Summary
Background
Male-specific products of the fruitless (fru) gene control the development and function of neuronal circuits that underlie male-specific behaviors in Drosophila, including courtship. Alternative splicing generates at least three distinct Fru isoforms, each containing a different zinc-finger domain. Here, we examine the expression and function of each of these isoforms.
Results
We show that most fru+ cells express all three isoforms, yet each isoform has a distinct function in the elaboration of sexually dimorphic circuitry and behavior. The strongest impairment in courtship behavior is observed in fruC mutants, which fail to copulate, lack sine song, and do not generate courtship song in the absence of visual stimuli. Cellular dimorphisms in the fru circuit are dependent on FruC rather than other single Fru isoforms. Removal of FruC from the neuronal classes vAB3 or aSP4 leads to cell-autonomous feminization of arborizations and loss of courtship in the dark.
Conclusions
These data map specific aspects of courtship behavior to the level of single fru isoforms and fru+ cell types—an important step toward elucidating the chain of causality from gene to circuit to behavior.
Highlights
-
•
fru A, B, and C isoforms have largely overlapping expression in the male fly CNS
-
•
All three fru isoforms contribute to male courtship, with fruC being the most critical
-
•
FruC specifies sexual dimorphisms in neuron number and arborizations
-
•
FruC is required in defined neuronal classes for male-specific anatomy and behavior
Drosophila express three male-specific isoforms (A, B, and C) of the putative transcription factor fruitless (fru) in an overlapping pattern. von Philipsborn et al. show that isoform-mutant flies exhibit defects in male courtship, copulation success, and song production. FruC specifies sexual dimorphisms in defined neuronal classes potentially crucial for pheromone processing.
Introduction
Males and females of sexually reproducing animal species typically display profound differences in their mating behaviors, reflecting the operation of sexually dimorphic neural circuits. Because most aspects of mating behaviors are innate, these sexual dimorphisms must be encoded in the genome and established during development. For several genetic model organisms, including flies and mice, the distinct behaviors of the two sexes and the initial molecular events that underlie sex determination are both well understood [1]. With the two endpoints thus well defined, the mating behaviors of these organisms provide an ideal opportunity to trace the long chain of causality from genes to behavior. This task involves defining the underlying neural circuitry at cellular resolution, relating specific cellular dimorphisms to specific behavioral dimorphisms, and understanding how these structural and functional dimorphisms are shaped by gene activity.
Progress toward this goal is currently most advanced for the male-specific courtship behavior of Drosophila melanogaster (reviewed in [2]). Sex in Drosophila is determined by the ratio of X chromosomes to autosomes. DNA-binding proteins have been identified that “count” chromosomes and trigger a cascade of gene regulatory events that results in female-specific expression of the transformer (tra) gene. tra determines almost all aspects of sexual differentiation, with the exception of the dosage compensation mechanisms that adjust expression levels of X-linked genes (reviewed in [3]). Thus, animals that are chromosomally female but lack tra function look and behave like males, whereas those that are chromosomally male but express tra look and behave like females [4]. The tra gene encodes a splicing factor with two known targets, doublesex (dsx) and fruitless (fru), both of which produce both male-specific (M) and female-specific (F) transcripts. fruF transcripts appear to be nonfunctional, whereas fruM, dsxM, and dsxF all encode predicted transcription factors essential for various aspects of sex-specific differentiation (reviewed in [5]).
The courtship behavior of Drosophila males consists of a series of discrete elements, including orientation toward the female, following the female, extending and vibrating one wing to produce a courtship song, licking the genitalia, and attempting copulation. Orientation, following, and singing are more common in the initial stages, whereas licking and attempted copulation are generally observed only during later stages of courtship with a sexually receptive female (reviewed in [6]). Multiple sensory inputs drive this behavior. Chemosensory and visual cues predominate, but neither is absolutely essential in single-pair assays performed under laboratory conditions. Chemosensory cues are thought to arouse the male and promote progression through courtship elements, whereas visual cues guide orientation and following [7].
Of the two distal genes in the sex determination pathway, fru plays the more prominent role in the establishment of sexually dimorphic neural circuitry and behavior. Males lacking fruM appear to be externally normal males yet are profoundly defective in most aspects of courtship behavior [8–10]. Conversely, females engineered to express fruM resemble normal females yet perform at least the initial stages of male courtship, albeit imperfectly [11, 12]. In contrast, the analogous mutations in dsx dramatically alter the animal’s appearance but have a comparatively milder impact on behavior, disrupting the song and reducing overall courtship levels [13, 14]. Tracing the causative links from genes to courtship behavior is thus most likely to be productive by following the fru branch of the sex determination pathway.
The sex-specific fru transcripts are expressed in ∼2,000 cells in the male nervous system [15], which have been subdivided on morphological and developmental criteria into ∼100 distinct classes and assembled into an anatomical atlas with cellular resolution [12, 16–18]. dsx is expressed in a subset of these cells [14, 19]. Neuronal silencing and activation experiments have demonstrated that the activity of the fru+ cells, collectively, is causally linked to the execution of courtship behavior [12, 16, 20]. Our working hypothesis is that many, perhaps even most, of these neurons contribute to some specific aspect of courtship behavior. For example, fru+ Or67d+ olfactory neurons detect the volatile inhibitory sex pheromone cis-vaccenyl acetate [21–23], fru+ IR84a+ olfactory neurons detect a plant volatile that stimulates courtship [24], and fru+ ppk23+ gustatory neurons detect the nonvolatile female aphrodisiac pheromone 7,11-heptacosadiene [25, 26]. Each of these chemosensory cues is likely to be further processed by fru+ neurons in the CNS [17, 27, 28]. Central fru+ neurons that contribute to various aspects of courtship song have also been identified [29–31], including the brain neurons P1/pMP4 and pIP10 and the thoracic neurons dPR1, vPR6, and vMS11. P1 is a critical node in the courtship circuitry, as it is stimulated by direct physical contact with a female [29] and its activity is both necessary and sufficient for song production [30].
If fruM expression indeed defines most of the neurons likely to have sex-specific functions in male courtship behavior, then the task now is to identify sexual dimorphisms within this circuit, understand how they contribute to sexually dimorphic information processing and behavior, and determine whether and how they are specified by fru itself. Studies using light microscopy have identified at least 12 distinct classes of fru+ neurons that are sexually dimorphic [16–18, 27, 32], and higher-resolution anatomical and physiological studies are likely to reveal many more. These differences include a few neuronal classes that are present in males but lacking in females, such as P1, pIP10, and vPR6, and several others that differ in cell numbers, projections, or arborizations, such as mAL/aDT2, aSP1, and aSP2. The existence of P1 in males but not females is attributable to dsx [33] and might explain why only males sing. Dimorphisms in some of the other cell types have been attributed to fru [27, 32, 34], but for most cellular dimorphisms, it is still unknown whether they depend on dsx or fru. Moreover, the behavioral significance of these dimorphisms remains obscure. Thus, for the most part, both the causes and consequences of anatomical dimorphisms among the fru+ neurons are unknown.
Mapping cellular dimorphisms to the fru gene is further complicated by its complex molecular architecture. In addition to the sex-specific transcripts, a set of common transcripts (fruCOM) is also produced from transcription that is initiated at alternative promoters, bypassing the target sequence for Tra and hence escaping sex-specific splicing [9, 10]. These fruCOM transcripts have essential functions during early embryonic development but are not expressed in adults and do not appear to contribute to sexual differentiation of the adult nervous system [35]. More importantly, the fru gene is additionally subject to alternative splicing at its 3′ end, resulting in at least four distinct variants (A–D) of both the fruM and fruCOM transcripts. The FruA–D isoforms each contain a distinct zinc-finger domain and thus potentially have distinct DNA-binding properties [9, 10, 36]. It is thus important to know for each cellular dimorphism in the fru circuit not only whether it is dependent upon fru itself, but also upon which isoform. Similarly, it is imperative to know which aspects of courtship behavior are dependent upon each of the fru isoforms.
This effort has been initiated with an analysis of a mutation affecting the fruC isoform, which disrupts courtship song and copulation, serotonergic innervations of the male reproductive system, and the central projections of foreleg gustatory neurons [34, 37]. Our primary objective in this work was to extend this study to a systematic investigation of all four major fru splice variants, mapping their expression to each of the distinct classes of fru+ neurons and assessing which cellular dimorphisms and which aspects of courtship behavior depend on each isoform. To the extent that currently available knowledge and genetic tools would allow, we also sought to correlate the cellular and behavioral functions of each fru isoform.
Results
Most fru Neurons Express Three FruM Isoforms
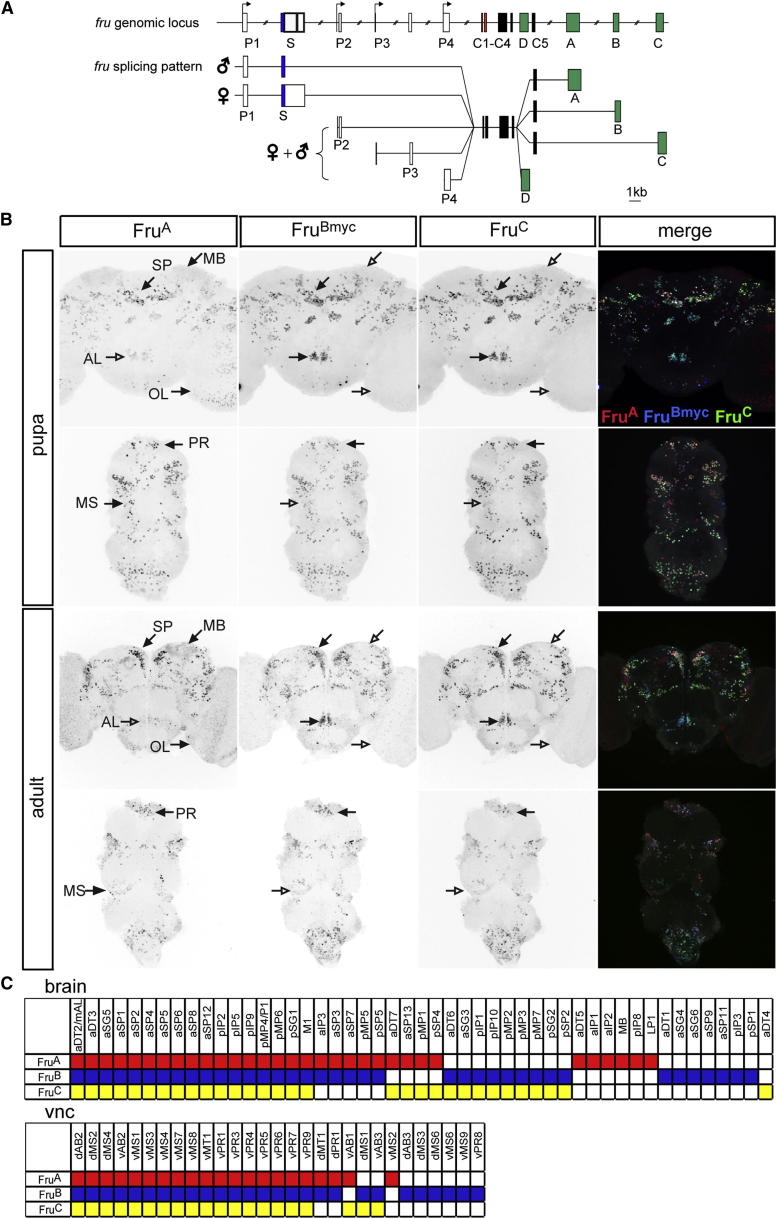
To determine the expression patterns of each of the four FruM isoforms, we used gene targeting by homologous recombination to independently attach c-myc epitope tags to the C termini of FruA, FruB, FruC, and FruD (Figure 1A). The tagged FruA, FruB, and FruC proteins could be detected in the nuclei of most, but not all, fru+ neurons in the adult male brain and nerve cord (see Figure S1A available online; data not shown). FruD could not be detected in the adult male CNS (data not shown), consistent with the reported absence of fruD mRNA in adult head tissue [9, 38]. As also expected, none of the isoforms could be detected in the adult female CNS (data not shown). In parallel, we generated antisera against protein domains encoded by the isoform-specific exons of FruA and FruC (Figure 1A) and confirmed their specificity by expressing fruMA, fruMB, and fruMC transgenes in larval CCAP neurons (Figure S1B). Applied to brains of the corresponding c-myc-tagged fru allele, these antisera labeled the c-myc+ nuclei in males, with no staining detected in females (Figures S1C and S1D).
Figure 1.
Overlapping Expression of FruM Isoforms in the Pupal and Adult CNS
(A) Schematics of the fru genomic locus and splicing pattern. P1–P4, alternative promoters; S, sex-specifically spliced exon; C1–C5, common exons (encoding BTB domain); A–D, isoform-specific exons (encoding zinc-finger domains). myc tags were located at the 5′ end of isoform-specific exons A–D; exons A and C encode the respective antibody epitopes.
(B) Brains and ventral nerve cords of male 48 hr pupa and 8 day adult flies triple labeled for FruA, FruBmyc, and FruC. Solid arrows indicate selected clusters showing expression of the indicated isoform; empty arrows the absence of staining. SP, medial superior protocerebrum; MB, mushroom body; AL, antennal lobes; OL, optic lobes; PR, ventral prothoracic ganglion; MS, mesothoracic ganglion.
(C) Overlapping and distinct isoform expression of a set of 78 anatomically characterized adult neuronal classes. Expression of FruA is marked in red, FruB in blue, and FruC in yellow.
Using the specific antisera for FruA and FruC and the c-myc-tagged allele for fruB, we performed triple stainings to simultaneously visualize all three isoforms in the adult and pupal male CNS (Figure 1B). In broad agreement with previous reports [12, 16, 17], we counted 1,604 ± 123 cells expressing one or more Fru isoforms in the adult (n = 5) and 1,573 ± 89 cells in the pupa (n = 5). In both stages, we saw a high but not complete overlap in the expression of the three isoforms (Figure 1C; Table S1). We further mapped the expression of each of the three isoforms in 78 of the previously characterized [17, 30] fru+ neuronal classes (Figures 1C and S2). Most of these cells expressed all three Fru isoforms, but some expressed only one or two (Figure S2). FruA was detected alone in seven cell types, FruB in thirteen, and FruC in one (Figures 1B and S2).
Isolation of Isoform-Specific fru Alleles
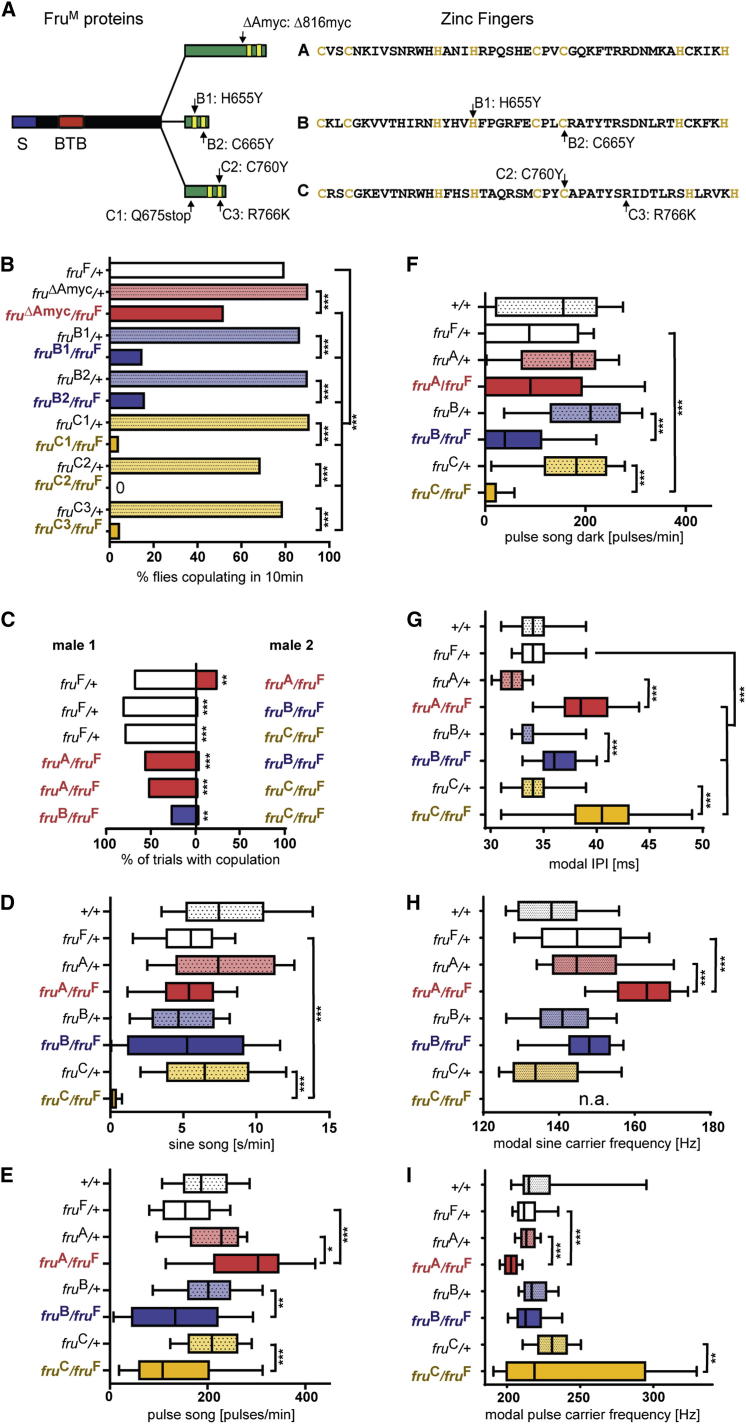
To assess the functions of each of the Fru isoforms, we next sought to generate a set of mutant alleles that selectively disrupt each of the FruA, FruB, or FruC isoforms. We first took advantage of the fact that fruM and fruΔtra are dominant sterile mutations in females [11]. We thus performed a chemical mutagenesis screen in the fruΔtra background to isolate fertile intragenic revertants, a self-selecting phenotype. We recovered 16 revertant alleles in this manner, two of which were associated with mutations in the B exon (fruB1 and fruB2) and three in the C exon (fruC1, fruC2, and fruC3) (Figure 2A). The remaining alleles all carried mutations in the common exons. We did not recover any mutations in the A or D exons, presumably because these isoforms do not account for the sterility of fruM or fruΔtra females. We therefore targeted mutations to the A isoform directly, using homologous recombination to generate the fruΔA allele, in which the nucleotides encoding the predicted zinc-finger DNA-binding domain of the FruA specific exon are replaced by nucleotides encoding c-myc epitope tags (Figure 2A). In all of these alleles, the zinc-finger domain is predicted to be fully incapacitated or deleted (Figure 2A), and hence we attribute any phenotypic differences to different functions of each isoform rather than to different allele strengths. We also have not observed any dominant phenotypes associated with any of these alleles, further suggesting that the mutant proteins do not interfere with other molecular processes, for example by forming inactive complexes.
Figure 2.
fru Isoform Mutants Display Distinct Impairments of Courtship Behavior
(A) FruM proteins and mutations analyzed in this study. Sex-specifically spliced exon S is shown in blue, BTB domain in red, and isoform-specific domains with zinc fingers in green. Conserved cysteine and histidine residues in the zinc-finger sequences are shown in yellow. In the fruΔA allele, a myc sequence is placed after aa 816, replacing the C-terminal 139 residues. Nucleotide changes in the ethyl methanesulfonate-induced mutations are: fruB1, CAT→TAT at codon 655; fruB2, TGC→TAC at codon 665; fruC1, CAG→TAG at codon 675; fruC2, TGC→TAC at codon 760; and fruC3, AGG→AAG at codon 766.
(B–I) Data for fruA mutants are shown in red, for fruB mutants in blue, and for fruC mutants in yellow; the respective controls are shown in lighter color. Mutant genotypes (isoform-mutant alleles/fruF) are indicated in bold colored type; controls are indicated in regular black type. fruA stands for fruΔAmyc, fruB for fruB2, and fruC for the fruC1 isoform-mutant allele.
(B) Copulation frequency of mutant males paired with wild-type virgin females in a 10 min courtship assay. n = 97–109 per genotype; ∗∗∗p < 0.0001 by Fisher’s exact test.
(C) Competitive mating assay, with one male of each genotype competing for a single wild-type virgin female. Bars show the fraction of males copulating within 30 min. n = 26–40 pairs; ∗∗∗p < 0.0001, ∗∗p < 0.001 by Fisher’s exact test.
(D–I) Box-and-whisker plots show 10th, 25th, 50th, 75th, and 90th percentiles. ∗∗∗p < 0.0001, ∗∗p < 0.001, ∗p < 0.05 by Kruskal-Wallis nonparametric ANOVA followed by Dunn’s multiple comparisons test.
(D) Amount of sine song. n = 50–75 flies per genotype.
(E) Amount of pulse song. n = 50–75 flies per genotype.
(F) Pulse song generation of mutant male flies in the dark. n = 72–75 flies per genotype.
(G) Modal IPI in fru isoform mutants. n = 47–75 flies per genotype, 50–1,500 IPIs per fly.
(H) Modal sine carrier frequencies. n = 40–64 flies per genotype.
(I) Modal pulse carrier frequencies. n = 49–74 flies per genotype.
Differential Contributions of Fru Isoforms to Mating Success and Courtship Song
The mutations in each of the isoform-specific fru alleles affect both the common and sex-specific transcripts. Indeed, like fru null mutants, both the fruB and fruC mutations are lethal in homozygotes (fruΔA homozygotes are viable without any obvious developmental abnormalities). To specifically assess the consequences of these mutations for the development and function of the courtship circuitry, we therefore examined males heterozygous for the fruF allele and one of our isoform-specific alleles. In such males, the common transcripts (derived from the fruF allele) retain the full isoform diversity, whereas the male-specific transcripts (derived from the isoform-specific allele) carry mutations in one of the zinc-finger domains. Hereafter, we refer to these fruΔA/fruF, fruB/fruF, and fruC/fruF males simply as fruA, fruB, and fruC mutants. fruA, fruB, and fruC mutants were fully viable and of normal size and did not show any obvious morphological abnormalities. fruC mutants had reduced fertility, consistent with previous reports [37]. Fertility was also reduced in fruB mutants, but not in fruA mutants (data not shown).
In standard single-pair courtship assays, males mutant for any of the three isoforms still courted virgin females, but with reduced copulation success compared to wild-type control males (Figure 2B). fruA mutants males were the least affected, and fruC mutant males the most affected. There was no significant difference in performance between the two fruB alleles (fruB1 and fruB2), nor between the two fruC alleles (fruC1, fruC2, and fruC3). We therefore focused subsequent analyses on just one allele for each isoform, fruB2 and fruC1, respectively.
The relative mating deficits of each allele were confirmed in a series of competitive mating assays in which we pitted two males against each other in chambers with a single wild-type female. In these assays, fruA males lost to fru+ (fruF/+) in about 75% of the cases but almost always outcompeted fruB or fruC males. fruB males always lost against fru+ males but mostly outcompeted fruC males. Finally, fruC males always lost, regardless of opponent. Thus, the overall mating ability retained in each of these alleles can be ranked: fru+ > fruA > fruB > fruC (Figure 2C).
One of the critical determinants of a male’s mating success is his courtship song, which consists of two distinct components, sine and pulse song. Sine song is a continuous 120–170 Hz vibration at low amplitude. Pulse song is a train of higher-amplitude 150–250 Hz pulses spaced at ∼35 ms intervals (the interpulse interval, or IPI). To assess courtship performance of isoform-mutant males in more detail, we quantified the amount and structure of the songs they produced. Sine song was normal for both fruA and fruB but almost completely absent in fruC mutants (Figure 2D). None of the isoform mutants produced significantly less pulse song than control males heterozygote for fruF (fruF/+) (Figure 2E). In contrast, pulse song of fruC mutants, but not fruA or fruB mutants, was dramatically reduced when the flies were deprived of visual cues (Figure 2F). For all three alleles, pulse songs had longer and more varied IPIs than those of control males (Figure 2G). The carrier frequencies of sine and pulse song also varied in an allele-specific manner: fruA mutants had higher-frequency sine song and lower-frequency pulse song, fruB mutants sang with normal carrier frequencies, and for fruC the carrier frequency of pulse song was highly variable but not significantly different than that of fruF/+ control males (Figures 2H and 2I). Each mutant allele thus results in a distinct spectrum of song deficits.
Differential Contributions of Fru Isoforms to Sexual Dimorphisms within the Courtship Circuit
We next asked how each of the fruA, fruB, and fruC mutations impairs the cellular substrate for courtship behavior, the fru circuit. We focused our attention on ten dimorphic fru+ cell types, several of which have been linked to song production. For seven of these cell types, cell number differs between the two sexes (Table 1); two are present in equal numbers in both males and females but have distinct arborization patterns (Figure 3), and one class differs in both cell number and arborizations (mAL/aDT2; Table 1 and Figure 3). For each of these cell types, we counted cells and compared their arborizations in wild-type males and females; fruF males and fruM females; and fruA, fruB, and fruC mutant males.
Table 1.
Cell Number Dimorphisms
| Neuronal Class | Cell Number Dependent on fruM? | Effect of Single Isoform? | Isoform Expression | ♂ fru+ | ♀ fru+ | ♀ fruM | ♂ fruF | ♂ fruΔA | ♂ fruB2 | ♂ fruC1 |
|---|---|---|---|---|---|---|---|---|---|---|
| pMP4/P1 | no | no | A, B, C | 17.3 ± 3.8 (n = 10) | 0.0 ± 0.0 (n = 10) | 0.0 ± 0.0 (n = 10) | 17.3 ± 3.3 (n = 10) | 17.7 ± 2.9 (n = 10) | 17.9 ± 3.9 (n = 10) | 17.1 ± 2.7 (n = 10) |
| pIP10 | yes | no | B, C | 0.7 ± 0.5 (n = 30) | 0.0 ± 0.0 (n = 30) | 1.0 ± 0.2 (n = 22)∗∗∗ | 0.0 ± 0.0 (n = 24)∗∗∗ | 0.8 ± 0.4 (n = 38) | 1.0 ± 0.2 (n = 24) | 0.9 ± 0.3 (n = 40) |
| dPR1 | yes | no | A, B | 0.9 ± 0.3 (n = 32) | 0.0 ± 0.0 (n = 20) | 0.8 ± 0.4 (n = 14)∗∗∗ | 0.0 ± 0.0 (n = 58)∗∗∗ | 0.9 ± 0.4 (n = 44) | 0.8 ± 0.4 (n = 32) | 0.9 ± 0.3 (n = 46) |
| vPR1 | yes | no | A, B, C | 3.1 ± 0.6 (n = 20) | 0.2 ± 0.5 (n = 20) | 2.8 ± 0.9 (n = 20)∗∗∗ | 0.6 ± 0.5 (n = 20)∗∗∗ | 3.2 ± 0.6 (n = 20) | 3.1 ± 1.2 (n = 20) | 2.8 ± 0.5 (n = 20) |
| vPR6 | partially | yes, C | A, B, C | 2.9 ± 0.7 (n = 18) | 0.0 ± 0.0 (n = 20) | 0.8 ± 0.8 (n = 16)∗∗∗ | 0.6 ± 0.9 (n = 20)∗∗∗ | 3.4 ± 0.6 (n = 20) | 3.0 ± 0.8 (n = 18) | 1.9 ± 1.0 (n = 20)∗∗∗ |
| aDT2/mAL | yes | yes, B and C | A, B, C | 11.1 ± 1.4 (n = 20) | 5.1 ± 1.7 (n = 20) | 12.3 ± 1.9 (n = 20)∗∗∗ | 5.1 ± 1.8 (n = 20)∗∗∗ | 10.2 ± 1.9 (n = 20) | 8.1 ± 2.1 (n = 20)∗∗∗ | 8.2 ± 2.6 (n = 20)∗∗∗ |
| aSP1 | yes | no | A, B, C | 13.3 ± 1.6 (n = 20) | 2.2 ± 1.1 (n = 20) | 14.5 ± 1.5 (n = 20)∗∗∗ | 5.0 ± 1.6 (n = 20)∗∗∗ | 15.0 ± 2.2 (n = 16) | 14.9 ± 2.0 (n = 18) | 15.2 ± 1.8 (n = 20) |
| aSP2 | yes | no | A, B, C | 62.1 ± 10.3 (n = 10) | 32.9 ± 3.7 (n = 10) | 63.0 ± 8.6 (n = 10)∗∗∗ | 34.3 ± 6.5 (n = 10)∗∗∗ | 67.0 ± 7.6 (n = 10) | 67.6 ± 11.1 (n = 10) | 62.9 ± 7.8 (n = 10) |
Cell bodies per hemisphere ± SD. n, number of hemispheres evaluated. Asterisks indicate significant increase of cells in ♀ fruM compared to ♀ fru+, significant decrease of cells in ♂ fruF compared to ♂ fru+, and significant decrease of cells in ♂ fruB2 or ♂ fruC1 compared to ♂ fru+ (∗∗∗p < 0.0001, Mann-Whitney test).
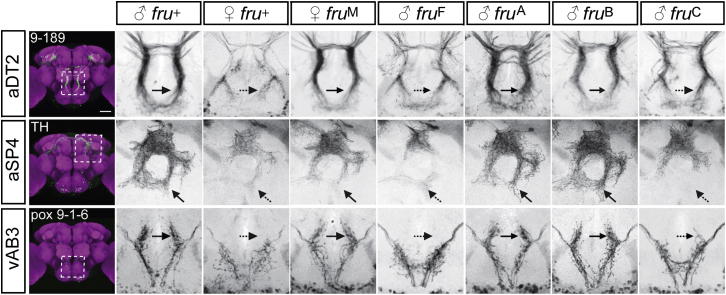
Figure 3.
FruC Specifies Sexual Arborization Patterns
Anatomical dimorphisms in the neuronal classes aDT2/mAL, aSP4, and vAB3. Each dimorphism was analyzed in the genetic backgrounds fru+, fruM, fruF, fruΔAmyc, fuB2, and fruC1, in each case in combination with fruFLP, the GAL4 driver line indicated in the leftmost panel, and a UAS>stop>mCD8-GFP reporter to label the neurons of interest. Leftmost panels show registered confocal images of fru+ male brains stained with anti-GFP (green) and nc82 (magenta). Other panels are higher-magnification views of the approximate region indicated by the dashed box, showing averaged projections of 7–10 registered brains per genotype, stained with anti-GFP (black) and nc82 (for registration, not shown). Arrows indicate sex-specific arborizations (solid arrow, male type; dotted arrow, female type).
As reported previously [33], the presence of P1/pMP4 neurons in males but not females is independent of fru (fruF males have as many P1 cells as fru+ males; fru+ and fruM females have none; Table 1). In contrast, for all other cell types that differ in number, this difference was either completely (pIP10, dPR1, vPR1, mAL/aDT2, aSP1, and aSP2) or partially (vPR6) dependent on fru. All of these cell types express multiple Fru isoforms, and for most of them we saw no differences in cell number in any of the fruA, fruB, and fruC mutants. The two exceptions were vPR6, with fewer cells in fruC mutants, and mAL/aDT2, for which slightly fewer cells were observed in both fruB and fruC mutants.
For each of the three cell types with dimorphic arborizations (mAL/aDT2, aSP4, and vAB3), we observed an apparently complete transformation to a male-like morphology in fruM females and to a female-like morphology in fruF males (Figure 3). These three cell types also each express multiple Fru isoforms, yet in each case, the male-specific arborization pattern was almost exclusively dependent upon fruC function, with female-like arborizations observed in fruC mutant males but relatively normal male-like arborizations in both fruA and fruB mutants (Figure 3).
Cell-Specific Requirements for FruC for Male-Specific Anatomy and Behavior
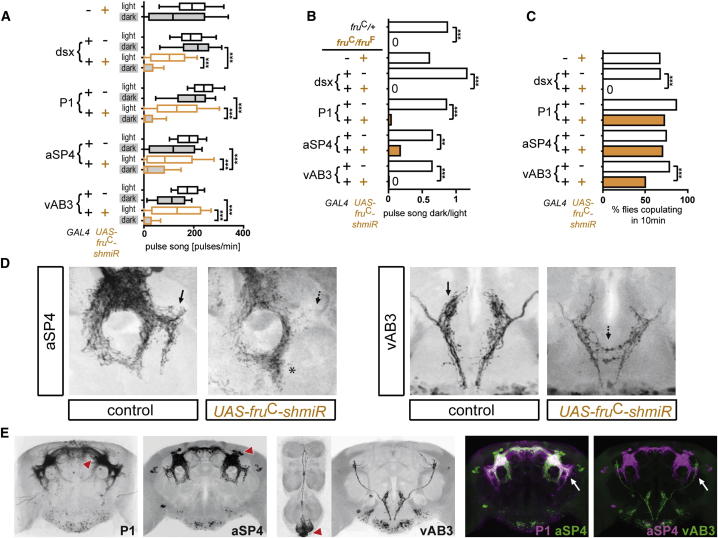
How do anatomical dimorphisms at the cellular level relate to behavioral dimorphisms at the organismal level? To begin to address this question, we needed to assess the behavioral consequences of selectively disrupting the function of a single fru isoform in specific cell types, leaving the rest of the fru circuit unperturbed. As our anatomical studies had revealed single-isoform requirements only for fruC, which was also the allele with the most profound behavioral deficits, we generated a short micro-RNAi construct to specifically disrupt fruC function in various cell types (fruC-shmiR). We confirmed that targeted expression of fruC-shmiR removed FruC, but not FruA or FruB, from specific fru neurons in the male brain (Figure S3A). Loss of the FruC isoform in dsx neurons recapitulated the loss of courtship in the dark (Figures 4A and 4B), as well as the defect in copulation seen in fruC mutant males (Figure 4C). One of the most prominent brain neuronal classes expressing both fruC and dsx is P1. We found that removing FruC in P1 by expressing fruC-shmiR under the NP2631 driver was sufficient to inhibit courtship in the dark (Figures 4A, 4B, and S3B) but did not affect copulation success in the light (Figure 4C).
Figure 4.
Cell-Specific Loss of FruC Impairs Courtship Behavior in the Absence of Visual Input
(A) Pulse song production by males of the indicated genotypes, assayed in the light or dark in pairings with single wild-type virgin females. GAL4 lines used to target fruC knockdown to the indicated cell types were dsx-GAL4 (dsx neurons), NP2631 (P1), TH-GAL4 (aSP4), or pox9-1-6-GAL4 (vAB3). n = 57–60 flies per genotype and condition. ∗∗∗p < 0.0001 by Kruskal-Wallis nonparametric ANOVA followed by Dunn’s multiple comparisons test. Box-and-whisker plots show 10th, 25th, 50th, 75th, and 90th percentiles. In (A)–(C), data of flies with UAS-fruC-shmiR-mediated knockdown is plotted in orange, and control data are plotted in black and white; gray boxes in (A) indicate data recorded in the dark.
(B) Ratio of median pulse song frequency in dark versus light conditions. ∗∗∗p < 0.0001, ∗∗p < 0.001 by permutation test (10,000 permutations).
(C) Copulation frequency of males depleted of FruC in dsx, P1, aSP4, or vAB3 neurons, paired with wild-type virgin flies in a 10 min courtship assay. GAL4 lines used for knockdown were the same as in (A). n = 74–88 per genotype; ∗∗∗p < 0.0001 by Fisher’s exact test.
(D) Registered projections of aSP4 and vAB3 in averaged images generated from 8–10 samples per genotype (UAS-fruC-shmiR, TH-GAL4,UAS>stop>mCD8-GFP,fruFLP for aSP4 and UAS-fruC-shmiR, pox1-9-6-GAL4,UAS>stop>mCD8-GFP,fruFLP for vAB3). Brain regions are shown as indicated in Figure 3, with solid and dotted arrows indicating male- and female-type arborizations, respectively. Asterisk indicates a GFP signal likely belonging to a neuronal type other than aSP4 (observed only after the prolonged aging required to detect the GFP signal in this genotype).
(E) Arborization areas of P1, aSP4, and vAB3, depicted in average images of male brains and ventral nerve cords (n = 4–10 registered brains) from flies expressing GFP in fru neurons under the control of NP2631, TH, or pox9-1-6, respectively (GAL4,UAS>stop>mCD8-GFP,fruFLP). Merged images show contacts of arborizations; arrows indicate the lateral extension of aSP4 lost upon knockdown of FruC as depicted in (D). Red arrowheads indicate the position of cell bodies for neuronal classes P1, aSP4, and vAB3.
We focused next on the aSP4 and vAB3 cells, which do not express dsx (data not shown), but for which we had observed a unique requirement for FruC in shaping their arborization patterns. Removal of FruC in either aSP4 or vAB3 by driving expression of fruC-shmiR with the TH and the pox9-1-6 driver, respectively (Figure S3B), also resulted in a specific and strong loss of courtship in the dark (Figures 4A and 4B). Copulation success in the light was not affected by knockdown of FruC in TH+ neurons but was moderately reduced upon knockdown of FruC in pox9-1-6+ neurons (Figure 4C). Loss of FruC in NP2631+, TH+, or pox9-1-6+ neurons did not affect sine song production in the light. Consistent with the moderate reduction of pulse song, loss of FruC in dsx+ neurons slightly reduced sine song but did not abolish it completely (Figure S3C). IPI distributions of pulse song were normal in each of these cell-specific FruC knockdowns (Figure S3D), implying that these behavioral deficits in fruC mutants must map to other cells.
When we targeted fruC-shmiR to either aSP4 or vAB3, using the TH-GAL4 and pox9-1-6-GAL4 drivers respectively, we observed the same feminization of their morphology that we had seen in the wholly mutant fruC males (Figure 4D). Accordingly, we conclude that fruC-dependent masculinization of both aSP4 and vAB3 arborizations might be essential for pulse song production in the absence of visual input. In light of the similar behavioral consequences of depleting FruC in P1, aSP4, and vAB3, it is interesting to note that the arbors of P1 and aSP4 overlap extensively in the dorsal protocerebrum, including in a lateral extension that is targeted by the dorsal arbor of vAB3 (Figure 4E). Notably, in the case of aSP4, this lateral extension is lost upon knockdown of FruC, suggesting that any connection between vAB3 and aSP4 is likely to be fruC dependent (Figure 4E).
Discussion
The primary goal of this study was to determine the expression pattern of each of the Fru isoforms (A–D) in the developing and adult male CNS, at cellular resolution, and to assess the contribution that each makes to both anatomical and behavioral dimorphisms. Such information is essential to the ultimate goal of understanding how fru sculpts the sex-specific neural circuitry, and hence the neural computations, that generate male courtship behavior. We confirmed previous reports that the D isoform is not expressed in the developing or adult CNS and did not examine this isoform further. Each of the other three isoforms is expressed in the CNS and makes some contribution to male courtship behavior.
FruA, FruB, and FruC are coexpressed in many of the fru+ neurons, although several cell types express only one or two of these isoforms. A similar pattern of substantial but incomplete overlap of Fru isoforms has also been observed in the embryonic nervous system [35]. Splicing at the 3′ end of the fru transcripts thus appears to be regulated in a cell-specific manner, independent of the sex-specific splicing that some transcripts undergo at their 5′ end.
Functionally, we found specific deficits in courtship behavior in flies lacking any one of the three isoforms. Thus, each isoform has some nonredundant contribution to courtship behavior and, presumably, the construction or function of the underlying circuitry. In general, however, the deficits observed upon eliminating just one isoform were relatively mild compared to those observed upon complete loss of the male-specific fru transcripts. All three isoform mutants performed male-female courtship, but with significantly reduced success. fruC mutants were the most affected, and fruA mutants the least. fruC mutants showed two striking defects in courtship behavior: the complete loss of sine song, and a dramatic reduction in pulse song in the absence of visual stimuli. The loss of sine song, which accounts for approximately half of the total song in control flies, is consistent with the significant reduction of wing extension observed previously in fruC mutants [37]. Deprived of visual cues, a male presumably becomes more reliant on volatile or contact pheromones [7]. Consistent with this, males unable to detect the female stimulatory pheromone 7,11-heptacosadiene show loss of courtship song in the dark, but not in the light [26, 39]. The similar defects in fruC mutants suggest that FruC might also be essential for detection or processing of this pheromone or other nonvisual stimuli from the female.
At the cellular level, we found that almost all sexual dimorphisms within the fru circuit depend upon the function of fru itself. It has been shown previously that the absence of P1 neurons in females is due to dsx and not fru [33]. On the other hand, dimorphisms in the number of mAL/aDT2 neurons, the size of the glomerular targets of Or67d+ OSNs, and the terminal arborizations of DA1 PNs had all been attributed to fru function [16, 27, 32]. Our analysis thus confirms and extends the general observation that most cellular dimorphisms among the fru+ neurons are indeed dependent upon fru. Moreover, we noticed an interesting pattern in the requirement for specific isoforms for each of these dimorphisms. For those cell types that are present in both sexes but differ in their arborization patterns, the FruC isoform was strictly required. In contrast, for those cell types that differ in number, no single isoform was essential. This might reflect redundancy among the distinct target genes of each isoform, or among their distinct binding sites at common target genes. Alternatively, Fru might regulate cell birth or survival by a mechanism that is independent of its zinc-finger domain.
Finally, in addition to determining how fru establishes all of these cellular dimorphisms, we also need to understand the impact that each has on neural processing and behavior. A useful strategy here might be to individually feminize each cell type and ask how this perturbs behavior and, as the tools become available, the underlying physiological processes. Here, we found that loss of FruC specifically in either aSP4 or vAB3 neurons feminizes their morphology and, we infer, changes their connectivity with pre- and postsynaptic partners. This might include connections between these two neurons, as aSP4 has FruC-dependent lateral arborizations that overlap with the processes of vAB3. Depleting FruC from aSP4 or vAB3 recapitulates one aspect of the courtship defect observed in mutants that lack FruC entirely: the pronounced loss of pulse song in the dark. The appropriate morphology and connectivity of aSP4 and vAB3 might therefore be essential for male-specific processing of pheromone signals. From their anatomy, both aSP4 and vAB3 appear to be candidate input neurons for P1, which is activated by the female gustatory pheromone [29].
As further cellular dimorphisms within the fru circuit are revealed, the tools generated here can be used to assess the genetic determinants and behavioral consequences of these dimorphisms. These analyses will guide the formulation of hypotheses about the role of specific cellular dimorphisms in the information processing that occurs within these circuits—hypotheses that can be readily tested as the physiological methods for circuit analysis advance. It may then ultimately be possible to establish mechanistic links from a single gene to the complex behavior that it specifies, encompassing the expression of the specific Fru isoforms and the target genes they regulate, the cellular properties these genes influence, the circuit-level information processing these properties enable, and ultimately the probabilistic mapping of sensory input to motor output that characterizes courtship behavior in the fly.
Experimental Procedures
Fly Stocks
UAS-lamin-GFP;fruGAL4 were as described in [16]; fruFLP and UAS>stop>mCD8-GFP were as described in [17]. fruF and fruM were as described in [11]. For validation of the isoform antibodies, CCAP-GAL4 [40] was crossed to UAS-FruMA, UAS-FruMB, and UAS-FruMC [35]. Enhancer trap GAL4 lines (obtained from the Drosophila Genetic Resource Center, Kyoto Institute of Technology and the collection of U. Heberlein) were as described in [17]. The VT collection of molecularly defined enhancer GAL4 lines was generated using the strategy of [41] (C. Masser, S.S. Bidaye, A. Stark, and B.J.D., unpublished data). GAL4 lines driving expression in P1, aSP4, and vAB3 (NP2631, TH-GAL4, and pox9-1-6) were as described in [17]. These drivers are also expressed in one (TH-GAL4 and pox9-1-6) or a few (NP2631) additional neuronal classes, none of which exhibited any morphological defects upon knockdown of FruC. The strategy for targeting the fruC transcripts was based on microRNA interference [42]. The targeting hairpin had the sequence ctagcagtCTGGCCATAAATCGCATCAGAtagttatattcaagcataTGTGATGCGAATTATGGCCAGgcg. For knockdown experiments, w+;UAS-fruC-shmiR virgins (CS background) were crossed to GAL4 lines with various genetic backgrounds.
Gene Targeting
fruAmyc, fruBmyc, fruCmyc, and fruDmyc alleles were generated by ends-in homologous recombination [43], adding c-myc epitope tags to the carboxyl terminus of the FruA, FruB, FruC, or FruD isoform. The donor construct contained a total of ∼7 kb of homology to the genomic sequence immediately upstream of the A, B, C, or D exon, with an I-SceI site approximately in the middle of the homology region. The homology region was followed by four in-frame c-myc epitope tags for fruA, fruB, and fruC and two c-myc tags for fruD (amino acid sequence EQKLISEEDLGS) without the deletion of endogenous sequences. A further ∼1.5 kb of homology following the endogenous stop codon, an I-CreI site, and the white+ marker were added. The duplication generated by ends-in targeting was removed by using hsI-CreI to introduce a double-stranded break at the I-CreI site and selecting progeny for the loss of the white+ marker. For generating the fruΔAmyc allele, the myc tag and stop codon were placed after codon G816 in the A exon (FBpp0083063; REFSEQ NP_732347), thereby deleting codons for the final 139 residues of FruA. All targeted alleles were validated by sequencing genomic PCR products extending across the targeted region. The recombinant flies were backcrossed for five generations to a w+;iso2;iso3 background prior to behavioral tests.
fruΔtra Reversion Screen
The fruΔtra allele was marked by recombining it with a mini-white insertion on the third chromosome (fruΔtra w+). fruΔtra w+/TM3 males were treated with ethyl methanesulfonate and crossed to Ly,hs-hid/TM3 virgins. The progeny were heat shocked during the late third instar. The eclosing males and females (∼85,000) were tested in small groups (ten females and males) for reversion of female fertility. Vials with more than ten pupae were kept, and the progeny were crossed inter se. Stocks were established from single males and tested for the presence of the fruΔtra insertion, a fru allele by failure to complement the deficiency Df(3R)Exel6179, and the male-specific allele fru4-40. All protein-coding exons were sequenced from these revertants. The five alleles containing a mutation in either the B or C zinc-finger domain were backcrossed to an isogenic background (w+;iso2;iso3) for at least five generations prior to behavioral analysis.
Antibody Generation
FruA and FruC antisera were obtained from rabbits and guinea pigs immunized with a GST fusion protein expressed from a cDNA containing the entire isoform-specific exon. The sera were purified against their antigen and dialyzed in PBS containing 50% glycerol. The reference sequences for the amino acids are FBpp0083063; REFSEQ NP_732347 for FruA and FBpp0083061; REFSEQ NP_732344 for FruC.
Behavioral Assays
Flies were raised on standard medium at 25°C in a 12:12 hr light: dark cycle and collected as virgins after eclosure. Females were kept in groups of up to 20, and males were housed individually. All behavioral experiments were conducted with 5- to 7-day-old males and 4- to 6-day-old females. For fertility tests, one male was kept with three females in a food vial that was checked after 4–5 days for progeny. Copulation frequency assays were performed in single-pair assays in chambers of 1 cm diameter for 10 min under constant light. Competitive courtship assays were carried out with an observation period of 30 min. The genotype of the competing males was distinguished by applying a terra cotta mark to the thorax at least 24 hr prior to testing. The mark itself did not affect courtship performance. Courtship song was recorded in beveled chambers of 11 mm diameter for 3.5 min or until copulation. The chambers were closed on one side with fine plastic mesh and placed on top of electret condenser microphones (CMP-5247TF-K, CUI Inc.). The signal was amplified with a custom-made circuit board and digitized with a multifunction data acquisition device (NI USB-6259 MASS Term, National Instruments) [44]. Tentative pulse and sine song were detected using a MATLAB script [44] and corrected manually in a custom-written user interface allowing fast annotation of sound oscillograms. For pulse/min counts, all pulses in trains of more than one pulse with IPIs of 15–150 ms were considered. For IPI analysis, all IPIs of 15–90 ms in trains of more than two pulses were considered. For IPI and carrier frequency analysis, only flies producing at least 50 of the respective events were considered. Mode values were determined by binning IPIs in 1 ms steps and carrier frequencies in 0.1 Hz steps. For comparing ratios of pulse song produced in the light versus in the dark, we employed a permutation test using a MATLAB script [45].
Immunohistochemistry and Image Analysis
Fly dissection and staining were carried out as described previously [17]. Antibodies used were rabbit anti-GFP (1:6,000, Torrey Pines), chicken anti-GFP (1:3,000, Abcam), mouse mAb nc82 (1:20, Developmental Studies Hybridoma Bank), rabbit anti-Myc (1:12,000, Abcam), rat anti-Myc (1:8,000, Abcam), rabbit anti-FruA (1:4,000, see above), rabbit anti-FruC (1:4,000, see above), guinea pig anti-FruC (1:8,000, see above), rabbit anti-DsRed (to detect mCherry; 1:500 or 1:1,000, Clontech), and secondary Alexa 488, 568, and 647 antibodies (1:500 or 1:1,000, Invitrogen). Confocal stacks of stained brains and ventral nerve cords were taken on a Zeiss LSM 510 or a Zeiss LSM 700 with a Plan NeoFluar 25×/0.8 multi-immersion objective and analyzed with Amira software (Visage Imaging). Cell number analysis of FruM isoform expression in the CNS was carried out with Imaris software (Bitplane) using a cell diameter of 3.5 μm for spot detection and correcting manually for wrongly detected spots. Nonrigid image registration onto an nc82 standard template brain and image averaging were performed as described in [17].
Acknowledgments
We thank K. Bartalska, B. Erdi, A. Gyorgy, M. Fellner, E. Viragh, and S. Wandl for technical assistance; C. Machacek and A. Seitinger for help with song analysis software; and M. Murthy for sharing a MATLAB script for fly song detection. S. Bidaye, T. Liu, and M. Palfreyman provided valuable discussions and critical feedback on the manuscript. This work was supported by a research grant from the European Research Council (B.J.D.) and by a postdoctoral fellowship from the European Molecular Biology Organization and a Stand-Alone grant from the Austrian Science Fund (FWF), project number P25087-B24 (A.C.v.P.). Basic research at the IMP is funded by Boehringer Ingelheim GmbH.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Anne C. von Philipsborn, Email: avp@mb.au.dk.
Barry J. Dickson, Email: dicksonb@janelia.hhmi.org.
Supplemental Information
References
- 1.Manoli D.S., Fan P., Fraser E.J., Shah N.M. Neural control of sexually dimorphic behaviors. Curr. Opin. Neurobiol. 2013;23:330–338. doi: 10.1016/j.conb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto D., Koganezawa M. Genes and circuits of courtship behaviour in Drosophila males. Nat. Rev. Neurosci. 2013;14:681–692. doi: 10.1038/nrn3567. [DOI] [PubMed] [Google Scholar]
- 3.Cline T.W., Meyer B.J. Vive la différence: males vs females in flies vs worms. Annu. Rev. Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- 4.Belote J.M., Baker B.S. Sexual behavior: its genetic control during development and adulthood in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 1987;84:8026–8030. doi: 10.1073/pnas.84.22.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billeter J.C., Rideout E.J., Dornan A.J., Goodwin S.F. Control of male sexual behavior in Drosophila by the sex determination pathway. Curr. Biol. 2006;16:R766–R776. doi: 10.1016/j.cub.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Greenspan R.J., Ferveur J.F. Courtship in Drosophila. Annu. Rev. Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- 7.Krstic D., Boll W., Noll M. Sensory integration regulating male courtship behavior in Drosophila. PLoS ONE. 2009;4:e4457. doi: 10.1371/journal.pone.0004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villella A., Gailey D.A., Berwald B., Ohshima S., Barnes P.T., Hall J.C. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics. 1997;147:1107–1130. doi: 10.1093/genetics/147.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryner L.C., Goodwin S.F., Castrillon D.H., Anand A., Villella A., Baker B.S., Hall J.C., Taylor B.J., Wasserman S.A. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- 10.Ito H., Fujitani K., Usui K., Shimizu-Nishikawa K., Tanaka S., Yamamoto D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl. Acad. Sci. USA. 1996;93:9687–9692. doi: 10.1073/pnas.93.18.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demir E., Dickson B.J. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Manoli D.S., Foss M., Villella A., Taylor B.J., Hall J.C., Baker B.S. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 13.Villella A., Hall J.C. Courtship anomalies caused by doublesex mutations in Drosophila melanogaster. Genetics. 1996;143:331–344. doi: 10.1093/genetics/143.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rideout E.J., Dornan A.J., Neville M.C., Eadie S., Goodwin S.F. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 2010;13:458–466. doi: 10.1038/nn.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee G., Foss M., Goodwin S.F., Carlo T., Taylor B.J., Hall J.C. Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. J. Neurobiol. 2000;43:404–426. doi: 10.1002/1097-4695(20000615)43:4<404::aid-neu8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Stockinger P., Kvitsiani D., Rotkopf S., Tirián L., Dickson B.J. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Yu J.Y., Kanai M.I., Demir E., Jefferis G.S.X.E., Dickson B.J. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr. Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Cachero S., Ostrovsky A.D., Yu J.Y., Dickson B.J., Jefferis G.S.X.E. Sexual dimorphism in the fly brain. Curr. Biol. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinett C.C., Vaughan A.G., Knapp J.M., Baker B.S. Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 2010;8:e1000365. doi: 10.1371/journal.pbio.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clyne J.D., Miesenböck G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 21.Ha T.S., Smith D.P. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J. Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtovic A., Widmer A., Dickson B.J. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 23.van der Goes van Naters W., Carlson J.R. Receptors and neurons for fly odors in Drosophila. Curr. Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosjean Y., Rytz R., Farine J.-P., Abuin L., Cortot J., Jefferis G.S.X.E., Benton R. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478:236–240. doi: 10.1038/nature10428. [DOI] [PubMed] [Google Scholar]
- 25.Thistle R., Cameron P., Ghorayshi A., Dennison L., Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toda H., Zhao X., Dickson B.J. The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell Rep. 2012;1:599–607. doi: 10.1016/j.celrep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Datta S.R., Vasconcelos M.L., Ruta V., Luo S., Wong A., Demir E., Flores J., Balonze K., Dickson B.J., Axel R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- 28.Ruta V., Datta S.R., Vasconcelos M.L., Freeland J., Looger L.L., Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- 29.Kohatsu S., Koganezawa M., Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011;69:498–508. doi: 10.1016/j.neuron.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 30.von Philipsborn A.C., Liu T., Yu J.Y., Masser C., Bidaye S.S., Dickson B.J. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Pan Y., Robinett C.C., Baker B.S. Turning males on: activation of male courtship behavior in Drosophila melanogaster. PLoS ONE. 2011;6:e21144. doi: 10.1371/journal.pone.0021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura K., Ote M., Tazawa T., Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- 33.Kimura K., Hachiya T., Koganezawa M., Tazawa T., Yamamoto D. Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron. 2008;59:759–769. doi: 10.1016/j.neuron.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Mellert D.J., Knapp J.-M., Manoli D.S., Meissner G.W., Baker B.S. Midline crossing by gustatory receptor neuron axons is regulated by fruitless, doublesex and the Roundabout receptors. Development. 2010;137:323–332. doi: 10.1242/dev.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song H.J., Billeter J.C., Reynaud E., Carlo T., Spana E.P., Perrimon N., Goodwin S.F., Baker B.S., Taylor B.J. The fruitless gene is required for the proper formation of axonal tracts in the embryonic central nervous system of Drosophila. Genetics. 2002;162:1703–1724. doi: 10.1093/genetics/162.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalton J.E., Fear J.M., Knott S., Baker B.S., McIntyre L.M., Arbeitman M.N. Male-specific Fruitless isoforms have different regulatory roles conferred by distinct zinc finger DNA binding domains. BMC Genomics. 2013;14:659. doi: 10.1186/1471-2164-14-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Billeter J.C., Villella A., Allendorfer J.B., Dornan A.J., Richardson M., Gailey D.A., Goodwin S.F. Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr. Biol. 2006;16:1063–1076. doi: 10.1016/j.cub.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 38.Goodwin S.F., Taylor B.J., Villella A., Foss M., Ryner L.C., Baker B.S., Hall J.C. Aberrant splicing and altered spatial expression patterns in fruitless mutants of Drosophila melanogaster. Genetics. 2000;154:725–745. doi: 10.1093/genetics/154.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu B., LaMora A., Sun Y., Welsh M.J., Ben-Shahar Y. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 2012;8:e1002587. doi: 10.1371/journal.pgen.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y.-J., Zitnan D., Galizia C.G., Cho K.H., Adams M.E. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr. Biol. 2006;16:1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 41.Pfeiffer B.D., Jenett A., Hammonds A.S., Ngo T.T., Misra S., Murphy C., Scully A., Carlson J.W., Wan K.H., Laverty T.R. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haley B., Hendrix D., Trang V., Levine M. A simplified miRNA-based gene silencing method for Drosophila melanogaster. Dev. Biol. 2008;321:482–490. doi: 10.1016/j.ydbio.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong W.J., Golic K.G. Ends-out, or replacement, gene targeting in Drosophila. Proc. Natl. Acad. Sci. USA. 2003;100:2556–2561. doi: 10.1073/pnas.0535280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arthur B.J., Sunayama-Morita T., Coen P., Murthy M., Stern D.L. Multi-channel acoustic recording and automated analysis of Drosophila courtship songs. BMC Biol. 2013;11:11. doi: 10.1186/1741-7007-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamyshev N.G., Iliadi K.G., Bragina J.V. Drosophila conditioned courtship: two ways of testing memory. Learn. Mem. 1999;6:1–20. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.