Abstract
Perivascular adipose tissue (PVAT) is an active endocrine and paracrine organ that modulates vascular function, with implications for the pathophysiology of cardiovascular disease (CVD). Adipocytes and stromal cells contained within PVAT produce mediators (adipokines, cytokines, reactive oxygen species and gaseous compounds) with a range of paracrine effects modulating vascular smooth muscle cell contraction, proliferation and migration. However, the modulatory effect of PVAT on the vascular system in diseases, such as obesity, hypertension and atherosclerosis, remains poorly characterized. AMP-activated protein kinase (AMPK) regulates adipocyte metabolism, adipose biology and vascular function, and hence may be a potential therapeutic target for metabolic disorders such as type 2 diabetes mellitus (T2DM) and the vascular complications associated with obesity and T2DM. The role of AMPK in PVAT or the actions of PVAT have yet to be established, however. Activation of AMPK by pharmacological agents, such as metformin and thiazolidinediones, may modulate the activity of PVAT surrounding blood vessels and thereby contribute to their beneficial effect in cardiometabolic diseases. This review will provide a current perspective on how PVAT may influence vascular function via AMPK. We will also attempt to demonstrate how modulating AMPK activity using pharmacological agents could be exploited therapeutically to treat cardiometabolic diseases.
Keywords: adipocytes, adiponectin, AMP-activated protein kinase, atherosclerosis, diabetes, leptin, perivascular fat, hypertension, vascular reactivity, metformin
Introduction
Cardiovascular disease (CVD) remains the most common cause of death worldwide. The inexorable rise in diabetes and obesity will continue to shorten many lives and be a huge burden on healthcare budgets throughout the world (Trujillo and Scherer, 2006). Evidence from several studies, including the Framingham Heart Study, has demonstrated that obesity is closely associated with increased vulnerability to insulin resistance, type 2 diabetes mellitus (T2DM), hypertension, coronary artery disease (CAD), myocardial infarction (MI) and sudden death, congestive heart failure and stroke (Henry et al., 2002; Galassi et al., 2006). However, the pathophysiological mechanisms underlying the relationship between obesity and CVD remain poorly understood. Dysfunctional perivascular adipose tissue (PVAT) is a good candidate as a link between obesity and cardiovascular risk due to the direct influence PVAT can exert on blood vessel health. AMP-activated protein kinase (AMPK) regulates adipocyte metabolism, adipose biology and vascular function, such that AMPK activation is an attractive therapeutic target for metabolic disorders such as T2DM and the vascular complications associated with obesity and T2DM. Recent studies have identified AMPK as a potential regulator of PVAT and also a target of PVAT action in the blood vessel.
Adipose tissue
Structurally, all adipose tissue consists of mature adipocytes containing lipid droplets, T lymphocytes, macrophages, collagen fibres, fibroblasts, preadipocytes, blood vessels and nerves. In general, adipose tissues have been classified into white adipose tissue (WAT) and brown adipose tissue (BAT) according to the size of lipid droplets and the number of mitochondria within the adipocytes. Adipocytes in WAT are small with single, large lipid droplets and variable numbers of mitochondria. Fat cells in BAT are larger in size with multiple small lipid droplets and larger numbers of mitochondria (Cinti, 2005; Nedergaard et al., 2007). WAT is widely distributed in subcutaneous areas and around visceral organs, whereas BAT, which was originally thought to be present mainly in the intrascapular region in rodents and human infants, has recently been discovered in supraclavicular, suprarenal, cervical and periaortic areas (Cinti, 2005). BAT is classically linked to thermogenesis and is associated with metabolically active adipocytes that contain uncoupling protein-1 within the mitochondria (Cannon and Nedergaard, 2004; Frontini et al., 2007). Whereas WAT can be considered to be a lipid storage depot, BAT is more vascularized and metabolically active, with greater noradrenergic innervation (Cannon and Nedergaard, 2004; Cinti, 2011).
Perivascular adipose tissue
PVAT is the adipose tissue surrounding the vasculature, which manifests a varied histological appearance according to the type of vessel it is surrounds. PVAT has been reported to be composed of both BAT and WAT, with different ratios depending on the location (Cinti, 2011). For instance, in Sv129 mice, PVAT around coronary arteries and thoracic aorta is composed mainly of BAT, PVAT in iliofemoral blood vessels has an equal amount of both types, whereas PVAT present around the abdominal aorta and mesenteric arteries consists mainly of WAT (Frontini and Cinti, 2010). In a similar manner, PVAT in Sprague-Dawley rats surrounding the thoracic aorta is mainly BAT, whereas PVAT in abdominal aorta and mesenteric vessels is predominantly WAT (Wang et al., 2009a). Findings from animal studies are consistent with data from human PVAT studies, in which PVAT surrounding the aorta and its main branches including subclavian, carotid, intercostals and renal arteries has been reported to contain BAT (Frontini and Cinti, 2010). However, data from coronary arteries have been conflicting, with PVAT dissected from the origin of human right coronary arteries expressing a high level of BAT-related genes, whereas others have reported gene expression around human coronary arteries to be related to WAT (Sacks et al., 2009). Such a disparity may be due to the method of harvesting the adipose tissue or to inter-individual variations in fat tissue distribution (Miao and Li, 2012). Interestingly, a very recent study has identified a strong correlation between presence of brown adipocytes and atherosclerotic plaque in 271 samples of human coronary artery (Salisbury et al., 2012). The authors speculate that as brown adipocytes are involved in neovascularization, they may have an important role to play in vascular remodelling in diseases such as atherosclerosis. However, another study, which investigated epicardial adipose tissue, found a positive correlation between the markers of BAT and circulating high-density lipoprotein (HDL) and a negative correlation with circulating triglycerides (Chechi et al., 2012). The data of Chechi et al. suggest that depots of BAT around the heart may have a beneficial effect on the atherogenic profile of circulating lipids. Thus, further investigation is required to determine the precise role of BAT on atherosclerotic disease.
PVAT: function and autocrine/paracrine activity
In addition to the release of free fatty acids (FFAs)/non-esterified fatty acids by lipolysis, adipose tissue secretes bioactive proteins that are collectively termed adipokines and have endocrine, autocrine and paracrine actions. PVAT-derived adipokines participate in the regulation of vascular function under normal physiological conditions. Despite the obvious paracrine effect of PVAT on vascular tissues, the extent of their endocrine effect remains elusive and they appear to have both beneficial and detrimental effects on vascular function and pathophysiology (see Mattu and Randeva, 2013).
More than 50 adipokines have since been identified and shown to have a wide spectrum of haemodynamic, metabolic and immunological effects (Trayhurn, 2005; Wood and Trayhurn, 2006). Adipokines can be classified according to their effect on cytokine levels as either pro-inflammatory, such as leptin, or anti-inflammatory, such as adiponectin and adrenomedullin. As emerging vascular modulators, adipokines including adiponectin, omentin, nesfatin, vaspin and chemerin have been proposed to play a role in the regulation of cardiovascular function (Table 1). The classification of some adipokines, such as resistin, can be somewhat blurred since it can be expressed by other cell types such as macrophages and participate in inflammation throughout the body (Jamaluddin et al., 2012). Others such as visfatin, originally considered as adipocytokines, may actually be produced in greater quantities by the stromal vascular tissue within PVAT (Stastny et al., 2012). Nevertheless, studies seem to indicate that generation of visfatin is elevated in obesity and metabolic syndrome and so it is likely to be an important pro-inflammatory mediator in cardiometabolic disease (Figure 2). In addition to release of adipokines, adipocytes in PVAT also produce classical chemokines (or cytokines) including IL-6, IL-8, CCL2 (MCP-1) and plasminogen-activator inhibitor-1 (Thalmann and Meier, 2007; Rajsheker et al., 2010). Furthermore, inflammatory cells such as macrophages and T lymphocytes, fibroblasts and capillary endothelial cells, which are normally found in PVAT or attracted in response to inflammatory chemokines released by adipocytes, have also been demonstrated to contribute to the secretory profile of adipose tissue (see Szasz and Webb, 2012).
Table 1.
PVAT-derived biologically active molecules with influence on vascular function and related cardiometabolic disorders
| Adipokines/Cytokines | Physiological effect | Effect on vasculature | Associated disease | PVAT dysfunction |
|---|---|---|---|---|
| Leptin | Pro-atherogenic Pro-inflammatory | Direct vasodilator (Nakagawa et al., 2002; Sahin and Bariskaner, 2007) ↑ VSMC proliferation/migration (Oda et al., 2001; Huang et al., 2010) ↓ VSMC proliferation (Bohlen et al., 2007) ↑ Vascular permeability (Cao et al., 2001) ↑ TNF-α, IL-6, IL-12, ROS (Fitzgibbons et al., 2011; Rittig et al., 2012) Indirect vasoconstrictor (Fruhbeck, 1999) | Obesity Hypertension Atherosclerosis Insulin resistance | ↑ Production in obesity (Marchesi et al., 2009; Ketonen et al., 2010) ↓ Production in hypertension (Galvez et al., 2006) Effect on VSMC contraction is lost in hypertension (Rodriguez et al., 2007) ↑ Production in atherosclerosis (Henrichot et al., 2005; Eiras et al., 2008) |
| Adiponectin | Anti-atherogenic Anti-inflammatory | Direct vasodilator (Fésüs et al., 2007; Zhu et al., 2008) ↓ VSMC proliferation/migration (Wang et al., 2005; Lamers et al., 2011) ↓ IFN-γ, IL-6, NF-κB, TNF-α phagocytosis, endothelial adhesion molecules (Ouchi et al., 1999; Engeli et al., 2003) ↑ IL-10, IL-1RA (Wolf et al., 2004) | Obesity Hypertension Atherosclerosis Insulin resistance T2DM | ↓ Production in obesity (Marchesi et al., 2009; Ketonen et al., 2010) ↓ Production in diabetes (Meijer et al., 2013) ↓ Atherosclerosis (Henrichot et al., 2005; Eiras et al., 2008) |
| Resistin | Pro-atherogenic | ↑ VSMC proliferation/migration (Shyu et al., 2011) ↑ Endothelial adhesion molecule ↑TNF-α, IL-6,NF-kB (Tilg and Moschen, 2006) | Atherosclerosis Insulin resistance T2DM | ↑ Endothelial injury (Shyu et al., 2011) |
| Visfatin | Pro-atherogenic Pro-inflammatory | ↑ VSMC proliferation/migration (Wang et al., 2009a) ↑ Endothelium-dependent vasodilatation (Yamawaki et al., 2009) ↑ TNF- α, IL-6, IL-8 (Tilg and Moschen, 2006) ↓ Apoptosis (Tilg and Moschen, 2006) | Atherosclerosis Insulin resistance T2DM | ↑ Production in atherosclerosis (Henrichot et al., 2005; Eiras et al., 2008) |
| Omentin | Anti-atherogenic Anti-inflammatory | ↑ eNOS (Tan et al., 2010) ↓ NF-κB (Tan et al., 2010) | Atherosclerosis Metabolic syndrome | ↓ Production in atherosclerosis, obesity and metabolic syndrome (Tan et al., 2010) |
| Chemerin | Anti-atherogenic Anti-inflammatory | ↓ TNF-α-induced VCAM-1 expression and ↓ Monocyte adhesion (Yamawaki et al., 2012a) | Atherosclerosis | ↑ Production in atherosclerosis (Yamawaki et al., 2012a) |
| Nefastin | Contractile | Impair NO donor and SNP induced smooth muscle relaxation (Yamawaki et al., 2012a) | Hypertension Obesity | ↑ Levels in hypertension and obesity (Ramanjaneya et al., 2010; Yamawaki et al., 2012b) |
| Vaspin | Anti-inflammatory Anti-atherogenic | ↓ SMC migration (Phalitakul et al., 2012) | Atherosclerosis T2DM Obesity | ↑ Levels in atherosclerosis, obesity and diabetes milletus (Youn et al., 2008; Phalitakul et al., 2012) |
| Apelin | Anti-contractile Angiogenic | ↑ Glucose utilization in skeletal muscle (Dray et al., 2008) Antagonize the effect of Ang II (Chun et al., 2008) ↑ NO production (Japp et al., 2008) | Obesity Insulin resistance | ↑ Levels in obesity and insulin resistance and heart failure (Chun et al., 2008; Dray et al., 2008) |
| Interleukins (IL-1, IL-6, IL-8), CCL2 | ↑ Endothelial proliferation (Henrichot et al., 2005) | Atherosclerosis | ↑ Production in atherosclerosis (Henrichot et al., 2005; Eiras et al., 2008) | |
| HGF | ↑ Endothelial proliferation (Rittig et al., 2012) ↑ Cytokines release from SMCs (Rittig et al., 2012) | Obesity | ↑ Production in obesity (Bell et al., 2006; Rittig et al., 2012) | |
| TNF-α | Pro-inflammatory | Vasodilator (Brian and Faraci, 1998) ↑ ROS ↑ Endothelial dysfunction (Zhang et al., 2009a) | Obesity Hypertension Atherosclerosis T2DM | ↑ Production in obesity, hypertension, atherosclerosis and T2DM (Zhang et al., 2009a) |
HGF, hepatic growth factor; NF-κB, nuclear factor kappa-light-chain enhancer of activated beta cells; PVAT, perivascular adipose tissue; ROS, reactive oxygen species; T2DM, type 2 diabetes mellitus; VSMCs, vascular smooth muscle cells.
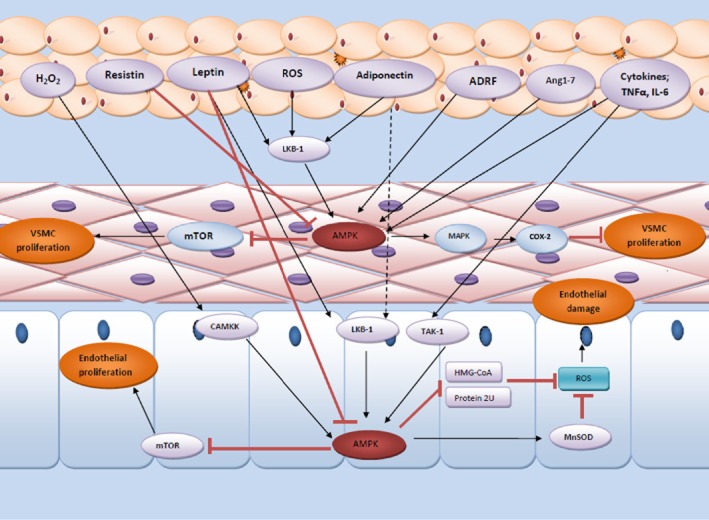
Figure 2.
Schematic presentation of how PVAT modulates the function of AMPK in both endothelium and vascular smooth muscle and how these affect vascular function. PVAT is a highly active organ secreting various adipokines and cytokines implicating in the regulation of vascular contractility via modulation of AMPK activity. ADRF, adipose tissue-derived relaxation factor; Ang1-7, angiotensin 1–7; BKCa, calcium-dependent big potassium channel; CAMKK, calcium/calmodulin-dependent protein kinase kinase; eNOS, endothelial NOS; HMG-COA, 3-hydroxy-3-methylglutaryl-coenzyme A; LKB, liver kinase B; MAPK, mitogen-activated protein kinases; MnSOD, manganese superoxide dismutase; mTOR, mammalian target of rapamycin; PGI2, prostacyclin; ROS, reactive oxygen species; TAK1, transforming growth factor β-activated kinase 1.
In addition to classical adipokines, PVAT can also produce angiotensin peptides, which, along with angiotensinogen, angiotensin-converting enzymes and receptors, are part of the renin–angiotensin–aldosterone system (Lu et al., 2010). PVAT also releases reactive oxygen species (ROS), including superoxide (Gao et al., 2006), H2O2 (Gao et al., 2007) and gaseous molecules such as H2S (Schleifenbaum et al., 2010). In denuded vessels, generation of H2O2 induces relaxation via phosphorylation of smooth muscle contractile proteins (Gao et al., 2007), whereas an enzyme present in PVAT, cystathionine γ-lyase, can generate H2S. The H2S induces relaxation by opening voltage-gated potassium channels in the vascular smooth muscle cells (SMCs) to cause hyperpolarization (Schleifenbaum et al., 2010). In contrast, superoxide and angiotensin can potentiate vasoconstriction to electrical field stimulation in vitro (Gao et al., 2006; Lu et al., 2010). Another adipokine, apelin, has been found to induce NO-dependent vasorelaxation of peripheral and splanchnic human arteries both in vitro and in vivo (Salcedo et al., 2007a; Japp et al., 2008). As PVAT can have a dual effect on vessel tone, work has focused on how the function of PVAT changes in disease states as this is a key to understanding the role of PVAT in disease progression. Table 1 summarizes the major adipokines that are proposed to influence cardiovascular function.
The recent interest in adipose tissue as an active endocrine and paracrine organ has resulted in a number of studies examining the properties of different fat depots in the body. Production of adipokines and cytokines from the PVAT surrounding small arteries, coronary arteries, aorta and systemic vessels can modulate inflammation, contractile activity of medial smooth muscle and endothelial function, all of which are likely to be important in diseases including atherosclerosis and hypertension (reviewed in Szasz et al., 2013) (Table 2011). The vasa vasorum present in the adventitia of the blood vessel promotes neovascularization following vascular damage and inflammation, facilitating transport of vasoactive factors released by PVAT to the inner layers of the vessel wall (Gossl et al., 2009). A close structural communication between the PVAT and inner vasculature has been demonstrated in studies showing luminal termination of vasa vasorum in the inner vasculature in vessels such as the saphenous vein (Dashwood et al., 2007).
Furthermore, the cellular composition of PVAT may vary according to age, nutritional status and environmental conditions. For instance, periaortic PVAT has been found to expand and contain more inflammatory cells in response to obesity (Skilton et al., 2009). PVAT adipocyte size also shows variation and heterogeneity in CVD. For example, the size of pericardial adipocytes near the proximal part of the right coronary artery in coronary heart disease (CAD) patients is increased in contrast with non-CAD patients (Silaghi et al., 2007). This implies that changes occur in PVAT in disease states, although the effect this has on the endocrine or paracrine functions of PVAT is still unclear.
PVAT in vascular diseases
Obesity accompanied by increased PVAT mass around blood vessels is a leading cause of vascular disease, leading to the hypothesis that dysfunctional PVAT in obese individuals may be an important inducer of vascular dysfunction (Figure 1). In vitro studies have shown that the vasoconstrictor effect of noradrenaline is attenuated in vessels with PVAT (Soltis and Cassis, 1991), whereas a study in lipoatrophic mice, which are characterized by loss of adipose tissue (including PVAT), showed spontaneous vascular dysfunction leading to hypertension (Guzik et al., 2007). However, it should be noted that lipoatrophic mice exhibit a number of phenotypes, which could account for changes within the vasculature. A recent study using lipoatrophic BAT-specific insulin receptor knockout mice found adiopocytokine overexpression, insulin resistance and inflammatory marker expression in the endothelium and vascular dysfunction at 1 year (Gomez-Hernandez et al., 2012). Treatment with an antibody to TNF-α reversed many of these effects, highlighting the importance of inflammatory adipocytokines in disease progression. Table 2011 summarizes the major adipokines that are proposed to influence cardiovascular function and correlated disease.
Figure 1.
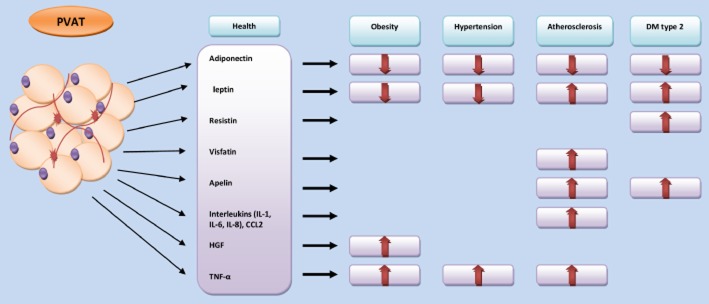
Changes in the PVAT production of adipokines and cytokines in cardiometabolic disease. The figure illustrates the alteration in the secretory profile of PVAT in obesity, hypertension and atherosclerosis. These diseases are associated with either reduction of adipokines release such as adiponectin or increased release of some other such as TNF-α and visfatin. DM, diabetes mellitus; HGF, hepatic growth factor; PVAT, perivascular adipose tissue.
PVAT in atherosclerosis
Atherosclerosis is characterized by a persistent inflammatory response that promotes development of atheromatous plaque in the tunica media and intima of the blood vessel wall (Ross, 1993). However, growing evidence suggests that the adventitia may also contribute to the development of atherosclerosis through accumulation of myofibroblasts, leading to constriction of the external elastic lamina and vascular remodelling (Scott et al., 1996). Inhibition of myofibroblast proliferation by, for example, intravascular brachytherapy can induce marked vessel thinning (Wilcox et al., 2001). Conversely, vascular proliferative disorders such as restenosis following balloon angioplasty are associated with inflammatory responses that extend into PVAT, suggesting an integral role of PVAT in remodelling and neointima formation (Okamoto et al., 2001; Wilcox et al., 2001). Wire or balloon injury to the lumen of mouse and rat vessels significantly increased the expression of pro-inflammatory adipocytokines and reduced adiponectin, an effect that was absent in TNF-α knockout (KO) mice and which could be replicated by TNF-α application to the perivascular area of the vessel (Takaoka et al., 2010). Furthermore, the same group showed that TNF-α KO mice had reduced neointimal formation, reinforcing the potential importance of PVAT-derived inflammatory mediators in vascular remodelling. A very recently published study has indicated that the adipokine C1q/TNF-related protein-9 attenuates neointima formation in the wire-injured obese mouse femoral artery (Uemura et al., 2013). The reduction in neointima was associated with reduced vascular SMC proliferation via a cAMP-PKA-dependent pathway. Conversely, neointimal area following femoral artery injury in both wild-type and leptin-deficit mice was enhanced by leptin and this was due to activation of the key regulator of protein and amino acid metabolism, mammalian target for rapamycin (mTOR) (Shan et al., 2008).
In PVAT from organ donors without atherosclerosis, perivascular adipocytes are characterized by a reduced level of adipocytic differentiation as compared with adipocytes derived from subcutaneous and visceral (perirenal) adipose depots. Secretion of anti-inflammatory adiponectin is markedly reduced, whereas that of pro-inflammatory cytokines IL-6, IL-8 and CCL2 is markedly increased in perivascular adipocytes. Likewise, PVAT harvested from murine aortic arch expresses lower levels of adipocyte-associated genes as compared with subcutaneous and visceral adipose tissues. Furthermore, 2 weeks of high-fat feeding caused further reductions in adipocyte-associated gene expression, while up-regulating pro-inflammatory gene expression, in PVATs. Changes in adipocyte differentiation and secretion were observed in the absence of macrophage recruitment to the PVAT, indicating that these properties are intrinsic to the adipocytes residing in this depot. Dysfunction of PVAT induced by fat feeding suggests that PVAT is capable of linking metabolic signals to inflammation in the blood vessel wall (Chatterjee et al., 2009). Furthermore, the accumulation of macrophage and T lymphocytes at the interface of PVAT and the adventitia of human atherosclerotic aorta suggests pro-chemotactic activity of PVAT (Henrichot et al., 2005). A recent study investigated the relationship between development of atherosclerosis and pro-inflammatory PVAT by transplantation of visceral fat to the mid-perivascular area of the carotid artery in apolipoprotein-E-deficient mice. Transplant of visceral WAT (inflammatory fat with a higher macrophage content) stimulated development of atherosclerotic lesions in carotid artery accompanied by an increased level of serum CCL2 and enhanced endothelial dysfunction. Such a detrimental effect was not seen with transplantation of subcutaneous fat and could be ameliorated by an antibody to P-selectin glycoprotein ligand (Ohman et al., 2011). This important study demonstrates very elegantly that the pro-inflammatory and pro-atherogenic properties of adipose tissue in the hypercholersterolaemic state can have an adverse influence on vascular function and plaque formation.
PVAT may also contribute to atherosclerosis through release of angiogenic factors such as hepatocyte growth factor (HGF), acidic fibroblast growth factor, thromboplastin-1, serpin-E1, CCL2 and insulin-like growth factor-binding protein-3 (Rittig et al., 2012). Indeed, as HGF is responsible for the stimulation of endothelial cell growth and cytokine secretion from SMCs, it is worth noting that PVAT mass, but not visceral or subcutaneous fat mass, is correlated with the level of HGF in patients with atherosclerosis, further reinforcing the link between increased PVAT, angiogenesis and remodelling in atherosclerosis (Rittig et al., 2012).
Anti-atherosclerotic properties of PVAT
Atherosclerosis is well known to be associated with impaired energy metabolism and endothelial dysfunction in addition to inflammation (Hennig and Chow, 1988). BAT is involved in the regulation of energy expenditure in humans, and since some adipocytes within PVAT possess the morphological features of BAT, it might be anticipated that the fat surrounding the blood vessels can control the local energy metabolism of the vessel wall. Studies have shown that PVAT can generate heat, which helps in maintaining intravascular temperature and correlates with increased activity of metabolic enzymes in mice housed at 16°C (Chang et al., 2012). In this study, development of atherosclerotic plaques in the mice was attenuated and serum triglyceride concentrations decreased markedly. In mice lacking smooth muscle PPARγ, an absence of PVAT was found, which caused endothelial dysfunction and temperature loss (Chang et al., 2012). Epidemiological studies have reported that both extreme hot and cold environments increase the rate of death due to heart attack (Medina-Ramon and Schwartz, 2007); however, there are no clinical data correlating cold exposure with protection from the risk of atherosclerosis in humans. PVAT, by regulating temperature and metabolic activity in the vessel, may well have an important role in protection against adverse cardiovascular events. Indeed, many studies have noted an inverse correlation between adipose-derived adiponectin and risk of CAD. As adiponectin has anti-inflammatory and anti-atherogenic properties, it may have a predictive value for the genesis and development of acute coronary syndrome (Luo et al., 2013). The role of PVAT-derived adiponectin compared with total serum adiponectin with respect to cardiovascular risk remains uncertain, however.
Effect of PVAT on vessel contractility and hypertension
Much evidence indicates that PVAT can exert anti-contractile effects in different vascular beds, an effect assumed to be due to release of PVAT-derived relaxant factors (PVRFs). Furthermore, the anti-contractile effect of PVAT is impaired in diseases such as hypertension (Lu et al., 2011; Galvez-Prieto et al., 2012) and metabolic syndrome (Payne et al., 2010; Ma et al., 2010b). However, to date, the identity of PVRF(s) has not been defined and the PVRFs may include adiponectin (Fésüs et al., 2007), H2S (Wojcicka et al., 2011), NO (Gao et al., 2007) and angiotensin (Ang) 1–7 (Lee et al., 2009a). Recent studies have also demonstrated that palmitic acid methyl esters released by PVAT exert anti-contractile effects via opening of voltage-dependent K+ channels in SMCs (Lee et al., 2011). Another study has indicated that prostacyclin secreted by PVAT may act as a PVRF and protect against endothelial dysfunction (Chang et al., 2012). Although it is well established that prostacyclin is synthesized by endothelial cells of blood vessels, PVAT-derived prostacyclin may act as a secondary source of this vasodilator when the endothelium is dysfunctional in diseases such as atherosclerosis (Chang et al., 2012). PVRFs may induce endothelium-dependent vasodilation by the release of NO followed by activation of smooth muscle K+ channels. A role for NO was supported by experiments where aortic rings devoid of PVAT were found to relax when transferred into an incubation solution containing PVAT in a manner sensitive to removal of the endothelium, NO synthase inhibition, high extracellular K+ or blockade of calcium-dependent K+ channels (Gao et al., 2007). PVRF may also induce an anti-contractile effect by an endothelium-independent mechanism involving release of H2O2 and activation of GC activity. This was observed in aortic rings denuded of endothelium where the effect of PVAT on the contraction to phenylephrine was attenuated by catalase or GC inhibitors and abolished by superoxide dismutase (SOD) and iron (Gao et al., 2007). In addition, Lu and colleagues found that SHRs had enhanced contractile responses to phenylephrine when PVAT was present. Based on pharmacological data, the vessels from SHRs were also less sensitive to the anti-contractile effects of Ang1-7 released from PVAT (Lu et al., 2011). In lipoatrophic mice, which also exhibit increases in BP, Takemori and co-workers also found enhanced contraction to phenylephrine and 5-HT and increased expression of angiotensin AT1 receptors (receptor nomenclature follows Alexander et al., 2013) in the aorta, all of which could contribute to the raised BP when PVAT is absent (Wartmann et al., 1995).
PVAT may also exert contractile effects through release of diffusible factors. It has been reported that all components of the renin–angiotensin system, except renin, can be found in PVAT (Hermenegildo et al., 2005). Moreover, it was reported that three isoforms of AT1a receptors exist in PVAT with low expression of AT1b receptors (Galvez-Prieto et al., 2008). An early study by Soltis and Cassis (1991) also demonstrated a reduced sensitivity to noradrenaline in rat vessels containing PVAT but noted that electrical stimulation induced contraction only in vessels with PVAT. This response was blocked using the non-selective α-adrenoceptor antagonist phentolamine and attenuated by an Ang II receptor antagonist, indicating that PVAT may release angiotensin (Soltis and Cassis, 1991). Enalaprilat, an ACE inhibitor has also been reported to attenuate the response of rat mesenteric arteries to electrical stimulation. Interestingly, exogenous Ang II was found to elicit vascular contraction in blood vessels without PVAT, but not in arteries with intact PVAT, suggesting that Ang II is significantly involved in PVAT-induced contractile effects (Lu et al., 2010). PVAT has also been reported to induce a contractile response to perivascular nerve stimulation via production of superoxide by NAD(P)H oxidase. This mechanism was proposed to involve activation of both tyrosine kinase and the ERK1/2 pathway (Gao et al., 2006).
PVAT in obesity and diabetes
The metabolic balance in normal adipose tissue favours a non-inflammatory state. However, with the development of obesity, the metabolic balance changes and favours chronic low-grade inflammation. The latter has been proposed to be the link between obesity, insulin resistance and CVD (Yudkin et al., 2005; Zhang and Zhang, 2009b). However, the cellular mechanisms leading to these changes are poorly understood. Obesity is defined as an increase in adipose tissue mass, and as this increases, there are also changes at morphological and functional levels in both BAT and WAT. Morphologically, there are changes in adipocyte type, fatty acid composition of lipid vesicles, cell size, cell number, inflammatory cell infiltration, extracellular matrix remodelling and structure. At a functional level, these changes result in an imbalance in adipokine production, oxidative stress, hypoxia and inflammation (Achike et al., 2011).
Total PVAT mass is increased in obese humans, which is accompanied by a diminished anti-contractile effect and the appearance of signs of local inflammation and hypoxia. The latter effects are mimicked by IL-6 and TNF-α and alleviated upon application of SOD, catalase and TNF-α antagonists (Lee et al., 2009b). Moreover, data from the Framingham Heart Study has demonstrated that periaortic fat mass is associated with diabetes, even if corrected for body mass index (Lehman et al., 2010). Reports from animal models of obesity show an increased PVAT mass and adipocyte size (Ketonen et al., 2010; Ma et al., 2010b), coupled with a noticeable loss of PVAT-mediated anti-contractile activity. The loss of the anti-contractile effect was proposed to be due to impaired adipokine release, inflammation and oxidative stress. Secretion of leptin is also uniformly increased in diet-induced obesity (DIO) in animal models (Takemori et al., 2007; Ketonen et al., 2010). In coronary arteries from pigs with metabolic syndrome, release of leptin appears to induce endothelial dysfunction via a PKC α-dependent mechanism (Payne et al., 2010). Obesity in mice, induced through either high-fat diet (HFD) or genetic modification, is also associated with reduced adiponectin, evidence of macrophage infiltration, ROS formation, decreased expression of SOD and signs of endothelial NOS (eNOS) uncoupling (Marchesi et al., 2009).
ROS appear to play a significant role in PVAT-induced vascular dysfunction in obesity (Ketonen et al., 2010). Attenuated endothelium-dependent relaxation in obese mice has been reported to be reversed by removal of PVAT or application of ROS scavengers and is associated with increased expression of leptin, CCL2 and NADPH oxidase in PVAT (Ketonen et al., 2010). In another study, chronic (6 months) high-fat feeding of rats was associated with endothelial dysfunction and correlated with a decrease in AMPK, eNOS and up-regulation of mTOR, which was reversed by removal of PVAT (Ma et al., 2010b).
Furthermore, an increased intake of fructose, as demonstrated in a study using the fructose-fed rat, induces multiple metabolic and endocrine alterations, which are concomitant with significant changes in PVAT fatty acyl composition and oxidative stress biomarkers, which are associated with changes in vascular reactivity. PVAT fatty acyl lipid composition showed significant changes in rats given a fructose-rich diet (FRD), mainly an increase in saturated fatty acids (FAs) and a decrease in monounsaturated (MUFA) and polyunsaturated fatty acids (PUFA), resulting in an increase of the saturated/MUFA and saturated/PUFA ratios. The PVAT redox state was also modified by the fructose overload, as shown by a reduced activity of antioxidant enzymes such as SOD and GSH-Px, which account for the excessive production of superoxide anion and organic peroxides, and a higher NO production, which may account for the activation of the inducible isoform of NO synthase in response to the augmented oxidative stress. It is likely that these findings account for the diminished anti-contractile effect of PVAT in the aortic vessels (Rebolledo et al., 2010).
It has been proposed that dysfunction of PVAT is associated with a reduction in insulin-stimulated glucose transport by reducing muscle perfusion (Meijer et al., 2011). Adiponectin released from PVAT has been reported to affect insulin sensitivity, inflammatory responses, appetite, atherosclerosis and haemostasis (see Tilg and Moschen, 2006). Adiponectin/ACRP30 deficiency and high TNF-α levels in adiponectin KO mice reduced muscle FATP-1 mRNA and IRS-1-mediated insulin signalling, resulting in severe diet-induced insulin resistance (Engeli et al., 2003). PPARγ agonists such as the thiazolidinediones (TZDs) may owe at least part of their beneficial effect on insulin sensitivity to increasing levels of adiponectin in vivo (Engeli et al., 2003). Indeed, adiponectin-deficient mice have reduced responsiveness to PPARγ agonists, implying that adiponectin is an important mediator for improving insulin responsiveness (Nawrocki et al., 2006). Furthermore, in obese animals, administration of adiponectin has been found to improve insulin sensitivity and to decrease hyperglycaemia and the level of fatty acids in the plasma (Berg et al., 2002; Fantuzzi, 2005). As adipose tissue mass increases, the expression of adiponectin is reduced and release of pro-inflammatory cytokines such as TNF-α and IL-6 increases (see Tilg and Moschen, 2006). Conversely, there is a correlation between increases in plasma leptin and insulin resistance and cardiac dysfunction in a rat model of T2DM (Chen et al., 2007). The effects of leptin may be indirectly mediated by the brain where it activates the sympathetic nervous system, thereby leading to elevation of BP via inducing vasoconstriction and Na+ retention, and also in the major metabolic organs where it induces insulin resistance (reviewed in Gu and Xu, 2013). Inflammatory cytokines such as TNF-α may be associated with obesity-related insulin resistance. Many studies have shown that ob/ob mice (leptin-deficient mice that have insulin resistance) also have a reduction of TNF-α or TNF receptors. In this study, the TNF-α-deficient obese mice had lower levels of circulating FFAs and were protected from the obesity-related reduction in the insulin receptor signalling in muscle and fat tissues. These findings demonstrated that TNF-α is an important mediator of insulin resistance in obesity through its effects on several important sites of insulin action (Fukada et al., 2005).
Moreover, C1q/TNF-related protein-12 (CTRP12), a novel adipokine with anti-diabetic effects, has recently been shown to improve insulin sensitivity in the liver and adipose tissue in ob/ob and DIO mice. In cultured adipocytes, CTRP12 activated the PI3K-Akt signalling pathway to promote glucose uptake (Wei et al., 2012). The study by Wei et al. provides further evidence linking adipose tissue to global glucose homeostasis and highlights the consequences of dysfunctional adipose tissue on insulin sensitivity and vascular function.
AMP-activated protein kinase (AMPK)
AMPK is a serine/threonine protein kinase that has been proposed to function as an intracellular energy sensor and is involved in the regulation of cellular and whole body metabolism (Hardie, 2011). AMPK is essential for the maintenance of cardiovascular health, has a regulatory role over vascular structure and function, and is activated by several pathological stimuli, including oxidative damage, ischaemia, hypoxia/anoxia and cardiac hypertrophy (Nagata and Hirata, 2010; Ewart and Kennedy, 2011). The mechanism of action of many drugs commonly used in cardiovascular and metabolic disease, such as statins, metformin and the TZDs, may also involve AMPK (see Ewart and Kennedy, 2011), although the precise upstream and downstream pathways remain poorly defined.
AMPK is a heterotrimeric protein complex comprising a catalytic α subunit and two regulatory β and γ subunits. Two or more isoforms of each subunit (α1, α2, β1, β2, γ1, γ2 and γ3) encoded by different genes give rise to 12 possible heterotrimeric combinations, with splice variants further increasing diversity. The α subunit of AMPK consists of an N-terminal catalytic kinase domain and a C-terminal regulatory domain. Within the activation loop of the kinase domain is a threonine residue (Thr172), whose phosphorylation by upstream kinases is required for activation (Hawley et al., 1996). The C-terminal region of the α subunit comprises an auto-inhibitory domain (Crute et al., 1998) and a region required for binding to the β and γ subunits. The β subunit comprises a C-terminal domain required for association with the α subunit (Wong and Lodish, 2006) and a central region for glycogen binding (Polekhina et al., 2003). The γ subunit comprises of tandem repeat cystathionine β-synthase motifs, known as Bateman domains, which are responsible for AMP, ADP or ATP binding (Cheung et al., 2000; Xiao et al., 2011).
The catalytic and regulatory subunit isoforms of AMPK differ in their tissue distribution, subcellular location and regulation (Cheung et al., 2000; Hardie, 2007). Thus, the tissue-specific subunit composition may be important to determine a specialized cellular and systemic response to different metabolic stimuli (Kahn et al., 2005). In adipose tissue and endothelium, the most predominant catalytic subunit isoform is α1 (Daval et al., 2005; Davis et al., 2006), whereas the α2 catalytic subunit is the most commonly found in skeletal and cardiac muscle. Equal distribution of both α1 and α2 isoforms has been shown in rat liver (Woods et al., 1996). Whereas the β1 subunit is ubiquitously expressed, AMPK containing the β2 subunit is abundant in skeletal muscle and heart. Notably, expression of the γ3 subunit appears highly specific to glycolytic skeletal muscle, whereas γ1 and γ2 show broad tissue distribution (Cheung et al., 2000).
Regulation of AMPK expression and activity
Under normal physiological conditions, ATP occupies some of the Bateman domains of the γ subunit and maintains AMPK in an inactive state (Adams et al., 2004; Scott et al., 2004). With depletion of ATP and elevation of AMP or ADP during periods of stress such as hypoxia or glucose shortage, ATP is displaced, leading to direct allosteric activation of the kinase (AMP). The degree of activation is determined by the subtypes of catalytic α and regulatory γ isoforms present (Cheung et al., 2000), and activation also induces conformational change in the kinase domain that protects AMPKα from dephosphorylation of Thr172 (Riek et al., 2008; Xiao et al., 2011). Such protection from dephosphorylation has been proposed to be the major physiological mechanism for activation of AMPK (Sanders et al., 2007). Two well-characterized upstream AMPKα-Thr172 kinases (AMPKK) have been identified to date. They are liver kinase B1 (LKB1) and Ca2+/calmodulin-dependent protein kinase kinase β. The combination of allosteric modulation and phosphorylation leads to a >1000-fold increase in kinase activity (Hawley et al., 2005; Xie et al., 2006). Another kinase, termed TGF-β-activated kinase 1 (TAK1), was recently identified as an upstream kinase for AMPK based on a genetic screen for mammalian kinases. TAK1 was shown to activate the Snf1 protein kinase in vivo and in vitro and co-expression of TAK1 and its binding partner TAB1 in HeLa cells stimulated phosphorylation of AMPK-Thr172 (Momcilovic et al., 2006). Mice carrying a cardiac-specific dominant negative mutation for TAK1 reported signs of the Wolff–Parkinson–White syndrome, which is similar to those associated with mutations in human AMPKγ2. Moreover, TAK1-deficient MEFs had reduced AMPK activation by oligomycin, metformin and 5-aminoimidazole-4-carboxamide 1-β-D-ribonucleoside (AICAR), leading the authors to propose that TAK1 has a pivotal role in the regulation of LKB1/AMPK signalling axis, an essential pathway in the regulation of cell metabolism (Xie et al., 2006).
Pharmacological activators of AMPK
In addition to physiological activation of AMPK by increases in the cellular AMP/ATP ratio (see Ewart and Kennedy, 2011), many pharmacological AMPK activators have been identified. Metformin is an oral biguanide that activates AMPK in intact cells and in vivo (Musi and Goodyear, 2002; Musi et al., 2002). Activation of AMPK by metformin is dependent on its uptake by organic cation transporter 1 (Shu et al., 2008), and recent studies utilizing AMPKγ mutants that are insensitive to AMP have shown that AMP increases are also essential for metformin-stimulated AMPK activity (Hawley et al., 2010). Other studies have suggested that ROS and reactive nitrogen species are also involved in metformin activation of AMPK (Zou et al., 2004; Fujita et al., 2010). The main site of therapeutic action of metformin is the liver, where stimulation of AMPK was originally thought to underlie a metformin-induced suppression of glucose production and increased fatty acid oxidation in hepatocytes (Zhou et al., 2001). However, more recent studies have demonstrated that AMPK activity is completely dispensable for the anti-gluconeogenic action (Foretz et al., 2010), yet it remains possible that AMPK activation underlies other effects of metformin. In the vasculature, metformin-induced AMPK activation has been reported to up-regulate eNOS phosphorylation, increase NO bioavailability (Calvert et al., 2008; Zhang et al., 2011) and reduce SMC proliferation, migration and inflammatory responses (Kim and Choi, 2012; Vigetti et al., 2011). In cardiac tissue, metformin-induced AMPK activity sustains energy balance, cardiomyocyte function and myocardial viability (Cha et al., 2010; Fu et al., 2011). The reported cardioprotective effect is principally achieved by the reduction of hypertrophic cell growth and endoplasmic reticulum (ER) stress (Dong et al., 2010a).
TZDs are a class of anti-diabetic drugs, including rosiglitazone, troglitazone and pioglitazone, which have been used to counteract insulin resistance in patients with T2DM by increasing the sensitivity of peripheral tissues to insulin (Marx et al., 2004). They act by binding to PPARγ, which promotes the synthesis of glucose transporters and proteins regulating lipid metabolism, leading to storage of lipids in adipocytes rather than hepatocytes and muscle (Semple et al., 2006; Burns and Vanden Heuvel, 2007). PPARγ receptors are nuclear hormone receptors that are expressed widely not only in adipose tissue but also in skeletal muscle (Hevener et al., 2003), liver (Gavrilova et al., 2003) and macrophages (Hevener et al., 2003). TZDs exert pleiotropic effects in a manner similar to statins, and some of these effects are observed acutely and do not require gene transcription. The acute effects of TZDs have been proposed to be via activation of AMPK in adipose tissue, skeletal muscle and liver (LeBrasseur et al., 2006). The mechanism of AMPK activation is reported to be due to an increase in intracellular AMP/ATP ratio (Fryer et al., 2002a). Similar to metformin and phenformin, TZDs have been demonstrated to act as inhibitors of complex 1 of the respiratory chain (Salcedo et al., 2007b); thus, an increase in the AMP/ATP ratio may be the mechanism for AMPK activation in response to these drugs. Notably, this effect was reported to be independent of PPARγ (Guh et al., 2010). TZDs can also activate AMPK by stimulating adiponectin release from adipose tissue (Yamauchi et al., 2002) and this effect may be important in PVAT. Both rosiglitazone and pioglitazone have been reported to have beneficial anti-atherosclerotic and anti-inflammatory effects (Stocker et al., 2007), as well as an additional beneficial influence on endothelium via AMPK-dependent and PPAR-γ-independent mechanisms (Polikandriotis et al., 2005; Ceolotto et al., 2007). However, it should be noted that TZD use is associated with the risk of fluid retention which may exacerbate heart failure (Hannan et al., 2003). There is also a concern that rosiglitazone is associated with additional cardiovascular (MI and stroke) risk in patients with T2DM (Soares de Moura et al., 2004).
Statins are HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase inhibitors used in the treatment of metabolic syndrome and hypercholesterolaemia. Besides their cholesterol-lowering effects, statins have also been reported to activate AMPK in human and bovine endothelial cells (Sun et al., 2006). Sun et al. also reported that while atorvastatin and lovastatin rapidly stimulate AMPK and eNOS in mouse myocardium and endothelial cells, they do not alter the cellular AMP/ATP ratio, suggesting a different pathway of AMPK activation (Sun et al., 2006; Goirand et al., 2007). Two days of treatment with fluvastatin (at a dose of 10 mM) has also been reported to stimulate eNOS and AMPK in human iliac endothelial cells. This effect was blocked by an eNOS inhibitor, implying that AMPK up-regulation was dependent on NO synthesis (Xenos et al., 2005). In another study conducted in rat aorta, simvastatin up-regulated both AMPK and the upstream kinase LKB1. They also reported that this activation of AMPK was dependent on PKCζ-mediated phosphorylation of LKB1 (Choi et al., 2008). However, another study using HeLa cells expressing mutant LKB1 reported that phosphorylation of Ser431 on LKB1 was not required for AMPK up-regulation (Fogarty and Hardie, 2009). In addition to the beneficial effects of statins on endothelial function, which are likely due to eNOS up-regulation (Laufs et al., 2000; 2002,), statins exert anti-inflammatory (Cahoon and Crouch, 2007) and anti-atherogenic effects (Nissen et al., 2005). These observations all indicate that AMPK activation might be a key to the pleiotropic effects of statins on cardiovascular protection.
6,7-Dihydro-4-hydroxy-3-(2′-hydroxy[1,1′-biphenyl]-4-yl)-6-oxo-thieno[2,3-b]pyridine-5-carbonitrile (A769662) is a member of the thienopyridone family that activates AMPK both allosterically and by inhibiting dephosphorylation of the kinase at Thr172 (Cool et al., 2006). A769662 is dependent on the β-subunit carbohydrate-binding module and γ subunit (Guh et al., 2010) of AMPK and, notably, exclusively activates trimers containing the β1 subunit (Scott et al., 2008). In in vitro studies, A769662 has been shown to stimulate phosphorylation of acetyl-CoA carboxylase (ACC) independently of the upstream kinases LKB1 and CaMKK (Cool et al., 2006; Goransson et al., 2007). In cell-free assays, it has no direct effect on the ability of LKB1 or CaMKK to phosphorylate AMPK. The mechanism of AMPK activation by A769662 is thought to be distinct from that of AMP, as A769662 can still activate AMPK containing a mutation in the γ subunit, whereas AMP cannot. In addition, A769662 activation of AMPK was inhibited by a mutation in the β1 AMPK subunit (Ser108 to Ala), which is an auto-phosphorylation site within the glycogen-binding domain; however, the same mutation only partially reduced AMPK activation by AMP (Cool et al., 2006; Sanders et al., 2007). A769662 stimulates AMPK directly in partially purified rat liver and suppresses fatty acid synthesis in primary rat hepatocytes. Short-term treatment of normal Sprague-Dawley rats with A769662 has been shown to reduce liver malonyl-CoA levels and the respiratory exchange ratio of CO2 production to O2 consumption, indicating an increased rate of whole-body fatty acid oxidation (Cool et al., 2006). Treatment with compound A769662 reduced plasma glucose, weight gain, and both plasma and liver triacylglycerol (triglyceride) levels in ob/ob mice (Cool et al., 2006).
Another widely used AMPK activator is AICAR, also known as Acadesine. AICAR is a pro-drug and analogue of adenosine that enters cells and stimulates AMPK, following its uptake and phosphorylation to its active nucleotide ZMP (5-aminoimidazole-4-carboxamide-1-β-D-furanosyl 5′-monophosphate), which mimics AMP (Merrill et al., 1997; Kwon et al., 1998). AICAR has been tested in human studies of ischaemic heart disease due its ability to block adenosine reuptake by cardiac cells, promoting stimulation of adenosine membrane receptors. In 1997, treatment with AICAR before and during surgery was shown to alleviate early cardiac death, MI and combined adverse cardiovascular consequences, although whether the effects were via AMPK was not investigated at that time (Mangano, 1997). AICAR has been demonstrated to reverse many aspects of the metabolic syndrome in animal models such as the ob/ob mouse, the fa/fa rat and high fat-fed rat (Buhl et al., 2002; Iglesias et al., 2002), and also in human subjects (Cuthbertson et al., 2007). AICAR has also been reported to stimulate release of adiponectin and inhibit release of cytokines such as TNF-α and IL-6, which have been implicated in the development of obesity-induced insulin resistance (Kern et al., 2001; Lihn et al., 2004). AICAR is not suitable for clinical use because of its short half-life, requirement for i.v. infusion and variable effectiveness. It also causes bradycardia and can lead to hypoglycaemia when administered intravenously (Young et al., 2005).
Downstream targets and role of AMPK
Once AMPK is activated, the enzyme phosphorylates several key proteins and mediates numerous downstream pathways that are involved in metabolic regulation. This includes decreasing gluconeogenesis, fatty acid and cholesterol synthesis, and increasing muscle glucose transport, fatty acid oxidation and mitochondrial biogenesis. The modulation of these processes ultimately leads to the inhibition of energy-consuming anabolic pathways and the stimulation of ATP producing pathways, thus regulating cellular energy balance. In the vasculature, activation of endothelial AMPK has been shown to phosphorylate eNOS at Ser1177 and Ser633, stimulating NO release (Chen et al., 2009; Mariman and Wang, 2010) and subsequent vasodilatation of both large conduit and resistance blood vessels (Wang et al., 2009b; Bradley et al., 2010). Purified AMPK has also been reported to phosphorylate eNOS at an inhibitory site (Thr495) in vitro (Hansen and Kristiansen, 2006). AMPK activation has been also found to exert anti-inflammatory effects in the endothelium by reducing TNF-α-stimulated monocyte adhesion to human aortic endothelial cells (Ewart et al., 2008). AMPK activators, such as AICAR (Mariman and Wang, 2010), metformin (Xie et al., 2008) and rosiglitazone (Boyle et al., 2008), have been reported to directly activate AMPK and enhance NO production in human endothelial cells. In eNOS knockout mice, shear stress- and bradykinin-mediated activation of AMPK is attenuated (Zhang et al., 2006; Wang et al., 2009a) and pharmacological inhibition of NOS prevents activation of AMPK by shear stress, bradykinin and calcium ionophores (Zhang et al., 2006). AMPK activation in vascular smooth muscle induces vasodilatation (Tirapelli et al., 2006) and attenuates smooth muscle contraction (Yamawaki et al., 2011). Metformin (O'Toole et al., 2008) and adiponectin (Deng et al., 2010) are other AMPK-activating agents that are reported to induce arterial vasorelaxation. Although this vasorelaxation in vivo may be at the level of the endothelium and is due to NO release, metformin was found to induce vasodilatation in blood vessels treated with an eNOS inhibitor. This suggests that AMPK in vascular smooth muscle can modulate vascular tone and this may be important where the endothelium is damaged or dysfunctional in terms of NO formation or release (Majithiya and Balaraman, 2006). At the level of the endothelium, AMPK activation with AICAR can also induce prostacyclin synthesis and subsequent vasodilatation (Chang et al., 2010). In the same study, Chang and co-workers demonstrated that AICAR increases expression of the pro-inflammatory enzyme COX-2. The later was assumed to be a result of activation of p38 MAPK and TAK1 phosphorylation (Chang et al., 2010).
In atherosclerosis, activation of AMPK by AICAR resulted in a reduction in ER stress and inhibition of macrophage proliferation, both induced by high levels of circulating oxidized low-density lipoprotein (LDL). This protects the endothelium from the pathological effect of modified LDL (Dong et al., 2010b). AMPK has been associated with anti-inflammatory effects in neutrophils, which are the primary source of myeloperoxidase (MPO) and involved in the modification of LDL (Alba et al., 2004). AMPK activation attenuated neutrophil activity and metformin also decreased MPO levels in lung tissue (Zhao et al., 2008; Tsoyi et al., 2011). In addition, AMPK activation has been shown to increase protein levels of the ATP-binding cassette transporters, ABCG1 and ABCA1, resulting in cholesterol efflux from macrophage-derived foam cells as well as reduced plaque formation in ApoE−/− mice (Li et al., 2010a; 2010b,).
AMPK has also been implicated in restenosis. AMPKα2 knockout mice had a dramatic increase in the formation of neointima after wire injury of the carotid artery compared with wild-type controls (Song et al., 2011). Local administration of the AMPK activator AICAR also reduced neointima formation 2 weeks after balloon injury in rat carotid arteries (Stone et al., 2012). Furthermore, endothelium-selective expression of constitutively active AMPK was protective against vascular injury and promoted re-endothelialization in a murine diabetic model (Li et al., 2012). Vascular smooth muscle proliferation is a critical event in the development and progression of vascular diseases, including atherosclerosis. It has been reported that AMPK suppresses vascular SMC proliferation associated with attenuated cell cycle regulation by p53 up-regulation (Dashwood et al., 2007).
Acute administration of the AMPK activator AICAR reduces mean arterial pressure (MAP) in both rodents and humans (Foley et al., 1989; Bosselaar et al., 2011). SHRs dosed with AICAR also showed an acute drop in MAP, which was not seen in the control group of Wistar-Kyoto rats, suggesting that AMPK could be a therapeutic target for novel antihypertensive agents (Ford et al., 2012). Long-term administration of AICAR or resveratrol, which has been described as an activator of AMPK, also caused a reduction in MAP in obese Zucker rats (Buhl et al., 2002; Rivera et al., 2009). Furthermore, short-term calorie restriction in the SHRs resulted in increased activity of AMPK, which led to a reduction in MAP (Dolinsky et al., 2010).
AMPK in obesity and inflammation
In many genetic models of rodent obesity, AMPK activity is down-regulated in peripheral tissues such as heart, skeletal muscle and liver (Barnes et al., 2002; Liu et al., 2006). However, it appears that AMPK activity is not altered in the hypothalamus (Fryer et al., 2002a) or skeletal muscle (Fryer et al., 2002a; 2002b,) of animals rendered obese by an HFD. It has been claimed that AMPK protein expression and activity are unaltered in obese human muscle (Minokoshi et al., 2002), although other studies have reported potential reductions (Zang et al., 2004). In human type 2 diabetic skeletal muscle, AMPK expression and activity are also unchanged in patients with a similar body mass (Shaw et al., 2005). The response of AMPK to allosteric activation by AMP (Minokoshi et al., 2002) and LKB1 activity and expression (Chen et al., 2005) is also unchanged in obesity and in obese type 2 diabetic skeletal muscle, suggesting that the muscle AMPK system is indeed intact at least in moderate obesity. Similarly, AICAR has been demonstrated to increase AMPK activity to a similar degree in obese skeletal muscle from rodents (Lin et al., 2000; Pold et al., 2005) and humans (Minokoshi et al., 2002) in comparison with lean controls. Notably, activation of AMPK by AICAR increases glucose uptake and fatty acid oxidation in obese diabetic rodents (Barnes et al., 2002) and humans (Minokoshi et al., 2002).
The role of AMPK in adipose tissue remains relatively poorly defined, although, given the anti-inflammatory and metabolic role of AMPK in other tissues, it is clear that the importance of the influence of this kinase on fat should not be discounted. The remainder of this review will focus on the impact of AMPK action in adipose tissue and, in particular, PVAT.
Several physiological stimuli are reported to activate AMPK in adipose tissue, including starvation, exercise, circulating HDL and the adipocyte-derived cytokine adiponectin. The principal catalytic subunit of AMPK expressed in adipose tissue is α1 (Salt et al., 2000; Daval et al., 2005), although an important role for α2 cannot be excluded as down-regulation of this isoform can have marked effects on adipocyte function (Wang et al., 2010). Whereas AMPK activation in skeletal muscle is proven to increase glucose transporter type 4 (GLUT-4) translocation and, therefore, both basal and insulin-stimulated glucose transport (Kramer et al., 2006; Fazakerley et al., 2010), AMPK activation in adipose tissue has yielded contrasting results. AICAR-stimulated AMPK activation has been reported to inhibit insulin-stimulated glucose uptake in adipocytes without affecting the early steps on the insulin signalling pathway (Salt et al., 2000; Wu et al., 2003) and is accompanied by a reduction in AS160 phosphorylation and reduced Rab-GAP activity (Gaidhu et al., 2010). In contrast, adiponectin was reported to activate AMPK and increase both basal and insulin-stimulated glucose uptake (Wu et al., 2003). Longer term AMPK activation (24–48 h) with metformin has been shown in 3T3-L1 adipocytes to increase basal glucose transport, thereby reducing the extent of stimulation in response to insulin (Boyle et al., 2011), and similar results were found in adipocytes derived from human fat (Fischer et al., 2010). In ex vivo experiments on human adipose tissue, GLUT-4 expression was found to be increased following prolonged incubation with AICAR (Lihn et al., 2008); however, levels were unaffected in 3T3-L1 adipocytes following prolonged AICAR or metformin treatment (Boyle et al., 2011). Biopsies from patients with type 2 diabetes treated for 10 weeks with metformin also showed no change in GLUT-4 expression levels (Boyle et al., 2011).
Abnormal lipid profiles and lipotoxicity, as seen in obesity and T2DM, predispose individuals to CVD. The AMPK pathway is a major regulator of lipid metabolism and has been shown to up-regulate fatty acid oxidation (muscle, adipose, hypothalamus) and uptake, inhibit cholesterol biosynthesis (liver) and reduce lipogenesis (liver, hypothalamus) (reviewed in Viollet et al., 2009). In adipocytes, AMPK phosphorylates and inhibits ACC and increases malonyl-CoA decarboxylase activity, resulting in lower malonyl-CoA levels and therefore promoting mitochondrial β-oxidation while simultaneously suppressing fatty acid synthesis (Viollet et al., 2009). However, the role of AMPK in lipolysis has precipitated much discussion, as AMPK has been reported to up-regulate or inhibit lipolysis via phosphorylation of hormone-sensitive lipase. In isolated human adipocytes, TZDs and biguanides inhibited lipolysis in a manner thought to be through AMPK cascade activation. These effects were all found to be inhibited by the AMPK inhibitor compound C (Flechtner-Mors et al., 1999). Experiments in rat adipocytes, however, have suggested that the impact of the biguanide metformin on lipolysis may, in fact, be due to PKA inhibition rather than AMPK activation (Zhang et al., 2009c). AICAR (Gaidhu et al., 2009) and A769662 (Hawley et al., 2012)-induced AMPK activation has also been reported to inhibit adipocyte lipolysis, although this was only observed following short-term AICAR incubations (Gaidhu et al., 2009). Overexpression of a constitutively active form of AMPK has been shown to inhibit β-adrenoceptor-induced lipolysis (Kwon et al., 1998; Daval et al., 2005). Notably, in AMPKα1 knockout mice, the size of adipocytes is smaller, and both basal and isoprenaline-induced lipolysis are higher than that of control adipocytes (Daval et al., 2005). Furthermore, deletion of the AMPKα2 subunit is also accompanied by modifications in adipose tissue. AMPKα2−/− mice fed an HFD exhibited increased body weight and fat mass in relation to control mice fed similarly. The increase in adipose tissue mass was due to the enlargement of the adipocytes with increased lipid accumulation. This study showed that deletion of AMPKα2 subunit may be a factor contributing to the development of obesity (Villena et al., 2004). Notably, it has been found that obesity increases PVAT mass and reduces its anti-contractile effect (Gao et al., 2005). In contrast, exercise-induced AMPK activation has been shown to stimulate lipolysis via β-adrenoceptors (Koh et al., 2007) and increased lipolysis was also observed after long-term stimulation with AICAR (Gaidhu et al., 2009). Puzzlingly, additional recent evidence has shown that reducing AMPK activity does not appear to have any significant effect on lipolysis at all (Chakrabarti et al., 2011), and it should also be noted that an increase in lipolysis itself may indirectly activate AMPK by increasing the AMP/ATP ratio (Gauthier et al., 2008).
AMPK in inflammation
Several studies have revealed an irrefutable relationship between AMPK activity and inflammation in different tissues and cell types, including adipocytes, endothelial cells and macrophages (Galic et al., 2011; Gauthier et al., 2011). Activation of AMPK down-regulates pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 in macrophages (Yang et al., 2010; Galic et al., 2011), and IL-6 and IL-8 in adipocytes (Lihn et al., 2008). AMPK activation has also been demonstrated to up-regulate the expression of the anti-inflammatory cytokine IL-10 in macrophages (Sag et al., 2008; Galic et al., 2011). In cultured human SCAT, AICAR was reported to attenuate TNF-α and IL-6 secretion, while stimulating adipose tissue AMPK α1 activity and adiponectin gene expression (Lihn et al., 2004). Berberine, an AMPK activator that inhibits pro-inflammatory signalling in isolated macrophages, has also been found to inhibit cytokine expression in adipose tissue of obese db/db mice (Jeong et al., 2009). The activity of the NLRP3 inflammasome is markedly increased in myeloid cells from patients with type 2 diabetes, and it is reported that treatment with metformin decreased the maturation and secretion of IL-1β and IL-18 in monocyte-derived macrophages. Metformin produces this effect through activation of AMPK; however, the mechanism remains elusive (Yang et al., 2010; Lee et al., 2013). Furthermore, it has been observed that IL-10 and TGF-β, two anti-inflammatory cytokines, induce a rapid phosphorylation and activation of AMPK, whereas a pro-inflammatory insult with LPS decreased the AMPK activity in both mouse and human macrophages (Sag et al., 2008).
Nutrient stress induced by high-fat feeding has been found to suppress cardiac AMPK activity and induces inflammation, which is characterized by increased number of macrophages and up-regulation of IL-6 levels in the mouse heart. Furthermore, acute infusion of IL-6 profoundly lowered the AMPK activity, decreased the glucose uptake by cardiac muscle and induced inflammation in the heart (Ko et al., 2009). Ko et al. also demonstrated that diet-induced insulin resistance and inflammation were alleviated in IL-6-deficient mice (Ko et al., 2009). However, IL-6 knockout in mice can be linked with the development of mature-onset obesity (Wallenius et al., 2002) and diet-induced atherosclerosis (Madan et al., 2008), an effect that can be attributed to the reduced AMPK activity in IL-6-deficient mice (Kelly et al., 2004).
Several studies have reported that the activation of AMPK down-regulates the NF-κB system, which is responsible for the development of low-grade chronic inflammation associated with obesity, T2DM and atherosclerosis (Rocha and Libby, 2009; Yang et al., 2010). AMPK suppresses NF-κB signalling indirectly via its downstream targets such as SIRT1 and PGC1α, forkhead Box (FoxO) family and PPARγ co-activator 1α (PGC-1α), which, in turn, activates the expression of inflammatory factors (Salminen et al., 2011). Generally, stimulation of macrophages by pro-inflammatory stimuli decreases sirtuin levels, which is associated with increased activation of RelA/p65 subunit of NF-κB and increased pro-inflammatory cytokine release (Yang et al., 2007). Constitutively active AMPKα1 has been found to mimic the effect of SIRT1 on de-acetylating NF-κB, which consequently inhibits LPS- and FFA-induced TNF-α expression and restores the anti-inflammatory phenotype of macrophage (Yang et al., 2010). Adenovirus-mediated overexpression of the PGC-1α gene in human aortic SMCs and EC alleviates intracellular and mitochondrial ROS production, NF-κB activity as well as CCL2 and VCAM-1 expression, supporting the possibility that signalling molecules stimulating PGC-1α in the vasculature, such as AMPK, will exert beneficial effects on vascular inflammation and development of atherosclerosis (Kim et al., 2007).
AMPK and autophagy
Autophagy is an essential mechanism not only for the recycling of amino acids and other macromolecules during periods of starvation but also for elimination of cellular components damaged by toxins, hypoxia or other noxious stimuli. Derangements in autophagy can lead to an exaggerated response to injury (Aki et al., 2013). Macrophages in advanced atherosclerotic lesions are prone to apoptosis, which can contribute to plaque destabilization and cardiovascular events. Autophagy in macrophages appears to be protective against atherosclerosis development in two ways: by preventing apoptosis of the macrophage and by enhancing efferocytosis – clearance of apoptotic cells in the plaque (Liao et al., 2012). The biochemical events involved in macrophage autophagy are still being actively investigated but, not surprisingly, energy sensing pathways such as AMPK and SIRT1 are involved. In human THP-1 cells, inhibition of SIRT1 with sirtinol was associated with up-regulation of pro-inflammatory genes, down-regulation of AMPK activity and impaired autophagy (Takeda-Watanabe et al., 2012). AMPK also plays a pivotal role in hypoxia-induced autophagy in human cancer cell lines (Papandreou et al., 2008) and human pulmonary artery SMCs exposed to hypoxia (Ibe et al., 2013). However, where the role of autophagy appears to be protective in macrophages, the situation is not so clear in vascular SMCs. In the study by Ibe et al., AMPK isoforms had different effects on pulmonary artery SMCs, with AMPKα1 inducing autophagy and promoting SMC survival. In terms of hypoxic pulmonary remodelling, this is an undesirable effect. Autophagy may also be responsible for removing the contractile proteins from vascular SMCs during their transition to a synthetic phenotype. In a recent study, spautin-1, an inhibitor of autophagy, prevented PDGF-induced disorganization of actin filaments, cell proliferation and migration, and production of extracellular matrix (Salabei et al., 2013). Thus, in vascular lesions, autophagy in response to oxidative stress and growth factors may initiate deleterious changes in vascular function, which ultimately lead to adverse vascular remodelling. The role of AMPK in autophagy-induced phenotypic transformation of vascular SMCs has not been determined as yet.
AMPK and micro-RNAs
Micro-RNAs (miR) are small, non-coding RNAs 18–25 nucleotides in length that negatively regulate gene expression in a sequence-specific manner. Recent evidence has indicated that AMPK targets specific miRs and this may be of importance in the regulation of vascular homeostasis. The miR-144/451 cluster, for example, is down-regulated by stretch on the smooth muscle, which then allows phosphorylation of AMPK, leading to contractile differentiation (Turczynska et al., 2013). At the level of the endothelial cell, shear stress was found to down-regulate ACE expression while activating AMPKα2 had the same effect via phosphorylation of p53 and up-regulation of miR-143/145 (Kohlstedt et al., 2013). Another miR that may impact on AMPK activity is miR-33. AMPK contains a predicted binding sequence for miR-33a/b and this miR appears to negatively regulate AMPK with the effect of increasing cellular cholesterol and triglyceride content (Fernandez-Hernando and Moore 2011). In terms of macrophage function, miR-223 appears to suppress the pro-inflammatory activation of macrophages in adipose tissue and thus may be a target to prevent adipose tissue inflammation and insulin resistance (Zhuang et al., 2012). Adipocytes themselves can secrete miRs such as miR-130b and this correlates with the degree of obesity in a human population. Interestingly, the same study showed that inflammatory mediators such as TGF-β could stimulate miR release by adipocytes, suggesting that inflammation within the adipose tissue in obesity can generate miRs (Wang et al., 2013). Taken together, these data indicate widespread generation of multiple miRs by cell types within the vasculature and surrounding adipose tissue, some of which may act through AMPK to influence vascular function. This may be mediated acutely through ACE, for example, or chronically via changes in vascular SMC differentiation or lipid content.
AMPK and PVAT
This review has shown that PVAT is implicated in vascular control (Figures 2 and 3) and function in health and disease and, as discussed earlier, the metabolic kinase AMPK is also known to have vasculoprotective properties. As such, targeting AMPK may have therapeutic potential in treating some of the effects of obesity and insulin resistance on PVAT. Recent investigations have proposed that AMPK-stimulating adipokines, such as adiponectin, are the main mediators of the modulatory effect of PVAT on the vasculature. Adiponectin release is markedly reduced in obese PVAT and this leads to inhibited vasorelaxation. Experiments with an adiponectin receptor antagonist and the AMPK inhibitor compound C were shown to mimic the effect of obesity on vascular relaxation, and globular adiponectin also failed to increase vasorelaxation in AMPK2α-deficient mice (Meijer et al., 2013; Weston et al., 2013).
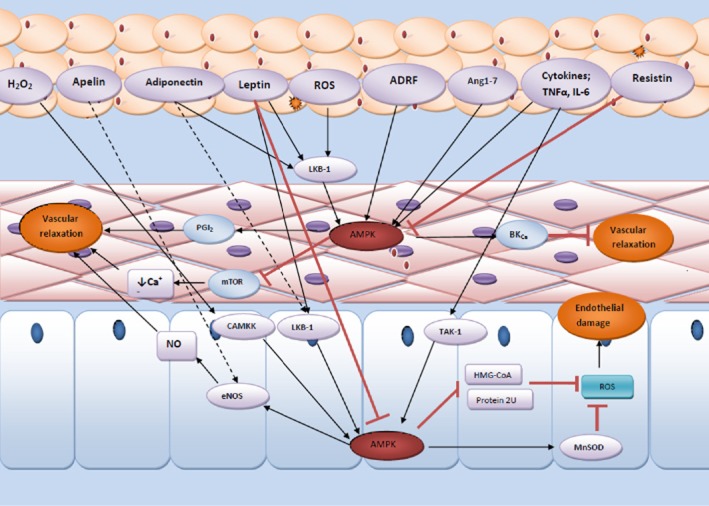
Figure 3.
Schematic presentation of how PVAT modulates the function of AMPK in both endothelium and vascular smooth muscle and how these effects lead to vascular proliferation and atherosclerosis. PVAT is a highly active organ secreting various active molecules implicating in the regulation of vascular reactivity via modulation of AMPK function. ADRF, adipose tissue-derived relaxation factor; Ang1-7, angiotensin 1–7; CAMKK, calcium/calmodulin-dependent protein kinase kinase; eNOS, endothelial NOS; HMG-COA, 3-hydroxy-3-methylglutaryl-coenzyme A;; IP3R, inositol trisphosphate receptor; LKB, liver kinase B; MAPK, mitogen-activated protein kinase; MnSOD, manganese superoxide dismutase; mTOR, mammalian target of rapamycin; PGI2, prostacyclin; ROS, reactive oxygen species; TAK1, transforming growth factor β-activated kinase 1.
It has been reported by Ma et al. (2010b) that PVAT can induce vascular dysfunction via dysregulation of the AMPK/mTOR pathway in diet-induced obese rats, with mesenteric arterial rings incubated with periaortic fat from rats on an HFD demonstrating lower endothelium-dependent relaxation and down-regulation of AMPK and eNOS in the aorta with a concurrent up-regulation of mTOR. This effect was absent in periaortic fat from rats on a chow diet. Co-culture of vascular SMCs with periaortic adipocytes from rats on an HFD also reduced AMPK phosphorylation and increased mTOR phosphorylation. In obese PVAT, release of TNF-α and FFA are also increased (Ma et al., 2010a), indicating further switching to a more pro-inflammatory vasoconstrictive phenotype. Adiponectin has been proposed to mediate crosstalk between PVAT and vascular endothelium and to contribute to the regulation of muscle perfusion (Bussey et al., 2011). Adiponectin could also exert a pro-angiogenic effect in ischaemic tissue by acting directly on the vascular endothelium. It has been shown that AMPK signalling mediates adiponectin-induced angiogenic and anti-apoptotic cellular responses in endothelial cells (Kobayashi et al., 2004; Ouchi et al., 2004). However, secretion of adiponectin and other adipokines has been reported to change in association with obesity (Tilg and Moschen, 2006; Eringa et al., 2007). Furthermore, activation of adipocyte β3-adrenoceptors has been reported to stimulate release of a substance, which is assumed to be adiponectin and which indirectly opens myocyte BKCa channels (Weston et al., 2013). This effect was reported to involve AMPK since it could be mimicked by the AMPK activator, A-769662, and blocked by the kinase inhibitor, dorsomorphin. This study has also demonstrated that glibenclamide and clotrimazole could block adipocyte-dependent myocyte hyperpolarization but not the response to a BKCa channel opener, NS1619, implying that AMPK may not activate BKCa directly (Weston et al., 2013).
Cytokines generated from within the PVAT may also induce changes in vascular reactivity indirectly through up-regulation of iNOS. A recent in vitro study in 3T3 L1 adipocytes demonstrated that TNF-α treatment induced a 200-fold increase in iNOS gene expression (Digby et al., 2010). AMPK activation within the adipose tissue may be beneficial in preventing the adverse effects of iNOS on vascular reactivity by inhibiting its up-regulation (Pilon et al., 2004). Recent studies have supported this by demonstrating inhibition of iNOS expression in adipose tissue in vivo and in adipocytes and myocytes in vitro in response to AMPK activation by the plant polyphenol resveratrol (Centeno-Baez et al., 2011). FFAs released by adipocytes may also be involved in changes in iNOS expression and vascular dysfunction. In human vascular SMCs, a combination of conditioned media from adipocytes and oleic acid induced not only iNOS up-regulation and NO formation but also a proliferative response (Lamers et al., 2011). It has also been hypothesized that one of the beneficial effects of adiponectin is via activation of AMPK and suppression of iNOS expression in the vascular adventitia (Cai et al., 2008). A follow-up study by the same group examined the effects of adiponectin on adventitial fibroblast transition and migration. Their data showed that adiponectin induced AMPK phosphorylation and reduced the migration of fibroblasts, the expression of iNOS and the peroxynitrite marker nitrotyrosine in response to LPS treatment (Cai et al., 2010). Taken together, these data indicate that PVAT cannot only modulate vascular reactivity via changes in iNOS and NO formation by adipocytes but also vascular structure through effects on VSMC and fibroblast proliferation and migration.
PVAT has also been reported to induce vasodilatation in muscle resistance arteries by increasing secretion of adiponectin and activation of AMPK α2 in the blood vessels. Furthermore, this study reported that in obese db/db mice, PVAT mass increased dramatically in the muscle and was associated with loss of its ability to induce insulin-mediated vasodilation. This was due to decreased release of adiponectin and disturbed insulin Akt activation. Notably, the effect of insulin-induced vascular reactivity can be restored by JNK (Meijer et al., 2013). All of these studies highlight the importance of PVAT in modulating the activity of the underlying layers of blood vessels. Crucially, evidence is now accumulating that some of the beneficial effects of PVAT may become deranged in obesity and this can predispose to CVDs. AMPK present in the PVAT is likely to be an important intermediate in the actions of some PVAT-derived mediators. Targeting AMPK may have therapeutic potential in treating some of the effects of obesity and insulin resistance on PVAT.
Conclusions
In summary, given the influence PVAT has on the vasculature, there has been much interest in PVAT as a potential link between obesity and the development of CVD. Although the mechanisms explaining these results have not yet been elucidated, it is clear that they heavily and independently affect CVD. Although many drugs currently in use are known to activate AMPK, this is not always wholly responsible for their therapeutic action. As knowledge of the structure, function and distribution of the different subunit isoforms of AMPK increases, targeting drugs more specifically should be achievable. Salicylate and A769662 are specific to complexes containing the β1 subunit, so developing pharmacological agents specific to other AMPK isoforms is likely to become a reality in the future.
Acknowledgments
The authors gratefully acknowledge support from Zawia University (Libya) and the Ministry of High Education (Libya) in the form of a PhD Studentship awarded to Dr Almabrouk.
Glossary
- ACC
acetyl-CoA carboxylase
- AICAR,
5-aminoimidazole-4-carboxamide 1-β-D-ribonucleoside
- AMPK
AMP-activated PK
- BAT
brown adipose tissue
- CAD
coronary artery disease
- CTRP12
C1q/TNF-related protein-12
- CVD
cardiovascular disease
- DIO
diet-induced obesity
- ER
endoplasmic reticulum
- GLUT4
glucose transporter type 4
- HDL
high-density lipoprotein
- HFD
high-fat diet
- HGF
hepatocyte growth factor
- HMG-CoA
3-hydroxy-3-methylglutaryl-coenzyme A
- LKB1
liver kinase B1
- MPO
myeloperoxidase
- mTOR
mammalian target of rapamycin
- MUFA
monounsaturated fatty acids
- PUFA
polyunsaturated fatty acids
- PVAT
perivascular adipose tissue
- PVRFs
PVAT-derived relaxant factors
- SMCs
smooth muscle cells
- SOD
superoxide dismutase
- T2DM
type 2 diabetes mellitus
- TZDs
thiazolidinediones
- WAT
white adipose tissue
Conflict of interest
None of the authors have any conflict of interest to declare.
References
- Achike FI, To NH, Wang H, Kwan CY. Obesity, metabolic syndrome, adipocytes and vascular function: a holistic viewpoint. Clin Exp Pharmacol Physiol. 2011;38:1–10. doi: 10.1111/j.1440-1681.2010.05460.x. [DOI] [PubMed] [Google Scholar]
- Adams J, Chen ZP, Van Denderen BJ, Morton CJ, Parker MW, Witters LA, et al. Intrasteric control of AMPK via the gamma1 subunit AMP allosteric regulatory site. Protein Sci. 2004;13:155–165. doi: 10.1110/ps.03340004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aki T, Funakoshi T, Unuma K, Uemura K. Impairment of autophagy: from hereditary disorder to drug intoxication. Toxicology. 2013;311:205–215. doi: 10.1016/j.tox.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Alba G, El Bekay R, Alvarez-Maqueda M, Chacon P, Vega A, Monteseirin J, et al. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS Lett. 2004;573:219–225. doi: 10.1016/j.febslet.2004.07.077. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BR, Ryder JW, Steiler TL, Fryer LG, Carling D, Zierath JR. Isoform-specific regulation of 5′ AMP-activated protein kinase in skeletal muscle from obese Zucker (fa/fa) rats in response to contraction. Diabetes. 2002;51:2703–2708. doi: 10.2337/diabetes.51.9.2703. [DOI] [PubMed] [Google Scholar]
- Bell LN, Ward JL, Degawa-Yamauchi M, Bovenkerk JE, Jones R, Cacucci BM, et al. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity. Am J Physiol Endocrinol Metab. 2006;291:E843–E848. doi: 10.1152/ajpendo.00174.2006. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- Bohlen F, Kratzsch J, Mueller M, Seidel B, Friedman-Einat M, Witzigmann H, et al. Leptin inhibits cell growth of human vascular smooth muscle cells. Vascul Pharmacol. 2007;46:67–71. doi: 10.1016/j.vph.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Bosselaar M, Smits P, van Loon LJ, Tack CJ. Intravenous AICAR during hyperinsulinemia induces systemic hemodynamic changes but has no local metabolic effect. J Clin Pharmacol. 2011;51:1449–1458. doi: 10.1177/0091270010382912. [DOI] [PubMed] [Google Scholar]
- Boyle JG, Logan PJ, Ewart MA, Reihill JA, Ritchie SA, Connell JM, et al. Rosiglitazone stimulates nitric oxide synthesis in human aortic endothelial cells via AMP-activated protein kinase. J Biol Chem. 2008;283:11210–11217. doi: 10.1074/jbc.M710048200. [DOI] [PubMed] [Google Scholar]
- Boyle JG, Logan PJ, Jones GC, Small M, Sattar N, Connell JM, et al. AMP-activated protein kinase is activated in adipose tissue of individuals with type 2 diabetes treated with metformin: a randomised glycaemia-controlled crossover study. Diabetologia. 2011;54:1799–1809. doi: 10.1007/s00125-011-2126-4. [DOI] [PubMed] [Google Scholar]
- Bradley EA, Eringa EC, Stehouwer CD, Korstjens I, van Nieuw Amerongen GP, Musters R, et al. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside in the muscle microcirculation increases nitric oxide synthesis and microvascular perfusion. Arterioscler Thromb Vasc Biol. 2010;30:1137–1142. doi: 10.1161/ATVBAHA.110.204404. [DOI] [PubMed] [Google Scholar]
- Brian JE, Jr, Faraci FM. Tumor necrosis factor-alpha-induced dilatation of cerebral arterioles. Stroke. 1998;29:509–515. doi: 10.1161/01.str.29.2.509. [DOI] [PubMed] [Google Scholar]
- Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, et al. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51:2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta. 2007;1771:952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey CT, Kolka CM, Rattigan S, Richards SM. Adiponectin opposes endothelin-1-mediated vasoconstriction in the perfused rat hindlimb. Am J Physiol Heart Circ Physiol. 2011;301:H79–H86. doi: 10.1152/ajpheart.00864.2010. [DOI] [PubMed] [Google Scholar]
- Cahoon WD, Jr, Crouch MA. Preprocedural statin therapy in percutaneous coronary intervention. Ann Pharmacother. 2007;41:1687–1693. doi: 10.1345/aph.1K248. [DOI] [PubMed] [Google Scholar]
- Cai XJ, Li CJ, Chen L, Rong YY, Zhang Y, Zhang M. A hypothesis: adiponectin mediates anti-atherosclerosis via adventitia-AMPK-iNOS pathway. Med Hypotheses. 2008;70:1044–1047. doi: 10.1016/j.mehy.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Cai XJ, Chen L, Li L, Feng M, Li X, Zhang K, et al. Adiponectin inhibits lipopolysaccharide-induced adventitial fibroblast migration and transition to myofibroblasts via AdipoR1-AMPK-iNOS pathway. Mol Endocrinol. 2010;24:218–228. doi: 10.1210/me.2009-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001;98:6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno-Baez C, Dallaire P, Marette A. Resveratrol inhibition of inducible nitric oxide synthase in skeletal muscle involves AMPK but not SIRT1. Am J Physiol Endocrinol Metab. 2011;301:E922–E930. doi: 10.1152/ajpendo.00530.2010. [DOI] [PubMed] [Google Scholar]
- Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, et al. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27:2627–2633. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- Cha HN, Choi JH, Kim YW, Kim JY, Ahn MW, Park SY. Metformin inhibits isoproterenol-induced cardiac hypertrophy in mice. Korean J Physiol Pharmacol. 2010;14:377–384. doi: 10.4196/kjpp.2010.14.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti P, English T, Karki S, Qiang L, Tao R, Kim J, et al. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J Lipid Res. 2011;52:1693–1701. doi: 10.1194/jlr.M014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–U1174. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MY, Ho FM, Wang JS, Kang HC, Chang Y, Ye ZX, et al. AICAR induces cyclooxygenase-2 expression through AMP-activated protein kinase-transforming growth factor-beta-activated kinase 1-p38 mitogen-activated protein kinase signaling pathway. Biochem Pharmacol. 2010;80:1210–1220. doi: 10.1016/j.bcp.2010.06.049. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechi K, Blanchard PG, Mathieu P, Deshaies Y, Richard D. Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int J Cardiol. 2012;167:2264–2270. doi: 10.1016/j.ijcard.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Chen MB, McAinch AJ, Macaulay SL, Castelli LA, O'Brien PE, Dixon JB, et al. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab. 2005;90:3665–3672. doi: 10.1210/jc.2004-1980. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ohmori K, Mizukawa M, Yoshida J, Zeng Y, Zhang L, et al. Differential impact of atorvastatin vs pravastatin on progressive insulin resistance and left ventricular diastolic dysfunction in a rat model of type II diabetes. Circ J. 2007;71:144–152. doi: 10.1253/circj.71.144. [DOI] [PubMed] [Google Scholar]
- Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346(Pt 3):659–669. [PMC free article] [PubMed] [Google Scholar]
- Choi HC, Song P, Xie Z, Wu Y, Xu J, Zhang M, et al. Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo. J Biol Chem. 2008;283:20186–20197. doi: 10.1074/jbc.M803020200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chun HJ, Ali ZA, Kojima Y, Kundu RK, Sheikh AY, Agrawal R, et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest. 2008;118:3343–3354. doi: 10.1172/JCI34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Cinti S. Between brown and white: novel aspects of adipocyte differentiation. Ann Med. 2011;43:104–115. doi: 10.3109/07853890.2010.535557. [DOI] [PubMed] [Google Scholar]
- Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- Cuthbertson DJ, Babraj JA, Mustard KJ, Towler MC, Green KA, Wackerhage H, et al. 5-Aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside acutely stimulates skeletal muscle 2-deoxyglucose uptake in healthy men. Diabetes. 2007;56:2078–2084. doi: 10.2337/db06-1716. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Dooley A, Shi-Wen X, Abraham DJ, Souza DS. Does periadventitial fat-derived nitric oxide play a role in improved saphenous vein graft patency in patients undergoing coronary artery bypass surgery. J Vasc Res. 2007;44:175–181. doi: 10.1159/000099833. [DOI] [PubMed] [Google Scholar]
- Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS pathway. Int J Obes (Lond) 2010;34:165–171. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- Digby JE, McNeill E, Dyar OJ, Lam V, Greaves DR, Choudhury RP. Anti-inflammatory effects of nicotinic acid in adipocytes demonstrated by suppression of fractalkine, RANTES, and MCP-1 and upregulation of adiponectin. Atherosclerosis. 2010;209:89–95. doi: 10.1016/j.atherosclerosis.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, et al. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56:412–421. doi: 10.1161/HYPERTENSIONAHA.110.154732. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, et al. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010a;121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, et al. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 2010b;59:1386–1396. doi: 10.2337/db09-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buleon M, et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008;8:437–445. doi: 10.1016/j.cmet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Eiras S, Teijeira-Fernandez E, Shamagian LG, Fernandez AL, Vazquez-Boquete A, Gonzalez-Juanatey JR. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine. 2008;43:174–180. doi: 10.1016/j.cyto.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, et al. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- Eringa EC, Bakker W, Smulders YM, Serne EH, Yudkin JS, Stehouwer CD. Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation. 2007;14:389–402. doi: 10.1080/10739680701303584. [DOI] [PubMed] [Google Scholar]
- Ewart MA, Kennedy S. AMPK and vasculoprotection. Pharmacol Ther. 2011;131:242–253. doi: 10.1016/j.pharmthera.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Ewart MA, Kohlhaas CF, Salt IP. Inhibition of tumor necrosis factor alpha-stimulated monocyte adhesion to human aortic endothelial cells by AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2008;28:2255–2257. doi: 10.1161/ATVBAHA.108.175919. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- Fazakerley DJ, Holman GD, Marley A, James DE, Stockli J, Coster AC. Kinetic evidence for unique regulation of GLUT4 trafficking by insulin and AMP-activated protein kinase activators in L6 myotubes. J Biol Chem. 2010;285:1653–1660. doi: 10.1074/jbc.M109.051185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Moore KJ. MicroRNA modulation of cholesterol homeostasis. Arterioscler Thromb Vasc Biol. 2011;31:2378–2382. doi: 10.1161/ATVBAHA.111.226688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fésüs G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, et al. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Fischer M, Timper K, Radimerski T, Dembinski K, Frey DM, Zulewski H, et al. Metformin induces glucose uptake in human preadipocyte-derived adipocytes from various fat depots. Diabetes Obes Metab. 2010;12:356–359. doi: 10.1111/j.1463-1326.2009.01169.x. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H1425–H1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechtner-Mors M, Ditschuneit HH, Jenkinson CP, Alt A, Adler G. Metformin inhibits catecholamine-stimulated lipolysis in obese, hyperinsulinemic, hypertensive subjects in subcutaneous adipose tissue: an in situ microdialysis study. Diabet Med. 1999;16:1000–1006. doi: 10.1046/j.1464-5491.1999.00189.x. [DOI] [PubMed] [Google Scholar]
- Fogarty S, Hardie DG. C-terminal phosphorylation of LKB1 is not required for regulation of AMP-activated protein kinase, BRSK1, BRSK2, or cell cycle arrest. J Biol Chem. 2009;284:77–84. doi: 10.1074/jbc.M806152200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JM, Adams GR, Meyer RA. Utility of AICAr for metabolic studies is diminished by systemic effects in situ. Am J Physiol. 1989;257(3 Pt 1):C488–C494. doi: 10.1152/ajpcell.1989.257.3.C488. [DOI] [PubMed] [Google Scholar]
- Ford RJ, Teschke SR, Reid EB, Durham KK, Kroetsch JT, Rush JW. AMP-activated protein kinase activator AICAR acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J Hypertens. 2012;30:725–733. doi: 10.1097/HJH.0b013e32835050ca. [DOI] [PubMed] [Google Scholar]
- Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–256. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Frontini A, Rousset S, Cassard-Doulcier AM, Zingaretti C, Ricquier D, Cinti S. Thymus uncoupling protein 1 is exclusive to typical brown adipocytes and is not found in thymocytes. J Histochem Cytochem. 2007;55:183–189. doi: 10.1369/jhc.6A7013.2006. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Pivotal role of nitric oxide in the control of blood pressure after leptin administration. Diabetes. 1999;48:903–908. doi: 10.2337/diabetes.48.4.903. [DOI] [PubMed] [Google Scholar]
- Fryer LG, Foufelle F, Barnes K, Baldwin SA, Woods A, Carling D. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem J. 2002a;363(Pt 1):167–174. doi: 10.1042/0264-6021:3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002b;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- Fu YN, Xiao H, Ma XW, Jiang SY, Xu M, Zhang YY. Metformin attenuates pressure overload-induced cardiac hypertrophy via AMPK activation. Acta Pharmacol Sin. 2011;32:879–887. doi: 10.1038/aps.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Hosokawa M, Fujimoto S, Mukai E, Abudukadier A, Obara A, et al. Metformin suppresses hepatic gluconeogenesis and lowers fasting blood glucose levels through reactive nitrogen species in mice. Diabetologia. 2010;53:1472–1481. doi: 10.1007/s00125-010-1729-5. [DOI] [PubMed] [Google Scholar]
- Fukada SY, Tirapelli CR, de Godoy MA, de Oliveira AM. Mechanisms underlying the endothelium-independent relaxation induced by angiotensin II in rat aorta. J Cardiovasc Pharmacol. 2005;45:136–143. doi: 10.1097/01.fjc.0000151929.34896.c3. [DOI] [PubMed] [Google Scholar]
- Gaidhu MP, Fediuc S, Anthony NM, So M, Mirpourian M, Perry RL, et al. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res. 2009;50:704–715. doi: 10.1194/jlr.M800480-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidhu MP, Perry RL, Noor F, Ceddia RB. Disruption of AMPKalpha1 signaling prevents AICAR-induced inhibition of AS160/TBC1D4 phosphorylation and glucose uptake in primary rat adipocytes. Mol Endocrinol. 2010;24:1434–1440. doi: 10.1210/me.2009-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Galic S, Fullerton MD, Schertzer JD, Sikkema S, Marcinko K, Walkley CR, et al. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011;121:4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26:1297–1302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- Galvez-Prieto B, Bolbrinker J, Stucchi P, Heras AI, Merino B, Arribas S, et al. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol. 2008;197:55–64. doi: 10.1677/JOE-07-0284. [DOI] [PubMed] [Google Scholar]
- Galvez-Prieto B, Somoza B, Gil-Ortega M, Garcia-Prieto CF, Heras AI, Gonzalez MC, et al. Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Front Pharmacol. 2012;3:103. doi: 10.3389/fphar.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zeng ZH, Teoh K, Sharma AM, Abouzahr L, Cybulsky I, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg. 2005;130:1130–1136. doi: 10.1016/j.jtcvs.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, et al. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, et al. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem. 2008;283:16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier MS, O'Brien EL, Bigornia S, Mott M, Cacicedo JM, Xu XJ, et al. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun. 2011;404:382–387. doi: 10.1016/j.bbrc.2010.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- Goirand F, Solar M, Athea Y, Viollet B, Mateo P, Fortin D, et al. Activation of AMP kinase alpha1 subunit induces aortic vasorelaxation in mice. J Physiol. 2007;581(Pt 3):1163–1171. doi: 10.1113/jphysiol.2007.132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Hernandez A, Otero YF, De las Heras N, Escribano O, Cachofeiro V, Lahera V, et al. Brown fat lipoatrophy and increased visceral adiposity through a concerted adipocytokines overexpression induces vascular insulin resistance and dysfunction. Endocrinology. 2012;153:1242–1255. doi: 10.1210/en.2011-1765. [DOI] [PubMed] [Google Scholar]
- Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, et al. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossl M, Herrmann J, Tang H, Versari D, Galili O, Mannheim D, et al. Prevention of vasa vasorum neovascularization attenuates early neointima formation in experimental hypercholesterolemia. Basic Res Cardiol. 2009;104:695–706. doi: 10.1007/s00395-009-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P, Xu A. Interplay between adipose tissue and blood vessels in obesity and vascular dysfunction. Rev Endocr Metab Disord. 2013;14:49–58. doi: 10.1007/s11154-012-9230-8. [DOI] [PubMed] [Google Scholar]
- Guh JH, Chang WL, Yang J, Lee SL, Wei S, Wang D, et al. Development of novel adenosine monophosphate-activated protein kinase activators. J Med Chem. 2010;53:2552–2561. doi: 10.1021/jm901773d. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol. 2007;58:591–610. [PubMed] [Google Scholar]
- Hannan RE, Davis EA, Widdop RE. Functional role of angiotensin II AT2 receptor in modulation of AT1 receptor-mediated contraction in rat uterine artery: involvement of bradykinin and nitric oxide. Br J Pharmacol. 2003;140:987–995. doi: 10.1038/sj.bjp.0705484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JB, Kristiansen K. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem J. 2006;398:153–168. doi: 10.1042/BJ20060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Adenosine monophosphate-activated protein kinase: a central regulator of metabolism with roles in diabetes, cancer, and viral infection. Cold Spring Harb Symp Quant Biol. 2011;76:155–164. doi: 10.1101/sqb.2011.76.010819. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Chow CK. Lipid peroxidation and endothelial cell injury: implications in atherosclerosis. Free Radic Biol Med. 1988;4:99–106. doi: 10.1016/0891-5849(88)90070-6. [DOI] [PubMed] [Google Scholar]
- Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- Henry P, Thomas F, Benetos A, Guize L. Impaired fasting glucose, blood pressure and cardiovascular disease mortality. Hypertension. 2002;40:458–463. doi: 10.1161/01.hyp.0000032853.95690.26. [DOI] [PubMed] [Google Scholar]
- Hermenegildo C, Oviedo PJ, Garcia-Perez MA, Tarin JJ, Cano A. Effects of phytoestrogens genistein and daidzein on prostacyclin production by human endothelial cells. J Pharmacol Exp Ther. 2005;315:722–728. doi: 10.1124/jpet.105.090456. [DOI] [PubMed] [Google Scholar]
- Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, et al. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- Huang F, Xiong X, Wang H, You S, Zeng H. Leptin-induced vascular smooth muscle cell proliferation via regulating cell cycle, activating ERK1/2 and NF-kappaB. Acta Biochim Biophys Sin (Shanghai) 2010;42:325–331. doi: 10.1093/abbs/gmq025. [DOI] [PubMed] [Google Scholar]
- Ibe JC, Zhou Q, Chen T, Tang H, Yuan JX, Raj JU, et al. AMPK is required for pulmonary artery smooth muscle cell survival and the development of hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2013;49:609–618. doi: 10.1165/rcmb.2012-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, et al. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165:622–632. doi: 10.1111/j.1476-5381.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japp AG, Cruden NL, Amer DA, Li VK, Goudie EB, Johnston NR, et al. Vascular effects of apelin in vivo in man. J Am Coll Cardiol. 2008;52:908–913. doi: 10.1016/j.jacc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–E964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, et al. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J. 2010;74:1479–1487. doi: 10.1253/circj.cj-09-0661. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park KG, Yoo EK, Kim YH, Kim YN, Kim HS, et al. Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Am J Physiol Endocrinol Metab. 2007;9:301–307. doi: 10.1089/ars.2006.1456. [DOI] [PubMed] [Google Scholar]
- Kim SA, Choi HC. Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochem Biophys Res Commun. 2012;425:866–872. doi: 10.1016/j.bbrc.2012.07.165. [DOI] [PubMed] [Google Scholar]
- Ko HJ, Zhang Z, Jung DY, Jun JY, Ma Z, Jones KE, et al. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58:2536–2546. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh HJ, Hirshman MF, He H, Li Y, Manabe Y, Balschi JA, et al. Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem J. 2007;403:473–481. doi: 10.1042/BJ20061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlstedt K, Trouvain C, Boettger T, Shi L, Fisslthaler B, Fleming I. AMP-activated protein kinase regulates endothelial cell angiotensin-converting enzyme expression via p53 and the post-transcriptional regulation of microRNA-143/145. Circ Res. 2013;112:1150–1158. doi: 10.1161/CIRCRESAHA.113.301282. [DOI] [PubMed] [Google Scholar]
- Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, et al. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- Kwon HM, Sangiorgi G, Ritman EL, Lerman A, McKenna C, Virmani R, et al. Adventitial vasa vasorum in balloon-injured coronary arteries: visualization and quantitation by a microscopic three-dimensional computed tomography technique. J Am Coll Cardiol. 1998;32:2079. doi: 10.1016/s0735-1097(98)00482-3. [DOI] [PubMed] [Google Scholar]
- Lamers D, Schlich R, Greulich S, Sasson S, Sell H, Eckel J. Oleic acid and adipokines synergize in inducing proliferation and inflammatory signalling in human vascular smooth muscle cells. J Cell Mol Med. 2011;15:1177–1188. doi: 10.1111/j.1582-4934.2010.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs U, Gertz K, Huang P, Nickenig G, Bohm M, Dirnagl U, et al. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31:2442–2449. doi: 10.1161/01.str.31.10.2442. [DOI] [PubMed] [Google Scholar]
- Laufs U, Gertz K, Dirnagl U, Bohm M, Nickenig G, Endres M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res. 2002;942:23–30. doi: 10.1016/s0006-8993(02)02649-5. [DOI] [PubMed] [Google Scholar]
- LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, et al. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291:E175–E181. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009a;27:782–790. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- Lee RM, Lu C, Su LY, Werstuck G, Gao YJ. Effects of hyperglycemia on the modulation of vascular function by perivascular adipose tissue. J Hypertens. 2009b;27:118–131. doi: 10.1097/HJH.0b013e3283163cc9. [DOI] [PubMed] [Google Scholar]
- Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, et al. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011;124:1160–1171. doi: 10.1161/CIRCULATIONAHA.111.027375. [DOI] [PubMed] [Google Scholar]
- Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210:656–661. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Wang D, Wang Y, Ling W, Feng X, Xia M. Adenosine monophosphate-activated protein kinase induces cholesterol efflux from macrophage-derived foam cells and alleviates atherosclerosis in apolipoprotein E-deficient mice. J Biol Chem. 2010a;285:33499–33509. doi: 10.1074/jbc.M110.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Zhang Y, Ma J, Ling W, Xia M. Adenosine monophosphate activated protein kinase regulates ABCG1-mediated oxysterol efflux from endothelial cells and protects against hypercholesterolemia-induced endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2010b;30:1354–1362. doi: 10.1161/ATVBAHA.110.204230. [DOI] [PubMed] [Google Scholar]
- Li FY, Lam KS, Tse HF, Chen C, Wang Y, Vanhoutte PM, et al. Endothelium-selective activation of AMP-activated protein kinase prevents diabetes mellitus-induced impairment in vascular function and re-endothelialization via induction of heme oxygenase-1 in mice. Circulation. 2012;126:1267–1277. doi: 10.1161/CIRCULATIONAHA.112.108159. [DOI] [PubMed] [Google Scholar]
- Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihn AS, Jessen N, Pedersen SB, Lund S, Richelsen B. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem Biophys Res Commun. 2004;316:853–858. doi: 10.1016/j.bbrc.2004.02.139. [DOI] [PubMed] [Google Scholar]
- Lihn AS, Pedersen SB, Lund S, Richelsen B. The anti-diabetic AMPK activator AICAR reduces IL-6 and IL-8 in human adipose tissue and skeletal muscle cells. Mol Cell Endocrinol. 2008;292:36–41. doi: 10.1016/j.mce.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wan Q, Guan Q, Gao L, Zhao J. High-fat diet feeding impairs both the expression and activity of AMPKa in rats' skeletal muscle. Biochem Biophys Res Commun. 2006;339:701–707. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- Lu C, Su LY, Lee RM, Gao YJ. Mechanisms for perivascular adipose tissue-mediated potentiation of vascular contraction to perivascular neuronal stimulation: the role of adipocyte-derived angiotensin II. Eur J Pharmacol. 2010;634:107–112. doi: 10.1016/j.ejphar.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Lu C, Su LY, Lee RM, Gao YJ. Alterations in perivascular adipose tissue structure and function in hypertension. Eur J Pharmacol. 2011;656:68–73. doi: 10.1016/j.ejphar.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Luo S, Lei H, Liu Q. Correlation between serum adiponectin and risk factors in patients with coronary artery disease. Clin Lab. 2013;59:121–126. doi: 10.7754/clin.lab.2012.120131. [DOI] [PubMed] [Google Scholar]
- Ma GE, Liu P, Lei H, Chen J, Liu ZJ. [Effect of liposuction on adipokines, inflammatory markers and insulin resistance] Zhonghua Zheng Xing Wai Ke Za. 2010a;26:26–28. [PubMed] [Google Scholar]
- Ma L, Ma S, He H, Yang D, Chen X, Luo Z, et al. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res. 2010b;33:446–453. doi: 10.1038/hr.2010.11. [DOI] [PubMed] [Google Scholar]
- Madan M, Bishayi B, Hoge M, Amar S. Atheroprotective role of interleukin-6 in diet- and/or pathogen-associated atherosclerosis using an ApoE heterozygote murine model. Atherosclerosis. 2008;197:504–514. doi: 10.1016/j.atherosclerosis.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majithiya JB, Balaraman R. Metformin reduces blood pressure and restores endothelial function in aorta of streptozotocin-induced diabetic rats. Life Sci. 2006;78:2615–2624. doi: 10.1016/j.lfs.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Mangano DT. Effects of acadesine on myocardial infarction, stroke, and death following surgery. A meta-analysis of the 5 international randomized trials. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. JAMA. 1997;277:325–332. doi: 10.1001/jama.277.4.325. [DOI] [PubMed] [Google Scholar]
- Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–1292. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx N, Duez H, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ Res. 2004;94:1168–1178. doi: 10.1161/01.RES.0000127122.22685.0A. [DOI] [PubMed] [Google Scholar]
- Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013;216:T17–T36. doi: 10.1530/JOE-12-0232. [DOI] [PubMed] [Google Scholar]
- Medina-Ramon M, Schwartz J. Temperature, temperature extremes, and mortality: a study of acclimatisation and effect modification in 50 US cities. J Occup Environ Med. 2007;64:827–833. doi: 10.1136/oem.2007.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer RI, Serne EH, Smulders YM, van Hinsbergh VW, Yudkin JS, Eringa EC. Perivascular adipose tissue and its role in type 2 diabetes and cardiovascular disease. Curr Diab Rep. 2011;11:211–217. doi: 10.1007/s11892-011-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer RI, Bakker W, Alta CL, Sipkema P, Yudkin JS, Viollet B, et al. Perivascular adipose tissue control of insulin-induced vasoreactivity in muscle is impaired in db/db mice. Diabetes. 2013;62:590–598. doi: 10.2337/db11-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273(6 Pt 1):E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Miao CY, Li ZY. The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol. 2012;165:643–658. doi: 10.1111/j.1476-5381.2011.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- Musi N, Goodyear LJ. Targeting the AMP-activated protein kinase for the treatment of type 2 diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2002;2:119–127. [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- Nagata D, Hirata Y. The role of AMP-activated protein kinase in the cardiovascular system. Hypertens Res. 2010;33:22–28. doi: 10.1038/hr.2009.187. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Higashi Y, Sasaki S, Oshima T, Matsuura H, Chayama K. Leptin causes vasodilation in humans. Hypertens Res. 2002;25:161–165. doi: 10.1291/hypres.25.161. [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- O'Toole TE, Conklin DJ, Bhatnagar A. Environmental risk factors for heart disease. Rev Environ Health. 2008;23:167–202. doi: 10.1515/reveh.2008.23.3.167. [DOI] [PubMed] [Google Scholar]
- Oda A, Taniguchi T, Yokoyama M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J Med Sci. 2001;47:141–150. [PubMed] [Google Scholar]
- Ohman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, et al. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2011;219:33–39. doi: 10.1016/j.atherosclerosis.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, et al. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ. 2008;15:1572–1581. doi: 10.1038/cdd.2008.84. [DOI] [PubMed] [Google Scholar]
- Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, et al. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalitakul S, Okada M, Hara Y, Yamawaki H. A novel adipocytokine, vaspin inhibits platelet-derived growth factor-BB-induced migration of vascular smooth muscle cells. Biochem Biophys Res Commun. 2012;423:844–849. doi: 10.1016/j.bbrc.2012.06.052. [DOI] [PubMed] [Google Scholar]
- Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem. 2004;279:20767–20774. doi: 10.1074/jbc.M401390200. [DOI] [PubMed] [Google Scholar]
- Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, et al. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes. 2005;54:928–934. doi: 10.2337/diabetes.54.4.928. [DOI] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, et al. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Polikandriotis JA, Mazzella LJ, Rupnow HL, Hart CM. Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor gamma-dependent mechanisms. Arterioscler Thromb Vasc Biol. 2005;25:1810–1816. doi: 10.1161/01.ATV.0000177805.65864.d4. [DOI] [PubMed] [Google Scholar]
- Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10:191–196. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanjaneya M, Chen J, Brown JE, Tripathi G, Hallschmid M, Patel S, et al. Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology. 2010;151:3169–3180. doi: 10.1210/en.2009-1358. [DOI] [PubMed] [Google Scholar]
- Rebolledo A, Rebolledo OR, Marra CA, Garcia ME, Roldan Palomo AR, Rimorini L, et al. Early alterations in vascular contractility associated to changes in fatty acid composition and oxidative stress markers in perivascular adipose tissue. Cardiovasc Diabetol. 2010;9:65. doi: 10.1186/1475-2840-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riek U, Scholz R, Konarev P, Rufer A, Suter M, Nazabal A, et al. Structural properties of AMP-activated protein kinase: dimerization, molecular shape, and changes upon ligand binding. J Biol Chem. 2008;283:18331–18343. doi: 10.1074/jbc.M708379200. [DOI] [PubMed] [Google Scholar]
- Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, Meile T, et al. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia. 2012;55:1514–1525. doi: 10.1007/s00125-012-2481-9. [DOI] [PubMed] [Google Scholar]
- Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Rocha VZ, Libby P. Obesity. Inflammation. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Fortuno A, Gomez-Ambrosi J, Zalba G, Diez J, Fruhbeck G. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology. 2007;148:324–331. doi: 10.1210/en.2006-0940. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94:3611–3615. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin AS, Bariskaner H. The mechanisms of vasorelaxant effect of leptin on isolated rabbit aorta. Fundam Clin Pharmacol. 2007;21:595–600. doi: 10.1111/j.1472-8206.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- Salabei JK, Cummins TD, Singh M, Jones SP, Bhatnagar A, Hill BG. PDGF-mediated autophagy regulates vascular smooth muscle cell phenotype and resistance to oxidative stress. Biochem J. 2013;451:375–388. doi: 10.1042/BJ20121344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo A, Garijo J, Monge L, Fernandez N, Luis Garcia-Villalon A, Sanchez Turrion V, et al. Apelin effects in human splanchnic arteries. Role of nitric oxide and prostanoids. Regul Pept. 2007a;144:50–55. doi: 10.1016/j.regpep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Salcedo A, Garijo J, Monge L, Sanchez A, Fernandez N, Garcia-Villalon AL, et al. Endothelium-dependent relaxation of isolated splanchnic arteries from cirrhotic patients: role of reactive oxygen species. Hepatol Res. 2007b;37:811–819. doi: 10.1111/j.1872-034X.2007.00135.x. [DOI] [PubMed] [Google Scholar]
- Salisbury E, Hipp J, Olmsted-Davis EA, Davis AR, Heggeness MH, Gannon FH. Histologic identification of brown adipose and peripheral nerve involvement in human atherosclerotic vessels. Hum Pathol. 2012;43:2213–2222. doi: 10.1016/j.humpath.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med. 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt IP, Connell JM, Gould GW. 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes. Diabetes. 2000;49:1649–1656. doi: 10.2337/diabetes.49.10.1649. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem. 2007;282:32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, et al. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28:1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, van Denderen BJ, Jorgensen SB, Honeyman JE, Steinberg GR, Oakhill JS, et al. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol. 2008;15:1220–1230. doi: 10.1016/j.chembiol.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, et al. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996;93:2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Nguyen TB, Totary-Jain H, Dansky H, Marx SO, Marks AR. Leptin-enhanced neointimal hyperplasia is reduced by mTOR and PI3K inhibitors. Proc Natl Acad Sci U S A. 2008;105:19006–19011. doi: 10.1073/pnas.0809743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83:273–280. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu KG, Lien LM, Wang BW, Kuan P, Chang H. Resistin contributes to neointimal formation via oxidative stress after vascular injury. Clin Sci (Lond) 2011;120:121–129. doi: 10.1042/CS20100226. [DOI] [PubMed] [Google Scholar]
- Silaghi A, Achard V, Paulmyer-Lacroix O, Scridon T, Tassistro V, Duncea I, et al. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. Am J Physiol Endocrinol Metab. 2007;293:E1443–E1450. doi: 10.1152/ajpendo.00273.2007. [DOI] [PubMed] [Google Scholar]
- Skilton MR, Serusclat A, Sethu AH, Brun S, Bernard S, Balkau B, et al. Noninvasive measurement of carotid extra-media thickness: associations with cardiovascular risk factors and intima-media thickness. JACC. Cardiovasc Imaging. 2009;2:176–182. doi: 10.1016/j.jcmg.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Soares de Moura R, Resende AC, Emiliano AF, Tano T, Mendes-Ribeiro AC, Correia ML, et al. The role of bradykinin, AT2 and angiotensin 1-7 receptors in the EDRF-dependent vasodilator effect of angiotensin II on the isolated mesenteric vascular bed of the rat. Br J Pharmacol. 2004;141:860–866. doi: 10.1038/sj.bjp.0705669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- Song P, Wang S, He C, Liang B, Viollet B, Zou MH. AMPKalpha2 deletion exacerbates neointima formation by upregulating Skp2 in vascular smooth muscle cells. Circ Res. 2011;109:1230–1239. doi: 10.1161/CIRCRESAHA.111.250423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Stastny J, Bienertova-Vasku J, Vasku A. Visfatin and its role in obesity development. Diabetes Metab Syndr. 2012;6:120–124. doi: 10.1016/j.dsx.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Stocker DJ, Taylor AJ, Langley RW, Jezior MR, Vigersky RA. A randomized trial of the effects of rosiglitazone and metformin on inflammation and subclinical atherosclerosis in patients with type 2 diabetes. Am Heart J. 2007;153:445. doi: 10.1016/j.ahj.2006.11.005. e441–446. [DOI] [PubMed] [Google Scholar]
- Stone JD, Narine A, Shaver PR, Fox JC, Vuncannon JR, Tulis D. AMP-activated protein kinase inhibits vascular smooth muscle cell proliferation and migration and vascular remodeling following injury. Am J Physiol Heart Circ Physiol. 2012;304:H369–381. doi: 10.1152/ajpheart.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Lee TS, Zhu M, Gu C, Wang Y, Zhu Y, et al. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114:2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (Lond) 2012;122:1–12. doi: 10.1042/CS20110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag. 2013;9:105–116. doi: 10.2147/VHRM.S33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, et al. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30:1576–1582. doi: 10.1161/ATVBAHA.110.207175. [DOI] [PubMed] [Google Scholar]
- Takeda-Watanabe A, Kitada M, Kanasaki K, Koya D. SIRT1 inactivation induces inflammation through the dysregulation of autophagy in human THP-1 cells. Biochem Biophys Res Commun. 2012;427:191–196. doi: 10.1016/j.bbrc.2012.09.042. [DOI] [PubMed] [Google Scholar]
- Takemori K, Gao YJ, Ding L, Lu C, Su LY, An WS, et al. Elevated blood pressure in transgenic lipoatrophic mice and altered vascular function. Hypertension. 2007;49:365–372. doi: 10.1161/01.HYP.0000255576.16089.b9. [DOI] [PubMed] [Google Scholar]
- Tan BK, Adya R, Randeva HS. Omentin: a novel link between inflammation, diabesity, and cardiovascular disease. Trends Cardiovasc Med. 2010;20:143–148. doi: 10.1016/j.tcm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Thalmann S, Meier CA. Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res. 2007;75:690–701. doi: 10.1016/j.cardiores.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Fukada SY, de Godoy MA, de Oliveira AM. Analysis of the mechanisms underlying the vasorelaxant action of angiotensin II in the isolated rat carotid. Life Sci. 2006;78:2676–2682. doi: 10.1016/j.lfs.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184:285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- Tsoyi K, Jang HJ, Nizamutdinova IT, Kim YM, Lee YS, Kim HJ, et al. Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice. Br J Pharmacol. 2011;162:1498–1508. doi: 10.1111/j.1476-5381.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turczynska KM, Bhattachariya A, Sall J, Goransson O, Sward K, Hellstrand P, et al. Stretch-sensitive down-regulation of the miR-144/451 cluster in vascular smooth muscle and its role in AMP-activated protein kinase signaling. PLoS ONE. 2013;8:e65135. doi: 10.1371/journal.pone.0065135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura Y, Shibata R, Ohashi K, Enomoto T, Kambara T, Yamamoto T, et al. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2013;27:25–33. doi: 10.1096/fj.12-213744. [DOI] [PubMed] [Google Scholar]
- Vigetti D, Clerici M, Deleonibus S, Karousou E, Viola M, Moretto P, et al. Hyaluronan synthesis is inhibited by adenosine monophosphate-activated protein kinase through the regulation of HAS2 activity in human aortic smooth muscle cells. J Biol Chem. 2011;286:7917–7924. doi: 10.1074/jbc.M110.193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena JA, Viollet B, Andreelli F, Kahn A, Vaulont S, Sul HS. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes. 2004;53:2242–2249. doi: 10.2337/diabetes.53.9.2242. [DOI] [PubMed] [Google Scholar]
- Viollet B, Guigas B, Leclerc J, Hebrard S, Lantier L, Mounier R, et al. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol. 2009;196:81–98. doi: 10.1111/j.1748-1716.2009.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res. 2009a;81:370–380. doi: 10.1093/cvr/cvn288. [DOI] [PubMed] [Google Scholar]
- Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009b;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, et al. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Bo QY, Zhao XH, Yang X, Chi ZF, Liu XW. Resveratrol pre-treatment reduces early inflammatory responses induced by status epilepticus via mTOR signaling. Brain Res. 2013;1492:122–129. doi: 10.1016/j.brainres.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, et al. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- Wartmann M, Campbell D, Subramanian A, Burstein SH, Davis RJ. The MAP kinase signal transduction pathway is activated by the endogenous cannabinoid anandamide. FEBS Lett. 1995;359:133–136. doi: 10.1016/0014-5793(95)00027-7. [DOI] [PubMed] [Google Scholar]
- Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, et al. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem. 2012;287:10301–10315. doi: 10.1074/jbc.M111.303651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston AH, Egner I, Porter EL, Dong Y, Heagerty AM, Edwards G. Stimulated release of a hyperpolarising factor (ADHF) from rat mesenteric artery perivascular adipose tissue; involvement of myocyte BK channels. Br J Pharmacol. 2013;169:1500–1509. doi: 10.1111/bph.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox JN, Okamoto EI, Nakahara KI, Vinten-Johansen J. Perivascular responses after angioplasty which may contribute to postangioplasty restenosis: a role for circulating myofibroblast precursors? Ann N Y Acad Sci. 2001;947:68–90. doi: 10.1111/j.1749-6632.2001.tb03931.x. dicussion 90–62. [DOI] [PubMed] [Google Scholar]
- Wojcicka G, Jamroz-Wisniewska A, Atanasova P, Chaldakov GN, Chylinska-Kula B, Beltowski J. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacol Res. 2011;63:68–76. doi: 10.1016/j.phrs.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- Wong KA, Lodish HF. A revised model for AMP-activated protein kinase structure: the alpha-subunit binds to both the beta- and gamma-subunits although there is no direct binding between the beta- and gamma-subunits. J Biol Chem. 2006;281:36434–36442. doi: 10.1074/jbc.M607410200. [DOI] [PubMed] [Google Scholar]
- Wood IS, Trayhurn P. Adipokines and the signaling role of adipose tissue in inflammation and obesity. Future Lipidol. 2006;1:81–89. [Google Scholar]
- Woods A, Salt I, Scott J, Hardie DG, Carling D. The alpha1 and alpha2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996;397:347–351. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]
- Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- Xenos ES, Stevens SL, Freeman MB, Cassada DC, Goldman MH. Nitric oxide mediates the effect of fluvastatin on intercellular adhesion molecule-1 and platelet endothelial cell adhesion molecule-1 expression on human endothelial cells. Ann Vasc Surg. 2005;19:386–392. doi: 10.1007/s10016-005-0011-7. [DOI] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117:952–962. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Hara N, Okada M, Hara Y. Visfatin causes endothelium-dependent relaxation in isolated blood vessels. Biochem Biophys Res Commun. 2009;383:503–508. doi: 10.1016/j.bbrc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–343. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Kameshima S, Usui T, Okada M, Hara Y. A novel adipocytokine, chemerin exerts anti-inflammatory roles in human vascular endothelial cells. Biochem Biophys Res Commun. 2012a;423:152–157. doi: 10.1016/j.bbrc.2012.05.103. [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Takahashi M, Mukohda M, Morita T, Okada M, Hara Y. A novel adipocytokine, nesfatin-1 modulates peripheral arterial contractility and blood pressure in rats. Biochem Biophys Res Commun. 2012b;418:676–681. doi: 10.1016/j.bbrc.2012.01.076. [DOI] [PubMed] [Google Scholar]
- Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physio. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn BS, Kloting N, Kratzsch J, Lee N, Park JW, Song ES, et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes. 2008;57:372–377. doi: 10.2337/db07-1045. [DOI] [PubMed] [Google Scholar]
- Young LH, Li J, Baron SJ, Russell RR. AMP-activated protein kinase: a key stress signaling pathway in the heart. Trends Cardiovasc Med. 2005;15:110–118. doi: 10.1016/j.tcm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Eringa E, Stehouwer CD. ‘Vasocrine’ signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, et al. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279:47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- Zhang CX, Pan SN, Meng RS, Peng CQ, Xiong ZJ, Chen BL, et al. Metformin attenuates ventricular hypertrophy by activating the AMP-activated protein kinase-endothelial nitric oxide synthase pathway in rats. Clin Exp Pharmacol Physiol. 2011;38:55–62. doi: 10.1111/j.1440-1681.2010.05461.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang C. Regulation of microvascular function by adipose tissue in obesity and type 2 diabetes: evidence of an adipose-vascular loop. Am J Biomed Sci. 2009b;1:133. doi: 10.5099/aj090200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009a;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, He J, Xu C, Zu L, Jiang H, Pu S, et al. Mechanisms of metformin inhibiting lipolytic response to isoproterenol in primary rat adipocytes. J Mol Endocrinol. 2009c;42:57–66. doi: 10.1677/JME-08-0130. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, et al. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, et al. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L497–L504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A. Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci (Lond) 2008;114:361–374. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WGT, Schlattner U, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]