Abstract
The biological equivalency of ergocalciferol (D2) and cholecalciferol (D3) has been debated; several comparisons have appeared in the adult literature but are scarce in pediatrics. The objective of this study was to compare increases in plasma 25-hydroxyvitamin D [25(OH)D] concentrations and attainment of 50 and 75 mol/L status cutoffs following 3 mo of daily supplementation with D2 compared with D3. Healthy, breast-fed, 1-mo-old infants (n = 52) received 10 μg (400 ic) of either D2 or D3 daily. At 1 and 4 mo of age, plasma 25-hydroxyergocalciferol and 25-hydroxycholecalciferol concentrations were determined by liquid chromatography tandem MS (LC-MS/MS) and total 25(OH)D by chemiluminescent immunoassay (DiaSorin Liaison). Data were analyzed using t tests and χ2 by intent to treat. A total of 23% of infants were deficient (≤24.9 nmol/L) at baseline and 2% at follow-up on the basis of LC-MS/MS. At 4 mo, 96% were breastfed and there were no differences in compliance, breastfeeding rates, or sun exposure among groups. The change in total 25(OH)D measured by LC-MS/MS did not differ between the D2 (17.6 ± 26.7 nmol/L) and D3 (22.2 ± 20.2 nmol/L) groups. In the combined groups, the baseline plasma 25(OH)D concentration was inversely related to the change in total 25(OH)D (r = −0.52; P < 0.001). Overall, 86% of infants met the 50 nmol/L cutoff at follow-up; however, fewer infants in the D2 group (75%) met this level compared with the D3 group (96%) (P < 0.05). Similar results were obtained by immunoassay. In conclusion, the increase in the 25(OH)D concentration among the D2 and D3 groups did not differ, suggesting daily intake of either isoform is acceptable for infants <4 mo.
Introduction
Most countries around the world recommend supplementing breast-fed infants with 10 μg (400 ic) of vitamin D daily (1–6). This dosage is thought to maintain circulating 25-hydroxyvitamin D [25(OH)D]8 concentrations of 40–50 nmol/L as suggested by the Institute of Medicine (IOM) for bone health (3); however, other organizations advise using a higher cutoff of 75 nmol/L (7). Standard infant preparations in Canada are mammalian cholecalciferol (D3), but most research studies tested plant-derived ergocalciferol (D2) (8–10). These isomers differ in side chain structure and were originally thought to exhibit identical biological responses (3, 11, 12). There is controversy in the adult literature regarding the equivalency of the 2 isoforms. A single dose of 1250 μg D3 increased 25(OH)D based on AUC during 28 d with 3–10 times greater efficacy than D2 (13). Furthermore, 100 μg/d D3 resulted in a 75% greater change in 25(OH)D compared with D2 for 14 d (14). In a study that followed adults for a longer period of 11 wk, a daily intake of 25 μg of either isoform maintained equivalent 25(OH)D status (15). Similar results were observed in elderly women taking supplements containing either D2 or D3 (<12.5 μg/d) for an average 5–6 y (16). It appears that dosage and sustained supplementation may explain the differences observed in these adult studies.
A recent meta-analysis concluded that D2 is less effective than D3 at raising the 25(OH)D concentration (17) and highlighted that too few studies exist to verify if this is the situation in children (18, 19). In neonates, there are additional physiological differences in intestinal absorption (19) and vitamin D metabolism (20) from adults. Whether differences in the absorption and binding to the vitamin D-binding protein between isoforms affect plasma 25(OH)D concentrations in infants is yet undetermined. A study was designed with the objective to compare the effectiveness of 10 μg/d of D2 compared with D3 to increase or maintain circulating 25(OH)D concentrations during a 3-mo period in healthy, breast-fed newborns. Our secondary objective was to compare the proportion of infants able to meet the 50 (3, 6) or 75 (7) nmol/L cutoffs at follow-up.
Participants and Methods
Trial design.
Infants were randomly assigned to receive a 10-μg/d oral dose of either D2 or D3 in a 1:1 ratio stratified by sex. Both the D3- (NPN no. 80001869) and D2- (NPN no. 80003406) based products are commercially available (Ddrops Company), eliminating the need for Health Canada approval as a phase IV trial. There were no differences in appearance and both products were tasteless and odorless. These products are oil based (coconut and palm) and dosages were delivered in 1-drop volumes (0.03 mL) using a standardized Eurodropper. Compliance was assessed by weighing unused portions in bottles and parent self-report. Compliance was calculated as the number of dosages taken divided by the days since last visit and “compliant” was defined as 80–100% of doses taken. The study was approved by the Institutional Review Board of McGill University. Parents gave written informed consent.
Participants.
Newborns were referred from a primary care hospital and birthing center located in greater Montréal between May 2010 and September 2011. To avoid oversampling in any one season, we aimed to recruit 50% of participants in the vitamin D-synthesizing period (April–October) for Montréal located at latitude 45°N (21). Infants were eligible to begin the study at 1 mo of age if they were healthy, singleton, term infants born the appropriate size for gestational age as assessed according to the WHO growth charts (between 5th and 95th percentile) and to healthy, breastfeeding women (consuming >80% of total feeds from breast milk). Exclusion criteria included infants of mothers with a history of gestational diabetes or hypertension in pregnancy, malabsorption syndromes (celiac and Crohn’s disease), or taking medications that interfere with vitamin D metabolism (anticonvulsants and corticosteroids). Mothers taking ≥50 μg/d of vitamin D from supplementation were not included, because this value is above the current nutrition recommendations (22). Demographic information, including race, education, and income (in Canadian dollars), was reported by the mother. At baseline and follow-up visits, weight, length, and head circumference were measured. Data are expressed in absolute units and Z-scores using data from the 2006 WHO growth charts (23) at each time point.
Dietary and endogenous vitamin D sources.
Information on infant breastfeeding status (feeds/day) and formula feeds (amount, frequency, brand) was collected at each visit. Skin pigmentation was measured on the constitutive upper underarm and facultative forehead, forearm, and outer lower leg using a portable computerized spectrophotometer (CM-600D, Konica Minolta). Based on the Commisssion Internationale de l’Eclairage colorimetry system (L*a*b*), the individual typological angle (ITA) {ITA° = [arc tangent (L* − 50)/b*) ] 180/3.14159} was calculated (24). Infants were classified into 5 skin phototypes: dark (≤10°), olive (10–28°), medium (28–41°), fair (41–55°), and very fair (>55°). The ITA difference between the exposed (forearm) and unexposed (inner arm) skin sites was used to assess exposure to sun during the trial.
Plasma 25(OH)D.
Capillary blood samples were collected from infants by heel or finger lance and mother’s fasted blood was collected by venipuncture (between 0800 and 1200 h). Samples were centrifuged (2235 × g for 20 min at 4°C) and stored frozen at −80°C for batch analysis. Plasma samples were analyzed using a sensitive (limit of quantification: 12 nmol/L) liquid chromatography tandem MS (LC-MS/MS) developed by Warnex Bioanalytical Services. This method uses derivatization of vitamin D metabolites with substituted triazolinediones in a Diels-Alder cycloaddition with chromatographic separation of epimers (25). The concentrations of 25-hydroxycholecalciferol [25(OH)D3] and 25-hydroxyergocalciferol [25(OH)D2] were calculated using Watson LIMS software, version 7.1.0.01. Pooled serum samples from the Vitamin D External Quality Assessment Scheme were used as quality control samples for 25(OH)D3. Deuterium-labeled 25(OH)D3 was used as internal standard. The intra-assay and inter-assay percent CVs for 25(OH)D2 and 25(OH)D3 were <6.2% and <10.1%, respectively, for all controls. The analytical ranges were 12.5–250.0 nmol/L (5–100 μg/L) for 25(OH)D3 and 25(OH)D2. At baseline, no subjects had detectable concentrations of 25(OH)D2. At follow-up, no subjects in the D3 group had detectable 25(OH)D2; therefore, the change in 25(OH)D for the D3 group was based only on 25(OH)D3 concentrations. For the D2 group, the sum of 25(OH)D2 and 25(OH)D3 at follow-up was subtracted from baseline 25(OH)D2 to calculate the change in 25(OH)D. Values below the quantification limit of the assay were replaced by zero (26, 27). Plasma 25(OH)D concentrations were categorized by different thresholds: ≤24.9 (deficiency), 25–49.9, 50–74.9, and ≥75 nmol/L.
To make this data usable by others using immunoassays, we also measured the total 25(OH)D concentration using an automated chemiluminescent immunoassay system (Liaison, DiaSorin). This assay measures total 25(OH)D in plasma and has a sensitivity of 10.0 nmol/L. The range of the assay was 10–375 nmol/L (4–150 μg/L). The intra-assay and inter-assay percent CVs were <4.9% and <10.7%, respectively, for the all controls. All analyses were completed in a laboratory meeting the performance targets set by the Vitamin D External Quality Assessment Scheme.
Sample size.
A previous study in infants (<6 wk old) that gave 10 μg of D2 daily found a Δ 35.7 ± 20.2 nmol/L during a 3-mo period (28). With a sample size of 26 participants/group, α = 0.05 and β = 0.20 (power 1 − β = 0.8) would allow the detection of a difference of 45% between treatments in the change in 25(OH)D during the 3-mo period This translates to an effect size of 16 nmol/L, representing ∼1 SD in the change in the 25(OH)D concentration (28).
Statistical methods.
Subject characteristics were tested for baseline differences among treatments using a t test for continuous variables and χ2 (with Fisher’s exact tests for small sample sizes) for categorical variables. Baseline characteristics with differences were included as covariates in the ANOVA model. The intent-to-treat principle was applied for all outcomes. The associations between mother’s and infant’s vitamin D status as well as the change in infant 25(OH)D concentrations and infant baseline vitamin D status were both tested using Pearson correlation. Plasma 25(OH)D concentrations at each time point as well as the mean change in 25(OH)D from baseline during the trial were compared between treatments using unpaired t tests. Differences in the change in 25(OH)D by treatment were also tested as a mixed-model ANOVA accounting for any baseline differences among the groups. To compare the proportion of infants above or below the 50 and 75 nmol/L cutoffs among treatment groups, sample proportions were evaluated by χ2 test (Fischer’s Exact for small sample size). To evaluate the effect of baseline status on the change in 25(OH)D, the change in 25(OH)D was plotted (mean ± SD) by baseline 25(OH)D category and analyzed by ANOVA. Tukey’s adjustment for multiple comparisons was used to detect significant differences among categories for the D2 and D3 groups separately and over time. Data were checked for normality and equal variances using Shapiro-Wilks and Levene’s tests; appropriate nonparametric tests were used if assumptions were violated. Significance was set at P ≤ 0.05; all tests presented are 2 tailed. Data were analyzed using SAS version 9.2 (SAS Institute).
Results
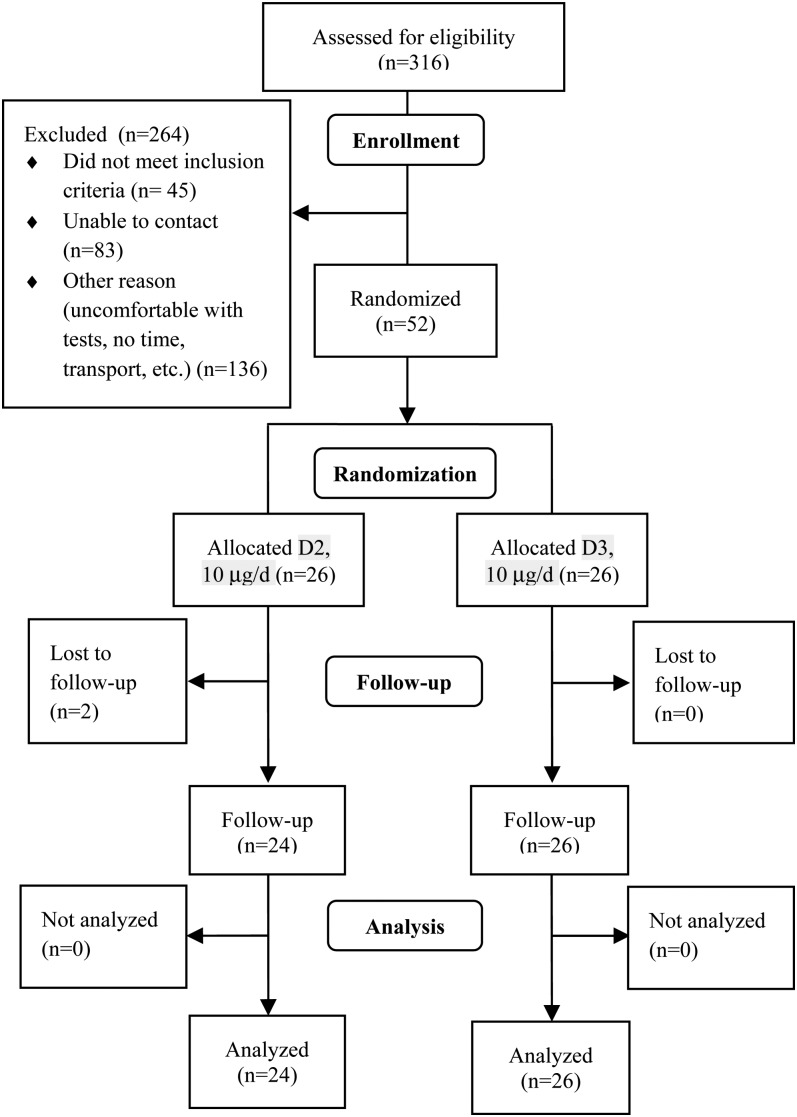
A total of 52 healthy infants were enrolled and 73% were taking a vitamin D supplement at baseline. There was a trend (P = 0.08) for differences in race among the groups (Table 1); however, skin color did not differ (P = 0.23). Two infants in the D2 group were lost to follow-up (Fig. 1). Infants were growing; the mean weight-for-age and length-for-age Z-scores were within 1 SD from the WHO standard. However, the D3 group had a significantly higher length-for-age Z-score than the D2 group at baseline and follow-up (Supplemental Table 1). The supplementation period tended (P = 0.09) to be 10% longer in the D3 group than in the D2 group. There were no differences in compliance among groups as assessed by both bottle weighing (median 89, range 32–100% of doses taken) and reported intake (95, 31–100% of doses taken). Maternal vitamin D status at baseline was associated with neonatal values at both baseline (r = 0.41; P = 0.003) and follow-up (r = 0.31; P = 0.03) as measured using LC-MS/MS; similar results were obtained by immunoassay (data not shown).
TABLE 1.
Infant and mother baseline characteristics1
| Treatments |
||
| Variable | D2 | D3 |
| Mothers | ||
| Plasma 25(OH)D3,2 nmol/L | 68.3 ± 21.4 | 69.5 ± 21.7 |
| Age at delivery, y | 31.0 ± 4.5 | 32.6 ± 4.2 |
| Income ≥75,000 Canadian $, n (%) | 16 (61.5) | 13 (50.0) |
| Education, ≥university, n (%) | 20 (76.9) | 17 (65.4) |
| Infants | ||
| Male, n (%) | 12 (46.2) | 13 (50.0) |
| Born during vitamin D-synthesizing period (April 1–October 31), n (%) | 15 (57.7) | 15 (57.7) |
| Taking a vitamin D supplement at baseline, n (%) | 17 (65.4) | 21 (80.8) |
| Age started vitamin D supplement, d | 4 (3, 7) | 5 (3, 10) |
| White, self-identified,3 n (%) | 14 (58.3) | 21 (80.8) |
| Skin color (based on ITA°),4 n (%) | ||
| Very fair | 2 (7.7) | 3 (11.5) |
| Fair | 12 (46.2) | 12 (46.2) |
| Medium | 7 (26.9) | 11 (42.3) |
| Olive | 3 (11.5) | 0 (0.0) |
| Dark | 2 (7.7) | 0 (0.0) |
Values are frequency and percent or mean ± SD, n = 26. Non-normally distributed data presented as median (25th, 75th percentile). D2, ergocalciferol; D3, cholecalciferol; ITA, individual typological angle; LC-MS/MS, liquid chromatography tandem MS; 25(OH)D, 25-hydroxyvitamin D.
Results were tested by LC-MS/MS. The results were similar to LC-MS/MS when tested by immunoassay: D2: 74.0 ± 21.7 vs. D3: 79.6 ± 27.4; P = 0.42.
Based on mother’s and father’s race.
ITA° = {arc tangent [(L* − 50)/b*]} 180/3.14159, classified in 5 skin phototypes: dark (≤10°), olive (10–28°), medium (28–41°), fair (41–55°), and very fair (>55°). 1 g vitamin D = 40 ic.
FIGURE 1.
Consort diagram. 1 μg vitamin D = 40 IU. D2, ergocalciferol; D3, cholecalciferol.
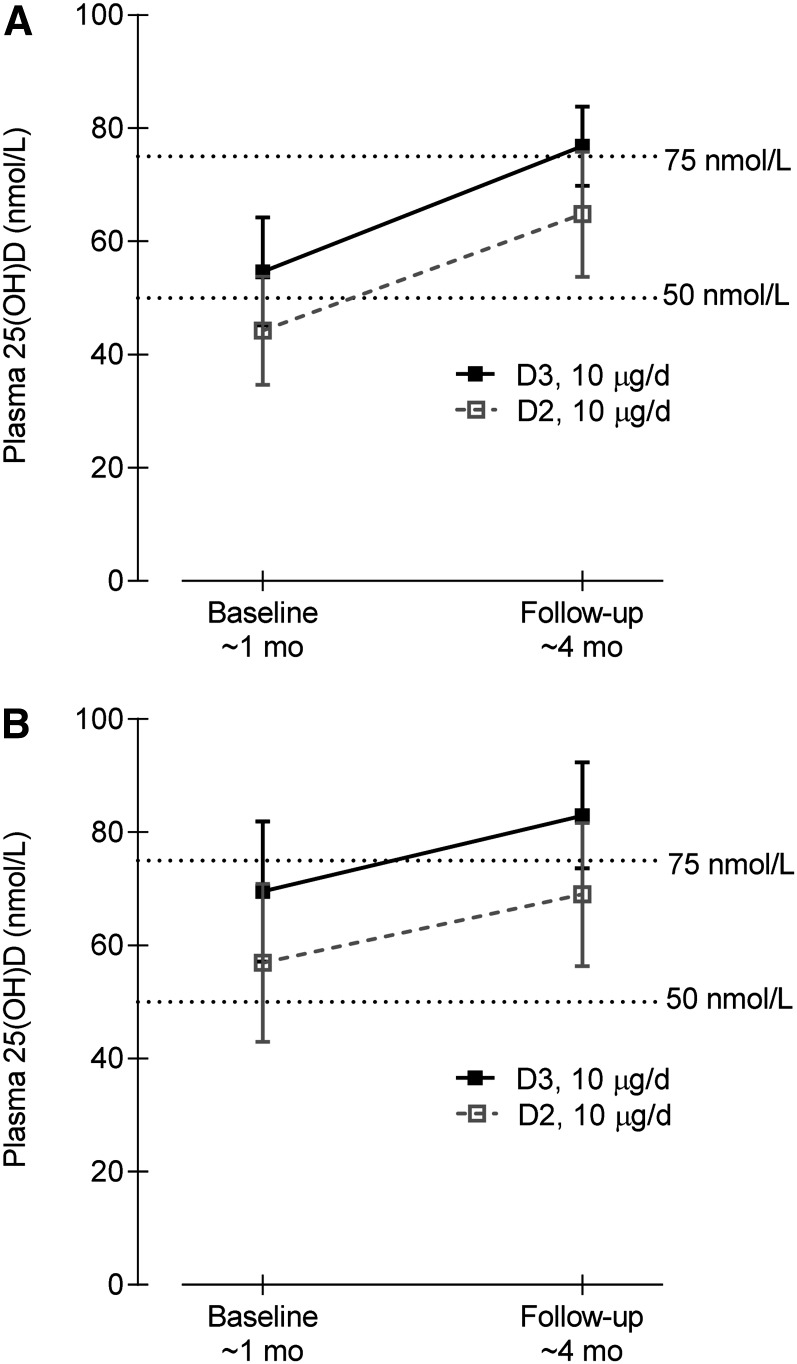
Overall, based on LC-MS/MS measurements, both supplements significantly increased the plasma 25(OH)D concentration during the 3-mo period, with a mean increase of 20.0 nmol/L (95% CI: 13.3–26.6 nmol/L) (Fig. 2). The mean difference between the increases (D2 − D3) was −4.5 nmol/L (95% CI: −17.9 to 8.9 nmol/L; P = 0.5). The difference between the slopes of the line [Δ in plasma 25(OH)D/Δ in time] of the 2 groups was not significant (D2: 24.4 ± 6.6 nmol/L; D3: 18.8 ± 6.2 nmol/L; P = 0.54). However, a higher (P = 0.05) proportion of infants in the D3 group (96.2%) than in the D2 group (75.0%) achieved the 50 nmol/L cutoff at follow-up (Table 2). No differences were noted among groups in the proportion that achieved the 75 nmol/L cutoff at follow-up. A larger proportion of infants in the D2 group (n = 8; 33%) had decreases in 25(OH)D concentration during the 3-mo period compared with infants in the D3 group (n = 2; 8%) (Fischer’s Exact test, P = 0.02). Similar results were observed using the immunoassay (Fig. 2; Table 2).
FIGURE 2.
Plasma 25(OH)D concentrations at baseline (∼1 mo) and follow-up (∼4 mo) in infants that received 10 μg/d D2 or D3 as assessed by LC-MS/MS (A) and immunoassay (B). Values are means and 95% CI, n = 24–26. 25(OH)D, 25-hydroxyvitamin D; D2, ergocalciferol; D3, cholecalciferol; LC-MS/MS, liquid chromatography tandem <S.
TABLE 2.
Plasma 25(OH)D concentrations and number (%) meeting 25(OH)D cutoffs of ≥50 and ≥75 nmol/L in infants that received 10 μg/d of D2 or D3 as assessed by immunoassay and LC-MS/MS1
| Treatments |
||||
| D2 | n | D3 | n | |
| Immunoassay | ||||
| Baseline 25(OH)D, nmol/L | 56.9 ± 33.7 | 25 | 69.5 ± 30.6 | 26 |
| Follow-up 25(OH)D, nmol/L | 69.0 ± 30.0 | 24 | 82.9 ± 23.1 | 26 |
| Δ Total 25(OH)D, nmol/L | 7.1 ± 36.7 | 23 | 13.4 ± 19.6 | 26 |
| (95% CI) | −8.8–22.9 | 5.5–21.4 | ||
| LC-MS/MS2 | ||||
| Baseline 25(OH)D3, nmol/L | 44.2 ± 23.8 | 26 | 54.6 ± 23.7 | 26 |
| Baseline 25(OH)D3, n (%) | ||||
| ≤24.9 nmol/L | 8 (30.8) | 4 (15.4) | ||
| 25–49.9 nmol/L | 5 (19.2) | 6 (23.1) | ||
| 50–74.9 nmol/L | 12 (46.2) | 13 (50.0) | ||
| ≥75 nmol/L | 1 (3.9) | 3 (11.5) | ||
| Follow-up | ||||
| Total 25(OH)D, nmol/L | 64.8 ± 26.2 | 24 | 76.8 ± 17.4 | 26 |
| Follow-up 25(OH)D2, nmol/L | 45.1 ± 30.7 | na | ||
| Follow-up 25(OH)D3, nmol/L | 19.6 ± 20.2 | 76.8 ± 17.4 | ||
| Δ Total 25(OH)D,2 nmol/L | 17.6 ± 26.7 | 24 | 22.2 ± 20.2 | 26 |
| (95% CI) | 6.4–28.9 | 14.0–30.3 | ||
| 25(OH)D ≥50 nmol/L at follow-up,3 n (%) | 18 (75.0) | 25 (96.2)* | ||
| 25(OH)D ≥75 nmol/L at follow-up,3 n (%) | 9 (37.5) | 14 (53.8) | ||
Values are frequency and percent or mean ± SD. *Different from D2 group, P ≤ 0.05. D2, ergocalciferol; D3, cholecalciferol; LC-MS/MS: liquid chromatography tandem MS; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D3, 25-hydroxycholecalciferol; 25(OH)D2, 25-hydroxyergocalciferol.
D2 group: total 25(OH)D = 25(OH)D2 + 25(OH)D3; D3 group: 25(OH)D3.
Results were similar to LC-MS/MS when tested by immunoassay: proportion of infants ≥50 nmol (D2: 79.2% vs. D3: 100%) or ≥75 nmol (D2: 37.5% vs. D3: 57.7%) cutoffs. 1 g vitamin D = 40 iu.
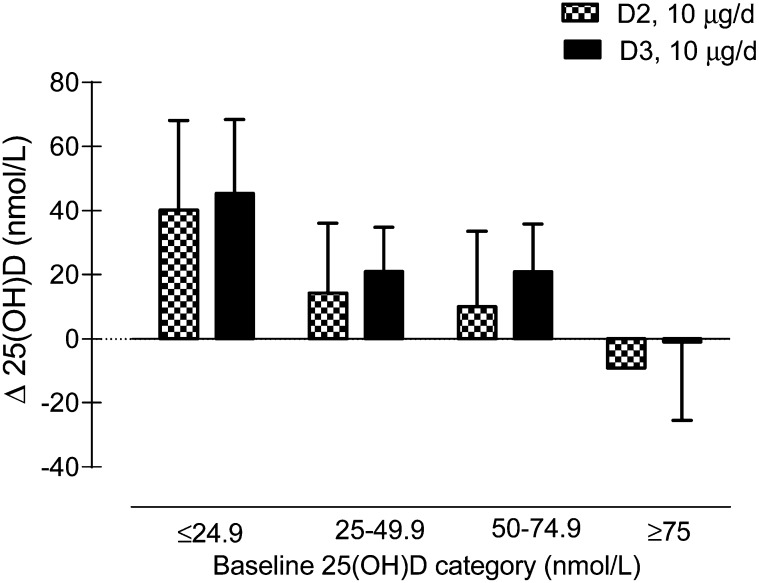
The baseline plasma 25(OH)D concentration based on the LC-MS/MS assay was inversely related to the change in 25(OH)D (r = −0.52; P < 0.001) (Fig. 3). The change in 25(OH)D concentrations did not significantly differ between groups when infants were categorized by baseline plasma 25(OH)D status (Fig. 3). The baseline 25(OH)D concentration, length-for-age Z-score, and supplementation period were included as covariates in the mixed-model ANCOVA. There were no differences in the change in plasma 25(OH)D between groups (P = 0.21). Similar results were observed using the immunoassay (data not shown).
FIGURE 3.
Plasma 25(OH)D concentration by LC-MS/MS from 1 to 4 mo of age in infants that received 10 μg/d D2 or D3 by baseline 25(OH)D category. Data are mean change ± SD. 25(OH)D, 25-hydroxyvitamin D; D2, ergocalciferol; D3, cholecalciferol; LC-MS/MS, liquid chromatography tandem MS.
As assessed by Bland-Altman analysis, the LC-MS/MS and immunoassay plasma 25(OH)D concentration limits of agreement ranged from −40 to +26% (Supplemental Fig. 1). The κ coefficient between methods was 0.57 when infants were categorized according to plasma 25(OH)D concentrations at follow-up.
Discussion
Daily supplementation of either vitamin D isoform at 10 μg/d elevated the plasma 25(OH)D concentration from 1 to 4 mo of age in healthy, breast-fed infants. This was important to know, because both D3 and D2 are common in the North American marketplace. These results are in accordance with the IOM’s Dietary Reference Intakes for vitamin D (3), which state “the two isoforms appear to be equivalent and adequate for almost all infants.” However, a significantly higher proportion of infants in the D3 group achieved the 50 nmol/L cutoff of plasma 25(OH)D by 3 mo compared with infants in the D2 group. Possible explanations include the fact that infants in the D2 group commenced the study with lower plasma 25(OH)D concentrations (P = 0.06) and the supplementation period was slightly shorter (P = 0.09). Our results were similar when 25(OH)D concentrations were measured by immunoassay or LC-MS/MS. We observed a significantly larger proportion of infants in the D2 group with decreases in 25(OH)D concentrations during the 3-mo period compared with infants in the D3 group. However, the mean decrease for 8 of the 24 infants in the D2 group was <10 nmol/L and compliance was questionable in 4 of these infants, who consumed <60% of doses (assessed either as reported or loss from bottle). Overall, the majority of infants (86%) achieved the 50 nmol/L cutoff at follow-up as suggested by the IOM for achievement of bone health (3). The results presented in this report complement the work of Gordon et al. (18), who found no differences in 25(OH)D concentrations after 6 wk of supplementation of 50 μg/d D2 compared with D3 for the treatment of low vitamin D status [25(OH)D ≤50 nmol/L] in older infants and toddlers. The increase in 25(OH)D after supplementation is largely dependent on the baseline 25(OH)D concentration and our sample consisted of 44% of infants with 25(OH)D ≤50 nmol/L at baseline. The observation that baseline 25(OH)D is a predictor of the change in 25(OH)D over time has been previously observed in adults (29, 30). This study shows that baseline status is an important factor in determining the response to supplementation in healthy infants. When infants were categorized by baseline status, there were no differences in the change in 25(OH)D concentration between treatment groups.
Previous studies in adults established a magnitude difference of 1.7-fold in the change in 25(OH)D concentration among groups taking D2 compared with D3; however, this was based on administration of an equivalent dosage of 100 μg/d (14). A lower dosage of 10 μg/d might result in more modest differences among groups. A similar pattern was observed in this dataset compared with Trang et al. (14) [30% higher change in 25(OH)D concentration in the D3 vs. D2 group]; however, the present study was perhaps underpowered for this analysis. A larger sample size would have provided more confidence to detect a smaller difference between treatments. Although most equivalency studies testing D2 compared with D3 had a similar number of subjects [Armas et al. (13), n = 30; Holick et al. (15), n = 68; Trang et al. (14), n = 72], future studies testing daily low-dose vitamin D (10 μg) should aim for an effect size in the range of 10–20% between the groups.
One of the challenges in assessing vitamin D status in infants includes the potential for a large proportion of 25(OH)D being in the C-3 α epimer form (31). To circumvent this issue and to quantify both 25(OH)D isoforms, 2 assays were used: one to quantify the isoforms and epimers individually (LC-MS/MS) and one that measured the total 25(OH)D without cross-reactivity to the epimer (chemiluminescence). Although moderate agreement was found between methods (limits of agreement, −40 to +26%), this is in line with other studies in adults (32) (limits of agreement, −57 to +48). In addition, replacing values below the level of quantification with zero or one-half the quantification level (26, 27) followed the same pattern. Furthermore, because both assays resulted in similar interpretations, either would be suitable for routine monitoring of vitamin D status in infants.
In our study, the infants were of healthy weight at birth and grew well according to the WHO standards. However, the generalizability of our vitamin D status results may pertain to only young infants (<4 mo), because vitamin D metabolism may change during the first year of life secondary to increases in growth, bone accretion, and maturation of enzymes and organs (3). This dataset was limited in sample size; thus, it would be important to conduct future, larger studies of longer duration to help establish whether older infants respond in a similar manner as well as to evaluate functional outcomes such as bone mass or calcium homeostasis.
In conclusion, this study provides novel findings regarding daily low-dose supplementation of the 2 vitamin D isoforms in breast-fed newborns. The increases in 25(OH)D concentration between the D2 and D3 groups did not differ, suggesting a sustained daily intake of either isoform is acceptable for infants 4 mo of age and younger.
Acknowledgments
The authors thank Dr. Line Duchesne, Manon Fabi, Louise LaCroix, and Christiane Leonard for their help with recruitment. S.G., C.R., and H.A.W. designed the research project; S.G., A.P., and C.A.V. conducted the research (collected data and conducted biochemistry); S.G. with the consultation of H.A.W. and C.R. performed all statistical analyses and wrote the paper; and C.A.V. and A.P. provided revisions. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: D2, ergocalciferol; D3, cholecalciferol; ITA, individual typological angle; IOM, Institute of Medicine; LC-MS/MS, liquid chromatography tandem MS; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D3, 25-hydroxycholecalciferol; 25(OH)D2, 25-hydroxyergocalciferol.
Literature Cited
- 1.European Commission Opinion on the Scientific Committee on Food on the tolerable upper intake level of vitamin D. Brussels: European Commission; 2003 [Google Scholar]
- 2. Vitamin D supplementation for breastfed infants; 2004. Health Canada Recommendations [cited 2012 Aug 14]. Available from: http://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/nutrition/vita_d_supp_e.pdf.
- 3.Institute of Medicine Dietary Reference Intakes for calcium and vitamin D. Washington, DC: National Academy Press; 2011 [Google Scholar]
- 4.Munns C, Zacharin MR, Rodda CP, Batch JA, Morley R, Cranswick NE, Craig ME, Cutfield WS, Hofman PL, Taylor BJ, et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Med J Aust. 2006;185:268–72 [DOI] [PubMed] [Google Scholar]
- 5.Mutlu GY, Kusdal Y, Ozsu E, Cizmecioglu FM, Hatun S. Prevention of vitamin D deficiency in infancy: daily 400 IU vitamin D is sufficient. Int J Pediatr Endocrinol. 2011;2011:4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52 [DOI] [PubMed] [Google Scholar]
- 7.Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health (Oxford). 2007;12:583–9 [PMC free article] [PubMed] [Google Scholar]
- 8.Greer FR, Searcy JE, Levin RS, Steichen JJ, Steichen-Asche PS, Tsang RC. Bone mineral content and serum 25-hydroxyvitamin D concentrations in breast-fed infants with and without supplemental vitamin D: one-year follow-up. J Pediatr. 1982;100:919–22 [DOI] [PubMed] [Google Scholar]
- 9.Specker BL, Ho ML, Oestreich A, Yin TA, Shui QM, Chen XC, Tsang RC. Prospective study of vitamin D supplementation and rickets in China. J Pediatr. 1992;120:733–9 [DOI] [PubMed] [Google Scholar]
- 10.Zeghoud F, Vervel C, Guillozo H, Walrant-Debray O, Boutignon H, Garabedian M. Subclinical vitamin D deficiency in neonates: definition and response to vitamin D supplements. Am J Clin Nutr. 1997;65:771–8 [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–231 [DOI] [PubMed] [Google Scholar]
- 13.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–91 [DOI] [PubMed] [Google Scholar]
- 14.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–8 [DOI] [PubMed] [Google Scholar]
- 15.Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapuri PB, Gallagher JC, Haynatzki G. Effect of vitamins D2 and D3 supplement use on serum 25OHD concentration in elderly women in summer and winter. Calcif Tissue Int. 2004;74:150–6 [DOI] [PubMed] [Google Scholar]
- 17.Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hypponen E, Berry J, Vieth R, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, Cox JE. Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab. 2008;93:2716–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollis BW, Lowery JW, Pittard WB III, Guy DG, Hansen JW. Effect of age on the intestinal absorption of vitamin D3-palmitate and nonesterified vitamin D2 in the term human infant. J Clin Endocrinol Metab. 1996;81:1385–8 [DOI] [PubMed] [Google Scholar]
- 20.Fujisawa Y, Kida K, Matsuda H. Role of change in vitamin D metabolism with age in calcium and phosphorus metabolism in normal human subjects. J Clin Endocrinol Metab. 1984;59:719–26 [DOI] [PubMed] [Google Scholar]
- 21.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8 [DOI] [PubMed] [Google Scholar]
- 22.Wren TA, Kim PS, Janicka A, Sanchez M, Gilsanz V. Timing of peak bone mass: discrepancies between CT and DXA. J Clin Endocrinol Metab. 2007;92:938–41 [DOI] [PubMed] [Google Scholar]
- 23.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards: growth velocity based on weight, length and head circumference: methods and development. Geneva: WHO; 2009 [Google Scholar]
- 24.Del Bino S, Sok J, Bessac E, Bernerd F. Relationship between skin response to ultraviolet exposure and skin color type. Pigment Cell Res. 2006;19:606–14 [DOI] [PubMed] [Google Scholar]
- 25.Aronov PA, Hall LM, Dettmer K, Stephensen CB, Hammock BD. Metabolic profiling of major vitamin D metabolites using Diels-Alder derivatization and ultra-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2008;391:1917–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole SR, Chu H, Nie L, Schisterman EF. Estimating the odds ratio when exposure has a limit of detection. Int J Epidemiol. 2009;38:1674–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langlois K, Greene-Finestone L, Little J, Hidiroglou N, Whiting S. Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2010;21:47–55 [PubMed] [Google Scholar]
- 28.Saadi HF, Dawodu A, Afandi B, Zayed R, Benedict S, Nagelkerke N, Hollis BW. Effect of combined maternal and infant vitamin D supplementation on vitamin D status of exclusively breastfed infants. Matern Child Nutr. 2009;5:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kluczynski MA, Wactawski-Wende J, Platek ME, Denysschen CA, Hovey KM, Millen AE. Changes in vitamin D supplement use and baseline plasma 25-hydroxyvitamin D concentration predict 5-y change in concentration in postmenopausal women. J Nutr. 2012;142:1705–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao LJ, Zhou Y, Bu F, Travers-Gustafson D, Ye A, Xu X, Hamm L, Gorsage DM, Fang X, Deng HW, et al. Factors predicting vitamin D response variation in non-Hispanic white postmenopausal women. J Clin Endocrinol Metab. 2012;97:2699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–61 [DOI] [PubMed] [Google Scholar]
- 32.Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–9 [DOI] [PubMed] [Google Scholar]