse hypertensive heart. Hypertension. 2012;59:1179–1187. doi: 10.1161/HYPERTENSIONAHA.111.190512. [DOI] [PubMed] [Google Scholar]
- Azibani F, Samuel JL, Delcayre C. Impact of gender and exercise on cardiac adaptation to pathological situations: sex hormones, exercise and cardiac adaptation. In: Ostadal B, Dhalla NS, editors. Cardiac Adaptations. New York: Springer; 2013. pp. 213–231. [Google Scholar]
- Babiker FA, De Windt LJ, van Eickels M, Thijssen V, Bronsaer RJ, Grohe C, et al. 17beta-estradiol antagonizes cardiomyocyte hypertrophy by autocrine/paracrine stimulation of a guanylyl cyclase A receptor-cyclic guanosine monophosphate-dependent protein kinase pathway. Circulation. 2004;109:269–276. doi: 10.1161/01.CIR.0000105682.85732.BD. [DOI] [PubMed] [Google Scholar]
- Baiardi G, Macova M, Armando I, Ando H, Tyurmin D, Saavedra JM. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regul Pept. 2005;124:7–17. doi: 10.1016/j.regpep.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Banka CL. On male-specific estrogen action: good for the gander? J Am Coll Cardiol. 2012;59:418–419. doi: 10.1016/j.jacc.2011.10.876. [DOI] [PubMed] [Google Scholar]
- Barouch LA, Cappola TP, Harrison RW, Crone JK, Rodriguez ER, Burnett AL, et al. Combined loss of neuronal and endothelial nitric oxide synthase causes premature mortality and age-related hypertrophic cardiac remodeling in mice. J Mol Cell Cardiol. 2003;35:637–644. doi: 10.1016/s0022-2828(03)00079-8. [DOI] [PubMed] [Google Scholar]
- Bendall JK, Damy T, Ratajczak P, Loyer X, Monceau V, Marty I, et al. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation. 2004;110:2368–2375. doi: 10.1161/01.CIR.0000145160.04084.AC. [DOI] [PubMed] [Google Scholar]
- Bhupathy P, Haines CD, Leinwand LA. Influence of sex hormones and phytoestrogens on heart disease in men and women. Womens Health (Lond Engl) 2010;6:77–95. doi: 10.2217/whe.09.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev. 2005;85:1255–1270. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- Brown DA, Lynch JM, Armstrong CJ, Caruso NM, Ehlers LB, Johnson MS, et al. Susceptibility of the heart to ischaemia-reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. J Physiol. 2005;564(Pt 2):619–630. doi: 10.1113/jphysiol.2004.081323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb KJ, Khambata RS, Ahluwalia A. Sexual dimorphism in rodent models of hypertension and atherosclerosis. Br J Pharmacol. 2012;167:298–312. doi: 10.1111/j.1476-5381.2012.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bupha-Intr T, Wattanapermpool J. Cardioprotective effects of exercise training on myofilament calcium activation in ovariectomized rats. J Appl Physiol. 2004;96:1755–1760. doi: 10.1152/japplphysiol.01227.2003. [DOI] [PubMed] [Google Scholar]
- Bupha-Intr T, Laosiripisan J, Wattanapermpool J. Moderate intensity of regular exercise improves cardiac SR Ca2+ uptake activity in ovariectomized rats. J Appl Physiol. 2009;107:1105–1112. doi: 10.1152/japplphysiol.00407.2009. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Lekontseva O, Peters A, Davidge ST. 17beta-Estradiol induces protein S-nitrosylation in the endothelium. Cardiovasc Res. 2010;85:796–805. doi: 10.1093/cvr/cvp368. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Shaul PW. Rapid activation of endothelial NO synthase by estrogen: evidence for a steroid receptor fast-action complex (SRFC) in caveolae. Steroids. 2002;67:413–419. doi: 10.1016/s0039-128x(01)00177-5. [DOI] [PubMed] [Google Scholar]
- Champion HC, Georgakopoulos D, Takimoto E, Isoda T, Wang Y, Kass DA. Modulation of in vivo cardiac function by myocyte-specific nitric oxide synthase-3. Circ Res. 2004;94:657–663. doi: 10.1161/01.RES.0000119323.79644.20. [DOI] [PubMed] [Google Scholar]
- Coimbra R, Sanchez LS, Potenza JM, Rossoni LV, Amaral SL, Michelini LC. Is gender crucial for cardiovascular adjustments induced by exercise training in female spontaneously hypertensive rats? Hypertension. 2008;52:514–521. doi: 10.1161/HYPERTENSIONAHA.108.114744. [DOI] [PubMed] [Google Scholar]
- Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res. 2007;74:456–465. doi: 10.1016/j.cardiores.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Cook CS, Zhang L, Ames GB, Fischer J, Zhang J, Levin S. Single- and repeated-dose pharmacokinetics of eplerenone, a selective aldosterone receptor blocker, in rats. Xenobiotica. 2003;33:305–321. doi: 10.1080/0049825021000049277. [DOI] [PubMed] [Google Scholar]
- Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res. 2011;71:1710–1720. doi: 10.1158/0008-5472.CAN-10-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello-Boerrigter LC, Boerrigter G, Redfield MM, Rodeheffer RJ, Urban LH, Mahoney DW, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–353. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, et al. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365–1367. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J, et al. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol. 2001;91:2205–2212. doi: 10.1152/jappl.2001.91.5.2205. [DOI] [PubMed] [Google Scholar]
- Deschepper CF, Llamas B. Hypertensive cardiac remodeling in males and females: from the bench to the bedside. Hypertension. 2007;49:401–407. doi: 10.1161/01.HYP.0000256279.49882.d8. [DOI] [PubMed] [Google Scholar]
- Douglas PS, Otto CM, Mickel MC, Labovitz A, Reid CL, Davis KB. Gender differences in left ventricle geometry and function in patients undergoing balloon dilatation of the aortic valve for isolated aortic stenosis. NHLBI Balloon Valvuloplasty Registry. Br Heart J. 1995;73:548–554. doi: 10.1136/hrt.73.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32:1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- van Eickels M, Grohe C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17beta-estradiol attenuates the development of pressure-overload hypertrophy. Circulation. 2001;104:1419–1423. doi: 10.1161/hc3601.095577. [DOI] [PubMed] [Google Scholar]
- Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- Fielitz J, Philipp S, Herda LR, Schuch E, Pilz B, Schubert C, et al. Inhibition of prolyl 4-hydroxylase prevents left ventricular remodelling in rats with thoracic aortic banding. Eur J Heart Fail. 2007;9:336–342. doi: 10.1016/j.ejheart.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, et al. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1597–R1606. doi: 10.1152/ajpregu.00825.2009. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Borlaug BA, Lewis GD, Hastings JL, Shafer KM, Bhella PS, et al. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation. 2013;127:55–62. doi: 10.1161/CIRCULATIONAHA.112.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JD, Brower GL, Janicki JS. Gender differences in cardiac remodeling secondary to chronic volume overload. J Card Fail. 2002;8:101–107. doi: 10.1054/jcaf.2002.32195. [DOI] [PubMed] [Google Scholar]
- Gardner JD, Brower GL, Voloshenyuk TG, Janicki JS. Cardioprotection in female rats subjected to chronic volume overload: synergistic interaction of estrogen and phytoestrogens. Am J Physiol Heart Circ Physiol. 2008;294:H198–H204. doi: 10.1152/ajpheart.00281.2007. [DOI] [PubMed] [Google Scholar]
- Garnier A, Bendall JK, Fuchs S, Escoubet B, Rochais F, Hoerter J, et al. Cardiac specific increase in aldosterone production induces coronary dysfunction in aldosterone synthase-transgenic mice. Circulation. 2004;110:1819–1825. doi: 10.1161/01.CIR.0000142858.44680.27. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol. 1998;275(1 Pt 2):H292–H300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- Green JS, Stanforth PR, Rankinen T, Leon AS, Rao DcD, Skinner JS, et al. The effects of exercise training on abdominal visceral fat, body composition, and indicators of the metabolic syndrome in postmenopausal women with and without estrogen replacement therapy: the HERITAGE family study. Metabolism. 2004;53:1192–1196. doi: 10.1016/j.metabol.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Grossini E, Molinari C, Mary DA, Uberti F, Caimmi PP, Surico N, et al. Intracoronary genistein acutely increases coronary blood flow in anesthetized pigs through beta-adrenergic mediated nitric oxide release and estrogenic receptors. Endocrinology. 2008;149:2678–2687. doi: 10.1210/en.2007-1361. [DOI] [PubMed] [Google Scholar]
- Gupte M, Thatcher SE, Boustany-Kari CM, Shoemaker R, Yiannikouris F, Zhang X, et al. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol. 2012;32:1392–1399. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagey AR, Warren MP. Role of exercise and nutrition in menopause. Clin Obstet Gynecol. 2008;51:627–641. doi: 10.1097/GRF.0b013e318180ba84. [DOI] [PubMed] [Google Scholar]
- Hagg U, Andersson I, Naylor AS, Gronros J, Jonsdottir IH, Bergstrom G, et al. Voluntary physical exercise-induced vascular effects in spontaneously hypertensive rats. Clin Sci (Lond) 2004;107:571–581. doi: 10.1042/CS20040171. [DOI] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590(Pt 9):2069–2079. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassager C, Christiansen C. Estrogen/gestagen therapy changes soft tissue body composition in postmenopausal women. Metabolism. 1989;38:662–665. doi: 10.1016/0026-0495(89)90104-2. [DOI] [PubMed] [Google Scholar]
- Hedlund PO, Damber JE, Hagerman I, Haukaas S, Henriksson P, Iversen P, et al. Parenteral estrogen versus combined androgen deprivation in the treatment of metastatic prostatic cancer: part 2. Final evaluation of the Scandinavian Prostatic Cancer Group (SPCG) Study No. 5. Scand J Urol Nephrol. 2008;42:220–229. doi: 10.1080/00365590801943274. [DOI] [PubMed] [Google Scholar]
- Higginbotham MB, Morris KG, Coleman RE, Cobb FR. Sex-related differences in the normal cardiac response to upright exercise. Circulation. 1984;70:357–366. doi: 10.1161/01.cir.70.3.357. [DOI] [PubMed] [Google Scholar]
- Horio T, Nishikimi T, Yoshihara F, Matsuo H, Takishita S, Kangawa K. Inhibitory regulation of hypertrophy by endogenous atrial natriuretic peptide in cultured cardiac myocytes. Hypertension. 2000;35(1 Pt 1):19–24. doi: 10.1161/01.hyp.35.1.19. [DOI] [PubMed] [Google Scholar]
- Howlett SE. Age-associated changes in excitation-contraction coupling are more prominent in ventricular myocytes from male rats than in myocytes from female rats. Am J Physiol Heart Circ Physiol. 2010;298:H659–H670. doi: 10.1152/ajpheart.00214.2009. [DOI] [PubMed] [Google Scholar]
- Ibanez J, Izquierdo M, Arguelles I, Forga L, Larrion JL, Garcia-Unciti M, et al. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care. 2005;28:662–667. doi: 10.2337/diacare.28.3.662. [DOI] [PubMed] [Google Scholar]
- Irigoyen MC, Paulini J, Flores LJ, Flues K, Bertagnolli M, Moreira ED, et al. Exercise training improves baroreflex sensitivity associated with oxidative stress reduction in ovariectomized rats. Hypertension. 2005;46:998–1003. doi: 10.1161/01.HYP.0000176238.90688.6b. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Rachelska G, Donghao W, McCann SM, Gutkowska J. Estrogen receptors activate atrial natriuretic peptide in the rat heart. Proc Natl Acad Sci U S A. 2001;98:11765–11770. doi: 10.1073/pnas.201394198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, et al. Myocyte turnover in the aging human heart. Circ Res. 2010;107:1374–1386. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- Kanashiro-Takeuchi RM, Heidecker B, Lamirault G, Dharamsi JW, Hare JM. Sex-specific impact of aldosterone receptor antagonism on ventricular remodeling and gene expression after myocardial infarction. Clin Transl Sci. 2009;2:134–142. doi: 10.1111/j.1752-8062.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappert K, Bohm M, Schmieder R, Schumacher H, Teo K, Yusuf S, et al. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: analysis of the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial (ONTARGET) Circulation. 2012;126:934–941. doi: 10.1161/CIRCULATIONAHA.111.086660. [DOI] [PubMed] [Google Scholar]
- Kararigas G, Bito V, Tinel H, Becher E, Baczko I, Knosalla C, et al. Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J Am Coll Cardiol. 2012;59:410–417. doi: 10.1016/j.jacc.2011.09.054. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komukai K, Mochizuki S, Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol. 2010;24:687–698. doi: 10.1111/j.1472-8206.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol. 2004;287:H2768–H2776. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CS, Cheng S, Choong K, Larson MG, Murabito JM, Newton-Cheh C, et al. Influence of sex and hormone status on circulating natriuretic peptides. J Am Coll Cardiol. 2011;58:618–626. doi: 10.1016/j.jacc.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laribi S, Aouba A, Nikolaou M, Lassus J, Cohen-Solal A, Plaisance P, et al. Trends in death attributed to heart failure over the past two decades in Europe. Eur J Heart Fail. 2012;14:234–239. doi: 10.1093/eurjhf/hfr182. [DOI] [PubMed] [Google Scholar]
- Lavoie JM, Gauthier MS. Regulation of fat metabolism in the liver: link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci. 2006;63:1393–1409. doi: 10.1007/s00018-006-6600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legget ME, Kuusisto J, Healy NL, Fujioka M, Schwaegler RG, Otto CM. Gender differences in left ventricular function at rest and with exercise in asymptomatic aortic stenosis. Am Heart J. 1996;131:94–100. doi: 10.1016/s0002-8703(96)90056-3. [DOI] [PubMed] [Google Scholar]
- Leri A, Malhotra A, Liew CC, Kajstura J, Anversa P. Telomerase activity in rat cardiac myocytes is age and gender dependent. J Mol Cell Cardiol. 2000;32:385–390. doi: 10.1006/jmcc.1999.1084. [DOI] [PubMed] [Google Scholar]
- Lopez-Ruiz A, Sartori-Valinotti J, Yanes LL, Iliescu R, Reckelhoff JF. Sex differences in control of blood pressure: role of oxidative stress in hypertension in females. Am J Physiol Heart Circ Physiol. 2008;295:H466–H474. doi: 10.1152/ajpheart.01232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyer X, Damy T, Chvojkova Z, Robidel E, Marotte F, Oliviero P, et al. 17beta-estradiol regulates constitutive nitric oxide synthase expression differentially in the myocardium in response to pressure overload. Endocrinology. 2007a;148:4579–4584. doi: 10.1210/en.2007-0228. [DOI] [PubMed] [Google Scholar]
- Loyer X, Oliviero P, Damy T, Robidel E, Marotte F, Heymes C, et al. Effects of sex differences on constitutive nitric oxide synthase expression and activity in response to pressure overload in rats. Am J Physiol Heart Circ Physiol. 2007b;293:H2650–H2658. doi: 10.1152/ajpheart.00883.2007. [DOI] [PubMed] [Google Scholar]
- Loyer X, Gomez AM, Milliez P, Fernandez-Velasco M, Vangheluwe P, Vinet L, et al. Cardiomyocyte overexpression of neuronal nitric oxide synthase delays transition toward heart failure in response to pressure overload by preserving calcium cycling. Circulation. 2008;117:3187–3198. doi: 10.1161/CIRCULATIONAHA.107.741702. [DOI] [PubMed] [Google Scholar]
- Lu H, Melendez GC, Levick SP, Janicki JS. Prevention of adverse cardiac remodeling to volume overload in female rats is the result of an estrogen-altered mast cell phenotype. Am J Physiol Heart Circ Physiol. 2012;302:H811–H817. doi: 10.1152/ajpheart.00980.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchner A, Brockel U, Muscholl M, Hense HW, Doring A, Riegger GA, et al. Gender-specific differences of cardiac remodeling in subjects with left ventricular dysfunction: a population-based study. Cardiovasc Res. 2002;53:720–727. doi: 10.1016/s0008-6363(01)00510-7. [DOI] [PubMed] [Google Scholar]
- Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol. 2009;71:1–18. doi: 10.1146/annurev.physiol.010908.163156. [DOI] [PubMed] [Google Scholar]
- Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz-Zagrosek V. 17beta-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc Res. 2010;85:719–728. doi: 10.1093/cvr/cvp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric-Bilkan C, Manigrasso MB. Sex differences in hypertension: contribution of the renin-angiotensin system. Gend Med. 2012;9:287–291. doi: 10.1016/j.genm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Marques CM, Nascimento FA, Mandarim-de-Lacerda CA, Aguila MB. Exercise training attenuates cardiovascular adverse remodeling in adult ovariectomized spontaneously hypertensive rats. Menopause. 2006;13:87–95. doi: 10.1097/01.gme.0000191209.13115.46. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Montalvo C, Villar AV, Merino D, Garcia R, Ares M, Llano M, et al. Androgens contribute to sex differences in myocardial remodeling under pressure overload by a mechanism involving TGF-beta. PLoS ONE. 2012;7:e35635. doi: 10.1371/journal.pone.0035635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res. 2007;75:478–486. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Nauta ST, Deckers JW, van Domburg RT, Akkerhuis KM. Sex-related trends in mortality in hospitalized men and women after myocardial infarction between 1985 and 2008: equal benefit for women and men. Circulation. 2012;126:2184–2189. doi: 10.1161/CIRCULATIONAHA.112.113811. [DOI] [PubMed] [Google Scholar]
- Oh H, Wang SC, Prahash A, Sano M, Moravec CS, Taffet GE, et al. Telomere attrition and Chk2 activation in human heart failure. Proc Natl Acad Sci U S A. 2003;100:5378–5383. doi: 10.1073/pnas.0836098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostadal B, Netuka I, Maly J, Besik J, Ostadalova I. Gender differences in cardiac ischemic injury and protection – experimental aspects. Exp Biol Med (Maywood) 2009;234:1011–1019. doi: 10.3181/0812-MR-362. [DOI] [PubMed] [Google Scholar]
- Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, et al. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, et al. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- Petrov G, Regitz-Zagrosek V, Lehmkuhl E, Krabatsch T, Dunkel A, Dandel M, et al. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation. 2010;122(11 Suppl):S23–S28. doi: 10.1161/CIRCULATIONAHA.109.927764. [DOI] [PubMed] [Google Scholar]
- Picard F, Deshaies Y, Lalonde J, Samson P, Labrie C, Belanger A, et al. Effects of the estrogen antagonist EM-652.HCl on energy balance and lipid metabolism in ovariectomized rats. Int J Obes Relat Metab Disord. 2000;24:830–840. doi: 10.1038/sj.ijo.0801240. [DOI] [PubMed] [Google Scholar]
- Pighon A, Barsalani R, Yasari S, Prud'homme D, Lavoie JM. Does exercise training prior to ovariectomy protect against liver and adipocyte fat accumulation in rats? Climacteric. 2010;13:238–248. doi: 10.3109/13697130903009203. [DOI] [PubMed] [Google Scholar]
- Powers SK, Demirel HA, Vincent HK, Coombes JS, Naito H, Hamilton KL, et al. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol. 1998;275(5 Pt 2):R1468–R1477. doi: 10.1152/ajpregu.1998.275.5.R1468. [DOI] [PubMed] [Google Scholar]
- Rakpongsiri K, Sawangkoon S. Protective effect of creatine supplementation and estrogen replacement on cardiac reserve function and antioxidant reservation against oxidative stress in exercise-trained ovariectomized hamsters. Int Heart J. 2008;49:343–354. doi: 10.1536/ihj.49.343. [DOI] [PubMed] [Google Scholar]
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Brokat S, Tschope C. Role of gender in heart failure with normal left ventricular ejection fraction. Prog Cardiovasc Dis. 2007;49:241–251. doi: 10.1016/j.pcad.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Dworatzek E, Kintscher U, Dragun D. Sex and sex hormone-dependent cardiovascular stress responses. Hypertension. 2013;61:270–277. doi: 10.1161/HYPERTENSIONAHA.111.189233. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR, White RE, Roy T, Cox BE. Calcium-activated potassium channels and nitric oxide coregulate estrogen-induced vasodilation. Am J Physiol Heart Circ Physiol. 2000;279:H319–H328. doi: 10.1152/ajpheart.2000.279.1.H319. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Ryan AS, Ortmeyer HK, Sorkin JD. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2012;302:E145–E152. doi: 10.1152/ajpendo.00618.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengsirisuwan V, Pongseeda S, Prasannarong M, Vichaiwong K, Toskulkao C. Modulation of insulin resistance in ovariectomized rats by endurance exercise training and estrogen replacement. Metabolism. 2009;58:38–47. doi: 10.1016/j.metabol.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Saito T, Ciobotaru A, Bopassa JC, Toro L, Stefani E, Eghbali M. Estrogen contributes to gender differences in mouse ventricular repolarization. Circ Res. 2009;105:343–352. doi: 10.1161/CIRCRESAHA.108.190041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012;345:e6409. doi: 10.1136/bmj.e6409. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Chen K, Emmett T, Kargacin G. Aldosterone and the autocrine modulation of potassium currents and oxidative stress in the diabetic rat heart. Br J Pharmacol. 2008;154:675–687. doi: 10.1038/bjp.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol. 2006;100:286–293. doi: 10.1152/japplphysiol.00869.2005. [DOI] [PubMed] [Google Scholar]
- Song D, Arikawa E, Galipeau DM, Yeh JN, Battell ML, Yuen VG, et al. Chronic estrogen treatment modifies insulin-induced insulin resistance and hypertension in ovariectomized rats. Am J Hypertens. 2005;18(9 Pt 1):1189–1194. doi: 10.1016/j.amjhyper.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Celis H, Fagard R. The epidemiology of the association between hypertension and menopause. J Hum Hypertens. 1998;12:587–592. doi: 10.1038/sj.jhh.1000670. [DOI] [PubMed] [Google Scholar]
- Staffileno BA, Braun LT, Rosenson RS. The accumulative effects of physical activity in hypertensive post-menopausal women. J Cardiovasc Risk. 2001;8:283–290. doi: 10.1177/174182670100800507. [DOI] [PubMed] [Google Scholar]
- Straface E, Vona R, Gambardella L, Ascione B, Marino M, Bulzomi P, et al. Cell sex determines anoikis resistance in vascular smooth muscle cells. FEBS Lett. 2009;583:3448–3454. doi: 10.1016/j.febslet.2009.09.052. [DOI] [PubMed] [Google Scholar]
- Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, et al. Myocardial AKT: the omnipresent nexus. Physiol Rev. 2011;91:1023–1070. doi: 10.1152/physrev.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank AM, Funk DC, Manire JT, Allard AL, Denny DM. Effect of resistance training and aerobic conditioning on muscular strength and submaximal fitness for individuals with chronic heart failure: influence of age and gender. J Strength Cond Res. 2010;24:1298–1305. doi: 10.1519/JSC.0b013e3181d82e5d. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- Torto R, Boghossian S, Dube MG, Kalra PS, Kalra SP. Central leptin gene therapy blocks ovariectomy-induced adiposity. Obesity (Silver Spring) 2006;14:1312–1319. doi: 10.1038/oby.2006.149. [DOI] [PubMed] [Google Scholar]
- Valdiviezo C, Lawson S, Ouyang P. An update on menopausal hormone replacement therapy in women and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2013;20:148–155. doi: 10.1097/MED.0b013e32835ed58b. [DOI] [PubMed] [Google Scholar]
- Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism. 2004;53:377–381. doi: 10.1016/j.metabol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Weinberg EO, Thienelt CD, Katz SE, Bartunek J, Tajima M, Rohrbach S, et al. Gender differences in molecular remodeling in pressure overload hypertrophy. J Am Coll Cardiol. 1999;34:264–273. doi: 10.1016/s0735-1097(99)00165-5. [DOI] [PubMed] [Google Scholar]
- Weinberg EO, Mirotsou M, Gannon J, Dzau VJ, Lee RT, Pratt RE. Sex dependence and temporal dependence of the left ventricular genomic response to pressure overload. Physiol Genomics. 2003;12:113–127. doi: 10.1152/physiolgenomics.00046.2002. [DOI] [PubMed] [Google Scholar]
- Wendler A, Albrecht C, Wehling M. Nongenomic actions of aldosterone and progesterone revisited. Steroids. 2012;77:1002–1006. doi: 10.1016/j.steroids.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Poss J, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52:470–482. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Witt H, Schubert C, Jaekel J, Fliegner D, Penkalla A, Tiemann K, et al. Sex-specific pathways in early cardiac response to pressure overload in mice. J Mol Med. 2008;86:1013–1024. doi: 10.1007/s00109-008-0385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin HB, Senbonmatsu T, Cheng DS, Wang YX, Copello JA, Ji GJ, et al. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature. 2002;416:334–338. doi: 10.1038/416334a. [DOI] [PubMed] [Google Scholar]
- Xu Y, Arenas IA, Armstrong SJ, Davidge ST. Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc Res. 2003;57:388–394. doi: 10.1016/s0008-6363(02)00705-8. [DOI] [PubMed] [Google Scholar]
- Zarins ZA, Johnson ML, Faghihnia N, Horning MA, Wallis GA, Fattor JA, et al. Training improves the response in glucose flux to exercise in postmenopausal women. J Appl Physiol. 2009;107:90–97. doi: 10.1152/japplphysiol.91568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]