Abstract
Background and Purpose
Despite ample evidence supporting the N-methyl-d-aspartate receptor (NMDAR) hypofunction hypothesis of schizophrenia, progress in the development of effective therapeutics based on this hypothesis has been limited. Facilitation of NMDA receptor function by co-agonists (d-serine or glycine) only partially alleviates the symptoms in schizophrenia; other means to facilitate NMDA receptors are required. NMDA receptor sub-types differ in their subunit composition, with varied GluN2 subunits (GluN2A-GluN2D) imparting different physiological, biochemical and pharmacological properties. CIQ is a positive allosteric modulator that is selective for GluN2C/GluN2D-containing NMDA receptors (Mullasseril et al.).
Experimental Approach
The effect of systemic administration of CIQ was tested on impairment in prepulse inhibition (PPI), hyperlocomotion and stereotypy induced by i.p. administration of MK-801 and methamphetamine. The effect of CIQ was also tested on MK-801-induced impairment in working memory in Y-maze spontaneous alternation test.
Key Results
We found that systemic administration of CIQ (20 mg·kg−1, i.p.) in mice reversed MK-801 (0.15 mg·kg−1, i.p.)-induced, but not methamphetamine (3 mg·kg−1, i.p.)-induced, deficit in PPI. MK-801 increased the startle amplitude to pulse alone, which was not reversed by CIQ. In contrast, methamphetamine reduced the startle amplitude to pulse alone, which was reversed by CIQ. CIQ also partially attenuated MK-801- and methamphetamine-induced hyperlocomotion and stereotyped behaviours. Additionally, CIQ reversed the MK-801-induced working memory deficit in spontaneous alternation in a Y-maze.
Conclusion and Implications
Together, these results suggest that facilitation of GluN2C/GluN2D-containing receptors may serve as an important therapeutic strategy for treating positive and cognitive symptoms in schizophrenia.
Keywords: GluN2C, GluN2D, CIQ, NMDA receptor, schizophrenia, GRIN2C, GRIN2D, prepulse inhibition, working memory in Y-maze
Introduction
Schizophrenia is characterized by a constellation of positive and negative symptoms and cognitive deficits. Two prominent hypotheses propose that schizophrenia arises due to dopamine transmission hyperfunction or due to glutamate hypofunction. Although dopaminergic signalling has an undisputed role in the pathophysiology of the disease, a growing body of evidence implicates hypofunction of NMDA receptors as a major pathological factor for schizophrenia that may more broadly account for the symptoms (Lisman et al., 2008; Kantrowitz and Javitt, 2010; Gilmour et al., 2012; receptor and subunit nomenclature follows Alexander et al., 2013). This hypothesis is originally based on the finding that NMDA receptor channel blockers, such as phencyclidine, ketamine or MK-801, produce a robust psychotomimetic state that is clinically indistinguishable from schizophrenia (Javitt and Zukin, 1991; Krystal et al., 1994). More recently, converging evidence from human genetic association and expression studies, and studies in animal models have strengthened the notion that impaired NMDA receptor function may lead to emergence of symptoms of schizophrenia (Lin et al., 2012; Moghaddam and Javitt, 2012; Snyder and Gao, 2013).
NMDA receptors are tetrameric receptors composed of two obligatory GluN1 and two GluN2 subunits. Most of the therapeutic agents acting on NMDA receptors tested for schizophrenia have targeted the obligatory GluN1 subunit to facilitate receptor function. Nonetheless, these agents, including glycine and d-serine, are only partially effective and are accompanied by pharmacokinetic limitations (Tsai and Lin, 2010; Lin et al., 2012). Furthermore, the effectiveness of these glycine-site agonists depends upon the extent to which that site is not already saturated in vivo. As these agents target the common GluN1 subunit, they cannot be readily modified to distinguish different NMDA receptor subtypes that differ in their GluN2 composition.
The four GluN2 subtypes (GluN2A through GluN2D) confer distinct physiological and pharmacological properties to the receptor complex. Interestingly, GluN2C/GluN2D-containing receptors display lower sensitivity to channel blockade by extracellular Mg2+ (Monyer et al., 1994) and are therefore more susceptible to channel blockers such as ketamine (Kotermanski and Johnson, 2009). Expression analysis suggest that GluN2C/GluN2D are localized to the interneurons in corticolimbic circuitry (Monyer et al., 1994; Rudolf et al., 1996; Standaert et al., 1996; Scherzer et al., 1998; Munoz et al., 1999; Xi et al., 2009), and therefore, the higher sensitivity of GluN2C/GluN2D receptors for NMDA receptor channel blockers may lead to disinhibition of the corticolimbic circuitry, which is a pathological feature in schizophrenia supported by observation of reduced expression of inhibitory markers such as glutamate decarboxylase 67 (GAD67) and parvalbumin (Akbarian and Huang, 2006; Gonzalez-Burgos et al., 2010; Nakazawa et al., 2012). This is also consistent with the finding that selective knockdown of NMDA receptors from corticolimbic interneurons leads to the emergence of schizophrenia-like phenotypes in mice (Belforte et al., 2010; Korotkova et al., 2010). Based on these findings, it has been postulated that preferential blockade of GluN2C/GluN2D-containing receptors may underlie some of the psychotomimetic effects elicited by acute administration of NMDA receptor channel blockers (O'Donnell, 2012).
Positive allosteric modulators represent an alternative approach for the reversal of NMDA receptor hypofunction. Recent drug discovery efforts have identified a novel GluN2C/GluN2D positive allosteric modulator, CIQ (Mullasseril et al., 2010). CIQ was shown to selectively potentiate currents through rat recombinant GluN2C- and GluN2D-containing NMDA receptors expressed in Xenopus laevis oocytes in a concentration-dependent manner, but not through other NMDA receptor subtypes or AMPA or kainate receptors (Mullasseril et al., 2010). The EC50 value of CIQ at GluN2C/GluN2D receptors was ∼3 μM and this compound produced approximately twofold potentiation of currents through GluN2C/GluN2D receptors both in oocyte and mammalian heterologous expression systems and subthalamic neurons that express GluN2D receptors (Mullasseril et al., 2010). The potentiation by CIQ was independent of glycine or glutamate concentrations. A recent study also identified that CIQ was fairly selective with off-target activity or binding to only 8 of the 73 targets tested. CIQ modestly inhibited nicotinic receptors. The radioligand binding assay of CIQ in heterologous systems displaced ligands for κ-opioid receptors with a Ki of 3.6 ± 0.6 μM, 5-HT2B receptors with a Ki of 2.8 ± 0.2 μM and 5-HT6 receptors with a Ki of 0.51 ± 0.04 μM, whereas the Ki of CIQ for the noradrenaline transporter (NET) and 5-HT2A receptors was >10 μM (Ogden et al., 2013), suggesting affinity towards κ-opioid receptor, 5-HT2A, 5-HT2B, 5-HT6 and NET, although the functional effect of binding of CIQ to these targets remains unknown. Overall, the effect of CIQ has been interpreted as primarily being mediated by GluN2C/GluN2D receptors and local infusion of CIQ into the amygdala was found to enhance fear retention and fear extinction by potentiation of GluN2C/GluN2D receptors (Ogden et al., 2013).
In the present work, we have tested the effect of systemic administration of CIQ on schizophrenia-like behaviours elicited by MK-801 and methamphetamine. Our data demonstrate reversal/attenuation of schizophrenia-like phenotypes by CIQ, suggesting that GluN2C/GluN2D-containing receptors may serve as important therapeutic targets for schizophrenia.
Methods
Animals and housing
All animal care and experimental procedures were approved by the Creighton University Institutional Animal Care and Use Committee and Institutional Animal Committee for the SLT Institute of Pharmaceutical Sciences and conformed to the 2011 NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and reduce the number of animals used. Studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 290 mice were used for the experiments described here that were generated by breeding at own facility. We used 6- to 8-week-old male C57BL/6 mice. Mice were age-matched (within 1 week of age) for different behavioural tests. Animals were group-housed on a 12:12 light/dark cycle with ad libitum access to food and water. Separate animals were used for each behavioural test. Upon completion of behavioural testing, animals were killed by CO2 asphyxiation followed by cervical dislocation.
Drug dosage
(+)MK-801 hydrogen maleate (M107; Sigma-Aldrich, St. Louis, MO, USA) and methamphetamine (M8750; Sigma-Aldrich) were dissolved in sterile isotonic saline and administered via i.p. injection as specified below. MK-801 and methamphetamine and their respective vehicles were injected at a volume of 0.1 mL. CIQ [(3-chlorophenyl)(6,7-dimethoxy-1-((4-methoxyphenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)methanone] was a gift from Dr Stephen Traynelis (Emory University, Atlanta, GA, USA), dissolved in DMSO and injected at a volume of 1 mL·kg−1. The use of this volume of DMSO for i.p. injection was based on previous publications (Atkins et al., 1998; Selcher et al., 1999; Rossi et al., 2008; Delawary et al., 2010). Control animals received the same volume of DMSO vehicle. The dose and time of administration of CIQ was based on the affinity (Mullasseril et al., 2010), pharmacokinetic properties and previous behavioural studies (Ogden et al., 2013). CIQ has a half-life of 46 and 81 min after i.v. and i.p. administration respectively. Moreover, CIQ (i.p.) shows bioavailability of 0.42 and its brain-to-plasma concentration ratio is 5.4 (Ogden et al., 2013). The administration of CIQ, 30 min prior to behavioural testing, is based on the pharmacokinetic data, which indicate that 30 min after i.p. injection, the plasma concentration reaches ∼4 μM, whereas the brain concentration reaches approximately 1.1 × 104 ng·g−1 brain tissue (Ogden et al., 2013). The time point for testing after MK-801 administration was based on previous publications, which generally used 15–20 min of interval before testing (e.g. Csernansky et al., 2005).
Prepulse inhibition (PPI)
Startle activity was measured using an SR-LAB startle chamber (San Diego Instruments, San Diego, CA, USA). The animal enclosure to measure acoustic startle response (ASR) was a transparent acrylic cylinder (size 12.7 cm × 3.81 cm for mice) fixed on a platform connected to a piezoelectric accelerometer that measures animal movements with an ultra-stable, hermetically sealed motion sensor using a 12 bit resolution. Above the cylinder was a speaker capable of producing noise up to 120 dB attached to programmable audio controls. The animal enclosure was localized within a sound-attenuating isolation cabinet (38.1 cm × 35.56 cm × 45.72 cm) illuminated by a LED (San Diego Instruments).
Mice were given either vehicle (DMSO) or CIQ (5, 10 or 20 mg·kg−1, i.p.) and placed back in the home cage. Fifteen minutes later, mice were injected with saline, MK-801 (0.15 or 0.3 mg·kg−1, i.p.) or methamphetamine (1 or 3 mg·kg−1, i.p.) and placed back in the home cage. In the case of MK-801, the effect of three doses of CIQ (5, 10 and 20 mg·kg−1, i.p.) was tested, whereas in the case of methamphetamine, the effect of a single dose of CIQ (20 mg·kg−1, i.p.) was tested. Animals were placed in the testing chamber 15 min after saline, MK-801 or methamphetamine administration. After a 5 min acclimation period to the startle environment, the response to the startle stimulus alone (120 dB noise, 20 ms duration) and the effect of prepulse stimuli (74, 78 and 84 dB noise, 20 ms duration) delivered 100 ms before the onset of the startle stimulus (120 dB noise) were measured. The acoustic stimuli were superimposed on a 70 dB background noise. Each PPI session consisted of a total of 54 trials subdivided into four bocks. Blocks 1 and 4 were pulse alone trials (120 dB) consisting of four stimuli presentation. Blocks 2 and 3 consisted of prepulse and pulse alone trials. A total of 23 trials were presented during each of blocks 2 and 3 with five prepulse trials for each decibel and eight pulse alone trial. Trials within each block were presented in a pseudorandom order and were separated by an intertrial interval of, on average, 15 s (ranging from 9 to 21 s). Measures of prepulse inhibition were assessed referencing to the startle stimulus alone presentation as follows:
 |
Locomotor activity and stereotypy
Locomotor activity was assessed in a custom-made circular open-field chamber (27.9 cm diameter × 35.6 cm wall height) bisected by two photobeams. Locomotion was counted via an automated photobeam break counter, indicating spatial movement when each photobeam was interrupted (Med Associates, Inc., St. Albans, VT, USA). After the 15 min acclimation, animals were briefly removed from the chamber and injected (i.p.) with CIQ (10, 20 or 30 mg·kg−1) or vehicle (DMSO) and then placed back into the chamber. Fifteen minutes after injection with CIQ or vehicle, animals were once again removed from the open-field chamber and injected with MK-801 (0.15 or 0.3 mg·kg−1), methamphetamine (1 or 3 mg·kg−1) or vehicle (saline), after which animals were reintroduced to the open-field chamber, and locomotor behaviour was recorded for 1 h. In the case of MK-801, the effect of three doses of CIQ (10, 20 and 30 mg·kg−1, i.p.) was tested, whereas in the case of methamphetamine, the effect of a single dose of CIQ (20 mg·kg−1, i.p.) was tested. The total beam breaks after the administration of saline, MK-801 or methamphetamine was measured. At the end of the open-field session, animals were returned to their home cages and stereotypic behaviours were measured. Between animals, the open field was thoroughly cleaned with 70% ethanol and allowed to dry before placing another animal into the chamber. The following behaviours were judged as stereotypic behaviours; head swings, 360° circular turn, rearing or wall leaping, rapid circular movements, and digging and grooming.
Spontaneous alternation in Y-maze
Spontaneous alternation in the Y-maze was performed as previously described (Yadav et al., 2013). A custom-made Y-maze with three identical Plexiglas arms (40 × 4.5 × 12 cm, 120° apart) was placed at the centre of a room under dim lighting conditions. The walls of each arm had a distinct design to provide visual cues. Mice were administered either vehicle (DMSO) or CIQ (20 mg·kg−1, i.p.) and placed back in the home cage. Fifteen minutes later, mice were administered saline or MK-801 (0.15 mg·kg−1, i.p.) and placed back in the home cage. Y-maze testing was carried out 15 min after saline or MK-801 administration. At the beginning of the test, each mouse was placed at the end of one arm facing the centre and allowed to explore the maze for 8 min. Sessions were video-recorded and arm entries were scored by a trained observer, unaware of the treatment group. A mouse was excluded from analysis if it did not have new entries for a period of 2 min or had less than 12 arm entries during the 8 min procedure. Successful alternation was defined as consecutive entries into a new arm before returning to the two previously visited arms. Percent alternation was calculated as
Rotarod test
Motor coordination and balance were evaluated in a rotarod apparatus (Rolex Scientific Instruments, Haryana, India). Mice were given three practice trials on a rotating cylinder at the rotational speed of 6 rpm. Mice were given i.p. injections of MK-801 (0.15 mg·kg−1), CIQ (20–30 mg·kg−1) or vehicle. After 30 min, mice were placed on the rotarod at a rotating speed of 6 rpm and rotational speed was gradually increased over a 5 min test session up to a maximum rotational speed of 40 rpm and latency to fall was recorded.
Data analyses
For PPI data, manova and repeated measures anova (two-way anova) with drug treatment as a between-subject factor and prepulse intensity as a within-subject factor was used to determine main effects. Whenever the drug treatment factor or the drug treatment factor × prepulse intensity interaction was significant, post hoc analysis was carried out. The post hoc individual comparisons were carried out for each decibel level using Dunnett's multiple comparison. The startle amplitude, locomotor activity, stereotyped behaviour Y-maze and rotarod data were compared by one-way anova, followed by Dunnett's multiple comparisons. Data were analysed using sas software, version 9.2 of the SAS system for Windows (SAS Institute Inc., Cary, NC, USA) or Prism 4 (GraphPad Software Inc., San Diego, CA, USA).
Results
CIQ reverses MK-801-induced deficit in PPI but not the startle amplitude to pulse alone
PPI of the startle response is a measure of sensorimotor gating, which is impaired in certain psychiatric disorders, and specifically in schizophrenia (Braff and Geyer, 1990; Perry et al., 1999; Swerdlow et al., 2001). We first assessed the dose-dependent effect of CIQ on the MK-801-induced deficit in PPI. In preliminary studies, we assessed the effect of two doses of MK-801 [0.15 and 0.3 mg·kg−1 (Figure 1A) and for 0.3 mg·kg−1; 74 dB = 29.3 ± 5.6, 78 dB = 41.3 ± 3.5, 84 dB = 56.6 ± 5.3, n = 3] on PPI. MK-801 produced robust deficit in PPI at both doses. Moreover, there was no difference in the degree of reduction in PPI at these prepulse levels, and therefore, we used the lower dose (0.15 mg·kg−1) for further PPI experiments. Next, we assessed the effect of three doses of CIQ 5, 10 and 20 mg·kg−1 on the MK-801 (0.15 mg·kg−1)-induced impairment in PPI. The PPI observed in the vehicle–vehicle group was indistinguishable from naïve animals that did not receive any injection (74 dB = 53.4 ± 3.7, 78 dB = 63.5 ± 2.8, 84 dB = 73.0 ± 2.3, n = 4), suggesting that vehicle injections by themselves did not induce any deficit in PPI.
Figure 1.
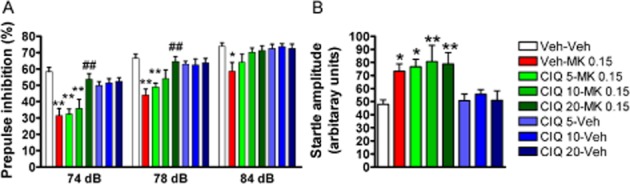
CIQ attenuates MK-801-induced impairment in PPI but not the startle response. (A) MK-801 was administered 15 min before the PPI session. MK-801 (0.15 mg·kg−1, i.p.) induced significant impairment in PPI. **P < 0.01, *P < 0.05 compared with Veh-Veh group. CIQ at 20 mg·kg−1 administered 15 min before MK-801 injection significantly attenuated the impairment in PPI by MK-801. ##P < 0.01 compared with Veh-MK-801 0.15 group. (B) MK-801 significantly increased the startle amplitude. *P < 0.05 compared with Veh-Veh group. CIQ (5–20 mg·kg−1, i.p.) had no effect by itself or on the enhanced startle response due to MK-801. n = 6–10 for each group.
Repeated measures anova was used to evaluate the effect of drug treatment using prepulse intensity as a repeated factor (Figure 1A). The effect of drug treatment, prepulse intensity and the drug treatment × prepulse intensity interaction were all found to be significant (F7,57 = 7.81, P < 0.0001; F2,114 = 221.0, P < 0.0001; F14,114 = 2.36, P = 0.0065; respectively, n = 6–10 per group). The manova of four groups (CIQ 0-MK-801, 0.15; CIQ 5-MK-801, 0.15; CIQ 10-MK-801, 0.15; and CIQ 20-MK-801, 0.15) revealed a significant effect (Wilks' lambda = 0.22, P = 0.0017). Moreover, the subsequent analysis using contrast (polynomial) showed that CIQ significantly attenuated MK-801-induced PPI impairment at 74 dB (P = 0.0016) and 78 dB (P = 0.0018) in a dose-dependent manner. Post hoc analysis of all data at each decibel intensity revealed that a dose of 20 mg·kg−1 CIQ attenuated the deficit in PPI induced by MK-801 at 74 and 78 dB (Dunnett's post hoc; Veh-MK-801 vs. CIQ 20-MK-801 0.15, P < 0.01). CIQ at all the doses alone did not affect the PPI (Dunnett's post hoc; Veh-Veh vs. CIQ 5-Veh, CIQ 10-Veh or CIQ 20-Veh, P > 0.05).
We next analysed the effect of treatment on startle amplitude. A significant main effect of treatment was observed on startle amplitude (one-way anova, F7,58 = 4.31, P < 0.001; Figure 1B). Post hoc analysis revealed that at a dose of 0.15 mg·kg−1, MK-801 produced a significant increase in the startle amplitude (Dunnett's post hoc; Veh-Veh vs. Veh-MK-801 0.15, P < 0.05). Enhanced startle amplitude by MK-801 persisted at all doses of CIQ [Dunnett's post hoc; Veh-Veh vs. CIQ 5-MK-801 0.15 (P < 0.05), CIQ 10-MK-801 0.15 (P < 0.01) or CIQ 20-MK-801 0.15 (P < 0.01)]. CIQ (5, 10 or 20 mg·kg−1, i.p.) alone did not affect the startle amplitude (Dunnett's post hoc; Veh-Veh vs. CIQ 5-Veh, CIQ 10-Veh or CIQ 20-Veh, P > 0.05).
CIQ reverses methamphetamine-induced reduction in the startle amplitude to pulse alone but not the PPI deficit
Two doses of methamphetamine were applied in the PPI test. A modest but insignificant deficit in PPI was observed at 74 dB by a dose of 1 mg·kg−1, which was not reversed by CIQ (20 mg·kg−1, i.p.) (Figure 2A). Repeated measures anova was used to evaluate the effect of drug treatment in PPI experiments with a 3 mg·kg−1 dose of methamphetamine; prepulse intensity was treated as a repeated factor (Figure 2C). The effects of drug treatment, prepulse intensity and the drug treatment × prepulse intensity interaction were all found to be significant (F3,21 = 6.74, P = 0.0023; F2,42 = 54.75, P < 0.0001; F6,42 = 3.54, P = 0.0063; respectively, n = 5–7 per group). A dose of 3 mg·kg−1 methamphetamine produced a significant deficit in PPI at all decibels (Figure 2C) (Dunnett's post hoc; Veh-Veh vs. Veh-Meth 3, P < 0.01 at 74 and 78 dB and P < 0.05 at 84 dB). CIQ (20 mg·kg−1) did not rescue the PPI deficit-induced by methamphetamine (Dunnett's post hoc; Veh-Meth 3 vs. CIQ 20-Meth 3, P > 0.05 at all decibels).
Figure 2.
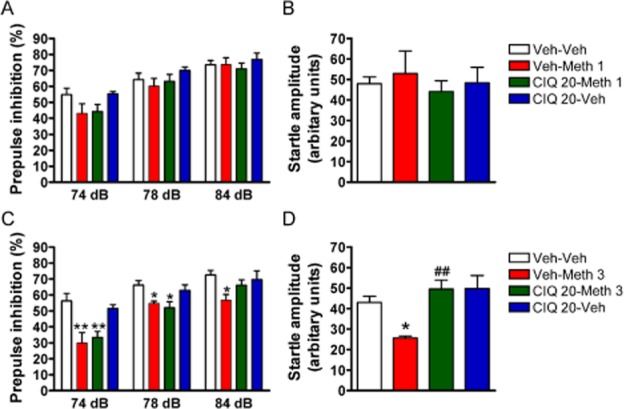
CIQ reverses the reduction in startle response by methamphetamine but not the impairment in PPI. (A, B) Methamphetamine was administered 15 min before the PPI session. Methamphetamine (1 mg·kg−1, i.p.) did not induce a significant deficit in PPI or in the startle amplitude. (C) A higher dose of methamphetamine (3 mg·kg−1 i.p.) impaired PPI. **P < 0.01, *P < 0.05 compared with Veh-Veh group. CIQ (20 mg·kg−1, i.p.) administered 15 min before methamphetamine injection was not able to attenuate the PPI impairment. **P < 0.01, *P < 0.05 compared with Veh-Veh group. (D) CIQ (20 mg·kg−1) reversed the reduction in startle amplitude by methamphetamine (3 mg·kg−1). *P < 0.05 compared with Veh-Veh group. ##P < 0.01 compared with Veh-Meth 3 group. n = 5–7 for each group.
The effect of treatments on startle amplitude was measured. A significant main effect of treatment on startle response was observed (one-way anova, F3,20 = 7.05, P < 0.01; Figure 2D). Post hoc analyses indicated that 3 mg·kg−1 methamphetamine produced a significant reduction in startle amplitude (Dunnett's post hoc; Veh-Veh vs. Veh-Meth 3, P < 0.05), which was reversed by CIQ (20 mg·kg−1, i.p) (Dunnett's post hoc; Veh-Meth 3 vs. CIQ 20-Meth 3, P < 0.01).
Effect of CIQ on MK-801-induced hyperlocomotion and stereotyped behaviour
In the initial studies, the effect of two doses of MK-801 (0.15 and 0.3 mg·kg−1, i.p.) was tested in hyperlocomotion. The dose of 0.15 mg·kg−1 (i.p.) was found to significantly increase locomotor activity and stereotyped behaviour (Figure 3) and was used for the following experiment. The dose of 0.3 mg·kg−1 (i.p.) did not lead to a significant increase in locomotion (total beam breaks = 1150 ± 221, n = 7, P > 0.05 compared with Veh-Veh) or stereotyped behaviour (26 ± 0.9, n = 7, P > 0.05 compared with Veh-Veh). We tested the effect of CIQ (10, 20 and 30 mg·kg−1, i.p.) on MK-801 (0.15 mg·kg−1, i.p.)-induced hyperlocomotion and stereotyped behaviour. A significant treatment effect was observed on locomotor activity (one-way anova, F7,49 = 4.56, P < 0.001, n = 5–10 per group; Figure 3B). A significant increase in locomotion was observed with the 0.15 mg·kg−1 dose of MK-801 (Dunnett's post hoc, Veh-Veh vs. Veh-MK-801 0.15 mg·kg−1, P < 0.01). Administration of CIQ at 10 mg·kg−1 (i.p.) did not reduce MK-801 0.15 mg·kg−1 (i.p.)-induced hyperlocomotion (Dunnett's post hoc; CIQ 10-MK-801 0.15 vs. Veh-Veh, P < 0.01). The locomotion in CIQ 20-MK-801 0.15 or CIQ 30-MK-801 0.15 group was not significantly different from that of MK-801 alone or vehicle alone groups (Dunnett's post hoc; CIQ 20-MK-801 0.15 or CIQ 30-MK-801 0.15 vs. Veh-MK-801 0.15, P > 0.05; CIQ 20-MK-801 0.15 or CIQ 30-MK-801 0.15 vs. Veh-Veh, P > 0.05).
Figure 3.
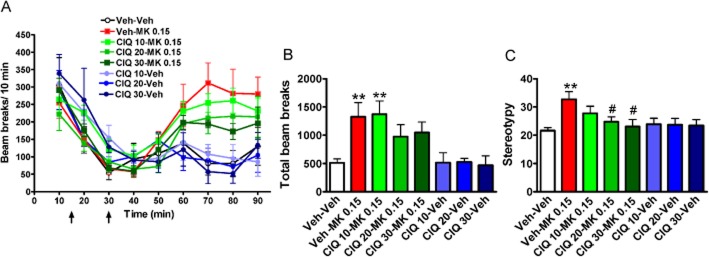
Effect of CIQ on MK-801-induced hyperlocomotion and stereotyped behaviours. After 15 min of acclimation, vehicle or CIQ was administered followed by vehicle or MK-801 15 min later. The effect of MK-801 (0.15 mg·kg−1, i.p.) was evaluated in the open-field test, followed by observation for stereotyped behaviours. (A) Raw beam breaks per 10 min. (B) Total beam breaks after MK-801 administration. MK-801 (0.15 mg·kg−1) induced an increase in locomotion in mice. **P < 0.01 compared with Veh-Veh group. Total beam breaks in CIQ (10 mg·kg−1)-MK-801 (0.15 mg·kg−1) was significantly higher than Veh-Veh group (**P < 0.01). Total beam break in CIQ (20 and 30 mg·kg−1)-MK-801 (0.15 mg·kg−1) group was not different from Veh-MK-801 (0.15 mg·kg−1) or Veh-Veh group (P > 0.05). (C) Stereotyped behaviours were measured after the open-field test. MK-801 (0.15 mg·kg−1) significantly increased the stereotyped behaviours in mice. **P < 0.01 compared with Veh-Veh group. CIQ (20 and 30 mg·kg−1) reduced the stereotypies induced by MK-801 (0.15 mg·kg−1). #P < 0.05 compared with Veh-MK-801 0.15 group. n = 5–10 for each group.
The role of treatment on stereotyped behaviours yielded a significant effect (one-way anova, F7,49 = 2.89, P < 0.05; Figure 3C). MK-801-induced stereotyped behaviours in mice at a dose of 0.15 mg·kg−1 (Dunnett's post hoc, Veh-Veh vs. Veh-MK-801 0.15, P < 0.01) and CIQ at 20 and 30 mg·kg−1 significantly attenuated the occurrence of stereotyped behaviour (Dunnett's post hoc; Veh-MK-801 0.15 vs. CIQ 20-MK-801 0.15 and CIQ 30-MK-801 0.15, P < 0.05).
CIQ reverses methamphetamine-induced hyperlocomotion and stereotyped behaviours
We tested the effect of CIQ (20 mg·kg−1, i.p.) on methamphetamine (1 and 3 mg·kg−1, i.p.)-induced hyperlocomotion and stereotyped behaviour. A significant main effect of treatment on locomotor activity was observed (one-way anova, F5,38 = 20.80, P < 0.0001, n = 6–8 per group; Figure 4B). Both doses of methamphetamine induced significant hyperlocomotion in mice (Dunnett's post hoc; Veh-Veh vs. Veh-Meth 1, P < 0.01; Veh-Veh vs. Veh-Meth 3, P < 0.01). The locomotion following treatment with CIQ alone was not different from the vehicle group (Dunnett's post hoc; Veh-Veh vs. Veh-CIQ 20, P > 0.05). CIQ significantly attenuated the hyperlocomotion induced by 3 mg·kg−1 methamphetamine (Dunnett's post hoc; Veh-Meth 3 vs. CIQ 20-Meth 3, P < 0.05). CIQ had no significant effect on the locomotor activity at 1 mg·kg−1 methamphetamine (Dunnett's post hoc; Veh-Meth 1 mg·kg−1 vs. CIQ 20-Meth 1, P > 0.05).
Figure 4.
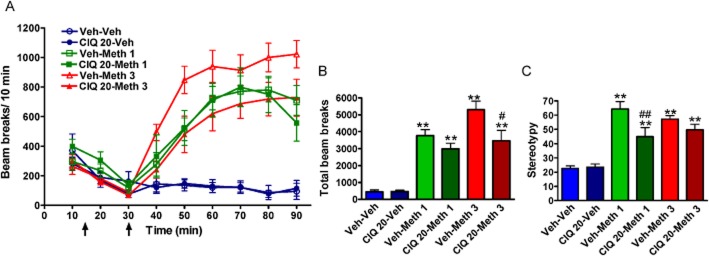
CIQ reverses methamphetamine-induced hyperlocomotion and stereotyped behaviours. After 15 min of acclimation, vehicle or CIQ was delivered followed 15 min later by vehicle or methamphetamine. The effect of two doses of methamphetamine (1 and 3 mg·kg−1, i.p.) was evaluated in open-field test and subsequently stereotyped behaviours were measured in home cage. (A) Raw beam breaks per 10 min. (B) Total beam breaks after methamphetamine administration. Methamphetamine (1 and 3 mg·kg−1) with pretreatment with vehicle or CIQ (20 mg·kg−1, i.p.) had higher locomotion compared with Veh-Veh group. **P < 0.01 compared with Veh-Veh group. Total beam break in CIQ 20-Meth 3 group was significantly lower than Veh-Meth 3 group. #P < 0.05. (C) Stereotyped behaviours were measured after the open-field test. Methamphetamine at both doses significantly increased the stereotyped behaviours in mice. **P < 0.01 compared with Veh-Veh group. CIQ (20 mg·kg−1) reduced the stereotypies induced by methamphetamine (1 mg·kg−1). ##P < 0.01 compared with Veh-Meth 1 group. n = 6–8 for each group.
A significant main effect of treatment on stereotyped behaviour was observed (one-way anova, F5,38 = 17.23, P < 0.0001; Figure 4C). Both doses of methamphetamine also produced robust stereotyped behaviours (Dunnett's post hoc; Veh-Veh vs. Veh-Meth 1, P < 0.01; Veh-Veh vs. Veh-Meth 3, P < 0.01). CIQ significantly attenuated the stereotyped behaviour induced by 1 mg·kg−1 methamphetamine (Dunnett's post hoc; Veh-Meth 1 vs. CIQ 20-Meth 1, P < 0.01). CIQ had no significant effect on the stereotyped behaviour at 3 mg·kg−1 methamphetamine (Dunnett's post hoc; Veh-Meth 3 vs. CIQ 20-Meth 3, P > 0.05).
CIQ reverses MK-801-induced deficit in Y-maze spontaneous alternation test
We tested the effect of CIQ (20 mg·kg−1, i.p.) on the MK-801 (0.15 mg·kg−1, i.p.)-induced deficit in the Y-maze spontaneous alternation test used as a test for working memory. A significant main effect of treatment was observed (one-way anova, F3,29 = 4.89, n = 7–9 per group; Figure 5). MK-801 significantly reduced spontaneous alternation in mice (Dunnett's post hoc; Veh-Veh vs. Veh-MK-801 0.15, P < 0.01). CIQ completely reversed the MK-801-induced deficit in spontaneous alternation in the Y-maze test (Dunnett's post hoc; Veh-MK-801 0.15 vs. CIQ 20-MK-801 0.15, P < 0.05). CIQ alone did not have a significant effect on spontaneous alternation (Dunnett's post hoc; Veh-Veh vs. CIQ 20-Veh, P > 0.05).
Figure 5.
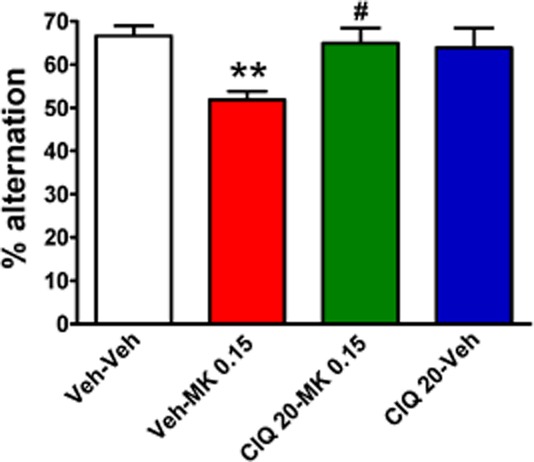
CIQ reverses the MK-801-induced deficit in working memory in the Y-maze spontaneous alternation test. MK-801 was administered 15 min before the Y-maze test. MK-801 (0.15 mg·kg−1, i.p.) induced a significant impairment in % alternation in Y-maze. *P < 0.01 compared with Veh-Veh group. CIQ at 20 mg·kg−1 administered 15 min before MK-801 injection significantly attenuated the impairment in the Y-maze by MK-801. #P < 0.05 compared with Veh-MK-801 0.15 group. n = 7–9 for each group.
Effect of drug treatment on motor coordination in rotarod test
One-way anova followed by Dunnett's test did not demonstrate any significant impairment of motor coordination by MK-801 (0.15 mg·kg−1) or CIQ (20 mg·kg−1) as compared to vehicle group (F3,18 = 3.54, P > 0.05, n = 5–6; Table 1). However, Dunnett's test showed that CIQ (30 mg·kg−1) significantly improved (P < 0.05) motor coordination as compared to control vehicle-treated animals. Overall, our results demonstrate that the reversal of MK-801 (0.15 mg·kg−1)-induced deficits by CIQ (20 mg·kg−1) is unlikely due to any effect on motor coordination.
Table 1.
Effect of drug treatment on motor coordination in rotarod test
| Treatment | Latency to fall (s) |
|---|---|
| Vehicle | 116.3 ± 26.90 |
| MK 801 (0.15 mg·kg−1) | 127.5 ± 44.59 |
| CIQ (20 mg·kg−1) | 179.7 ± 20.22 |
| CIQ (30 mg·kg−1) | 233.0 ± 21.64* |
Effect of vehicle, MK 801 (0.15 mg·kg−1) and CIQ (20–30 mg·kg−1) on latency to fall in rotarod test in mice. Data are mean ± SEM from n = 5–6 mice per group.
P < 0.05 versus vehicle.
Discussion
We tested the effect of a novel NMDA receptor potentiator that has selectivity for GluN2C/GluN2D-containing receptors (Mullasseril et al., 2010) on schizophrenia-like behaviours induced by acute administration of MK-801 and methamphetamine. The results from our studies demonstrate for the first time that potentiation of GluN2C/GluN2D-containing receptors with CIQ can reverse/or attenuate the impairment in PPI, expression of stereotyped behaviour and working memory deficit induced by MK-801. Additionally, CIQ was also effective in partially or fully normalizing the reduction in startle amplitude, hyperlocomotion and stereotyped behaviour induced by methamphetamine.
Selective effect of CIQ on MK-801 and methamphetamine-induced deficits in PPI and startle response
Both the nuclei that mediate the startle response as well as those that mediate and modulate PPI are relevant to the expression pattern of GluN2C/GluN2D subunits, which may underlie the ability of CIQ in reversing/attenuating impairment in startle response or PPI induced by methamphetamine and MK-801. The ASR is mediated by a set of nuclei, including the caudal nucleus of the pontine reticular formation, ventrolateral tegmental nucleus and ventral cochlear nucleus (Davis et al., 1982; Koch and Schnitzler, 1997). Reduction of startle response with a prepulse or PPI is influenced in rodents after specific manipulations, such as lesions of the limbic cortex, striatum, pallidal or pontine tegmentum. In addition, manipulation of prefrontal cortex and ventral hippocampus also modulate PPI (Swerdlow et al., 2001). In healthy human volunteers, PPI leads to activation of striatum extending to hippocampus and thalamus, inferior frontal and inferior parietal regions and there is reduced activation of these regions in schizophrenia patients (Campbell et al., 2007; Kumari et al., 2007; Hazlett et al., 2008). In the forebrain, GluN2C subunits are expressed in the pontine nucleus, including the caudal nucleus of pontine reticular formation, mediodorsal thalamus, nucleus reticularis and the PV-positive interneurons in the medial prefrontal cortex (Monyer et al., 1994; Wenzel et al., 1995; Karavanova et al., 2007; Xi et al., 2009) which are relevant to startle and schizophrenia circuitry. The GluN2D subunit on the other hand is expressed in the striatum, pallidum and thalamus as well as in interneurons in cortico-limbic regions (Buller et al., 1994; Monyer et al., 1994; Wenzel et al., 1995; 1996,; Dunah et al., 1996), which are also relevant to dopaminergic and startle circuitry. The expression of GluN2C and GluN2D in key brain regions that mediate startle reflex and PPI suggest that these receptors may at least partly regulate these processes.
Our data indicate that MK-801 (0.15 mg·kg−1, i.p) and methamphetamine (3 mg·kg−1, i.p.) have contrasting effects on the startle reflex, with MK-801 increasing the startle amplitude, while methamphetamine reduces the startle amplitude (Figures 1 and 2). These results are in agreement with previous reports on MK-801 and amphetamine effect on startle response (Kinney et al., 1999; Long et al., 2006; Arai et al., 2008; Egashira et al., 2008; Larrauri and Levin, 2010). Thus, at these doses, it appears that potentiation of dopaminergic signalling and inhibition of glutamate signalling may have distinct effects on the neurocircuitry involved in startle response. CIQ alone did not affect startle responses, suggesting that GluN2C/GluN2D receptors have limited, if any, role in the expression of startle responses or that the GluN2C/GluN2D component is already saturated. However, CIQ reversed the reduction in startle response to methamphetamine, suggesting that GluN2C/GluN2D receptors may be able to modulate the dopaminergic circuitry involved in startle responses. This is also consistent with the ability of CIQ to reverse methamphetamine-induced hyperlocomotion and stereotyped behaviour (Figure 4). Expression of GluN2D subunits in the striatum and ventral pallidum suggests that this may be the site of action of CIQ in reversing methamphetamine effects. CIQ did not affect the increase in startle response to MK-801, suggesting that the pathway that mediates or modulates the effects of glutamatergic input to startle response does not involve GluN2C/GluN2D receptor activation or the GluN2C/GluN2D component is saturated.
In agreement with the previous studies (Lipina et al., 2005; Long et al., 2006; Ishii et al., 2010), we found that 0.15 mg·kg−1 MK-801 produced impairment in PPI. Methamphetamine at 3 mg·kg−1 (i.p.), but not at 1 mg·kg−1, produced significant deficits in PPI, which is also consistent with a previous finding (Arai et al., 2008). CIQ attenuated the impairment in PPI by MK-801 but not that due to methamphetamine (Figures 1 and 2). This finding contrasts with the effect of CIQ on MK-801- and methamphetamine-induced changes in startle response. This suggests that the neurocircuitry involved in MK-801 and methamphetamine-induced PPI impairment is likely to be different and that the role of GluN2C/GluN2D differs between these circuits.
Efficacy of CIQ for deficit in spontaneous alternation in Y-maze test as a model for working memory
Impairment in working memory is one of the hallmarks of schizophrenia (Perlstein et al., 2001) and MK-801 led to a reduction in working memory as measured in the Y-maze spontaneous alternation test. CIQ reversed the deficit in spontaneous alternation in the Y-maze test induced by MK-801 (Figure 5). Although additional tests for working memory will be necessary to fully explore the efficacy of CIQ in cognitive deficits across different models; nonetheless, our preliminary findings in the Y-maze test are promising. The dorsolateral prefrontal cortex in humans or medial prefrontal cortex in rodents is an important neural substrate for the maintenance of working memory (Perlstein et al., 2001). MK-801 may lead to disinhibition of the medial prefrontal cortex circuit by preferential blockade of interneurons (Homayoun and Moghaddam, 2007) and an associated increase in glutamate release in the medial prefrontal cortex (Moghaddam et al., 1997), which may be partly responsible for the working memory deficit. The expression of GluN2C/GluN2D in interneurons in corticolimbic regions (Monyer et al., 1994; Rudolf et al., 1996; Standaert et al., 1996; Scherzer et al., 1998; Munoz et al., 1999; Xi et al., 2009) and the higher sensitivity of these receptors to NMDA receptor channel blockers (Kotermanski and Johnson, 2009) suggest that these receptors may be partly responsible for disinhibition in prefrontal cortex, in response to NMDA receptor channel blockers. Thus, CIQ, by overcoming GluN2C/GluN2D inhibition by MK-801, may be able to normalize working memory. We have previously shown that genetic deletion of GluN2C in mouse leads to certain characteristic features observed in schizophrenia, such as working memory deficit and deficit in associative contextual fear (Hillman et al., 2011). In addition, the efficacy of d-cycloserine in schizophrenia symptoms has been proposed to arise partly due to its higher efficacy at GluN2C-containing receptors (Dravid et al., 2010; Goff, 2012). Thus, the report of a reduction in GluN2C expression in the prefrontal cortex of human schizophrenia patients (Weickert et al., 2012) supports the idea that GluN2C receptors in the medial prefrontal cortex may serve as important targets for cognitive deficits in schizophrenia.
In conclusion, our data strongly indicate that facilitation of GluN2C/GluN2D receptors can attenuate schizophrenia-like deficits induced by MK-801 and methamphetamine. Thus, further development of potentiators of GluN2C/GluN2D receptors may lead to compounds useful in treatments for schizophrenia. Additional studies will be required to confirm the molecular target of the effects of CIQ and to determine whether facilitation of GluN2C or GluN2D, or both, is essential for the effect of CIQ. Although facilitation of GluN2C/GluN2D receptors represents the prominent action of CIQ, the involvement of κ-opioid, 5-HT2A, 5-HT2B, 5-HT6 receptors and NET in these actions of CIQ needs to be critically assessed. Thus, studies using genetic knockout models together with more selective potentiators may shed light on the specific roles of GluN2C and GluN2D receptors in neuronal circuits, which are dysregulated in schizophrenia and other related disorders.
Acknowledgments
This work was supported by the Health Future Foundation (S. M. D). The authors would also like to thank Dr Stephen F. Traynelis for providing CIQ, Chris Wichman for helping with statistical analysis and Dr Daniel T. Monaghan for his critical comments. The project was also supported by G20RR024001 from National Center for Research Resources. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Glossary
- ASR
acoustic startle response
- GAD67
glutamate decarboxylase 67
- NET
noradrenaline transporter
- PPI
prepulse inhibition
Conflict of interest
None.
References
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Takahashi K, et al. Involvement of pallidotegmental neurons in methamphetamine- and MK-801-induced impairment of prepulse inhibition of the acoustic startle reflex in mice: reversal by GABAB receptor agonist baclofen. Neuropsychopharmacology. 2008;33:3164–3175. doi: 10.1038/npp.2008.41. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Buller AL, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT. The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci. 1994;14:5471–5484. doi: 10.1523/JNEUROSCI.14-09-05471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LE, Hughes M, Budd TW, Cooper G, Fulham WR, Karayanidis F, et al. Primary and secondary neural networks of auditory prepulse inhibition: a functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. Eur J Neurosci. 2007;26:2327–2333. doi: 10.1111/j.1460-9568.2007.05858.x. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Martin M, Shah R, Bertchume A, Colvin J, Dong H. Cholinesterase inhibitors ameliorate behavioral deficits induced by MK-801 in mice. Neuropsychopharmacology. 2005;30:2135–2143. doi: 10.1038/sj.npp.1300761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delawary M, Tezuka T, Kiyama Y, Yokoyama K, Inoue T, Hattori S, et al. NMDAR2B tyrosine phosphorylation regulates anxiety-like behavior and CRF expression in the amygdala. Mol Brain. 2010;3:37. doi: 10.1186/1756-6606-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, et al. Structural determinants of d-cycloserine efficacy at the NR1/NR2C NMDA receptors. J Neurosci. 2010;30:2741–2754. doi: 10.1523/JNEUROSCI.5390-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Yasuda RP, Wang YH, Luo J, Davila-Garcia M, Gbadegesin M, et al. Regional and ontogenic expression of the NMDA receptor subunit NR2D protein in rat brain using a subunit-specific antibody. J Neurochem. 1996;67:2335–2345. doi: 10.1046/j.1471-4159.1996.67062335.x. [DOI] [PubMed] [Google Scholar]
- Egashira N, Okuno R, Harada S, Matsushita M, Mishima K, Iwasaki K, et al. Effects of glutamate-related drugs on marble-burying behavior in mice: implications for obsessive-compulsive disorder. Eur J Pharmacol. 2008;586:164–170. doi: 10.1016/j.ejphar.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T, et al. NMDA receptors, cognition and schizophrenia – testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology. 2012;62:1401–1412. doi: 10.1016/j.neuropharm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Goff DC. d-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophr Bull. 2012;38:936–941. doi: 10.1093/schbul/sbs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Zhang J, Newmark RE, Glanton CF, Zelmanova Y, et al. Frontal-striatal-thalamic mediodorsal nucleus dysfunction in schizophrenia-spectrum patients during sensorimotor gating. Neuroimage. 2008;42:1164–1177. doi: 10.1016/j.neuroimage.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman BG, Gupta SC, Stairs DJ, Buonanno A, Dravid SM. Behavioral analysis of NR2C knockout mouse reveals deficit in acquisition of conditioned fear and working memory. Neurobiol Learn Mem. 2011;95:404–414. doi: 10.1016/j.nlm.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D, Matsuzawa D, Kanahara N, Matsuda S, Sutoh C, Ohtsuka H, et al. Effects of aripiprazole on MK-801-induced prepulse inhibition deficits and mitogen-activated protein kinase signal transduction pathway. Neurosci Lett. 2010;471:53–57. doi: 10.1016/j.neulet.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavanova I, Vasudevan K, Cheng J, Buonanno A. Novel regional and developmental NMDA receptor expression patterns uncovered in NR2C subunit-beta-galactosidase knock-in mice. Mol Cell Neurosci. 2007;34:468–480. doi: 10.1016/j.mcn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments. The ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney GG, Wilkinson LO, Saywell KL, Tricklebank MD. Rat strain differences in the ability to disrupt sensorimotor gating are limited to the dopaminergic system, specific to prepulse inhibition, and unrelated to changes in startle amplitude or nucleus accumbens dopamine receptor sensitivity. J Neurosci. 1999;19:5644–5653. doi: 10.1523/JNEUROSCI.19-13-05644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats – circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer's drug memantine. J Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Geyer MA, Ffytche D, Williams SC, Sharma T. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:463–477. doi: 10.1017/S1461145706007139. [DOI] [PubMed] [Google Scholar]
- Larrauri JA, Levin ED. PPI deficit induced by amphetamine is attenuated by the histamine H1 antagonist pyrilamine, but is exacerbated by the serotonin 5-HT2 antagonist ketanserin. Psychopharmacology (Berl) 2010;212:551–558. doi: 10.1007/s00213-010-2005-6. [DOI] [PubMed] [Google Scholar]
- Lin CH, Lane HY, Tsai GE. Glutamate signaling in the pathophysiology and therapy of schizophrenia. Pharmacol Biochem Behav. 2012;100:665–677. doi: 10.1016/j.pbb.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Lipina T, Labrie V, Weiner I, Roder J. Modulators of the glycine site on NMDA receptors, d-serine and ALX 5407, display similar beneficial effects to clozapine in mouse models of schizophrenia. Psychopharmacology (Berl) 2005;179:54–67. doi: 10.1007/s00213-005-2210-x. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LE, Malone DT, Taylor DA. Cannabidiol reverses MK-801-induced disruption of prepulse inhibition in mice. Neuropsychopharmacology. 2006;31:795–803. doi: 10.1038/sj.npp.1300838. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan H, Kurtkaya NL, et al. A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat Commun. 2010;1:90. doi: 10.1038/ncomms1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz A, Woods TM, Jones EG. Laminar and cellular distribution of AMPA, kainate, and NMDA receptor subunits in monkey sensory-motor cortex. J Comp Neurol. 1999;407:472–490. doi: 10.1002/(sici)1096-9861(19990517)407:4<472::aid-cne2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. Cortical disinhibition in the neonatal ventral hippocampal lesion model of schizophrenia: new vistas on possible therapeutic approaches. Pharmacol Ther. 2012;133:19–25. doi: 10.1016/j.pharmthera.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Ogden KK, Khatri A, Traynelis SF, Heldt SA. Potentiation of GluN2C/D NMDA receptor subtypes in the amygdala facilitates the retention of fear and extinction learning in mice. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.241. [Epub ahead of print; doi: 10.1038/npp.2013.241] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Perry W, Geyer MA, Braff DL. Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Arch Gen Psychiatry. 1999;56:277–281. doi: 10.1001/archpsyc.56.3.277. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Kusayanagi H, Mataluni G, Bernardi G, et al. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci. 2008;28:7284–7292. doi: 10.1523/JNEUROSCI.5346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf GD, Cronin CA, Landwehrmeyer GB, Standaert DG, Penney JB, Jr, Young AB. Expression of N-methyl-D-aspartate glutamate receptor subunits in the prefrontal cortex of the rat. Neuroscience. 1996;73:417–427. doi: 10.1016/0306-4522(96)00048-6. [DOI] [PubMed] [Google Scholar]
- Scherzer CR, Landwehrmeyer GB, Kerner JA, Counihan TJ, Kosinski CM, Standaert DG, et al. Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: hippocampus and cortex. J Comp Neurol. 1998;390:75–90. doi: 10.1002/(sici)1096-9861(19980105)390:1<75::aid-cne7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder MA, Gao WJ. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci. 2013;7:31. doi: 10.3389/fncel.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert DG, Landwehrmeyer GB, Kerner JA, Penney JB, Jr, Young AB. Expression of NMDAR2D glutamate receptor subunit mRNA in neurochemically identified interneurons in the rat neostriatum, neocortex and hippocampus. Brain Res Mol Brain Res. 1996;42:89–102. doi: 10.1016/s0169-328x(96)00117-9. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Fung SJ, Catts VS, Schofield PR, Allen KM, Moore LT, et al. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol Psychiatry. 2012;18:1185–1192. doi: 10.1038/mp.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Scheurer L, Kunzi R, Fritschy JM, Mohler H, Benke D. Distribution of NMDA receptor subunit proteins NR2A, 2B, 2C and 2D in rat brain. Neuroreport. 1995;7:45–48. [PubMed] [Google Scholar]
- Wenzel A, Villa M, Mohler H, Benke D. Developmental and regional expression of NMDA receptor subtypes containing the NR2D subunit in rat brain. J Neurochem. 1996;66:1240–1248. doi: 10.1046/j.1471-4159.1996.66031240.x. [DOI] [PubMed] [Google Scholar]
- Xi D, Keeler B, Zhang W, Houle JD, Gao WJ. NMDA receptor subunit expression in GABAergic interneurons in the prefrontal cortex: application of laser microdissection technique. J Neurosci Methods. 2009;176:172–181. doi: 10.1016/j.jneumeth.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Hillman BG, Gupta SC, Suryavanshi P, Bhatt JM, Pavuluri R, et al. Deletion of glutamate delta-1 receptor in mouse leads to enhanced working memory and deficit in fear conditioning. PLoS ONE. 2013;8:e60785. doi: 10.1371/journal.pone.0060785. [DOI] [PMC free article] [PubMed] [Google Scholar]


