Abstract
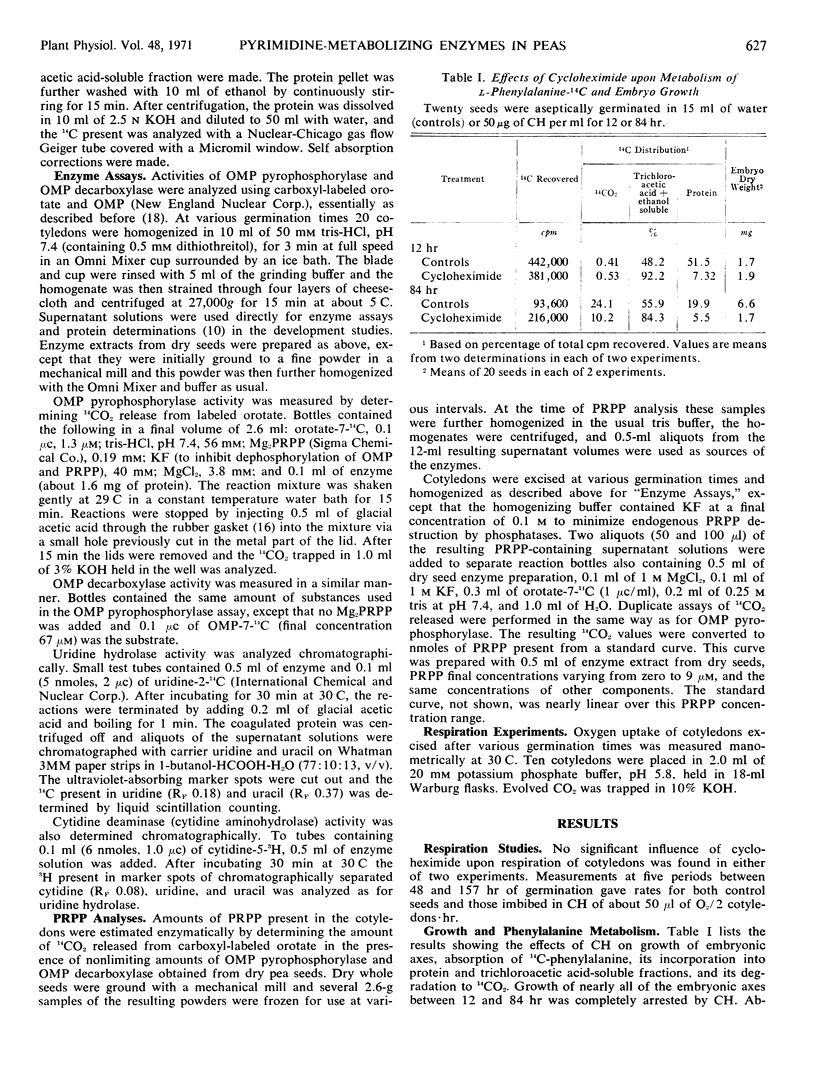
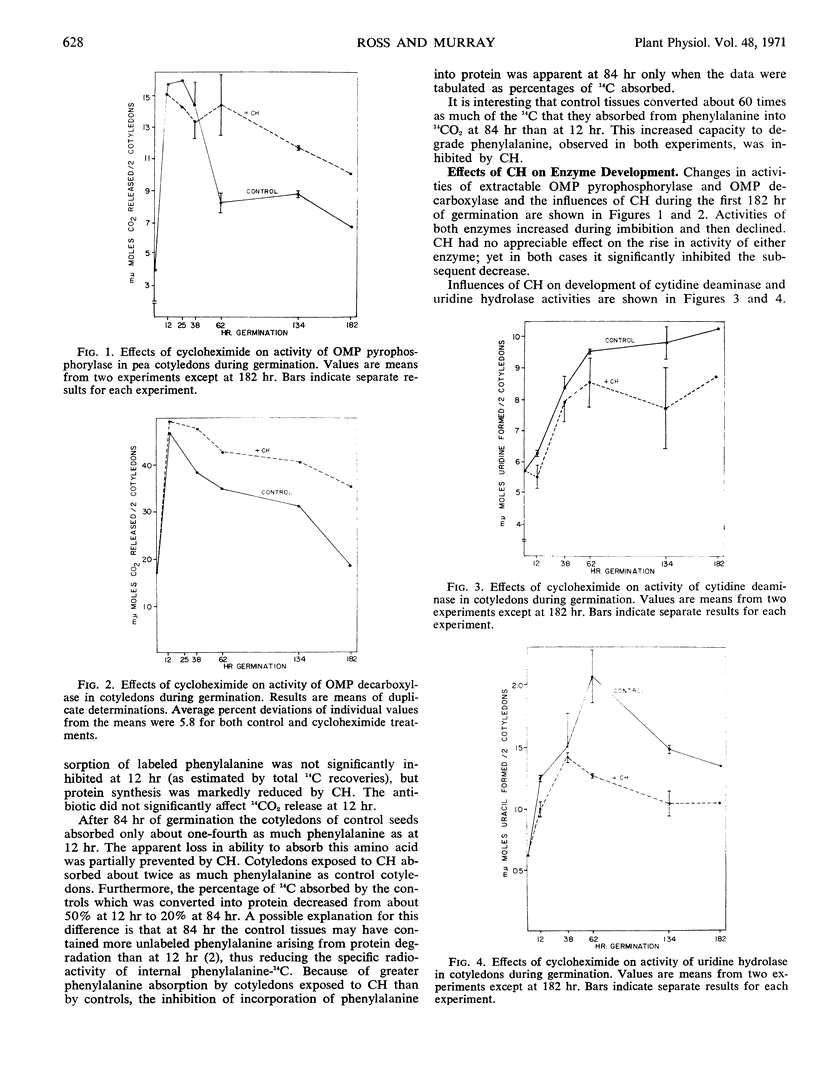
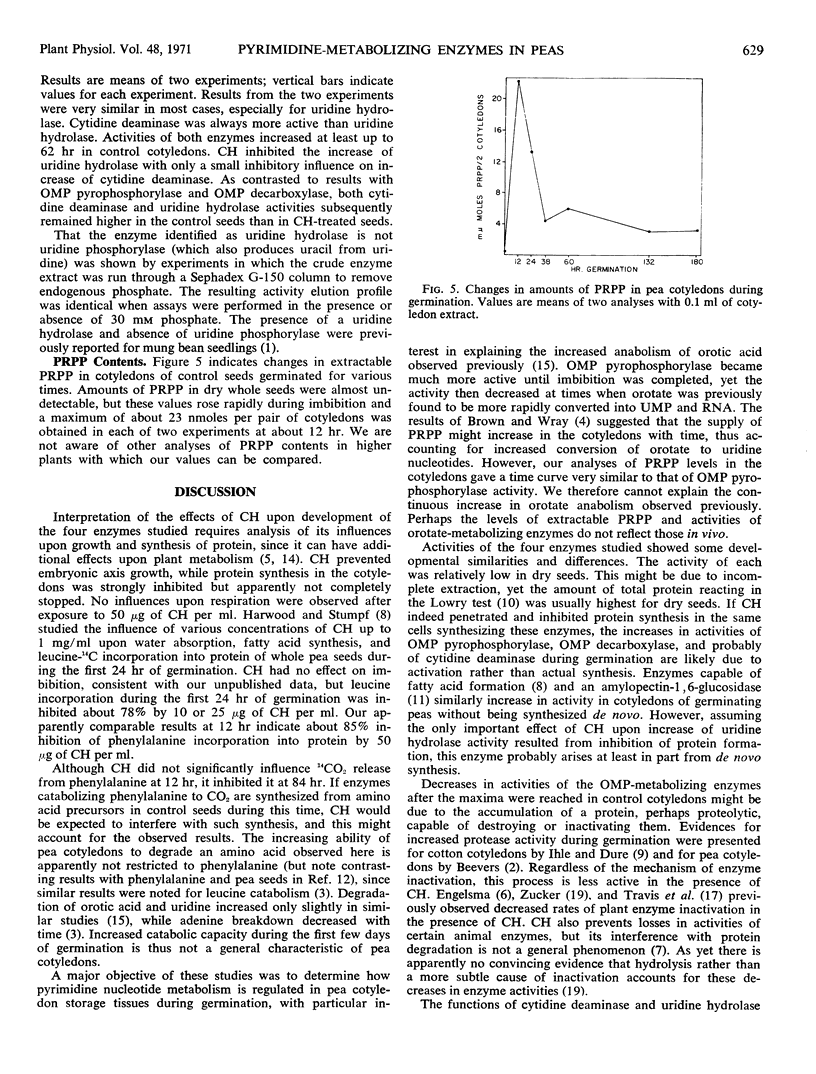
Mechanisms controlling conversion of orotic acid-6-14C to uridine-5′-phosphate in cotyledons of germinating Alaska peas (Pisum sativum L.) were investigated. The content of 5-phosphoribosyl-1-pyrophosphate was very low in dry seeds, increased to a maximum after about 12 hours of imbibition, and then rapidly declined. Orotidine-5′-phosphate pyrophosphorylase and orotidine-5′-phosphate decarboxylase activities more than doubled during the first 24 hours of germination and then also decreased. These results do not account for the continuous increases of orotate anabolism in such cotyledons as we observed previously. The initial increases in activities of these two enzymes were unaffected by cycloheximide, while the subsequent decreases were less rapid in the presence of this inhibitor. Activities of cotyledonary cytidine deaminase and uridine hydrolase also increased during imbibition, but the activity of only the latter showed a decrease after imbibition was completed. Cycloheximide inhibited the initial rapid increase in uridine hydrolase activity but had little effect on its subsequent decline. Cycloheximide had only slight inhibitory effects on the development of cytidine deaminase activity during the first 62 hours. The evidence suggests that uridine hydrolase might be synthesized de novo during the first few days of germination, but that the other three enzymes might not be.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achar B. S., Vaidyanathan C. S. Purification and properties of uridine hydrolase from mung-bean (Phaseolus radiatus) seedlings. Arch Biochem Biophys. 1967 Mar;119(1):356–362. doi: 10.1016/0003-9861(67)90465-1. [DOI] [PubMed] [Google Scholar]
- Brown A. P., Wray J. L. Correlated changes of some enzyme activities and cofactor and substrate contents of pea cotyledon tissue during germination. Biochem J. 1968 Jul;108(3):437–444. doi: 10.1042/bj1080437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., Macdonald I. R. Specificity of cycloheximide in higher plant systems. Plant Physiol. 1970 Aug;46(2):227–232. doi: 10.1104/pp.46.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M., Yagil G. Does cycloheximide interfere with protein degradation? Biochem Biophys Res Commun. 1969 Oct 8;37(2):198–203. doi: 10.1016/0006-291x(69)90719-0. [DOI] [PubMed] [Google Scholar]
- Harwood J. L., Stumpf P. K. Fat Metabolism in Higher Plants: XL. Synthesis of Fatty Acids in the Initial Stage of Seed Germination. Plant Physiol. 1970 Oct;46(4):500–508. doi: 10.1104/pp.46.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Dure L., 3rd Synthesis of a protease in germinating cotton cotyledons catalzed by mRNA synthesized during embryogenesis. Biochem Biophys Res Commun. 1969 Aug 22;36(5):705–710. doi: 10.1016/0006-291x(69)90667-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mayer A. M., Shain Y. Zymogen granules in enzyme liberation and activation in pea seeds. Science. 1968 Dec 13;162(3859):1283–1284. doi: 10.1126/science.162.3859.1283. [DOI] [PubMed] [Google Scholar]
- Nozzolillo C., Paul K. B., Godin C. The Fate of l-Phenylalanine Fed to Germinating Pea Seeds, Pisum sativum (L.) var. Alaska, during Imbibition. Plant Physiol. 1971 Jan;47(1):119–123. doi: 10.1104/pp.47.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS C. W. INFLUENCE OF 6-AZAURACIL ON PYRIMIDINE METABOLISM OF COCKLEBUR LEAF DISCS. Biochim Biophys Acta. 1964 Aug 12;87:564–573. doi: 10.1016/0926-6550(64)90274-9. [DOI] [PubMed] [Google Scholar]
- Ross C., Coddington R. L., Murray M. G., Bledsoe C. S. Pyrimidine metabolism in cotyledons of germinating alaska peas. Plant Physiol. 1971 Jan;47(1):71–75. doi: 10.1104/pp.47.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. Influence of cycloheximide (Actidione) upon pyrimidine nucleotide metabolism and rna synthesis in cocklebur leaf discs. Biochim Biophys Acta. 1968 Aug 23;166(1):40–47. doi: 10.1016/0005-2787(68)90488-7. [DOI] [PubMed] [Google Scholar]
- STARR R. I., ROSS C. W. A METHOD FOR DETERMINATION OF CARBON IN PLANT TISSUE. Anal Biochem. 1964 Oct;9:243–246. doi: 10.1016/0003-2697(64)90109-5. [DOI] [PubMed] [Google Scholar]
- Travis R. L., Jordan W. R., Huffaker R. C. Evidence for an Inactivating System of Nitrate Reductase in Hordeum vulgare L. during Darkness That Requires Protein Synthesis. Plant Physiol. 1969 Aug;44(8):1150–1156. doi: 10.1104/pp.44.8.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolcott J. H., Ross C. Orotidine-5'-phosphate decarboxylase and pyrophosphorylase of bean leaves. Plant Physiol. 1967 Feb;42(2):275–279. doi: 10.1104/pp.42.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Sequential Induction of Phenylalanine Ammonia-lyase and a Lyase-inactivating System in Potato Tuber Disks. Plant Physiol. 1968 Mar;43(3):365–374. doi: 10.1104/pp.43.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]


