Abstract
Background and Purpose
Paclitaxel (PAC) is associated with chemotherapy-induced neuropathic pain (CIPN) that can lead to the cessation of treatment in cancer patients even in the absence of alternate therapies. We previously reported that chronic administration of the non-psychoactive cannabinoid cannabidiol (CBD) prevents PAC-induced mechanical and thermal sensitivity in mice. Hence, we sought to determine receptor mechanisms by which CBD inhibits CIPN and whether CBD negatively effects nervous system function or chemotherapy efficacy.
Experimental Approach
The ability of acute CBD pretreatment to prevent PAC-induced mechanical sensitivity was assessed, as was the effect of CBD on place conditioning and on an operant-conditioned learning and memory task. The potential interaction of CBD and PAC on breast cancer cell viability was determined using the MTT assay.
Key Results
PAC-induced mechanical sensitivity was prevented by administration of CBD (2.5 – 10 mg·kg−1) in female C57Bl/6 mice. This effect was reversed by co-administration of the 5-HT1A antagonist WAY 100635, but not the CB1 antagonist SR141716 or the CB2 antagonist SR144528. CBD produced no conditioned rewarding effects and did not affect conditioned learning and memory. Also, CBD + PAC combinations produce additive to synergistic inhibition of breast cancer cell viability.
Conclusions and Implications
Our data suggest that CBD is protective against PAC-induced neurotoxicity mediated in part by the 5-HT1A receptor system. Furthermore, CBD treatment was devoid of conditioned rewarding effects or cognitive impairment and did not attenuate PAC-induced inhibition of breast cancer cell viability. Hence, adjunct treatment with CBD during PAC chemotherapy may be safe and effective in the prevention or attenuation of CIPN.
Keywords: cannabidiol, paclitaxel, chemotherapy-induced neuropathic pain, CIPN, 5-HT1A, breast cancer, cannabinoid, mechanical sensitivity
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a serious dose-limiting side effect associated with several commonly used chemotherapeutic agents, including taxanes, platinum agents and vinca alkaloids. CIPN occurs in 30–40% of patients but incidences can approach 75% with certain regimens. Common peripheral sensory symptoms include paresthesias and dysesthesias, pain, numbness and tingling, and sensitivity to touch and temperature. Motor symptoms include weakness and gait and balance disturbances (Visovsky et al., 2007). In most cases, CIPN is only partially reversible with cessation of treatment and in the worst cases damage can be permanent. To date, no one drug or drug class is considered to be safe and effective for treatment of CIPN (Lynch et al., 2004), making the identification of alternative effective analgesics a crucial medical need.
The exact mechanism of CIPN has not been fully elucidated and can differ across classes of chemotherapeutic agents. In general, these agents can affect cellular microtubules, disrupt mitochondrial function or impair DNA synthesis. Such assaults on peripheral nerves can lead to sensitization and spontaneous activity of these fibres (Xiao and Bennett, 2008), alteration of voltage-gated sodium and transient receptor potential vanilloid (TRPV) channel activity and expression (Adelsberger et al., 2000; Gauchan et al., 2009), dorsal column ascending fibre pathology (Cavaletti et al., 1995), and infiltration of activated microglia and release of pro-inflammatory cytokines (Hu and McLachlan, 2002), ultimately leading to ascending pain pathway sensitization (Peters et al., 2007). Functional changes to the descending inhibitory pain pathway can also result, altering noradrenaline and 5-HT signalling and further amplifying the effects of central sensitization (Baron et al., 2010).
Cannabinoids suppress neuropathic pain induced by traumatic nerve injury, toxic insults and metabolic changes (for review, see Guindon and Hohmann, 2008). The mixed CB1/CB2 agonist WIN55,212-2 suppresses neuropathic nociception induced by the chemotherapeutic agent paclitaxel (PAC) through a CB1-specific mechanism (Pascual et al., 2005). WIN55,212-2 also suppresses vincristine-induced neuropathy through activation of both CB1 and CB2 receptors (Rahn et al., 2007). Activation of CB2 receptors partially attenuates vincristine-induced neuropathy (Rahn et al., 2007) and fully attenuates PAC-induced neuropathy (Rahn et al., 2008; Deng et al., 2012) in rats. In humans, several studies have demonstrated anti-neuropathic effects of whole cannabis, Δ9-tetrahydrocannabinol (THC), or its synthetic analogues nabilone or dronabinol (Pinsger et al., 2006; Skrabek et al., 2008; Ware et al., 2010). However, several reports describe these effects as modest, while others have reported negative results (Wade et al., 2004; Johnson et al., 2010). Importantly, patients in the vast majority of studies also report several adverse events such as dizziness, dryness, sedation, disorientation and decreased concentration, and while these were not categorized as serious they probably limit the tolerability and compliance with such treatments.
One of the more successful cannabis-based pharmaceuticals for the treatment of pain is the buccal spray Sativex [1:1 formulation of THC and the phytocannabinoid cannabidiol (CBD)], approved in the EU and Canada for treatment of multiple sclerosis spasticity, with an additional license in Canada for use in multiple sclerosis-associated neuropathic pain and cancer pain. Sativex has recently entered directly into US late-stage trials because of its promising therapeutic uses, and has shown pain-relieving effects in two recent clinical trials: one for cancer pain (Johnson et al., 2010) and one for neuropathic pain associated with multiple sclerosis (Langford et al., 2013). However, the psychoactive side effects of Sativex mediated by THC may limit its broader utility in the clinic. For example, THC and Sativex have been determined to produce similar subjective and physiological effects (Johnson et al., 2010; Karschner et al., 2011). However, mounting preclinical evidence now demonstrates that CBD alone has anti-neuropathic effects (Costa et al., 2007; Toth et al., 2010; Xiong et al., 2012; see Fernández-Ruiz et al., 2013 for review). To date, no clinical trials have yet commenced to study the efficacy of the non-psychoactive CBD as a monotherapy for the treatment of neuropathic pain. We have recently reported that 14 days of administration of CBD prevents the onset of PAC-induced mechanical and thermal sensitivity in a female mouse model of CIPN (Ward et al., 2011).
In the present set of experiments, we aimed to determine whether sub-chronic dosing regimen of CBD would prevent PAC-induced mechanical sensitivity while also determining whether this effect is mediated by activation of 5-HT1A receptors. CBD binds to the 5-HT1A receptor as an agonist with micromolar affinity (Russo et al., 2005), and research has demonstrated potent anti-neuropathic effects with 5-HT1A agonists (e.g. Colpaert, 2006). Indeed, intra-periaqueductal grey injection of CBD produces dose-dependent antinociception that is blocked by co-administration of the 5-HT1A antagonist WAY100635 (Maione et al., 2011). Lastly, we also sought to determine whether treatment with CBD would have any effects on conditioned reward, learning and memory, and the inhibitory activity of PAC on breast cancer cell viability.
Methods
Animals. Female C57Bl/6 mice weighing 16–20 g (Taconic Farms, Cranbury, NJ, USA; Jackson Labs, Chicago, IL, USA) were acclimatized to the temperature- and humidity-controlled vivarium and housed in groups of four for at least 5 days before initiation of behavioural studies. Artificial lighting provided a reverse 12 h light/dark cycle (lights off 10:00 h). The animals had free access to dietary food and water except where noted. The total number of animals used was 240 and the procedures used were as humane as possible and complied with the guidelines of the Temple University Institutional Animal Care and Use Committee. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Drugs. PAC solution [Teva Parenteral Medicines: dissolved in 1:1 mixture of alcohol and cremophor (CRM)] was obtained from Temple University Hospital Cancer Center (Philadelphia, PA, USA). For cell viability studies in breast cancer cell lines, PAC was obtained from Sigma (St. Louis, MO, USA). CBD, morphine sulfate, and the CB1 (SR141716A) and CB2 receptor (SR144528) antagonist were provided by the National Institute on Drug Abuse drug supply program (Bethesda, MD, USA). WAY100635 was purchased from RBI. PAC was diluted in 0.9% saline. CBD was dissolved in a 1:1 mixture of ethanol and CRM (Sigma-Aldrich, St. Louis, MO, USA) and diluted with saline to a final ratio of 1:1:18 (ethanol : CRM : saline). Morphine and WAY100635 were dissolved in 0.9% saline. All injections were given i.p. in a volume of 10 μL·g−1 of body weight.
Mechanical allodynia
In the first set of experiments, mechanical allodynia was assessed in five groups of mice (n = 8 per group) using von Frey monofilaments of varying forces (0.07–4.0 g) applied to the mid-plantar surface of the right hind paw, with each application held in c-shape for 6 s using the up-down method of Dixon (1980). Mice were placed in individual Plexiglas compartments (Med Associates, St. Albans, VT, USA) on top of a wire grid floor suspended 20 cm above the laboratory bench top and acclimatized to the environment for 15 min before each test session. Baseline sensitivity to the monofilaments was assessed 1 day before the start of drug administration and continued weekly for 10 weeks. On experimental days 1, 3, 5 and 7, mice received the following two i.p. injections, spaced 15 min apart: group 1 – CRM vehicle, CRM vehicle; group 2 – CRM vehicle, 4.0 mg·kg−1 PAC; group 3 – CRM vehicle, 8.0 mg·kg−1 PAC; group 4 – 2.5 mg·kg−1 CBD, 8.0 mg·kg−1 PAC; 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC. Mechanical allodynia was not assessed on injection days. PAC and CBD doses were based on significant findings from Ward et al. (2011).
In the second set of experiments, mechanical allodynia was assessed in an identical manner to that described above. Four groups of mice were treated on experimental days 1, 3, 5 and 7 with three i.p. injections spaced 15 min apart: group 1 – saline, CRM vehicle, CRM vehicle; group 2 – saline, CRM vehicle, 8.0 mg·kg−1 PAC; group 3 – saline, 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC; group 4 – 1.0 mg·kg−1 WAY100635, 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC. Dose of WAY100635 was based on several studies investigating blockade of 5-HT1A agonist-mediated behavioural pharmacological effects (e.g. Hagiwara et al., 2008).
In the third set of experiments, mechanical allodynia was assessed 1 day before the start of drug administration and on day 15 following the first injections. Five groups of mice were treated on experimental days 1, 3, 5 and 7 with three i.p. injections spaced 15 min apart: group 1 – saline, CRM vehicle, CRM vehicle; group 2 – saline, CRM vehicle, 8.0 mg·kg−1 PAC; group 3 – saline, 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC; group 4 – 3.0 mg·kg−1 SR141716, 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC; group 5 – 3.0 mg·kg−1 SR144528, 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC. Doses of SR141716 and SR144528 were based on several studies investigating blockade of CB1 and CB2 agonist-mediated effects respectively (Rahn et al., 2007; 2008,).
Place conditioning
The conditioned rewarding effects of CBD and morphine were assessed using a standard mouse place conditioning procedure and Med Associates mouse three compartment place conditioning chambers (MED-CPP-3013). Mice received vehicle or morphine (2.5–10 mg·kg −1, i.p.; 15 min pretreatment) or vehicle or CBD (2.5–10 mg·kg−1, i.p.; 30 min pretreatment) on alternate days for 30 min conditioning sessions for 6 successive days. Vehicle injections were paired with the black compartment and the drug injections with the white compartment of the conditioned place preference (CPP) apparatus. On day 7, test sessions were conducted where mice in a drug-free state had 30 min free access to all chambers following an initial 5 min acclimation in the central grey compartment. The time spent in the drug- and vehicle-paired compartments was recorded on the test day and the data are presented as time spent in the drug-paired compartment.
Autoshaping
The effect of CBD (2.0–20 mg·kg−1, i.p.) on acquisition and retention of a conditioned learning task was assessed using a modified autoshaping procedure and Med Associates mouse operant conditioning chambers (ENV 307W) as described in Foley et al. (2008). Briefly, mice were weighed and food-restricted for 24 h before the experimental session. On the acquisition day, mice were placed inside a standard mouse experimental chamber, and the availability of a sweet liquid reinforcer (50% vanilla Ensure in tap water; Abbott Laboratories, Columbus, OH, USA) under a variable interval schedule was signalled by a tone. The mouse was reinforced with the vanilla Ensure if it made a nose-poke response into a centre dipper receptacle during an 8 s period following the tone. Each acquisition session lasted for 2 h or until 20 reinforced nose pokes were recorded. For the retention test, mice were placed back into the chambers 24 h following the acquisition session under the same conditions. In the present experiment, mice were pretreated with vehicle or CBD 30 min before the acquisition session.
Cell culture and treatments
The mouse and human breast cancer cell lines used were 4T1 (obtained from ATCC) and MDA-MB231-luc-D3H2LN (obtained from Caliper; Jenkins et al., 2005) cells respectively. Cell lines were maintained at 37°C and 5% CO2. In all experiments, the different cell populations were first cultured in RPMI media containing 10% FBS. Cells were then seeded into 96-well plates in 10% FBS and on the first day of treatment the media was replaced with vehicle control or drug in RPMI and 0.1% FBS as previously reported (McAllister et al., 2005). The media with the appropriate compounds were replaced every 24 h.
MTT assay
Assays were performed as previously described (McAllister et al., 2007). Cell viability (%) was calculated as the MTT absorbance of the treated cells/control cells × 100.
Pharmacological and statistical analyses
IC50 values were calculated using CompuSyn (Paramus, NJ, USA). To test for synergism, the combination index (CI) was also calculated using Compusyn where CI <1, = 1 and >1 indicates synergism, additive effect and antagonism, respectively, as previously described (Chou et al., 1993; Chou, 2006) and as previously published by our group (Marcu et al., 2010). Based on the classic isobologram for mutually exclusive effects relative to the end point of measurement, the CI value for x % inhibition is calculated as: CI = (D)1/(Dx)1 + (D)2/(Dx)2.
(D)1 PAC; (D)2 represents CBD; (Dx)1 and (Dx)2 are the doses for x% growth that can be obtained using the ICF equation described above. (D)1 and (D)2 are the concentrations in the combination which also inhibit cell growth by x % (Chou et al., 1993).
Results
Mechanical allodynia
Treatment with either 4.0 or 8.0 mg·kg−1 PAC on alternating days for a total of four injections produced mechanical sensitivity in female C57Bl/6 mice. Peak sensitivity was achieved by week 2 post-treatment and lasted for the full 10 weeks of the study for the 8.0 mg·kg−1 PAC dose. Co-administration of either 2.5 or 5.0 mg·kg−1 CBD 15 min prior to each PAC injection prevented PAC-induced mechanical sensitivity. Two-way anova revealed significant main effects of treatment [F(4, 310) = 27.71, P < 0.0001] and time [F(9, 310) = 5.001, P < 0.001] and no significant interaction (F <1.0). Bonferroni post-tests revealed a significant increase in sensitivity in both the 4.0 and 8.0 mg·kg−1 PAC groups compared with Veh/Veh. In contrast, the PAC groups pretreated with either 2.5 or 5.0 mg·kg−1 CBD were not significantly different from Veh/Veh in their mechanical sensitivity (Figure 1).
Figure 1.
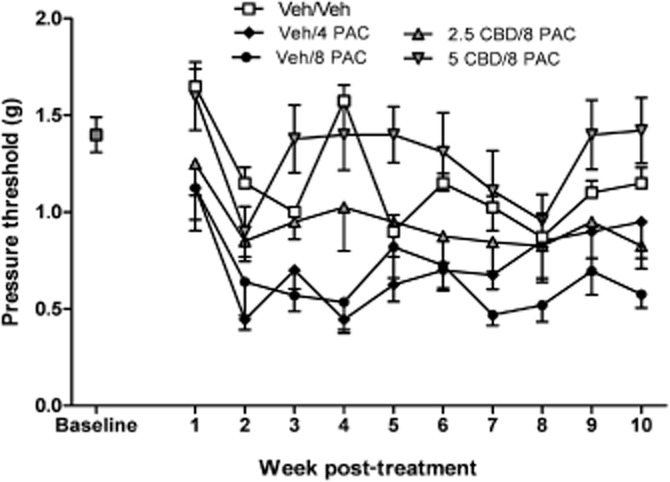
Effect of CBD pretreatment (2.5, 5.0 mg·kg−1, i.p.) on PAC-induced mechanical allodynia in female C57Bl/6 mice. Baseline sensitivity to von Frey filaments was assessed on the day before drug administration and continued weekly for 10 weeks. Mice received the following two i.p. injections spaced 15 min apart on days 1, 3, 5 and 7: CRM vehicle, CRM vehicle; CRM vehicle, 4.0 mg·kg−1 PAC; CRM vehicle, 8.0 mg·kg−1 PAC; 2.5 mg·kg−1 CBD, 8.0 mg·kg−1 PAC; 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC. Two-way anova revealed significant main effects of treatment [F(4, 310) = 27.71, P < 0.0001] and time [F(9, 310) = 5.001, P < 0.001] and no significant interaction (F < 1.0). Bonferroni post-tests revealed a significant increase in sensitivity in both the 4.0 and 8.0 mg·kg−1 PAC groups compared with Veh/Veh. In contrast, the PAC groups pretreated with either 2.5 or 5.0 mg·kg−1 CBD were not significantly different from Veh/Veh in their mechanical sensitivity. X-axis: time points pre- or post-day first injection. Y-axis: threshold pressure to elicit hind paw withdrawal from von Frey filament. Data points represent the mean and SEM, n = 8 per group.
Additional administration of the 5-HT1A antagonist WAY 100635 (1.0 mg·kg−1) before PAC and CBD treatment attenuated the reversal of PAC-induced mechanical sensitivity by CBD. Two-way anova revealed significant effects of treatment [F(3, 280) = 24.66, P < 0.0001] and time [F(9, 280) = 5.058, P < 0.001] and no significant interaction (F <1.0). Bonferroni post-test revealed a significant increase in the sensitivity of the PAC group and the WAY/CBD/PAC groups compared with Veh/Veh/Veh. In contrast, the Veh/CBD/PAC group did not differ significantly from the Veh/Veh/Veh group on mechanical sensitivity (Figure 2).
Figure 2.
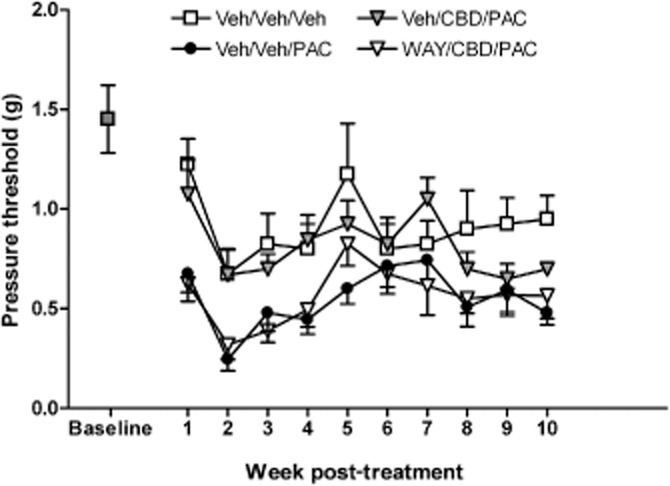
Effect of WAY100635 pretreatment (1.0 mg·kg−1, i.p.) on CBD prevention of PAC-induced mechanical allodynia in female C57Bl/6 mice. Baseline sensitivity to von Frey filaments was assessed on the day before drug administration and continued weekly for 10 weeks. Mice received the following three i.p. injections spaced 15 min apart on days 1, 3, 5 and 7: saline, CRM vehicle, CRM vehicle; saline, CRM vehicle, 8.0 mg·kg−1 PAC; saline, 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC; 1.0 mg·kg−1 WAY100635, 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC. Two-way anova revealed significant effects of treatment [F(3, 280) = 24.66, P < 0.0001] and time [F(9, 280) = 5.058, P < 0.001] and no significant interaction (F <1.0). Bonferroni post-test revealed a significant increase in the sensitivity of the PAC group and the WAY/CBD/PAC groups compared with Veh/Veh/Veh. In contrast, the Veh/CBD/PAC group did not differ significantly from the Veh/Veh/Veh group on mechanical sensitivity. X-axis: time points pre- or post-day first injection. Y-axis: threshold pressure to elicit hind paw withdrawal from von Frey filament. Data points represent the mean and SEM, n = 8 per group.
Conversely, additional administration of either the CB1 antagonist SR141716 (3.0 mg·kg−1) or the CB2 antagonist SR144528 (3.0 mg·kg−1) had no effect on the reversal of PAC-induced mechanical sensitivity by CBD as measured on day 15 post-initiation of treatment. One-way anova revealed a significant effect of treatment [F(8, 79) = 7.647, P < 0.05]. Dunnett's multiple comparison test determined that only the Veh/Veh/PAC, WAY/CBD/PAC, SR1/Veh/PAC and SR2/Veh/PAC groups were statistically different from the Veh/Veh/Veh control group (P < 0.05), showing significant mechanical allodynia (Figure 3). Furthermore, the ability of WAY to block CBD's anti-allodynic effect could not be attributed to the effect of WAY alone on PAC-induced mechanical sensitivity, as WAY itself did not potentiate the effect of PAC alone (WAY/Veh/PAC).
Figure 3.
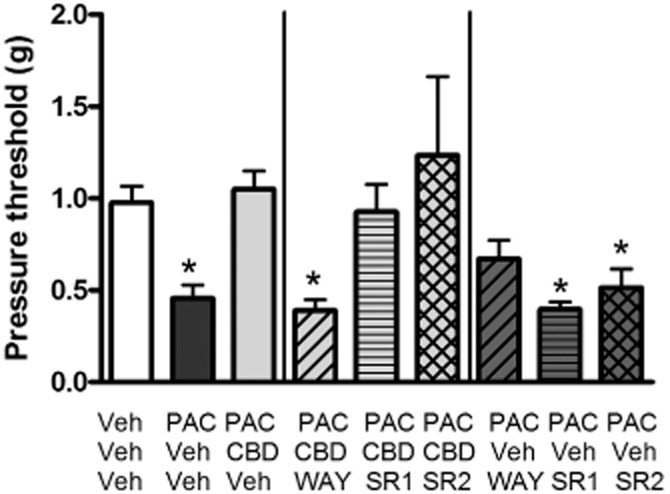
Effect of CB1 (SR141716; SR1) or CB2 (SR144528; SR2) receptor antagonism on CBD prevention of PAC-induced mechanical allodynia in female C57Bl/6 mice. Sensitivity to von Frey filaments was assessed on day 15 post-treatment. Mice received the following three i.p. injections spaced 15 min apart on days 1, 3, 5 and 7: saline, CRM vehicle, CRM vehicle; saline, CRM vehicle, 8.0 mg·kg−1 PAC; saline, 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC; 1.0 mg·kg−1 WAY, 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC; 3.0 mg·kg−1 SR141716, 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC; 3.0 mg·kg−1 SR144528, 5.0 mg·kg−1 CBD, 8.0 mg·kg−1 PAC; 1.0 mg·kg−1 WAY, CRM, 8.0 mg·kg−1 PAC; 3.0 mg·kg−1 SR141716, CRM, 8.0 mg·kg−1 PAC; 3.0 mg·kg−1 SR144528, CRM, 8.0 mg·kg−1 PAC. One-way anova revealed a significant effect of treatment [F(8, 79) = 7.647, P < 0.05]. Dunnett's multiple comparison test determined that only the Veh/Veh/PAC, WAY/CBD/PAC, SR1/Veh/PAC and SR2/Veh/PAC groups were statistically different from the Veh/Veh/Veh control group (P < 0.05). X-axis: treatment. Y-axis: threshold pressure to elicit hind paw withdrawal from von Frey filament. Data points represent the mean and SEM, n = 8 per group.
Place conditioning and autoshaping
There was no effect of CBD on time spent in the white, CBD-paired compartment compared with CRM vehicle control, although there was a trend towards a decrease in the time spent in the CBD-paired compartment at the highest dose tested. One-way anova revealed no significant effect of treatment [F(3, 31) = 2.477, n.s.]. By comparison, morphine treatment significantly increased the time spent in the white, morphine-paired compartment compared with saline vehicle control [F(3, 30) = 15.66, P < 0.0001] (Figure 4). Furthermore, CBD treatment had no effect on the time to earn 10 reinforcers during the acquisition [F(3, 32) <1] or retention [F(3, 25) = 1.692, n.s.] sessions (Figure 5).
Figure 4.
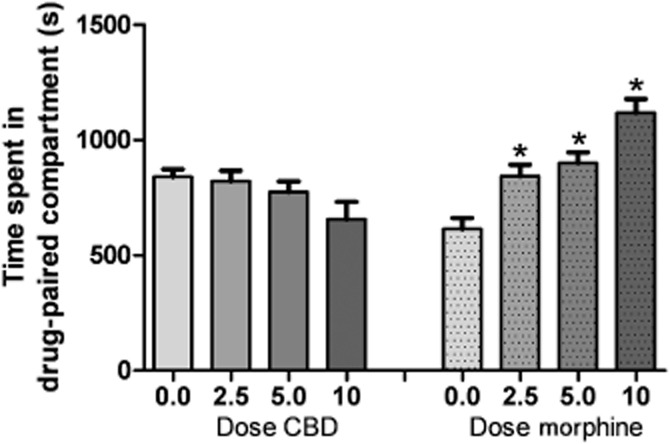
Ability of CBD (2.5–10 mg·kg−1, i.p.) or morphine (2.5–10 mg·kg−1, i.p.) to produce place conditioning in female C57Bl/6 mice. Mice received vehicle or morphine (2.5–10 mg·kg−1, i.p.; 15 min pretreatment) or vehicle or CBD (2.5–10 mg·kg−1, i.p.; 30 min pretreatment) on alternate days for 30 min conditioning sessions for 6 successive days. One-way anovas revealed no significant effect of CBD treatment [F(3, 31) = 2.477, n.s.] and a significant effect of morphine treatment on time spent in the white compartment compared with saline vehicle control [F(3, 30) = 15.66, P < 0.0001]. X-axis: treatment. Y-axis: the time spent in the drug-paired (white) compartment on the treatment-free test day.
Figure 5.
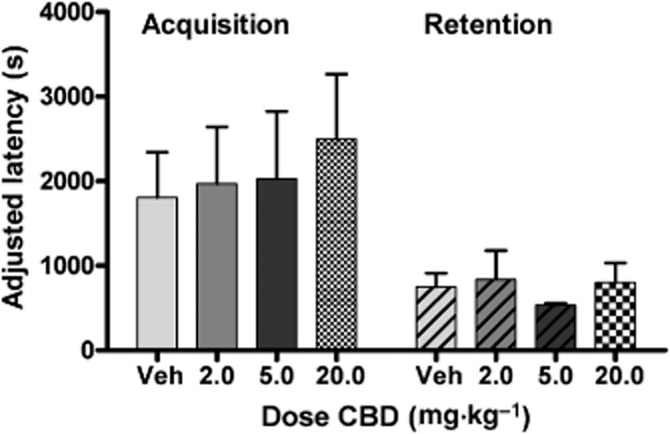
Effect of CBD administration (2.5–20 mg·kg−1, i.p.) on acquisition and retention of a conditioned food reward task. Nose-poke responses are reinforced when made within 8 s following a tone signalling availability of the sweet liquid reinforcer (50% vanilla Ensure in tap water). Each session lasted for 2 h or until 20 reinforced nose pokes were recorded. CBD treatment had no effect on the time to earn 10 reinforcers during the acquisition [F(3, 32) = <1] or retention [F(3, 25) = 1.692, n.s.] sessions. X-axis: treatment. Y-axis: the time elapsed between the first earned reinforcer and the tenth reinforcer.
CBD enhances PAC inhibition of breast cancer cell viability
Multiple studies now show that CBD can act as a direct antitumor agent against aggressive cancers (Massi et al., 2013). Therefore, there is the potential for CBD to produce synergistic, additive or antagonist effects when combined with PAC. We studied these potential interactions by evaluating the effects of the drugs alone and in combination on breast cancer cell viability. 4T1 and luciferase-labelled MDA-MB231-luc-D3H2LN (LN 231) cells were treated for 2 days with a range of concentrations of either PAC or CBD and the ability of the drugs to inhibit cell viability was assessed using the MTT assay (Figure 6A). In this assay, CBD was more potent than PAC at inhibiting cell viability and CBD acted as a full agonist whereas PAC acted as a partial agonist. PAC could not fully inhibit cell viability even up to concentration of 50 μM. PAC began to precipitate out of solution in the MTT assay at the higher concentration range which precluded us from further concentrating the drug. The average values from the concentration response curves which were then used to derive medium-effect plot parameters including the dose-reduction index were calculated (Table 1). Using the calculated IC50 values, various dose ratios of CBD and PAC were combined in both 4T1 and LN 231 cells and viability was evaluated (Figure 6B and C). The use of higher dose ratios was limited by the solubility of PAC; however, this did not affect the calculation of a CI. As shown in Figure 6D, the combination of CBD and PAC led to an additive and synergistic inhibition of cell viability in 4T1 and LN 231 cells respectively.
Figure 6.
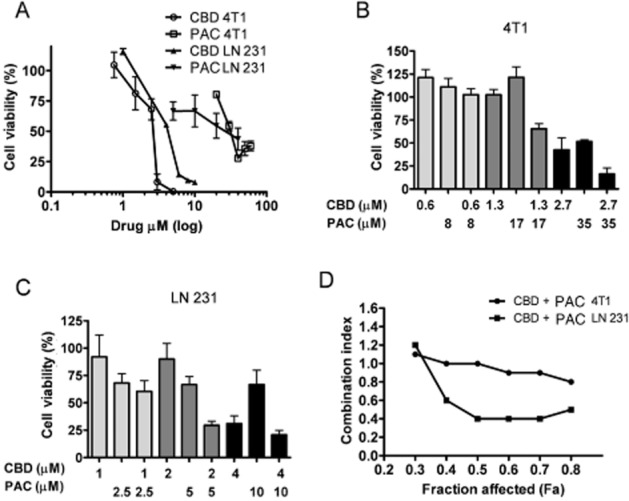
Treatments combining CBD and PAC produce additive to synergistic inhibition of breast cancer cell viability. Cell viability was measured using the MTT assay. (A) 4T1 and MDA-MB231-luc-D3H2LN (LN 231) cells were treated for 2 days with vehicle, CBD or PAC. Specific dose ratios of CBD and Pac where then combined in (B) 4T1 and (C) MDA-MB231-luc-D3H2LN cells. Cell viability (%) was calculated as the MTT product absorbance in the treated cells/control cells × 100. These data were used to calculate (D) CI values as described in Methods. A CI value of <1, 1 and >1 indicates synergism, additivity and antagonism respectively (Chou et al., 1993). Data are the mean of at least three independent experiments; bars, ±SEM.
Table 1.
Calculated median-effect plot parameters and DRI for drugs and drug combinations
| Median-effect plot parameters | DRI | ||||
|---|---|---|---|---|---|
| Cell line | Chemotherapy | Dm | m | r | 50% inhibition |
| 4T1 | CBD | 2.7 μM | 2.5 | 0.98 | |
| PAC | 35 μM | 1.8 | 0.86 | 2.0 | |
| CBD + PAC | 18 μM | 3.0 | 0.99 | ||
| LN 231 | CBD | 4.1 μM | 3.2 | 0.99 | |
| Pac | 51 μM | 0.3 | 0.92 | 15 | |
| CBD + PAC | 5.0 μM | 2 | 1.0 | ||
The median-effect dose (Dm), slope (m), linear correlation coefficient (r) and DRI (dose-reduction index) for drugs were calculated using Compusyn.
Discussion
We had previously reported that a 14 day dosing regimen of CBD (5.0 and 10 mg·kg−1) prevented the onset of PAC-induced mechanical and thermal sensitivity (Ward et al., 2011). In the present study, we determined that both 2.5 and 5 mg·kg−1 CBD treatment, administered only before each of the four PAC injections of a standard dosing regimen for inducing CIPN in rodents, also prevents the development of PAC-induced mechanical sensitivity in female C57Bl/6 mice. The present study further demonstrated that 5-HT1A receptors are partially involved in the neuroprotective effect of CBD in this model, in that co-administration of the 5-HT1A antagonist blocked the preventive effect of CBD on PAC-induced mechanical sensitivity. In contrast, neither the CB1 antagonist SR141716 nor the CB2 antagonist SR144528 affected the efficacy of CBD, suggesting its neuroprotective effect was not mediated by activation of CB1 or CB2 receptors. Furthermore, treatment with the antagonists alone did not further exacerbate PAC-induced mechanical sensitivity. In addition, CBD did not produce conditioned rewarding effects using the place conditioning procedure, nor did it produce deficits in acquisition or retention of an operant learning task using the autoshaping procedure. Lastly, CBD did not attenuate the anti-neoplastic effect of PAC on breast cancer cells in culture. Indeed, at optimal concentrations, CBD + PAC combinations produce additive to synergistic inhibition of breast cancer cell viability.
Cannabinoids represent a promising pharmacotherapeutic strategy for treatment of neuropathic pain considering that available alternatives are not always successful in the clinic. A putative role for cannabinoids in the amelioration of established PAC-induced CIPN has recently been demonstrated. Pascual et al. (2005) showed that the non-selective cannabinoid agonist WIN 55,212-2 reduced an established thermal hyperalgesia and tactile allodynia 22 days post-PAC treatment in rats, and that this effect was blocked by the CB1 antagonist SR141716, suggesting the involvement of the CB1 receptor; the potential participation of the CB2 receptor in mediating this effect, however, was not investigated. The anti-neuropathic efficacy of non-selective CB agonist therapies, including the THC : CBD combination Sativex, appears promising; nonetheless, unwanted side effects, mainly the production of psychoactivity produced through activation of CB1 receptors, remain a hindrance to their wider use (Johnson et al., 2010; Karschner et al., 2011; but see Langford et al., 2013). The efficacy and safety of CB2 selective agents in humans for treatment of neuropathic pain remain to be determined. Activation of CB2 receptors has been shown to suppress established chemotherapy-induced CIPN in rats (Naguib et al., 2008; Rahn et al., 2008; Deng et al., 2012). In the study of Rahn et al, CB2 agonist administration was most effective at 30 min post-injection, with mechanical sensitivity re-emerging 60 min following agonist administration, suggesting that repeated administration would be necessary to treat the CIPN symptoms in the long term.
Based on growing preclinical literature, the myriad of CBD's pharmacological effects, from anti-neuropathic to anxiolytic and antipsychotic, may be mediated through either CB receptor-dependent and independent mechanisms or combinations thereof (Izzo et al., 2009). It is important from both a basic science mechanistic as well as drug discovery perspective to identify which of these are necessary and/or sufficient for CBD's anti-neuropathic effects specifically. In the present study, we demonstrated that activation of 5-HT1A receptors is necessary for the protective effect of CBD against PAC-induced neuropathic pain, in that pretreatment with WAY100635 blocked this effect. CBD acts as a direct agonist at 5-HT1A receptors (Russo et al., 2005; Alves et al., 2010), and activation of the 5-HT1A receptor in the rostroventromedial medulla plays an important role in modulating the descending inhibitory pain pathway (Colpaert, 2006; Viisanen and Pertovaara, 2010). Importantly, 5-hydroxytryptaminergic drugs presently represent one of the only drug classes showing efficacy in the treatment of neuropathic pain in human clinical trials (Finnerup et al., 2010). 5-HT1A agonism has also been shown to be neuroprotective via attenuation of microglial activation and oxidative stress (Collier et al., 2011a,b2011b), two immune alterations relevant to CIPN. Results from the present study failed to show a role for CB1 or CB2 receptor activation in CBD's anti-neuropathic effect. Although CBD has no appreciable affinity for CB1 or CB2 receptors, some evidence suggests that it can act as an indirect CB agonist via enhancement of eCB levels (Bisogno et al., 2001; Campos et al., 2013). However, our results are in agreement with the previous report by Comelli et al. (2008) demonstrating that CBD's anti-hyperalgesic effect did not involve CB1 and CB2 receptors. Others have shown that neither CB1 nor CB2 receptor activation was involved in CBD's neuroprotective (Sagredo et al., 2007; 2011,) or anti-inflammatory (Costa et al., 2004) effects in other rodent models, whereas CBD-induced tail flick analgesia was blocked by co-administration of the CB1 antagonist SR141716 (Maione et al., 2011). CB1 receptor involvement in the pharmacological effects of CBD on non-nociceptive behaviours has also been reported (Casarotto et al., 2010; Do Monte et al., 2013). Additionally, CBD binds with moderate affinity to TRPV1 (vanilloid) receptors and important nociceptive modulators, and anti-neuropathic effects of CBD have been shown to depend upon TRPV1 activation (Comelli et al., 2008), while acute antinociceptive effects have not (Maione et al., 2011). Taken together with these other findings, our results suggest that specific pharmacological effects of CBD, such as its activity at 5-HT1A and TRPV1 receptors, mediate CBD's anti-neuropathic effects, while its activity at other targets, including CB receptors, may be more important for other actions.
A novel strategy investigated in the present study is that of assessing the ability of CB-based pharmacotherapy to prevent the development of PAC-induced mechanical sensitivity as opposed to acutely reversing it. Other studies have demonstrated the ability of agents from other drug classes, including anticonvulsants (Xiao et al., 2007), antidepressants (Xiao et al., 2008) and opioids (Rahn et al., 2008), to reduce CIPN symptoms in rodents, but to date no one drug or drug class is considered to be effective for reversal of CIPN (Lynch et al., 2004). CIPN represents a neuropathic pain state with the unique possibility of aiming to prevent its onset with effective adjunctive treatment, as opposed to only attempting to reverse its symptoms following its onset. However, such investigations into prevention of PAC-induced CIPN in rodents are few. Interestingly, CBD has also recently been reported to protect against the onset of type I diabetic peripheral neuropathic pain (Toth et al., 2010), hepatic ischaemia/reperfusion injury (Mukhopadhyay et al., 2011), and retinal inflammation and degeneration (El-Remessy et al., 2008) in rodent models. While clinical trials are ongoing investigating the anti-inflammatory effects of CBD as a monotherapy in disease states such as inflammatory bowel disease and graft versus host disease, its efficacy at preventing the onset of neuropathic pain in humans remains to be determined.
CBD represents a significant improvement in CB-based pharmacotherapy, in that CBD represents a cannabinoid that is regarded as being devoid of psychoactive euphoric effects. Surprisingly, however, a few preclinical studies to date have investigated CBD in reward models (e.g. Parker et al., 2004). Here we demonstrated across a wider range of doses that CBD does not produce a CPP in C57Bl/6 mice using parameters that readily detect the conditioned rewarding properties of the same doses of morphine (Figure 4). CBD does, however, bind to several brain receptors and its anxiolytic and antipsychotic actions have been well characterized in animals and more recently in humans, so it is worth investigating whether CBD produces other CNS effects that would be considered adverse. An important pharmacological effect of CB receptor activation in addition to euphoria that has been extensively studied is disruption of learning and memory processes (see Lichtman et al., 2002 for review). In the present study, we demonstrated that CBD across a wide range of doses did not impair acquisition or retention of an instrumental learning task. Interestingly, others have reported that CBD actually enhances certain types of learning, specifically extinction (Bitencourt et al., 2008) and reconsolidation (Stern et al., 2012). Determination of the effect of a putative anti-CIPN pharmacotherapy on learning and memory is important because cancer chemotherapeutics themselves are associated with a form of cognitive impairment in many cancer patients also known as ‘chemofog’ or ‘chemobrain’ (Argyriou et al., 2011). CB agonists are likely to exacerbate these effects, while in contrast CBD should not affect cognition, and may therefore prove to be a more tolerable alternative as an adjuvant chemotherapy agent. In fact, as oxidative stress is a leading hypothesis regarding the mechanism underlying chemotherapy-associated cognitive impairment, the ability of CBD to reverse this phenomenon should also be investigated.
Finally, CBD has direct antitumor activity in multiple types of cancer (Massi et al., 2013). We determined that at optimal concentrations, CBD in combination with PAC produces additive to synergistic inhibition of breast cancer cell viability. Our results in breast cancer cells are in agreement with a recent investigation demonstrating CBD could enhance the activity of first-line agents targeting prostate cancer in culture and in vivo (Aviello et al., 2011). The doses that prevent PAC-induced allodynia in our model overlap with doses of CBD that attenuate breast cancer metastasis in vivo (McAllister et al., 2011). This integrated approach to using CBD to prevent CIPN while directly and indirectly targeting tumour progression makes it a potential valuable therapeutic for the treatment of cancer patients undergoing treatments with first-line agents.
In summary, our data suggest that CBD is protective against PAC-induced neurotoxicity and that this effect is in part mediated by the 5-HT1A receptor system. Furthermore, CBD treatment is devoid of other nervous system effects such as conditioned reward or cognitive impairment. CBD also did not attenuate the efficacy of PAC in inhibiting breast cancer cell viability. Taken together, adjunct treatment with CBD during PAC chemotherapy treatment may be safe and effective in the prevention or attenuation of CIPN.
Acknowledgments
We acknowledge Khristina Pavlenko and Mak Sarich.
Glossary
- CB
cannabinoid
- CBD
cannabidiol
- CI
combination index
- CIPN
chemotherapy-induced peripheral neuropathy
- CPP
conditioned place preference
- CRM
cremophor
- PAC
paclitaxel
- THC
tetrahydrocannabinol
- TRPV
transient receptor potential vanilloid
Conflict of interest
There are no conflicts of interest present.
References
- Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na(+) channel kinetics on rat sensory neurons. Eur J Pharmacol. 2000;406:25–32. doi: 10.1016/s0014-2999(00)00667-1. [DOI] [PubMed] [Google Scholar]
- Alves FH, Crestani CC, Gomes FV, Guimarães FS, Correa FM, Resstel LB. Cannabidiol injected into the bed nucleus of the stria terminalis modulates baroreflex activity through 5-HT1A receptors. Pharmacol Res. 2010;62:228–236. doi: 10.1016/j.phrs.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Assimakopoulos K, Iconomou G, Giannakopoulou F, Kalofonos HP. Either called ‘chemobrain’ or ‘chemofog,’ the long-term chemotherapy-induced cognitive decline in cancer survivors is real. J Pain Symptom Manage. 41:126–139. doi: 10.1016/j.jpainsymman.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Aviello G, Romano B, Borrelli F, Capasso R, Gallo L, Piscitelli F, et al. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J Mol Med (Berl) 2011-2012;90:925–934. doi: 10.1007/s00109-011-0856-x. [DOI] [PubMed] [Google Scholar]
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. 2008;18:849–859. doi: 10.1016/j.euroneuro.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Campos AC, Ortega Z, Palazuelos J, Fogaça MV, Aguiar DC, Díaz-Alonso J. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Int J Neuropsychopharmacol. 2013;16:1407–1419. doi: 10.1017/S1461145712001502. [DOI] [PubMed] [Google Scholar]
- Casarotto PC, Gomes FV, Resstel LB, Guimarães FS. Cannabidiol inhibitory effect on marble-burying behaviour: involvement of CB1 receptors. Behav Pharmacol. 2010;21:353–358. doi: 10.1097/fbp.0b013e32833b33c5. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Tredici G, Braga M, Tazzari S. Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exp Neurol. 1995;133:64–72. doi: 10.1006/exnr.1995.1008. [DOI] [PubMed] [Google Scholar]
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- Chou TC, Tan QH, Sirotnak FM. Quantitation of the synergistic interaction of edatrexate and cisplatin in vitro. Cancer Chemother Pharmacol. 1993;31:259–264. doi: 10.1007/BF00685668. [DOI] [PubMed] [Google Scholar]
- Collier RJ, Wang Y, Smith SS, Martin E, Ornberg R, Rhoades K. Complement deposition and microglial activation in the outer retina in light-induced retinopathy: inhibition by a 5-HT1A agonist. Invest Ophthalmol Vis Sci. 2011a;52:8108–8116. doi: 10.1167/iovs.10-6418. [DOI] [PubMed] [Google Scholar]
- Collier RJ, Patel Y, Martin EA, Dembinska O, Hellberg M, Krueger DS. Agonists at the serotonin receptor (5-HT(1A)) protect the retina from severe photo-oxidative stress. Invest Ophthalmol Vis Sci. 2011b;52:2118–2126. doi: 10.1167/iovs.10-6304. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. 5-HT(1A) receptor activation: new molecular and neuroadaptive mechanisms of pain relief. Curr Opin Investig Drugs. 2006;7:40–47. [PubMed] [Google Scholar]
- Comelli F, Giagnoni G, Bettoni I, Colleoni M, Costa B. Antihyperalgesic effect of a Cannabis sativa extract in a rat model of neuropathic pain: mechanisms involved. Phytother Res. 2008;22:1017–1024. doi: 10.1002/ptr.2401. [DOI] [PubMed] [Google Scholar]
- Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143:247–250. doi: 10.1038/sj.bjp.0705920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556:75–83. doi: 10.1016/j.ejphar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Deng L, Guindon J, Vemuri VK, Thakur GA, White FA, Makriyannis A, et al. The maintenance of cisplatin- and paclitaxel-induced mechanical and cold allodynia is suppressed by cannabinoid CB2 receptor activation and independent of CXCR4 signaling in models of chemotherapy-induced peripheral neuropathy. Mol Pain. 2012;8:71. doi: 10.1186/1744-8069-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Do Monte FH, Souza RR, Bitencourt RM, Kroon JA, Takahashi RN. Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behav Brain Res. 2013;250:23–27. doi: 10.1016/j.bbr.2013.04.045. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Tang Y, Zhu G, Matragoon S, Khalifa Y, Liu EK, et al. Neuroprotective effects of cannabidiol in endotoxin-induced uveitis: critical role of p38 MAPK activation. Mol Vis. 2008;14:2190–2203. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fernández-Ruiz J, Sagredo O, Pazos MR, García C, Pertwee R, Mechoulam R, et al. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol. 2013;75:323–333. doi: 10.1111/j.1365-2125.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Foley JJ, Raffa RB, Walker EA. Effects of chemotherapeutic agents 5-fluorouracil and methotrexate alone and combined in a mouse model of learning and memory. Psychopharmacology (Berl) 2008;199:527–538. doi: 10.1007/s00213-008-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchan P, Andoh T, Ikeda K, Fujita M, Sasaki A, Kato A, et al. Mechanical allodynia induced by paclitaxel, oxaliplatin and vincristine: different effectiveness of gabapentin and different expression of voltage-dependent calcium channel alpha(2)delta-1 subunit. Biol Pharm Bull. 2009;32:732–734. doi: 10.1248/bpb.32.732. [DOI] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153:319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara H, Fujita Y, Ishima T, Kunitachi S, Shirayama Y, Iyo M, et al. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the antipsychotic drug perospirone: role of serotonin 5-HT1A receptors. Eur Neuropsychopharmacol. 2008;8:448–454. doi: 10.1016/j.euroneuro.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Hu P, McLachlan EM. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience. 2002;112:23–38. doi: 10.1016/s0306-4522(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Jenkins DE, Hornig YS, Oei Y, Dusich J, Purchio T. Bioluminescent human breast cancer cell lines that permit rapid and sensitive in vivo detection of mammary tumors and multiple metastases in immune deficient mice. Breast Cancer Res. 2005;7:R444–R454. doi: 10.1186/bcr1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC : CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39:167–179. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Karschner EL, Darwin WD, McMahon RP, Liu F, Wright S, Goodwin RS, et al. Subjective and physiological effects after controlled Sativex and oral THC administration. Clin Pharmacol Ther. 2011;89:400–407. doi: 10.1038/clpt.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260:984–997. doi: 10.1007/s00415-012-6739-4. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Varvel SA, Martin BR. Endocannabinoids in cognition and dependence. Prostaglandins Leukot Essent Fatty Acids. 2002;66:269–285. doi: 10.1054/plef.2001.0351. [DOI] [PubMed] [Google Scholar]
- Lynch JJ, 3rd, Wade CL, Zhong CM, Mikusa JP, Honore P. Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. Pain. 2004;110:56–63. doi: 10.1016/j.pain.2004.03.010. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Chan C, Taft RJ, Luu T, Abood ME, Moore DH, et al. Cannabinoids selectively inhibit proliferation and induce death of cultured human glioblastoma multiforme cells. J Neurooncol. 2005;74:31–40. doi: 10.1007/s11060-004-5950-2. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Christian RT, Horowitz MP, Garcia A, Desprez PY. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol Cancer Ther. 2007;6:2921–2927. doi: 10.1158/1535-7163.MCT-07-0371. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Murase R, Christian RT, Lau D, Zielinski AJ, Allison J, et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat. 2011;129:37–47. doi: 10.1007/s10549-010-1177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Piscitelli F, Gatta L, Vita D, De Petrocellis L, Palazzo E, et al. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br J Pharmacol. 2011;162:584–596. doi: 10.1111/j.1476-5381.2010.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu JP, Christian RT, Lau D, Zielinski AJ, Horowitz MP, Lee J, et al. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol Cancer Ther. 2010;9:180–189. doi: 10.1158/1535-7163.MCT-09-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Solinas M, Cinquina V, Parolaro D. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303–312. doi: 10.1111/j.1365-2125.2012.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Horváth B, Bátkai S, Park O, Tanchian G, et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med. 2011;50:1368–1381. doi: 10.1016/j.freeradbiomed.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib M, Diaz P, Xu JJ, Astruc-Diaz F, Craig S, Vivas-Mejia P, et al. MDA7: a novel selective agonist for CB2 receptors that prevents allodynia in rat neuropathic pain models. Br J Pharmacol. 2008;155:1104–1116. doi: 10.1038/bjp.2008.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R. Effect of low doses of delta9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology (Berl) 2004;175:360–366. doi: 10.1007/s00213-004-1825-7. [DOI] [PubMed] [Google Scholar]
- Pascual D, Goicoechea C, Suardíaz M, Martín MI. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain. 2005;118:23–34. doi: 10.1016/j.pain.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Peters CM, Jimenez-Andrade JM, Jonas BM, Sevcik MA, Koewler NJ, Ghilardi JR, et al. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol. 2007;203:42–54. doi: 10.1016/j.expneurol.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Pinsger M, Schimetta W, Volc D, Hiermann E, Riederer F, Pölz W. Benefits of an add-on treatment with the synthetic cannabinomimetic nabilone on patients with chronic pain–a randomized controlled trial. Wien Klin Wochenschr. 2006;118:327–335. doi: 10.1007/s00508-006-0611-4. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Makriyannis A, Hohmann AG. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol. 2007;152:765–777. doi: 10.1038/sj.bjp.0707333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Zvonok AM, Thakur GA, Khanolkar AD, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J Pharmacol Exp Ther. 2008;327:584–591. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Sagredo O, Ramos JA, Decio A, Mechoulam R, Fernández-Ruiz J. Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur J Neurosci. 2007;26:843–851. doi: 10.1111/j.1460-9568.2007.05717.x. [DOI] [PubMed] [Google Scholar]
- Sagredo O, Pazos MR, Satta V, Ramos JA, Pertwee RG, Fernández-Ruiz J. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington's disease. J Neurosci Res. 2011;89:1509–1518. doi: 10.1002/jnr.22682. [DOI] [PubMed] [Google Scholar]
- Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain. 2008;9:164–173. doi: 10.1016/j.jpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Stern CA, Gazarini L, Takahashi RN, Guimarães FS, Bertoglio LJ. On disruption of fear memory by reconsolidation blockade: evidence from cannabidiol treatment. Neuropsychopharmacology. 2012;37:2132–2142. doi: 10.1038/npp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth CC, Jedrzejewski NM, Ellis CL, Frey WH., 2nd Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol Pain. 2010;6:16. doi: 10.1186/1744-8069-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viisanen H, Pertovaara A. Roles of the rostroventromedial medulla and the spinal 5-HT(1A) receptor in descending antinociception induced by motor cortex stimulation in the neuropathic rat. Neurosci Lett. 2010;476:133–137. doi: 10.1016/j.neulet.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Visovsky C, Collins M, Abbott L, Aschenbrenner J, Hart C. Putting evidence into practice: evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 2007;11:901–913. doi: 10.1188/07.CJON.901-913. [DOI] [PubMed] [Google Scholar]
- Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10:434–441. doi: 10.1191/1352458504ms1082oa. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Ramirez MD, Neelakantan H, Walker EA. Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesth Analg. 2011;113:947–950. doi: 10.1213/ANE.0b013e3182283486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182:E694–E701. doi: 10.1503/cmaj.091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Boroujerdi A, Bennett GJ, Luo ZD. Chemotherapy-evoked painful peripheral neuropathy: analgesic effects of gabapentin and effects on expression of the alpha-2-delta type-1 calcium channel subunit. Neuroscience. 2007;144:714–720. doi: 10.1016/j.neuroscience.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Naso L, Bennett GJ. Experimental studies of potential analgesics for the treatment of chemotherapy-evoked painful peripheral neuropathies. Pain Med. 2008;9:505–517. doi: 10.1111/j.1526-4637.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- Xiao WH, Bennett GJ. C-fiber spontaneous discharge evoked by chronic inflammation is suppressed by a long-term infusion of lidocaine yielding nanogram per milliliter plasma levels. Pain. 2008;137:218–228. doi: 10.1016/j.pain.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Xiong W, Cui T, Cheng K, Yang F, Chen SR, Willenbring D, et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J Exp Med. 2012;209:1121–1134. doi: 10.1084/jem.20120242. [DOI] [PMC free article] [PubMed] [Google Scholar]


