Abstract
Background and Purpose
The P2Y14 receptor is the newest member of the P2Y receptor family; it is Gi/o protein-coupled and is activated by UDP and selectively by UDP-glucose and MRS2690 (2-thiouridine-5′-diphosphoglucose) (7–10-fold more potent than UDP-glucose). This study investigated whether P2Y14 receptors were functionally expressed in porcine isolated pancreatic arteries.
Experimental Approach
Pancreatic arteries were prepared for isometric tension recording and UDP-glucose, UDP and MRS2690 were applied cumulatively after preconstriction with U46619, a TxA2 mimetic. Levels of phosphorylated myosin light chain 2 (MLC2) were assessed with Western blotting. cAMP concentrations were assessed using a competitive enzyme immunoassay kit.
Key Results
Concentration-dependent contractions with a rank order of potency of MRS2690 (10-fold) > UDP-glucose ≥ UDP were recorded. These contractions were reduced by PPTN {4-[4-(piperidin-4-yl)phenyl]-7-[4-(trifluoromethyl)phenyl]-2-naphthoic acid}, a selective antagonist of P2Y14 receptors, which did not affect responses to UTP. Contraction to UDP-glucose was not affected by MRS2578, a P2Y6 receptor selective antagonist. Raising cAMP levels and forskolin, in the presence of U46619, enhanced contractions to UDP-glucose. In addition, UDP-glucose and MRS2690 inhibited forskolin-stimulated cAMP levels. Removal of the endothelium and inhibition of endothelium-derived contractile agents (TxA2, PGF2α and endothelin-1) inhibited contractions to UDP glucose. Y-27632, nifedipine and thapsigargin also reduced contractions to the agonists. UDP-glucose and MRS2690 increased MLC2 phosphorylation, which was blocked by PPTN.
Conclusions and Implications
P2Y14 receptors play a novel vasocontractile role in porcine pancreatic arteries, mediating contraction via cAMP-dependent mechanisms, elevation of intracellular Ca2+ levels, activation of RhoA/ROCK signalling and MLC2, along with release of TxA2, PGF2α and endothelin-1.
Keywords: UDP-glucose, UDP, MRS2690, P2Y14 receptor, vasoconstriction, endothelium
Introduction
P2Y receptors are members of the superfamily of GPCRs; they are responsive to purine and pyrimidine nucleotides and nucleotide sugars [ADP, ATP, uridine-5′-triphosphate (UTP), uridine diphosphate (UDP) and UDP-glucose]. Eight mammalian subtypes of P2Y receptors have been identified: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14 receptors (Abbracchio et al., 2006; receptor nomenclature follows Alexander et al., 2013). The P2Y14 receptor was the most recently identified (Chambers et al., 2000). In contrast to other P2Y receptors, P2Y14 receptors are activated by nucleotide sugars such as UDP-glucose, in addition to UDP-galactose and UDP-glucuronic acid that are less potent than UDP-glucose (Abbracchio et al., 2003; Fricks et al., 2008; Harden et al., 2010). They are also activated by UDP and MRS2690 (2-thiouridine-5′-diphosphoglucose), which is more selective at P2Y14 receptors and 7–10-fold more potent than UDP-glucose (Carter et al., 2009; Jacobson et al., 2009; Gao et al., 2010). The P2Y14 receptor is involved in Gi-protein-mediated signalling, leading to the inhibition of AC activity and, accordingly, is sensitive to Pertussis toxin (PTX) (Jacobson et al., 2009). Gi-protein-derived Gβγ dimers can initiate PLCβ signalling pathways, which leads to the stimulation of DAG and inositol 1,4,5-triphosphate (IP3) and subsequent activation of RhoA/ROCK (Rho-associated protein kinase) signalling, PKC and myosin light chain (MLC) kinase, besides elevation of the intracellular calcium level (Amano et al., 1996; Hartshorne and Gorecka, 2011; Sesma et al., 2012).
Pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) and suramin are non-selective antagonists at most of the P2Y receptors, but some P2Y receptors are insensitive to these antagonists (Chootip et al., 2005). There is currently no report of antagonist sensitivity of P2Y14 receptors for suramin and PPADS. Recently, a novel antagonist at P2Y14 receptors was identified; this antagonist, 4-[4-(piperidin-4-yl)phenyl]-7-[4-(trifluoromethyl)phenyl]-2-naphthoic acid (PPTN) (Barrett et al., 2013), was characterized in HEK cells through its ability to inhibit UDP-glucose-stimulated Ca2+ mobilization (Robichaud et al., 2011). In addition, it showed good affinity for the P2Y14 receptor (Ki = 1.9 nM in a chimpanzee P2Y14 binding assay) (Robichaud et al., 2011). When it was studied in human C6 glioma cells, PPTN showed selectivity for P2Y14 receptors with a KB of 434 pM, with no agonist or antagonist affinity at P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12 or P2Y13 receptors (Barrett et al., 2013).
P2Y14 receptor mRNA and protein have a varied expression in the body; they have been found in the spleen, placenta, lung, heart, adipose tissue, gastrointestinal smooth muscle, endothelial cells and immune cells (Chambers et al., 2000; Scrivens and Dickenson, 2005; Umapathy et al., 2010). Relatively little is known, however, about the functional expression of the P2Y14 receptor. The best characterized role is in regulation of the immune system, as a number of studies have described an involvement of P2Y14 receptors in modulation of the function of human neutrophils and T-lymphocytes, and in secretion of the pro-inflammatory cytokine IL-8 in airway epithelial cells (Scrivens and Dickenson, 2005; 2006,; Jacobson et al., 2009). UDP-glucose can be released in a constitutive manner and during shear stress, to act as an extracellular signalling molecule (Lazarowski et al., 2003); its release from multiple cell types suggests potentially broad role(s) of the P2Y14 receptor.
Although the exact mechanisms remain to be established, an increase in pancreatic endocrine cell activity during hormone secretion corresponds to an increase in blood flow, to meet metabolic demand. Thus, alterations in blood flow can influence pancreatic function. The role of exogenous purine and pyrimidine di- and triphosphate nucleotides in controlling the functions of endocrine and exocrine components of the pancreas are well described (Novak, 2008; Burnstock and Novak, 2012). Within the pancreatic vasculature, P2X receptors were suggested to mediate vasoconstriction, and P2Y receptors vasodilatation, in response to ATP (Hillaire-Buys et al., 1991). P2X1, P2Y2, P2Y4 and P2Y6 receptors, with different sensitivities to ATP, UDP and UTP, mediate vasoconstriction in a number of blood vessels (Ralevic and Burnstock, 1998), and there is some evidence for a vasocontractile function of the P2Y12 receptor (Wihlborg et al., 2004; Högberg et al., 2010). In the current study, we describe the pharmacological characterization of P2Y14 receptor-mediated contractile responses of porcine isolated pancreatic artery preparations. A preliminary account of some of these data has previously been presented to the British Pharmacological Society (Alsaqati et al., 2010).
Methods
Tissue preparation
Pancreases from pigs (either sex, age less than 6 months, weighing ∼50 kg) were obtained on ice from a local abattoir (G Wood & Sons Ltd, Mansfield, UK). A crude dissection was conducted to isolate the pancreatic arteries (greater pancreatic artery), which were located in the body of the pancreas. The vessels were dissected out and placed in Krebs–Henseleit buffer containing 2% (w/v) Ficoll (type 70) and refrigerated overnight at 4°C. The next day, fine dissection was performed, and the arteries were cut into rings of 0.5 cm in length and suspended in Krebs–Henseleit buffer (gassed, 95% O2, 5% CO2).
The endothelium of some arteries was removed by gently rubbing their innermost surface with forceps before attaching them to the set-up (Rayment et al., 2007a). Successful removal of endothelium was tested using substance P (10 nM). Endothelium-denuded arteries relaxed in response to substance P to less than 10% of the U46619 (11α,9α-epoxymethano-PGH2)-induced contraction, while in endothelium-intact arteries, the relaxation to substance P was 36 ± 8% (n = 7).
Responses in the porcine isolated pancreatic artery
Arterial rings were mounted onto wires in tissue baths (20 mL) containing warm (37°C), oxygenated Krebs–Henseleit solution and were connected via isometric force transducers (ADInstruments, Sydney, Australia) to a PC running the computer program LabChart (ADInstruments). Rings were put under tension (15 g) and allowed to equilibrate for 60 min before assessing viability with two challenges of 75 mM KCl. The tissues were then allowed to equilibrate for 60 min, after which U46619 (10–100 nM), a TxA2 mimetic, was used to contract the tissues to between 40 and 80% of the second KCl response. This ensured that if there was a vasodilator component to the response, for example, due to activation of multiple P2 receptor subtypes, this could be detected. Once an appropriate level of U46619 response had been achieved, cumulative addition of UDP-glucose, UDP or MRS2690 was applied. Antagonists or inhibitors were applied 10 min prior to the addition of U46619, allowing incubation with the tissues for a minimum of 30 min before the application of agonists. Desensitization of the contraction to UDP-glucose was generated by exposing the arteries to UDP or UDP-glucose, 10 min before the addition of U46619. An exception to preconstriction with U46619 were experiments with L-655,240 {1-[(4-chlorophenyl)methyl]-5-fluoro-α,α,3-trimethyl-1H-indole-2-propanoic acid}, in which arteries were preconstricted with endothelin-1. In experiments using DMSO as the solvent (see Materials), DMSO was added to the arteries (vehicle control).
Effect of forskolin on subsequent UDP-glucose or UTP responses
Tissues were exposed to U46619 (10–100 nM) and relaxed with forskolin (1 μM), involving elevation of cAMP, back to the baseline; cumulative concentration–response curves were then constructed for UDP-glucose (1 μM–1 mM) or UTP (10 μM–1 mM). Responses to UDP-glucose or UTP obtained under these conditions were compared with control responses in which drugs were added at basal tone without exposing to either U46619 or forskolin. The tissue was allowed to recover for 20 min before a concentration–response curve to UDP-glucose or UTP was constructed.
Western blotting
Segments of porcine pancreatic arteries were set up in the organ baths under a tension of 15 g and then left for approximately 60 min to reach a new baseline of resting tension. Tissues were incubated with 100 μM UDP-glucose for 30, 60, 120, 180, 240 and 300 s. In addition, in other experiments, the tissues were incubated with UDP-glucose (100 μM) or MRS2690 (10 μM) for 30 s in the presence or absence of PPTN. Segments were quickly removed from the organ baths and immediately frozen on dry ice. Control tissues were not exposed to any compound (basal conditions). Segments were then homogenized in lysis buffer [20 mM Tris, 1 mM EGTA, 0.1% (v/v) Triton X-100, 1 mM NaF, 10 mM β-glycerophosphate, pH 7.6], containing protease inhibitor cocktail tablets, EDTA free. Samples with solubilization buffer 6 × SB [24% (w/v) SDS, 30% (v/v) glycerol, 5% (v/v) β-mercaptoethanol, 2.5% (v/v) bromophenol blue, 1.5 M Tris–HCl, pH 6.8] were heated at 95°C for 5 min. Subsequently, electrophoresis was carried out on 4–20% Tri-Glycine (PAGE) Gold Precast Gels (Bio-Rad, Hercules, CA, USA), 15 μg protein per lane. Samples were transferred to nitrocellulose membranes. Next, blots were incubated in blocking solution [5% (w/v) powdered milk in Tris-buffered saline containing 0.1% (v/v) Tween 20; Fisher Scientific UK Ltd., Loughborough, UK] for 60 min at room temperature. Blots were incubated overnight at 4°C with primary antibody against phosphorylated myosin light chain kinase 2 (pMLC2, 1:500) or total (MLC2, 1:1000) diluted in blocking solution. After washing in Tris-buffered saline containing 0.1% (v/v) Tween 20, the blots were incubated with an appropriate IRDye®-conjugated secondary antibody (Li-Cor Biosciences, Biotechnology, Lincoln, NE, USA). Proteins were visualized using the Li-Cor/Odyssey infrared imaging system. Bands were analysed by densitometry using the Odyssey application software and expressed as phosphorylated MLC2 normalized to total MLC2.
cAMP measurement in porcine pancreatic arteries
Pancreatic artery rings were stimulated with 75 mM KCl followed by U46619 (10 nM) and forskolin (1 μM), and then the arteries were challenged for 3 min with UDP-glucose (1 mM), MRS2690 (10 μM), UTP (1 mM) or distilled water (control group). Pancreatic artery rings were collected and immediately frozen on dry ice and then stored in vials at −80°C until their study. The tissues were homogenized in 5% (w/v) trichloroacetic acid (TCA) in water with a borosilicate glass homogenizer and then centrifuged for 10 min at 1500× g. TCA was extracted from the supernatant samples using water-saturated ether and evaporated for 5 min to remove the residual ether from the aqueous fractions. Samples were diluted (1:2) in ether-extracted 5% (w/v) TCA. cAMP concentration was measured using a competitive enzyme immunoassay (EIA) kit (Cayman Chemical Co., Ann Arbor, MI, USA). The working range of the cAMP assay was 0.1–1000 pmol·mL−1. cAMP concentration was expressed as a percentage of forskolin-induced [cAMP] elevation.
Data analysis
Data were expressed as log concentration–response plots. The contraction to all agonists was expressed in g, and measured from the stabilized U46619 response. Values for all figures refer to mean ± SEM with 95% confidence. Results were compared by two-way anova or Student's unpaired t-test (Prism; GraphPad, San Diego, CA, USA). Differences were considered to be significant when the P-value was <0.05. n expresses the number of animals.
Materials
Krebs–Henseleit buffer was of the following composition (mM): NaCl 118, KCl 4.8, CaCl2·H2O 1.3, NaHCO3 25.0, KH2PO4 1.2, MgSO4·1.2 and glucose 11.1. Suramin, nordihydroguaiaretic acid (NDGA), nifedipine, thapsigargin, UTP, U46619, sodium nitroprusside (SNP), zafirlukast, BQ788 (N-cis-2,6-dimethylpiperidinocarbonyl-L-gmethylleucyl-D-1-methoxycarboyl-D-norleucine), UDP-glucose and UDP were purchased from Sigma (Poole, Dorset, UK), while DUP 697, MRS2578 {N,N″-1,4-butanediyl bis[N′-(3-isothiocynatophenyl)] thiourea}, PPADS and substance P, MRS2690, L-655,240, endothelin-1, BQ123 [cyc(DTrp-DAsp-Pro-D-Val-Leu)], Y-27632 {trans-4-[(1R)-1-aminoethyl]-N-4-pyridinyl-cyclohexane carboxamide} and forskolin were from Tocris Biosciences Ltd. (Bristol, UK). PPTN, a selective high-affinity antagonist of P2Y14 receptor, was a gift from Merck Frosst Centre for Therapeutic Research. Primary antibodies for Western blotting were purchased from Cell Signaling Technology (Danvers, MA, USA) for phosphorylated MLC2 antibody (Cat No. 3674S) and total MLC2 antibody (Cat No. 3672). cAMP EIA kit (Cat No. 581001) was purchased from Cayman Chemical Company. U46619 was dissolved in ethanol at 10 mM stock concentration. DUP 697, PPTN, BQ788, MRS2578, L-655,240, nifedipine, thapsigargin, zafirlukast and forskolin were dissolved in DMSO. All other drugs were dissolved in distilled water.
Results
Effect of UDP-glucose, UDP and MRS2690 in porcine isolated pancreatic arteries
To investigate the possible functional expression of P2Y14 receptors and their role in porcine pancreatic arteries, agonists for this receptor were applied as cumulative concentrations. MRS2690, a selective P2Y14 receptor agonist (0.1–30 μM), UDP-glucose (1 μM–1 mM) and UDP (1 μM–1 mM) were added after preconstriction with U46619. All of the agonists induced a concentration-dependent contraction with a rank order of potency of MRS2690 > UDP-glucose ≥ UDP. MRS2690 was significantly more potent, by approximately 10-fold, than UDP-glucose (P < 0.01, two-way anova; Figure 1A), while UDP-glucose and UDP responses were equipotent (Figure 1A). In other experiments, pre-exposure of the arteries to both UDP-glucose and UDP (P2Y14 receptor agonists) separately induced significant attenuation of the contraction to UDP-glucose; for instance, the response to 100 μM UDP-glucose was decreased by 55 ± 7% in the presence of 100 μM UDP-glucose and by 53 ± 7% in the presence of 100 μM UDP (P < 0.001, n = 10–13; Figure 1B).
Figure 1.
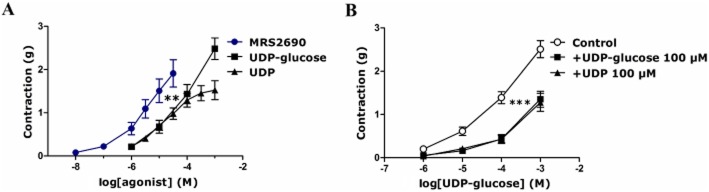
(A) Concentration-dependent contractions evoked by UDP-glucose, UDP and MRS2690 in U46619-preconstricted porcine pancreatic arteries (**P < 0.01, two-way anova, MRS2690 response vs. UDP-glucose and UDP responses, F = 13.74, 16.03; n = 9–12). (B) Attenuation of UDP-glucose-induced contraction (the control) in the presence of UDP-glucose (100 μM) and UDP (100 μM) (added 10 min prior to U46619 addition). Both UDP-glucose and UDP significantly attenuated the contraction evoked by UDP-glucose (***P < 0.001, two-way anova, UDP-glucose contraction in the absence or presence of UDP-glucose or UDP, F = 63.11, 56.48; n = 10–13). Data are presented as mean ± SEM.
Effect of PPADS and suramin on responses to UDP-glucose, UDP and MRS2690 in porcine isolated pancreatic arteries
Responses to UDP-glucose, UDP and MRS2690 were characterized using the non-selective P2 receptor antagonists PPADS (10 μM) and suramin (100 μM) (Rayment et al., 2007b). Both PPADS and suramin significantly enhanced the contractions evoked by UDP-glucose and UDP (Figure 2A,B). The contraction to 100 μM UDP-glucose was enhanced by 121 ± 38% (P < 0.001, n = 7) and 100 ± 23% (P < 0.001, n = 8) in the presence of PPADS and suramin respectively (Figure 2A). The contraction to 1 mM UDP was enhanced by 180 ± 46% (P < 0.001, n = 8) and 154 ± 30% (P < 0.001, n = 8) in the presence of PPADS and suramin respectively (Figure 2B). Suramin and PPADS failed to alter the contraction to MRS2690 (Figure 2C). In contrast, suramin and PPADS blocked the contraction to UTP, a P2Y2 and P2Y4 receptor agonist (von Kügelgen, 2006; M. Alsaqati, unpubl. obs.). UDP is a ligand at P2Y6 receptors as well as P2Y14 receptors. Therefore, the effect of UDP was examined in the presence of MRS2578 (10 μM), a P2Y6 receptor selective antagonist (Mamedova et al., 2004). The contraction evoked by UDP was unaffected at lower concentrations but was augmented at higher concentrations of UDP (Supporting Information), while MRS2578 did not alter the responses to UDP-glucose or MRS2690 (Supporting Information).
Figure 2.
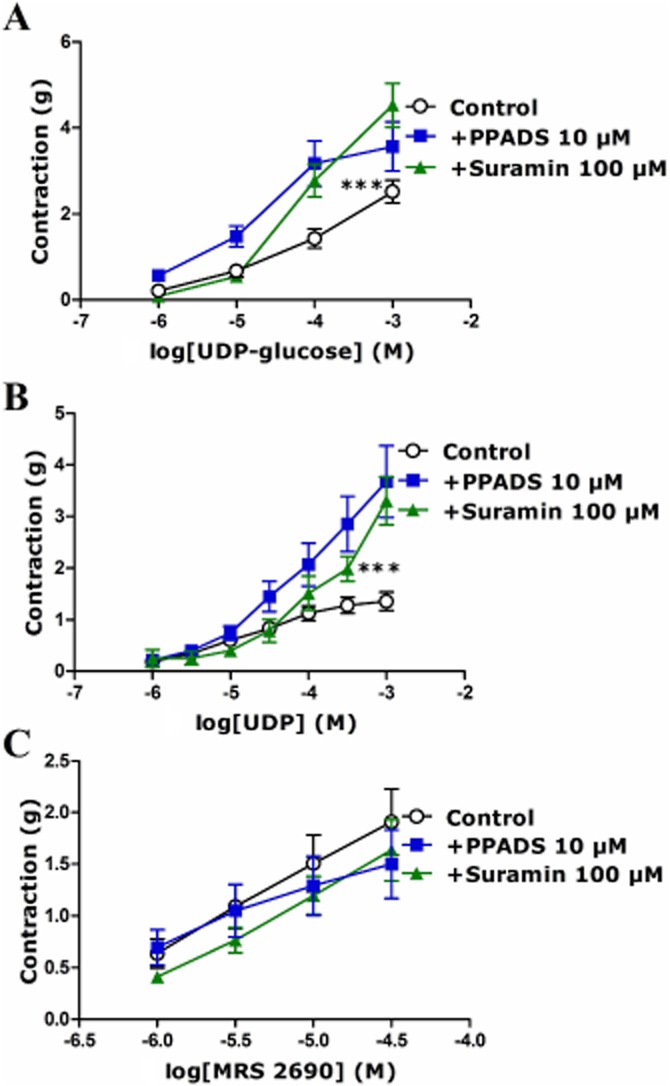
Effect of PPADS (10 μM) and suramin (100 μM) on responses to (A) UDP-glucose, (B) UDP and (C) MRS2690 in U46619-preconstricted porcine pancreatic arteries. (A) Suramin and PPADS enhanced the effects of UDP-glucose (***P < 0.001, two-way anova, UDP-glucose with suramin or PPADS vs. UDP-glucose alone, F = 19.85, 23.07; n = 6–10). (B) Suramin and PPADS enhanced the effects of UDP (***P < 0.001, two-way anova, UDP with suramin or PPADS vs. UDP alone, F = 16.83, 45.24; n = 8–16). (C) Suramin and PPADS had no effect on the contraction to MRS2690 (n = 5–9). Data are presented as mean ± SEM.
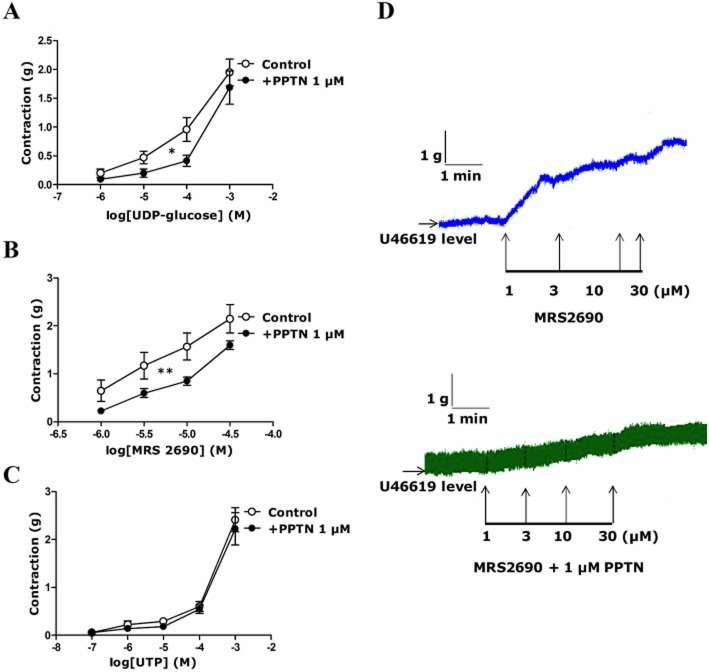
Effect of PPTN, a selective high-affinity antagonist of P2Y14 receptor, on responses to UDP-glucose and MRS2690 in porcine isolated pancreatic arteries
The responses to UDP-glucose and MRS2690 were examined in the presence of PPTN (1 μM), a selective high-affinity antagonist of P2Y14 receptors (Robichaud et al., 2011; Barrett et al., 2013). This compound significantly reduced the contraction evoked by UDP-glucose at basal tone (Supporting Information) and that by UDP-glucose and MRS2690 at raised tone (Figure 3A,B). PPTN inhibited the contraction to 100 μM UDP-glucose and 10 μM MRS2690 by 55 ± 10% (P < 0.05, n = 7) and 46 ± 9% (P < 0.01, n = 9) respectively (Figure 3A,B). The contraction to UTP was not altered in the presence of PPTN (Figure 3C). Typical traces, showing the effect of MRS2690 in the absence and presence of PPTN, are shown in Figure 3D.
Figure 3.
Effect of PPTN (1 μM), a P2Y14 receptor antagonist, on responses to (A) UDP-glucose, (B) MRS2690 and (C) UTP in U46619-preconstricted porcine pancreatic arteries. (A) PPTN inhibited the effect of UDP-glucose (*P < 0.05, two-way anova, F = 6.56; n = 7). (B) PPTN inhibited the effect of MRS2690 (**P < 0.01, two-way anova, F = 12.85; n = 9). (C) PPTN had no effect on the response to UTP (n = 8–10). Data are presented as mean ± SEM. (D) Typical traces showing the effect of MRS2690 in the absence and presence of PPTN.
Effect of endothelium removal on responses to UDP-glucose, UDP and MRS2690 in porcine isolated pancreatic arteries
The responses of UDP-glucose, UDP and MRS2690 were studied after the endothelium had been removed. The contractions induced by UDP-glucose, UDP and MRS2690 were significantly attenuated in the endothelium-denuded arteries (Figure 4). Removal of endothelium reduced the contractions to 1 mM UDP-glucose by 50 ± 7% (P < 0.001, n = 12), that to 1 mM UDP by 61 ± 11% (P < 0.001, n = 15) and that to 30 μM MRS2690 by 41 ± 5% (P < 0.01, n = 5) (Figure 4).
Figure 4.
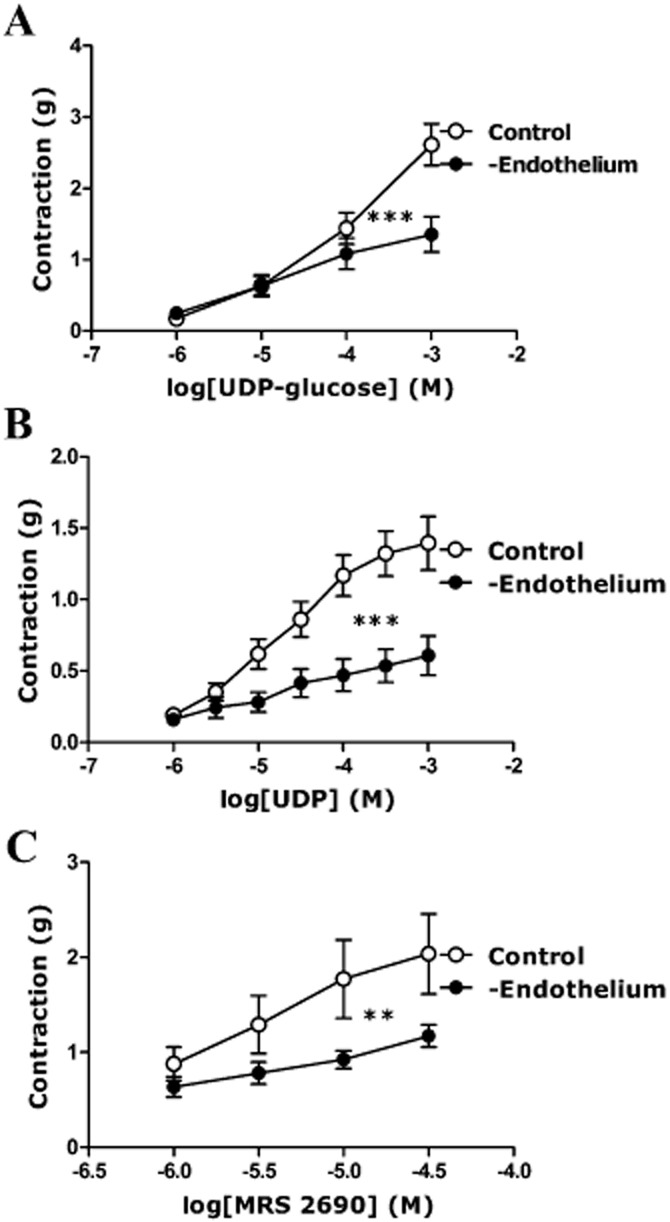
Effect of removal of the endothelium on responses to (A) UDP-glucose, (B) UDP and (C) MRS2690 in U46619-preconstricted porcine pancreatic arteries. The removal of endothelium reduced the contractions evoked by (A) UDP-glucose, (B) UDP and (C) MRS2690 (**P < 0.01, ***P < 0.001, two-way anova, F = 8.15, 51.24, 9.48; n = 4–15). Data are presented as mean ± SEM.
Effect of DUP 697 on responses to UDP-glucose, UDP and MRS2690 in porcine isolated pancreatic arteries
Because the contractions to P2Y14 receptor agonists were mainly endothelium dependent, these were studied in the presence of DUP 697, a COX-2 inhibitor, as COX-2 facilitates the release of agents that are responsible for endothelium-dependent contraction. DUP 697 (3 μM) diminished the responses to UDP-glucose, UDP and MRS2690 to a similar extent as removal of the endothelium (Figure 5), while DUP 697 did not alter the contraction to U46619 (the preconstriction agent) or the contraction to ATP (data not shown). DUP 697 reduced the contraction to 1 mM UDP-glucose by 34 ± 10% (P < 0.01, n = 11), that to 1 mM UDP by 38 ± 7.5% (P < 0.001, n = 15) and that to 30 μM MRS2690 by 22.5 ± 13% (P < 0.01, n = 4) (Figure 5).
Figure 5.
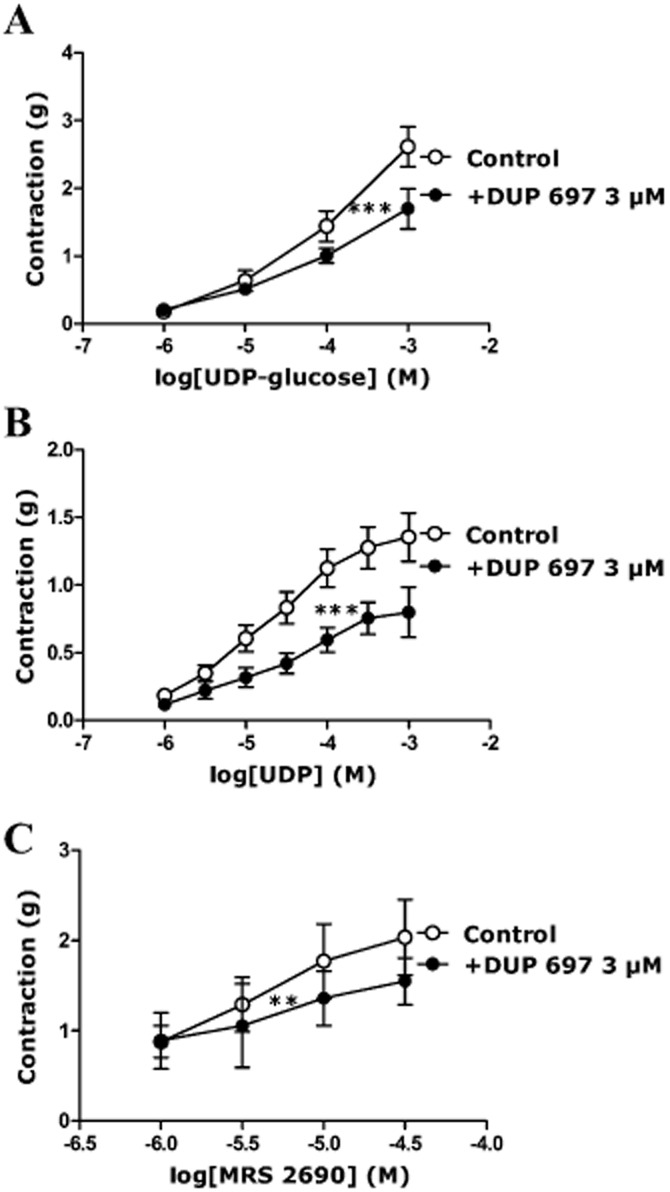
Effect of DUP 697 (3 μM) on responses to (A) UDP-glucose, (B) UDP, (C) MRS2690 in U46619-preconstricted porcine pancreatic arteries. DUP 697 inhibited the contractions evoked by (A) UDP-glucose, (B) UDP and (C) MRS2690 (** P < 0.01, *** P < 0.001, two-way anova, F = 7.85, 35.31, 4.95; n = 4–15). Data are presented as mean ± SEM.
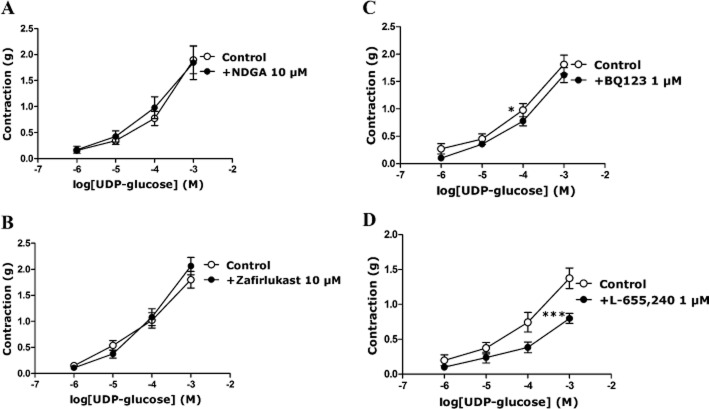
The role of endothelium-derived contracting factors in the response to UDP-glucose in porcine isolated pancreatic arteries
The following experiments were carried out using mainly UDP-glucose or UDP due to the cost considerations involved with use of MRS2690. The possible involvement of TxA2, LTs, endothelin-1 and PGF2α, which can be released from endothelial cells (Mombouli and Vanhoutte, 1993; Kurahashi et al., 2003; Wong et al., 2009), in endothelially mediated contraction to UDP-glucose was investigated. The contraction to UDP-glucose was not altered in the presence of NDGA (10 μM), a lipoxygenase inhibitor (Figure 6A), or zafirlukast (10 μM), an LT receptor inhibitor (Figure 6B). The contraction to UDP-glucose was reduced in the presence of BQ123 (1 μM), a selective ETA receptor antagonist; the response to 100 μM UDP-glucose was attenuated by 10 ± 3% (P < 0.05, n = 14) in the presence of BQ123 (Figure 6C), which was only effective in arteries with intact endothelium (Supporting Information). The contraction to UDP-glucose was unaltered in the presence of BQ788 (1 μM), a selective ETB receptor antagonist (Supporting Information). In addition, UDP-glucose induced-contraction was reduced in the presence of L-655,240 (1 μM), a selective TxA2/PG endoperoxide receptor antagonist. The response to 100 μM UDP-glucose was inhibited by 38 ± 5% (P < 0.001, n = 9) in the presence of L-655,240 (Figure 6D). These data indicate that UDP-glucose-mediated contraction occurs mainly via thromboxane and PGs, with a lesser involvement of endothelin-1.
Figure 6.
The contraction evoked by UDP-glucose in the presence of (A) NDGA (10 μM), (B) zafirlukast (10 μM), (C) BQ123 (1 μM) in U46619-preconstricted porcine pancreatic arteries, and (D) L-655,240 (1 μM) in endothelin-1-preconstricted porcine pancreatic arteries. (A, B) NDGA and zafirlukast did not alter the response to UDP-glucose (n = 7–10). (C, D) BQ-123 and L-655,240 inhibited the response evoked by UDP-glucose (*P < 0.05, ***P < 0.001, two-way anova, F = 4.97, 19.03; n = 9–14). Data are presented as mean ± SEM.
Effect of inhibition of calcium release and calcium entry on the response to UDP-glucose and UDP in porcine isolated pancreatic arteries
Binding of agonist to the recombinant P2Y14 receptor leads to increase Ca2+ flux in some cells (Skelton et al., 2003; Gao et al., 2010). To test this in porcine pancreatic arteries, responses to UDP-glucose and UDP were examined in the presence and absence of nifedipine (1 μM), an L-type voltage-gated calcium channel blocker, and thapsigargin (100 nM), a potent inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases, which leads to depletion of intracellular calcium. Both of these inhibitors reduced the contraction evoked by 100 μM UDP-glucose, by 39 ± 5% (P < 0.001, n = 15) in the presence of nifedipine and by 25 ± 8% (P < 0.05, n = 12–15) in the presence of thapsigargin (Figure 7A,B). Responses to 100 μM UDP were inhibited by 53 ± 2% (P < 0.001, n = 9) in the presence of nifedipine and by 36 ± 6% (P < 0.001, n = 9) in the presence of thapsigargin (data not shown). Typical traces showing the effect of UDP-glucose in the absence and presence of nifedipine are shown in Figure 7C.
Figure 7.

Effect of (A) nifedipine (1 μM) and (B) thapsigargin (100 nM) on the response to UDP-glucose in U46619-preconstricted porcine pancreatic arteries. Both inhibitors, nifedipine and thapsigargin, inhibited the contraction evoked by UDP-glucose (*P < 0.05, ***P < 0.001, two-way anova, F = 32.5, 5.84; n = 12–15). Data are presented as mean ± SEM. (C) Typical traces showing the responses of UDP-glucose in the absence and presence of nifedipine.
Effect of inhibition of the Rho-kinase pathway on the responses to UDP-glucose, UDP and MRS2690 in porcine isolated pancreatic arteries
Activation of P2Y14 receptors may cause stimulation of RhoA/ROCK signalling (Sesma et al, 2012). To test the possible involvement of this pathway, experiments were conducted to study the contractions to UDP-glucose, UDP and MRS2690 in the presence of Y-27632 (5 μM), a selective inhibitor of the Rho-associated protein kinase, p160ROCK. Y-27632 significantly inhibited the contraction evoked by UDP (Figure 8A), MRS2690 (Figure 8B) and UDP-glucose (data not shown). For instance, the response to 300 μM UDP was reduced by 50 ± 4% (P < 0.001, n = 15; Figure 8A), that to 30 μM MRS2690 by 50 ± 15% (P < 0.001, n = 9; Figure 8B) and that to 1 mM UDP-glucose by 52 ± 8% (P < 0.001, n = 9, data not shown). The response to UDP-glucose was associated with an increase in the level of MLC2 phosphorylation, after 30–60 s of treatment with 100 μM UDP-glucose, and returned to the basal level of phosphorylated MLC2 from 120 to 300 s (Figure 8C), which suggested an involvement of MLC2 activation in the response to UDP-glucose P2Y14 receptor signalling pathway. The ability of UDP-glucose (100 μM) or MRS2690 (10 μM) to elevate the MLC2 phosphorylation (after 30 s of treatment) was abolished in the presence of PPTN (1 μM), which shows that the ability of UDP-glucose or MRS2690 to elevate MLC2 phosphorylation in porcine pancreatic arteries is mediated by P2Y14 receptors (Figure 9).
Figure 8.
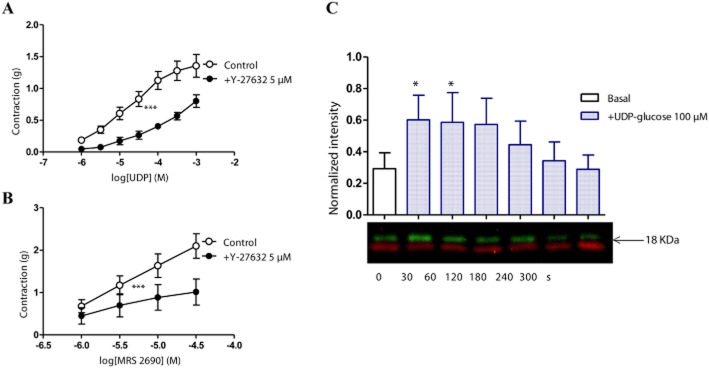
Effect of Y-27632 (5 μM), a selective inhibitor of the Rho-associated protein kinase, on responses to (A) UDP and (B) MRS2690 in U46619-preconstricted porcine pancreatic arteries. Y-27632 reduced the contraction to (A) UDP and (B) MRS2690 (***P < 0.001, two-way anova, F = 53.07, 10.32; n = 9–15). Data are presented as mean ± SEM. (C) MLC2 phosphorylation induced by 100 μM UDP-glucose in porcine pancreatic arteries. A time course (above) and a representative blot (below) of phospho- (green) and total- (red) MLC2 (*P < 0.05, one-way anova, n = 4).
Figure 9.
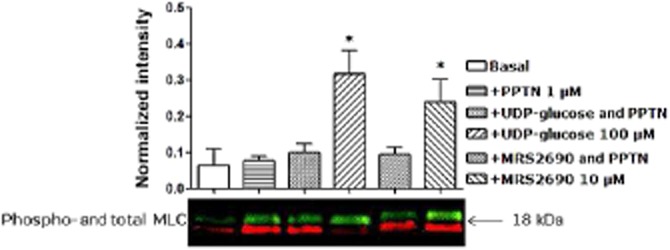
PPTN, a selective high-affinity antagonist of P2Y14 receptor, abolished the ability of UDP-glucose and MRS2690 to elevate the level of MLC phosphorylation (*P < 0.05, Student's unpaired t-test, n = 3). Data are presented as mean ± SEM. Representative immunoblots of phospho- (green) and total- (red) MLC2 from three separate experiments in the absence or presence of PPTN.
Effect of pre-constriction with U46619, and relaxation with forskolin, on the response to UDP-glucose in porcine isolated pancreatic arteries
Agonist-promoted activation of Gi and subsequent inhibition of AC is one of the signalling responses of P2Y14 receptors. Therefore, the response to UDP-glucose was tested after exposure to U46619 and subsequent relaxation by forskolin (back to the baseline), involving elevation of intracellular cAMP. UDP-glucose induced a greater contraction compared with the control, in which UDP-glucose was applied at basal tone without the tissues being exposed to U46619 or forskolin (Figure 10). The contraction to 100 μM UDP-glucose was enhanced by 930 ± 108% (P < 0.001, n = 11) after the exposure to U46619 and forskolin (Figure 10A). In contrast, when responses to UTP, an agonist at P2Y2 and P2Y4 receptors (Gq-protein coupled receptors), were investigated in vessels exposed to U46619 and forskolin, there was no change in the contractions (Figure 10B). In addition, the response to UDP-glucose was not altered after contraction with U46619 and relaxation with SNP (100 μM), which elevates intracellular cGMP (Roberts et al., 1999). Typical traces showing the effect of UDP-glucose on basal tone and after the tissues had been contracted by U46619 and then relaxed with forskolin are shown in Figure 10C.
Effect of preconstriction with U46619 followed by relaxation with forskolin (1 μM) on the responses to (A) UDP-glucose and (B) UTP in porcine pancreatic arteries. (A) Exposing the tissues to U46619 followed by forskolin significantly enhanced the contraction evoked by UDP-glucose (***P < 0.001, two-way anova, F = 54.34; n = 8–13). Data are presented as mean ± SEM. (B) Exposing the tissues to U66619 followed by forskolin failed to affect the response to UTP. (C) Typical traces showing the effect of UDP-glucose on basal tone (inset) and after the tissues were preconstricted with U46619 and then relaxed with forskolin (main).
Effect of UDP-glucose and MRS2690 on the cAMP level in porcine isolated pancreatic arteries
On the basis that cAMP is involved in the contraction to P2Y14 receptor agonists, we measured the cellular levels of this second messenger in pancreatic artery rings. We investigated the effects of UDP-glucose, MRS2690 and UTP (as a negative control, as it is coupled to Gq protein) on cAMP levels in the presence of U46619 + forskolin (to mimic the raised tone condition of the pharmacology experiments). UDP-glucose (1 mM) and MRS2690 (10 μM) induced a significant decrease in the cAMP level relative to the control (U46619 + forskolin only, expressed as 100%) (*P < 0.05, n = 4), while UTP had no significant effect on cAMP levels (Figure 11).
Figure 11.
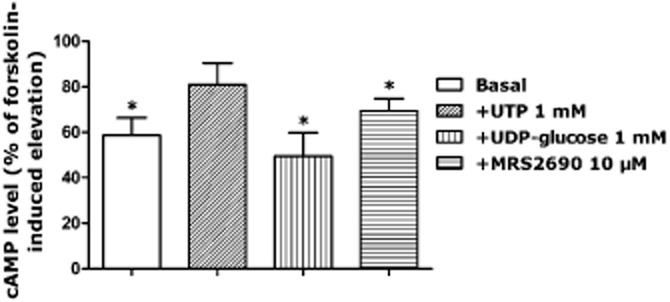
Effect of UTP (1 mM), UDP-glucose 1 (mM) and MRS2690 (10 μM) on the cAMP concentrations in porcine pancreatic arteries exposed to U46619 followed by forskolin. UTP had no significant effect on cAMP level, while UDP-glucose and MRS2690 significantly reduced the cAMP level (*P < 0.05, Student's unpaired t-test, the response to UTP or UDP-glucose or MRS2690 vs. their respective controls, n = 4). Basal cAMP level represents the level of cAMP in the absence of forskolin, which was also significantly different from that in the presence of forskolin (*P < 0.05, Student's unpaired t-test, n = 4). Data are presented as mean ± SEM.
Discussion
The current study presents evidence for the functional expression of contractile P2Y14 receptors, sensitive to the endogenous nucleotides UDP-glucose and UDP in porcine pancreatic arteries. Evidence from the contractile studies and the cAMP immunoassay is consistent with the ability of this receptor to inhibit AC, while immunoblotting indicates the downstream involvement of MLC2. The contractile response was mediated largely by the endothelium with an involvement of endothelin, TxA2 and PGF2α.
Contractile studies showed that contractions to UDP-glucose and UDP were almost equipotent, whereas MRS2690, a selective P2Y14 receptor agonist, was approximately 10-fold more potent than UDP-glucose at the P2Y14 receptor (Figure 11A). This is consistent with previous reports, which suggest a 7–10-fold greater potency of MRS2690 over UDP-glucose (Jacobson et al., 2009; Gao et al., 2010). In the present study, MRS2690 activity was observed at ≤10 μM; at 10 μM, MRS2690 is inactive at P2Y2 receptors (Ko et al., 2009).
PPTN is a non-nucleotide, high-affinity competitive antagonist at P2Y14 receptors. It was assessed in HEK cells using a calcium mobilization assay and it inhibited UDP-glucose-mediated signalling, and showed no effect on other P2Y receptors at concentrations up to 10 μM (Robichaud et al., 2011; Barrett et al., 2013). The responses to the P2Y14 receptor agonists were examined in the presence of this antagonist. PPTN (1 μM) blocked the contractions induced by UDP-glucose and MRS2690, which confirms the involvement of P2Y14 receptors in our arteries (Figure 3A,B).
The responses to the P2Y14 receptor agonists were examined in the presence of the non-selective P2 receptor antagonists, suramin and PPADS. The non-selective antagonists induced a small increase in the contractions to UDP and UDP-glucose (Figure 2A,B). However, they failed to change the response to MRS2690 (Figure 2C). The lack of effect of suramin and PPADS appears to rule out an involvement of P2Y2 and/or P2Y4 receptors as we have shown that they blocked the responses to UTP in porcine pancreatic arteries (M. Alsaqati, unpubl. obs.) and other tissues (Rayment et al., 2007b). It is unclear why these antagonists enhanced the effects of P2Y14 receptor agonists. However, it is clear that suramin and PPADS had different effects on responses to UTP from those on responses to MRS2690 and UDP-glucose, indicating actions at different receptors.
Contractile responses to UDP-glucose, UDP and MRS2690 were significantly inhibited after the endothelium was removed (Figure 4), which indicated that the main expression of P2Y14 receptors may be on the endothelial layer of porcine pancreatic arteries. Similarly, the P2Y14 receptor is expressed in endothelial cells of porcine coronary artery, human lung microvascular endothelial cells and pulmonary artery vasa vasorum endothelial cells (Umapathy et al., 2010; Abbas et al., 2011; Lyubchenko et al., 2011). P2Y14 receptor expression was barely detectable in mouse thoracic aorta (Kauffenstein et al., 2010); in contrast, rat aortic smooth muscle cells show robust expression of the receptor, as observed in freshly isolated and cultured cells (Govindan et al., 2010).
To investigate the mechanism underlying the contraction mediated by P2Y14 receptors in pancreatic arteries, responses to UDP-glucose, UDP and MRS2690 were examined in the presence of DUP 697. As seen in Figure 5, the endothelium-dependent contractions were attenuated in the presence of the selective COX-2 inhibitor. Endothelial cells can release contractile factors (EDCFs), which may include TxA2, PGF2α, LTs and endothelin-1. TxA2 and PGF2α are released from the endothelium as products of COX-2 (Mombouli and Vanhoutte, 1993; Wong et al., 2009). To characterize EDCFs involved in the contraction to UDP-glucose, experiments were conducted to study the responses to UDP-glucose in the presence of zafirlukast, BQ123, BQ788 and L-655,240. Only BQ123 and L-655,240 were able to inhibit the contraction evoked by UDP-glucose, which indicated an involvement of TxA2, PGs and endothelin-1 (Figure 6). These agents, after being released from the endothelium, may act on their receptors on vascular smooth muscle cells (Wong et al., 2009).
The involvement of extracellular calcium influx and calcium released from sarcoplasmic reticulum (SR) as a part of the response to P2Y14 receptor activation has been reported (Verin et al., 2011). In addition, calcium-induced release of calcium from SR and influx of external Ca2+ in excitation-contraction in response to some activated receptors have been also reported (Fabiato, 1983; Li et al., 2003). In porcine pancreatic arteries, contractions to UDP-glucose (Figure 7A,B) and UDP (data not shown) were significantly decreased in the presence of nifedipine or thapsigargin, which identified an involvement of elevated intracellular calcium level. Collectively, our results suggest that when UDP-glucose, UDP and MRS2690 act at the P2Y14 receptors, which are expressed mainly on the endothelium, the intracellular Ca2+ levels may be elevated; this may then lead to the release of endothelin-1 and activation of PLA2, which liberates arachidonic acid, which is then converted by the activity of COX-2 to produce TxA2 and PGs, which bind to receptors on vascular smooth muscle cells to induce contraction.
A number of studies have considered the signalling mechanism underlying the functional response of P2Y14 receptors (Harden et al., 2010; Sesma et al., 2012). Recent reports claimed that UDP-glucose promotes concentration-dependent activation of RhoA in isolated human neutrophils (Sesma et al., 2012). This was tested in porcine pancreatic arteries when responses were investigated in the presence of Y-27632; this compound inhibited the contraction evoked by UDP-glucose, UDP and MRS2690 (Figure 8A,B), which showed an involvement of RhoA in the response to P2Y14 receptor agonists. Activation of RhoA leads to phosphorylation of MLC and subsequently contraction of arteries in a calcium-independent manner (Amano et al., 1996; Hartshorne and Gorecka, 2011). Accordingly, when UDP-glucose or MRS2690 were incubated with the tissues, the level of phosphorylated MLC2 was elevated, but this elevation was abolished in the presence of PPTN, confirming that it is happening through the activation of P2Y14 receptors (Figures 8 and 9).
It is well established that the P2Y14 receptor is coupled to Gi protein, leading to the inhibition of AC activity, and hence inhibition of the cAMP level. Accordingly, the P2Y14 receptor is PTX-sensitive (Jacobson et al., 2009). It is notoriously difficult to successfully block Gi coupled receptors with PTX in isolated blood vessels, as we have also found. However, in tissues that had been preconstricted with U46619 and relaxed with forskolin (to elevate the cAMP levels), subsequent addition of UDP-glucose produced a significantly greater contraction compared with the controls (UDP-glucose added at basal tone, or UTP applied after preconstriction with U46619 and relaxation with forskolin) (Figure 10), consistent with an involvement of Gi coupled receptors. Other purine receptors, namely P2Y1, P2Y2, P2Y4 and P2Y6 receptors, are generally Gq protein-coupled. Furthermore, relaxation with SNP (to elevate cGMP levels) after preconstriction with U46619 had no effect on the contractile response to UDP-glucose (data not shown). These findings, together with the data obtained from cAMP assay (Figure 1), indicate that the enhanced contraction to UDP-glucose is mainly dependent on the agonist's ability to lower cAMP levels.
Reduction in pancreatic blood flow has been observed in acute and chronic pancreatitis and some other pancreatic diseases (Satoh et al., 2000; Nguyen et al., 2010), implicating pancreatic tissue perfusion as an important factor in pathogenesis of pancreatic diseases and symptoms. There is increasing evidence for the role of purinergic signalling in the pathophysiology of the pancreas (Burnstock and Novak, 2012). Drugs designed to target specific components of the purinergic system may be of relevance to the management of pancreatitis, cystic fibrosis, pancreatic cancer and diabetes.
In conclusion, this study has shown a novel vasocontractor action of UDP-glucose, which appears to be mediated by the P2Y14 receptor in porcine isolated pancreatic arteries.P2Y14 receptors induce contraction via an involvement of endothelin-1, TxA2 and PGs released from the endothelium. In addition, P2Y14-mediated contraction involves the activation of Rho kinase and the subsequent phosphorylation of MLC2. The ability of P2Y14 receptor agonist to inhibit cAMP levels indicates P2Y14 receptor coupling to Gi proteins. This study identifies a novel role for the P2Y14 receptor as a mediator of vasoconstriction.
Acknowledgments
We would like to thank Merck Frosst Centre for Therapeutic Research for the generous gift of PPTN, Dr. W Cameron Black for his helpful discussion, Dr. Richard Roberts for his generous donation of BQ123 and Damascus University, Syria for funding the project.
Glossary
- IP3
inositol 1,4,5-triphosphate
- MLC2
myosin light chain 2
- NDGA
nordihydroguiaretic acid
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid)
- PPTN
4-(4-(Piperidin-4-yl)phenyl)-7-(4-(trifluoromethyl)phenyl)-2-naphthoic acid
- RhoA
ras homolog gene family member A
- ROCK
Rho-associated protein kinase
- SNP
sodium nitroprusside
- UDP-glucose
uridine diphosphate glucose
Conflict of interest
The authors declare that they have not any conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site: http://dx.doi.org/10.1111/bph.12473
Effect of BQ123 (1 μM) in endothelium-denuded arteries and the effect of endothelium removal on responses to UDP-glucose in U46619-preconstricted porcine pancreatic arteries. BQ123 in endothelium-denuded arteries did not induce further reduction of the contraction to UDP-glucose (***P < 0.001, two-way ANOVA, F > 8.149, 9.294; n > 14). Data are presented as mean ± SEM.
Effect of BQ788 (1 μM) on response to UDPglucose in U46619-preconstricted porcine pancreatic arteries. BQ788 had no effect on the contraction to UDP-glucose (n > 7– 8). Data are presented as mean ± SEM.
Effect of MRS2578 (10 μM) on responses to UDP, UDP-glucose and MRS2690 in U46619-preconstricted porcine pancreatic arteries. (A) MRS2578 enhanced significantly the contraction evoked by UDP (**P < 0.01, two-way ANOVA, F > 9.953; n > 8– 13). (B, C) MRS2578 failed to alter the contraction to UDP-glucose and MRS2690 (n > 6– 13). Data are presented as mean ± SEM.
Concentration-dependent contraction evoked by UDP-glucose, in porcine pancreatic arteries in the presence and absence of PPTN (1 μM) (***P < 0.001, two-way ANOVA, F > 24.37; n > 12).
References
- Abbas Z, Latif L, Ralevic V. 2011. Effects of P2Y14 receptor agonists, UDP-glucose and MRS2690, on porcine coronary artery contractility. Available at: http://www.pA2online.org/abstracts/Vol9Issue3abst051p.pdf (accessed 4/9/2012)
- Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, et al. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G, Boeynaems J-M, Barnard EA, Boyer JL, Kennedy C, et al. International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaqati M, Latif LL, Chan SLF, Ralevic R. 2010. Identification of the novel P2Y14 receptor in porcine isolated pancreatic arteries. Available at: http://www.pa2online.org/abstracts/vol8issue1abst028p.pdf (accessed 4/9/2012)
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Barrett MO, Sesma JI, Ball CB, Jayasekara PS, Jacobson KA, Lazarowski ER, et al. Selective high-affinity antagonist of the P2Y14 receptor inhibits UDP-glucose-stimulated chemotaxis of human neutrophils. Mol Pharmacol. 2013;84:41–49. doi: 10.1124/mol.113.085654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Novak I. Purinergic signalling in the pancreas in health and disease. J Endocrinol. 2012;213:123–141. doi: 10.1530/JOE-11-0434. [DOI] [PubMed] [Google Scholar]
- Carter RL, Fricks IP, Barrett MO, Burianek LE, Zhou Y, Ko H, et al. Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor. Mol Pharmacol. 2009;76:1341–1348. doi: 10.1124/mol.109.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, et al. A G protein-coupled receptor for UDP-glucose. J Biol Chem. 2000;275:10767–10771. doi: 10.1074/jbc.275.15.10767. [DOI] [PubMed] [Google Scholar]
- Chootip K, Gurney AM, Kennedy C. Multiple P2Y receptors couple to calcium-dependent, chloride channels in smooth muscle cells of the rat pulmonary artery. Respir Res. 2005;6:124. doi: 10.1186/1465-9921-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fricks IP, Maddileti S, Carter RL, Lazarowski ER, Nicholas RA, Jacobson KA, et al. UDP is a competitive antagonist at the human P2Y14 receptor. J Pharmacol Exp Ther. 2008;325:588–594. doi: 10.1124/jpet.108.136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Ding Y, Jacobson KA. UDP-glucose acting at P2Y14 receptors is a mediator of mast cell degranulation. Biochem Pharmacol. 2010;79:873–879. doi: 10.1016/j.bcp.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan S, Taylor EJ, Taylor CW. Ca(2+) signalling by P2Y receptors in cultured rat aortic smooth muscle cells. Br J Pharmacol. 2010;160:1953–1962. doi: 10.1111/j.1476-5381.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden TK, Sesma JI, Fricks IP, Lazarowski ER. Signalling and pharmacological properties of the P2Y receptor. Acta Physiol (Oxf) 2010;199:149–160. doi: 10.1111/j.1748-1716.2010.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne DJ, Gorecka A. Biochemistry of the contractile proteins of smooth muscle. In: Terjung R, editor. Comprehensive Physiology. Hoboken: John Wiley & Sons; 2011. pp. 93–120. Chapter 4. [Google Scholar]
- Hillaire-Buys D, Chapal J, Petit P, Loubatières-Mariani M-M. Dual regulation of pancreatic vascular tone by P2X and P2Y purinoceptor subtypes. Eur J Pharmacol. 1991;199:309–314. doi: 10.1016/0014-2999(91)90494-b. [DOI] [PubMed] [Google Scholar]
- Högberg C, Svensson H, Gustafsson R, Eyjolfsson A, Erlinge D. The reversible oral P2Y12 antagonist AZD6140 inhibits ADP-induced contractions in murine and human vasculature. Int J Cardiol. 2010;142:187–192. doi: 10.1016/j.ijcard.2008.12.091. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Ivanov AA, de Castro S, Harden TK, Ko H. Development of selective agonists and antagonists of P2Y receptors. Purinergic Signal. 2009;5:75–89. doi: 10.1007/s11302-008-9106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D'Orléans-Juste P, et al. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Das A, Carter RL, Fricks IP, Zhou Y, Ivanov AA, et al. Molecular recognition in the P2Y(14) receptor: probing the structurally permissive terminal sugar moiety of uridine-5′-diphosphoglucose. Bioorg Med Chem. 2009;17:5298–5311. doi: 10.1016/j.bmc.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Kurahashi K, Nishihashi T, Trandafir CC, Wang AM, Murakami S, Ji X. Diversity of endothelium-derived vasocontracting factors – arachidonic acid metabolites. Acta Pharmacol Sin. 2003;24:1065–1069. [PubMed] [Google Scholar]
- Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential extracellular signalling molecule. Mol Pharmacol. 2003;63:1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- Li N, Sul JY, Haydon PG. A calcium-induced calcium influx factor, nitric oxide, modulates the refilling of calcium stores in astrocytes. J Neurosci. 2003;23:10302–10310. doi: 10.1523/JNEUROSCI.23-32-10302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubchenko T, Woodward H, Veo KD, Burns N, Nijmeh H, Liubchenko GA, et al. P2Y1 and P2Y13 purinergic receptors mediate Ca2+ signaling and proliferative responses in pulmonary artery vasa vasorum endothelial cells. Am J Physiol Cell Physiol. 2011;300:C266–C275. doi: 10.1152/ajpcell.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamedova LK, Joshi BV, Gao ZG, von Kugelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–1770. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM. Purinergic endothelium-dependent and -independent contractions in rat aorta. Hypertension. 1993;22:577–583. doi: 10.1161/01.hyp.22.4.577. [DOI] [PubMed] [Google Scholar]
- Nguyen NC, Taalab K, Osman MM. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin Cancer Res. 2010;16:367. doi: 10.1158/1078-0432.CCR-09-2512. Author reply 567. [DOI] [PubMed] [Google Scholar]
- Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal. 2008;4:237–253. doi: 10.1007/s11302-007-9087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rayment SJ, Ralevic V, Barrett DA, Cordell R, Alexander SP. A novel mechanism of vasoregulation: ADP-induced relaxation of the porcine isolated coronary artery is mediated via adenosine release. FASEB J. 2007a;21:577–585. doi: 10.1096/fj.06-7050com. [DOI] [PubMed] [Google Scholar]
- Rayment SJ, Latif ML, Ralevic V, Alexander SP. Evidence for the expression of multiple uracil nucleotide-stimulated P2 receptors coupled to smooth muscle contraction in porcine isolated arteries. Br J Pharmacol. 2007b;150:604–612. doi: 10.1038/sj.bjp.0707120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Kendall DA, Wilson VG. α2-Adrenoceptor and NPY receptor-mediated contractions of porcine isolated blood vessels: evidence for involvement of the vascular endothelium. Br J Pharmacol. 1999;128:1705–1712. doi: 10.1038/sj.bjp.0702979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud J, Fournier JF, Gagne S, Gauthier JY, Hamel M, Han Y, et al. Applying the pro-drug approach to afford highly bioavailable antagonists of P2Y(14) Bioorg Med Chem Lett. 2011;21:4366–4368. doi: 10.1016/j.bmcl.2010.12.113. [DOI] [PubMed] [Google Scholar]
- Satoh A, Shimosegawa T, Satoh K, Ito H, Kohno Y, Masamune A, et al. Activation of adenosine A1-receptor pathway induces edema formation in the pancreas of rats. Gastroenterology. 2000;119:829–836. doi: 10.1053/gast.2000.16502. [DOI] [PubMed] [Google Scholar]
- Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in murine T-lymphocytes. Br J Pharmacol. 2005;146:435–444. doi: 10.1038/sj.bjp.0706322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in human neutrophils. Eur J Pharmacol. 2006;543:166–173. doi: 10.1016/j.ejphar.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Sesma JI, Kreda SM, Steinckwich-Besancon N, Dang H, García-Mata R, Harden TK, et al. The UDP-sugar-sensing P2Y14 receptor promotes Rho-mediated signalling and chemotaxis in human neutrophils. Am J Physiol Cell Physiol. 2012;303:C490–C498. doi: 10.1152/ajpcell.00138.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton L, Cooper M, Murphy M, Platt A. Human immature monocyte-derived dendritic cells express the G protein-coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol. 2003;171:1941–1949. doi: 10.4049/jimmunol.171.4.1941. [DOI] [PubMed] [Google Scholar]
- Umapathy NS, Hick RT, Yu Y, Verin AD. Functional expression of the purinergic receptor P2Y14 in the human lung microvascular endothelial cells (HLMVEC) FASEB J. 2010;24(Meeting Abstract Supplement):111.9. [Google Scholar]
- Verin A, Zemskov E, Umapathy N, Lucas R. P2Y receptors as regulators of lung endothelial barrier integrity. J Cardiovasc Dis Res. 2011;2:14–22. doi: 10.4103/0975-3583.78582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wihlborg AK, Wang L, Braun O, Eyjolfsson A, Ronny G, Gudbjartsson T, et al. ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:1810–1815. doi: 10.1161/01.ATV.0000142376.30582.ed. [DOI] [PubMed] [Google Scholar]
- Wong SL, Leung FP, Lau CW, Au CL, Yung LM, Yao X, et al. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ Res. 2009;104:228–235. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of BQ123 (1 μM) in endothelium-denuded arteries and the effect of endothelium removal on responses to UDP-glucose in U46619-preconstricted porcine pancreatic arteries. BQ123 in endothelium-denuded arteries did not induce further reduction of the contraction to UDP-glucose (***P < 0.001, two-way ANOVA, F > 8.149, 9.294; n > 14). Data are presented as mean ± SEM.
Effect of BQ788 (1 μM) on response to UDPglucose in U46619-preconstricted porcine pancreatic arteries. BQ788 had no effect on the contraction to UDP-glucose (n > 7– 8). Data are presented as mean ± SEM.
Effect of MRS2578 (10 μM) on responses to UDP, UDP-glucose and MRS2690 in U46619-preconstricted porcine pancreatic arteries. (A) MRS2578 enhanced significantly the contraction evoked by UDP (**P < 0.01, two-way ANOVA, F > 9.953; n > 8– 13). (B, C) MRS2578 failed to alter the contraction to UDP-glucose and MRS2690 (n > 6– 13). Data are presented as mean ± SEM.
Concentration-dependent contraction evoked by UDP-glucose, in porcine pancreatic arteries in the presence and absence of PPTN (1 μM) (***P < 0.001, two-way ANOVA, F > 24.37; n > 12).