Abstract
Unlike most other cell types, neurons preferentially metabolize glucose via the pentose phosphate pathway (PPP) to maintain their antioxidant status. Inhibiting the PPP in neuronal cell models causes cell death. In rodents, inhibition of this pathway causes selective dopaminergic cell death leading to motor deficits resembling parkinsonism. Using postmortem human brain tissue, we characterized glucose metabolism via the PPP in sporadic Parkinson's disease (PD), Alzheimer's disease (AD), and controls. AD brains showed increased nicotinamide adenine dinucleotide phosphate (NADPH) production in areas affected by disease. In PD however, increased NADPH production was only seen in the affected areas of late-stage cases. Quantifying PPP NADPH-producing enzymes glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase by enzyme-linked immunosorbent assay, showed a reduction in the putamen of early-stage PD and interestingly in the cerebellum of early and late-stage PD. Importantly, there was no decrease in enzyme levels in the cortex, putamen, or cerebellum of AD. Our results suggest that down-regulation of PPP enzymes and a failure to increase antioxidant reserve is an early event in the pathogenesis of sporadic PD.
Keywords: Parkinson's disease, Neurodegeneration, Glucose metabolism, Pentose-phosphate pathway, NADPH
1. Introduction
Regeneration of oxidized glutathione by glutathione reductase to its reduced form, glutathione (GSH) is vitally important in combating oxidative stress in neurons. To reduce oxidized glutathione, glutathione reductase uses nicotinamide adenine dinucleotide phosphate (NADPH) as its cofactor. As levels of reactive oxygen species accumulate in neurons, the demand for NADPH increases (Ben-Yoseph et al., 1994). The levels of GSH in cells in culture are directly related to the production of NADPH by the pentose phosphate pathway (PPP) (Salvemini et al., 1999) and it has been shown that the PPP is the primary source of NADPH in cells (Pandolfi et al., 1995). Importantly, in neurons the rate limiting glycolytic enzyme Phosphofructokinase B3 is translated but subsequently ubiquitylated and degraded by the proteasome, with the result that neurons preferentially metabolize glucose via the PPP as opposed to glycolysis, in contrast to most other cell types (Herrero-Mendez et al., 2009). Inhibiting glucose flux through the PPP in these experiments was shown to cause increased levels of oxidative stress and cell death, emphasizing the extent to which neurons rely on the PPP for survival.
The effects of inhibiting the pentose phosphate and gluthathione pathways are similar to many of the pathologic changes associated with Parkinson's disease (PD). Depleting glutathione in rats using L-buthionine sulfoximine, has been shown to cause complex I and IV damage (Heales et al., 1995), a phenomenon, which has been documented in postmortem PD brain tissue, with complex I damage in PD widely reported (Schapira et al., 1989). Similarly, decreased levels of total GSH have been measured in PD brains (Perry et al., 1982; Sofic et al., 1992). This loss of GSH is found in PD and Lewy body disorders, but not multiple system atrophy, progressive supranuclear palsy, or Huntington's disease (Sian et al., 1994). One important, but less reported finding is that pharmacologic inhibition of the PPP in rats using the irreversible inhibitor 6-aminonicotinamide, has been shown to cause selective dopaminergic cell death in the striatum and muscle rigidity resembling bradykinesia (Herken, 1990).
Recent studies investigating metabolism in PD have continued to emphasize the importance of glucose usage in the disease process, with glucose hypometabolism shown in PD using magnetic resonance imaging and fludeoxyglucose (18F) positron emission tomography studies (Borghammer, 2012). Genetic microarray studies of the substantia nigra in PD show a strong association with the transcriptional regulator PGC1α, which is involved in the control of glucose usage (Zheng et al., 2010) and in animal models, knocking down α-synuclein (α-syn) has been shown to cause increased glyoxylase-1 expression with accompanied glycation damage. A recent article-characterizing metabolism in cultured adipocytes suggests that glucose uptake may be regulated by α-syn signaling via the LPAR2/Gab1/PI3K/Akt pathway (Rodriguez-Araujo et al., 2013). Importantly, studies looking at the effect of glucagon-like-peptide 1 on animal models of PD, have shown that modulating glucose metabolism is able to reverse dopaminergic cell loss in rats (Harkavyi et al., 2008; Li et al., 2009) and clinical trials of the glucagon-like-peptide 1 agonist exendin-4 are currently at stage II for use in PD (Tom Foltyne, personal communication).
Through this study, we aim to characterize the activity of the PPP in PD. Comparison of these results with those from AD and control tissue; will allow any differences in NADPH production to be identified. We hypothesize that reduced glucose metabolism via the PPP in neurons, is an early event in sporadic PD pathogenesis.
2. Methods
2.1. Tissue
We studied flash-frozen tissue from the frontal cortex (Brodmann area 4), putamen, and cerebellar vermis. Cases with pathologically diagnosed PD were stratified according to the McKeith criteria (McKeith et al., 2005). “Mild” cases were grouped according to those with Lewy body pathology in the brain stem only (McKeith brainstem predominant). “Moderate and/or severe” cases were those with Lewy body pathology visible in the limbic and neocortical areas (McKeith limbic and diffuse neocortical Lewy body pathology). AD cases were similarly confirmed by postmortem analysis and cases with Braak and Braak stages IV-VI (Braak et al., 2003) were selected for these experiments. For each brain region 13 controls, 11 mild PD, 12 moderate and/or severe, and 13 AD cases were used. Where tissue was available, cortex, putamen, and cerebellum samples were taken from the same individual.
Age at death of all cases was 80 ± 5 years. Controls were age matched and showed no Lewy body pathology. Cases with Braak and Braak stages II and under were used for this study.
Tissue was homogenized in an isolation buffer of 320 mM sucrose, 1 mM ethylenediaminetetraacetic acid and 10 mM tris-base, pH 7.4 using a mechanical homogenizer. Homogenates were centrifuged for 15 minutes at 16,000 g at 4 °C and the supernatant removed for assaying. Samples were aliquoted into eppendorf tubes and stored at −80 °C before assay.
2.2. Assays
We characterized PPP in PD by assessing the total NADPH generated by glucose-6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate dehydrogenase (6PGD). This was done using a modified protocol, based on that described by (Ninfali et al., 1997). All readings were within the linear range.
Tissue homogenate was assayed under 2 separate conditions, 1 containing G6P and 6PG to calculate the total activity of the pentose phosphate pathway and 1 with 6PG alone. G6PD activity was calculated by subtracting the NADPH generated in the combined condition, from that in the 6PG condition. Reaction mixture contained a final concentration of 2 mM NADP, 1 mM glucose-6-phosphate, and 1 mM phosphogluconic acid reconstituted in 1 M tris-HCl, 5 mM ethylenediaminetetraacetic acid pH 7.6, 15% (vol/vol) WST-8 dye supplemented with 0.2 mM 1-methoxy phenazinium methylsulfate (All materials were purchased from Sigma-Aldrich, Poole, UK). Samples were added to the reaction mixture and incubated at 37 °C for 15 minutes. NADPH generated was calculated by measuring the increase in absorbance at 450 nm.
2.3. Enzyme-linked immunosorbent assays (ELISAs)
The levels of the NADPH-producing enzymes G6PD and 6PGD were calculated using ELISA kits specific for human G6PD and 6PGD, according to the manufacturer's instructions (Wuhan life sciences, Houston, TX, USA). Samples were run in duplicate.
2.4. Protein estimation
Protein concentration was estimated using the Bio-Rad DC protein assay kit according to the manufacturer's instructions (Bio-Rad, Hemel Hempstead, UK).
2.5. Stastistical analyses
All results in this study were analyzed using a 1-way analysis of variance (ANOVA) followed by a Bonferroni posttest. The significance level was set at p < 0.05.
3. Results
We set out to characterize utilization of the PPP in flash frozen postmortem PD brain tissue. To look at glucose metabolism via this pathway over the course of disease progression, we identified 2 groups of PD samples, which were classified according to the extent of their Lewy body pathology based on the McKeith Lewy body pathology criteria (McKeith et al., 2005). Cases assigned to the “mild” group were those with Lewy body pathology predominantly in the brain stem. “Moderate and/or severe” cases were those with Lewy body pathology that had progressed to the limbic and neocortical areas. All cases in the “mild” group showed a low level of Lewy body deposition in the putamen. AD cases were confirmed by postmortem neuropathological examination and cases with Braak and Braak stages IV-VI (Braak et al., 2003) were used for these experiments. Controls were age matched and confirmed to have Braak and Braak stages of II and under. Table 1 shows the postmortem delay for each group.
Table 1.
Postmortem delays for the samples used in this study. Flash-frozen tissue from the frontal cortex, putamen, and cerebellum were used from each case
| Group | Postmortem delay (mean in h ± s.d) | Number of cases |
|---|---|---|
| Control | 41.58 ± 25.60 | 15 |
| Mild PD | 12.82 ± 6.56 | 11 |
| Moderate and/or severe PD | 49.32 ± 18.65 | 16 |
| AD | 58.00 ± 30.56 | 16 |
Key: AD, Alzheimer's disease; PD, Parkinson's disease; s.d, standard deviation.
Total NADPH produced by the PPP was calculated by addition of NADPH production by G6PD and 6PGD. To assess the impact of enzyme expression on the production of NADPH, the levels of G6PD and 6PGD were also measured by ELISA. Data for 6PGD ELISA and enzyme activity levels are shown as Supplementary data.
3.1. Cortex
Increased NADPH production in the cortex has been shown to occur in response to increased levels of oxidative stress in AD (Martins et al., 1986). Calculation of NADPH production by the PPP in our samples showed that generation of NADPH is significantly increased in the AD and moderate and/or severe PD groups as compared with controls (Fig. 1A gray bars, 1-way ANOVA followed by Bonferroni posttest vs. control).
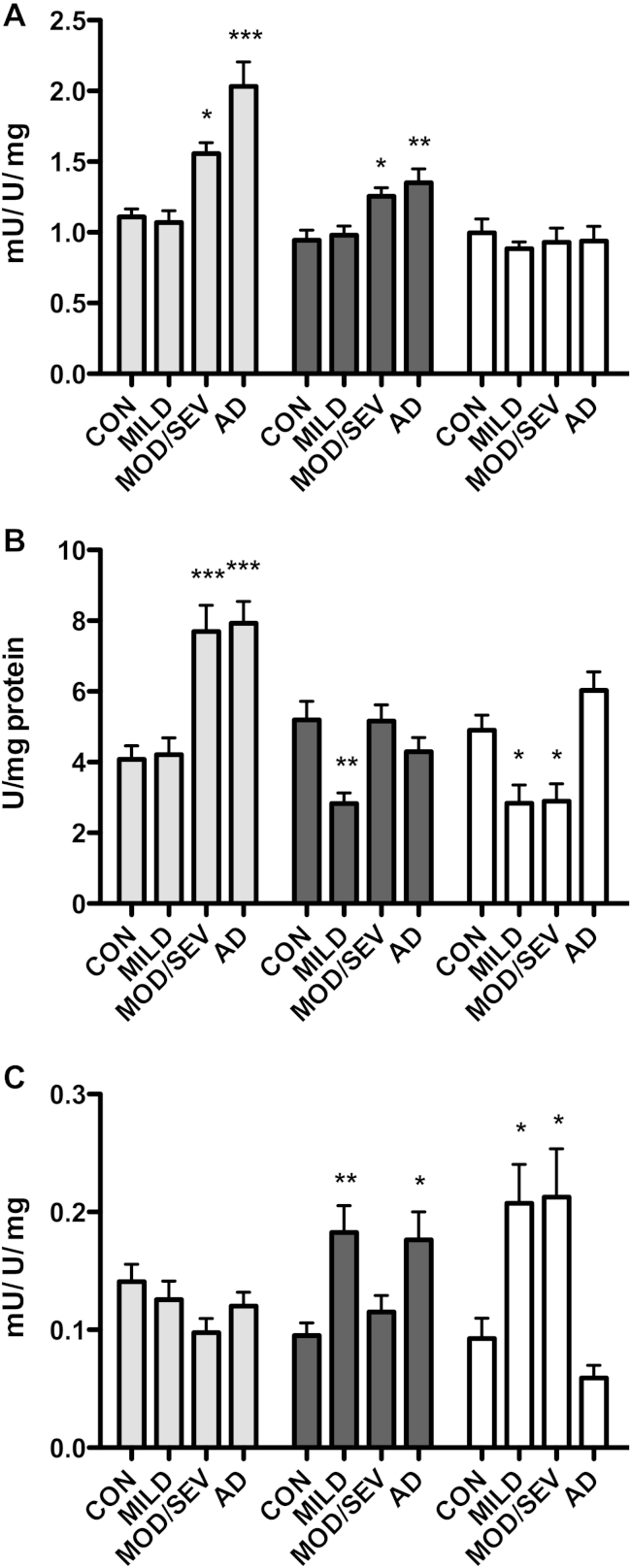
Fig. 1.
(A) Total nicotinamide adenine dinucleotide phosphate (NADPH) produced by the pentose phosphate pathway. The combined activity of G6PD and 6PGD is shown. One mU is defined as 1 μmole NADPH produced/min/unit of enzyme. Activity is shown as the mean NADPH produced per unit expressed per mg total protein ± SEM. (B) G6PD enzyme levels. Levels of the G6PD enzyme were measured by enzyme-linked immunosorbent assay (ELISA) and the combined total shown here per mg total protein. (C) Efficiency of G6PD. The efficiency of G6PD was calculated by expressing the total NADPH measured per mg total protein, per ELISA unit (U) of G6PD. Relative activities are shown as mean NADPH produced per ELISA unit of protein ± SEM. Levels are shown as mean ± SEM. Con- pathologically normal controls, n = 13. Mild-McKeith brainstem predominant Parkinson's disease (PD) cases n = 11. Mod/Sev-McKeith limbic and diffuse neocortical cases. PD cases n = 12. Alzheimer's disease (AD) Braak and Braak stages IV-VI n = 13. Gray-cortex Brodmann area 4. Black-putamen. White-cerebellar vermis. One-way analysis of variance (ANOVA) significance p < 0.05, with Bonferroni posttest versus control * p < 0.05, ** p < 0.01, *** p < 0.001. Abbreviations: AD, Alzheimer's disease; ANOVA, analysis of variance; ELISA, enzyme-linked immunosorbent assay; NADPH, nicotinamide adenine dinucleotide phosphate; PD, Parkinson's disease; SEM, standard error of the mean.
This increase was accompanied by higher levels of the rate-limiting enzyme G6PD (Fig. 1B, gray bars, 1-way ANOVA followed by Bonferroni posttest p < 0.05). In the mild PD group, that is PD cases that do not have pathology in the cortex, there was no increase in either the amount of NADPH produced or levels of G6PD enzymes (Fig. 1A and B gray bars). Calculating the efficiency of G6PD by expressing NADPH production per ELISA unit of G6PD protein showed that there was no significant change in any of the experimental groups characterized (Fig. 1C, gray bars, 1-way ANOVA, p = 0.8017).
3.2. Putamen
Next we looked at PPP usage in the putamen. Total NADPH produced by the PPP in the putamen was significantly increased in the brains of those with AD and in the moderate and/or severe PD group (Fig. 1A, black bars, 1-way ANOVA followed by Bonferroni posttest).
In AD, the levels of G6PD enzyme were not increased compared with controls. Similarly the moderate and/or severe PD group showed no significant increase in protein levels of the rate-limiting enzyme G6PD however, the mild PD group showed a significant decrease, suggesting a down-regulation of this pathway at the protein level (Fig. 1B, black bars, 1-way ANOVA followed by Bonferroni posttest). Calculating the efficiency of G6PD for all samples, showed an activation of G6PD in the mild PD and AD groups. In these groups, each ELISA unit of the enzyme produced approximately twice as much NADPH per minute compared with the control group (Fig. 1C, black bars, 1-way ANOVA followed by Bonferroni posttest vs. control). Importantly, AD cases with Braak and Braak stages IV-VI have amyloid beta and tau pathology in the putamen and the activation of G6PD in these samples suggests that an oxidative stress response is occurring.
While both the mild and moderate and/or severe PD cases used in this study showed Lewy body pathology in the putamen, it is only the moderate and/or severe group which shows increased NADPH production in response to this (Fig. 1A, black bars). This would suggest that although the mild cases are able to match control NADPH production levels, the ability to mount an oxidative stress responsive is decreased and that an increase in NADPH is not seen until PD pathology is more extensive.
3.3. Cerebellum
To assess the function of the PPP pathway in a region not affected in PD, we measured NADPH production and the enzyme levels of G6PD and 6PGD in the cerebellum.
NADPH production across all 3 experimental groups showed no significant change in the cerebellum when compared with controls (Fig. 1A, white bars, 1-way ANOVA p = 0.8692). Interestingly however, quantification of G6PD protein levels, again showed a down-regulation of the rate-limiting enzyme that was present in both PD groups, with enzyme levels showing a significant decrease compared with control (Fig. 1B, white bars, 1-way ANOVA followed by Bonferroni posttest). Calculating the efficiency of G6PD in these samples showed a 2-fold increase in NADPH production of about 2-fold in these samples when compared with controls (Fig. 1C, 1-way ANOVA followed by Bonferroni posttest). As shown in the putamen of samples in the mild PD group, despite a significant decrease in NADPH producing enzymes, net NADPH production in both PD groups remains level with that of the controls, because of each unit of G6PD producing more NADPH. Samples in the AD group showed no difference in the NADPH produced (Fig. 1A, white bars), or in the levels of PPP enzymes measured (Fig. 1B, white bars).
4. Discussion
Neurons are subjected to high levels of oxidative stress because of a relatively high level of oxidative phosphorylation and nitric oxide signaling from astrocytes (Bolanos and Heales, 2010.), leaving them vulnerable to damage. It has been shown that production of NADPH from the PPP is responsible for reduction of oxidative species and therefore reducing levels of oxidative stress, leaving neurons able to function normally. Inhibition of the PPP is deleterious to neurons (Filosa et al., 2003; Pandolfi et al., 1995). Increased levels of oxidative stress are important in the pathogenesis of PD (Alam et al., 1997; Dexter et al., 1989; Floor et al., 1998) and it has been suggested that oxidative stress could play an important role in the formation of Lewy body pathology (Jenner and Olanow, 1996). As the PPP is the main source of defense against oxidative stress in neurons, we thought it important to characterize the usage of this pathway in PD. Studies showing increased activity of the PPP in AD (Martins et al., 1986), neuromuscular diseases (Meijer, 1991), myocardial infarction (Gupte, 2008), and studies using hepatocyte cell models (Ursini et al., 1997) support the idea that oxidative stress is normally met with increased production of NADPH. However, characterization of the PPP in our experiments has shown that NADPH production is not increased in the putamen of samples with early stage PD and furthermore, PPP enzyme levels are actually decreased. This is despite widely documented evidence that dopaminergic cell death in the striatum of PD cases is accompanied by increased markers for oxidative stress and neuroinflammation in postmortem samples (Alam et al., 1997; Imamura et al., 2003; Marttila et al., 1988; Ouchi et al., 2005; Sanchez-Ramos et al., 1994) and in vivo, by positron emission tomography imaging studies (Ikawa et al., 2011). Similarly, although there are a number of published studies showing increased levels of oxidative stress in the frontal cortex of cases with both clinical and preclinical PD (Dalfo et al., 2005; Ferrer, 2009; Parker et al., 2008), the early stage cases in our experiments again did not show an increase in NADPH levels. This could be because of faulty oxidative stress-sensing mechanisms in affected neurons, or because of an active suppression mechanism that is stopping increased NADPH production from occurring.
An article looking at the PPP in ALS, showed that neuroblastoma-spinal cord hybrid cell lines (NSC34) carrying G93A or G37R SOD1 mutations display decreased levels of G6PD enzyme, which affects the production of NADPH by this pathway (Kirby et al., 2005). Similarly ataxia telangiectasia mutated knockout mice also show a reduction of G6PD in the cerebellum (Cosentino et al., 2011), suggesting that downregulation of the PPP in disease could be a key mechanism in some forms of neurodegeneration. Taking our results together with data from cell and animal models, suggests that dysregulation of the PPP could be causing oxidative stress because of less efficient GSH recycling (Heales et al., 1995; Herrero-Mendez et al., 2009) and that dysregulation of the PPP could be causative factor in the increased levels of oxidative stress seen in PD.
The results of our experiments suggest that there are 2 stages of PPP involvement with oxidative stress in PD. In the early stages of disease, lower levels of PPP enzymes in the putamen could be causing increased levels of oxidative stress. In response to this, G6PD is activated, potentially by posttranslational modification of the enzyme as shown in other studies (Cosentino et al., 2011; Ursini et al., 1997) and the increased demand for NADPH met by increased activity per ELISA unit of the protein. In the later stages of disease, where pathology has spread to the limbic system and neocortex, our results show that there is a switch from activation of G6PD at its existing levels, to upregulating the NADPH-producing enzymes at a protein level. Interestingly, the exact mechanisms for regulation seem to differ between brain regions and despite evidence of increased oxidative stress in the cerebellum in PD, the cerebellum does not show evidence of neurodegeneration. Elucidating the mechanisms underlying these differences could be important in understanding the vulnerability of certain cell types over others in different neurodegenerative diseases. The results of this study come with the caveat that the tissue was from postmortem brains and therefore only provides a snapshot of the changes occurring at that time. Despite this, further investigation into mechanisms that are potentially underlying our findings may prove important in understanding the pathogenesis of PD.
As discussed, the evidence for perturbed glucose metabolism in PD is increasing. The results of this study shed light on a potentially new mechanism in the pathogenesis of sporadic PD and when taken with further studies from cell and animal models, may lead to better understanding of these mechanisms. Our data suggest that down-regulation of the PPP in PD could be a primary event in disease progression and further research down this avenue may provide potential targets for future therapeutic avenues.
We hypothesize that mitochondrial damage in PD occurs as a direct result of PPP dysregulation and that α-syn plays an important role in the altered metabolism of glucose via the PPP in this disease.
Acknowledgements
The authors would like to thank those who donated the brain tissue, this study would not have been possible without them. Tissue from this study was prepared by the Queen Square Brain Bank, the Parkinson's UK Brain Bank, and the King's College Institute of Psychiatry Brain Bank. The authors thank Prof. John Hardy for critical comments on this manuscript and Dr Julia Fitzgerald and Dr Lee Stanyer for their help and support with grant writing and experiments. This study was funded by a Rapid Response Innovation Award from the Michael J. Fox Foundation for Parkinson's Research to RB and SJH and a Parkinson's UK grant to RB. LD was supported by the Michael J Fox Foundation. RB is supported by the Reta Lila Weston Trust. HL is supported by the Progressive Supranuclear Palsy (Europe) Association (6AMN) and the Reta Lila Weston Institute Fellowship. JLH is supported by Parkinson's UK, the Multiple System Atrophy Trust, Alzheimer's Research UK, the Progressive Supranuclear Palsy (Europe) Association and the Reta Lila Weston Institute for Neurological Studies. This research was supported in part by the National Institute for Health Research, Biomedical Research Unit based at University College London Hospitals, University College London. This work was supported in part by the Wellcome Trust and Medical Research Council Joint Call in Neurodegeneration award (WT089698) to the UK Parkinson's Disease Consortium whose members are from the University College London Institute of Neurology, the University of Sheffield, and the Medical Research Council Protein Phosphorylation Unit at the University of Dundee.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary data
References
- Alam Z.I., Jenner A., Daniel S.E., Lees A.J., Cairns N., Marsden C.D., Jenner P., Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph O., Boxer P.A., Ross B.D. Oxidative stress in the central nervous system: monitoring the metabolic response using the pentose phosphate pathway. Dev. Neurosci. 1994;16:328–336. doi: 10.1159/000112127. [DOI] [PubMed] [Google Scholar]
- Bolanos J.P., Heales S.J. Persistent mitochondrial damage by nitric oxide and its derivatives: neuropathological implications. Front Neuroenergetics. 2010;2 doi: 10.3389/neuro.14.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghammer P. Perfusion and metabolism imaging studies in Parkinson's disease. Dan. Med. J. 2012;59:B4466. [PubMed] [Google Scholar]
- Braak H., Del Tredici K., Rub U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Cosentino C., Grieco D., Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30:546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalfo E., Portero-Otin M., Ayala V., Martinez A., Pamplona R., Ferrer I. Evidence of oxidative stress in the neocortex in incidental Lewy body disease. J. Neuropathol. Exp. Neurol. 2005;64:816–830. doi: 10.1097/01.jnen.0000179050.54522.5a. [DOI] [PubMed] [Google Scholar]
- Dexter D.T., Carter C.J., Wells F.R., Javoy-Agid F., Agid Y., Lees A., Jenner P., Marsden C.D. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J. Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Ferrer I. Early involvement of the cerebral cortex in Parkinson's disease: convergence of multiple metabolic defects. Prog. Neurobiol. 2009;88:89–103. doi: 10.1016/j.pneurobio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Filosa S., Fico A., Paglialunga F., Balestrieri M., Crooke A., Verde P., Abrescia P., Bautista J.M., Martini G. Failure to increase glucose consumption through the pentose-phosphate pathway results in the death of glucose-6-phosphate dehydrogenase gene-deleted mouse embryonic stem cells subjected to oxidative stress. Biochem. J. 2003;370:935–943. doi: 10.1042/BJ20021614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floor E., Wetzel M.G. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 1998;70:268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- Gupte S.A. Glucose-6-phosphate dehydrogenase: a novel therapeutic target in cardiovascular diseases. Curr. Opin. Investig. Drugs. 2008;9:993–1000. [PubMed] [Google Scholar]
- Harkavyi A., Abuirmeileh A., Lever R., Kingsbury A.E., Biggs C.S., Whitton P.S. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease. J. Neuroinflammation. 2008;5 doi: 10.1186/1742-2094-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heales S.J., Davies S.E., Bates T.E., Clark J.B. Depletion of brain glutathione is accompanied by impaired mitochondrial function and decreased N-acetyl aspartate concentration. Neurochem. Res. 1995;20:31–38. doi: 10.1007/BF00995149. [DOI] [PubMed] [Google Scholar]
- Herken H. Neurotoxin-induced impairment of biopterin synthesis and function: Initial stage of a Parkinson-like dopamine deficiency syndrome. Neurochem. Int. 1990;17:223–238. doi: 10.1016/0197-0186(90)90145-j. [DOI] [PubMed] [Google Scholar]
- Herrero-Mendez A., Almeida A., Fernandez E., Maestre C., Moncada S., Bolanos J.P. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat. Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- Ikawa M., Okazawa H., Kudo T., Kuriyama M., Fujibayashi Y., Yoneda M. Evaluation of striatal oxidative stress in patients with Parkinson's disease using [62Cu]ATSM PET. Nucl. Med. Biol. 2011;38:945–951. doi: 10.1016/j.nucmedbio.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Imamura K., Hishikawa N., Sawada M., Nagatsu T., Yoshida M., Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Jenner P., Olanow C.W. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. 1996;47:S161–170. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- Kirby J., Halligan E., Baptista M.J., Allen S., Heath P.R., Holden H., Barber S.C., Loynes C.A., Wood-Allum C.A., Lunec J., Shaw P.J. Mutant SOD1 alters the motor neuronal transcriptome: implications for familial ALS. Brain. 2005;128:1686–1706. doi: 10.1093/brain/awh503. [DOI] [PubMed] [Google Scholar]
- Li Y., Perry T., Kindy M.S., Harvey B.K., Tweedie D., Holloway H.W., Powers K., Shen H., Egan J.M., Sambamurti K., Brossi A., Lahiri D.K., Mattson M.P., Hoffer B.J., Wang Y., Greig N.H. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl. Acad. Sci. U.S.A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins R.N., Harper C.G., Stokes G.B., Masters C.L. Increased cerebral glucose-6-phosphate dehydrogenase activity in Alzheimer's disease may reflect oxidative stress. J. Neurochem. 1986;46:1042–1045. doi: 10.1111/j.1471-4159.1986.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Marttila R.J., Lorentz H., Rinne U.K. Oxygen toxicity protecting enzymes in Parkinson's disease. Increase of superoxide dismutase-like activity in the substantia nigra and basal nucleus. J. Neurol. Sci. 1988;86:321–331. doi: 10.1016/0022-510x(88)90108-6. [DOI] [PubMed] [Google Scholar]
- McKeith I.G., Dickson D.W., Lowe J., Emre M., O'Brien J.T., Feldman H., Cummings J., Duda J.E., Lippa C., Perry E.K., Aarsland D., Arai H., Ballard C.G., Boeve B., Burn D.J., Costa D., Del Ser T., Dubois B., Galasko D., Gauthier S., Goetz C.G., Gomez-Tortosa E., Halliday G., Hansen L.A., Hardy J., Iwatsubo T., Kalaria R.N., Kaufer D., Kenny R.A., Korczyn A., Kosaka K., Lee V.M., Lees A., Litvan I., Londos E., Lopez O.L., Minoshima S., Mizuno Y., Molina J.A., Mukaetova-Ladinska E.B., Pasquier F., Perry R.H., Schulz J.B., Trojanowski J.Q., Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Meijer A.E. The pentose phosphate pathway in skeletal muscle under patho-physiological conditions. A combined histochemical and biochemical study. Prog. Histochem. Cytochem. 1991;22:1–118. doi: 10.1016/s0079-6336(11)80052-5. [DOI] [PubMed] [Google Scholar]
- Ninfali P., Aluigi G., Balduini W., Pompella A. Glucose-6-phosphate dehydrogenase activity is higher in the olfactory bulb than in other brain areas. Brain Res. 1997;744:138–142. doi: 10.1016/s0006-8993(96)00933-x. [DOI] [PubMed] [Google Scholar]
- Ouchi Y., Yoshikawa E., Sekine Y., Futatsubashi M., Kanno T., Ogusu T., Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann. Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Pandolfi P.P., Sonati F., Rivi R., Mason P., Grosveld F., Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995;14:5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker W.D., Jr., Parks J.K., Swerdlow R.H. Complex I deficiency in Parkinson's disease frontal cortex. Brain Res. 2008;1189:215–218. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T.L., Godin D.V., Hansen S. Parkinson's disease: a disorder due to nigral glutathione deficiency? Neurosci. Lett. 1982;33:305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Araujo G., Nakagami H., Hayashi H., Mori M., Shiuchi T., Minokoshi Y., Nakaoka Y., Takami Y., Komuro I., Morishita R., Kaneda Y. Alpha-synuclein elicits glucose uptake and utilization in adipocytes through the Gab1/PI3K/Akt transduction pathway. Cell Mol. Life Sci. 2013;70:1123–1133. doi: 10.1007/s00018-012-1198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini F., Franze A., Iervolino A., Filosa S., Salzano S., Ursini M.V. Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression. J. Biol. Chem. 1999;274:2750–2757. doi: 10.1074/jbc.274.5.2750. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J., Overik E., Ames B. A marker of oxyradical-mediated DNA damage (8-hydroxy-2′deoxyguanosine) is increased in nigro-striatum of Parkinson's disease brain. Neurodegeneration. 1994;3:197–204. [Google Scholar]
- Schapira A.H., Cooper J.M., Dexter D., Jenner P., Clark J.B., Marsden C.D. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Sian J., Dexter D.T., Lees A.J., Daniel S., Agid Y., Javoy-Agid F., Jenner P., Marsden C.D. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Sofic E., Lange K.W., Jellinger K., Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson's disease. Neurosci. Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- Ursini M.V., Parrella A., Rosa G., Salzano S., Martini G. Enhanced expression of glucose-6-phosphate dehydrogenase in human cells sustaining oxidative stress. Biochem. J. 1997;323:801–806. doi: 10.1042/bj3230801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Liao Z., Locascio J.J., Lesniak K.A., Roderick S.S., Watt M.L., Eklund A.C., Zhang-James Y., Kim P.D., Hauser M.A., Grunblatt E., Moran L.B., Mandel S.A., Riederer P., Miller R.M., Federoff H.J., Wullner U., Papapetropoulos S., Youdim M.B., Cantuti-Castelvetri I., Young A.B., Vance J.M., Davis R.L., Hedreen J.C., Adler C.H., Beach T.G., Graeber M.B., Middleton F.A., Rochet J.C., Scherzer C.R. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson's disease. Sci. Transl. Med. 2010;2:52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.