Abstract
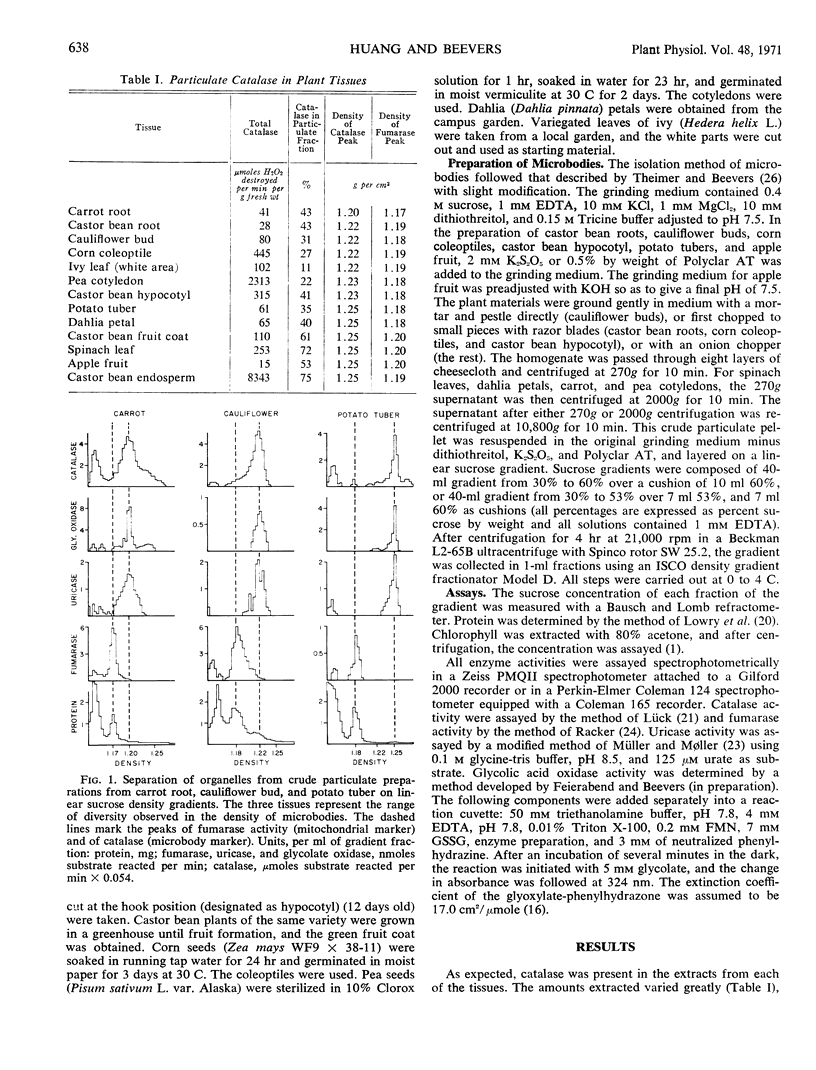
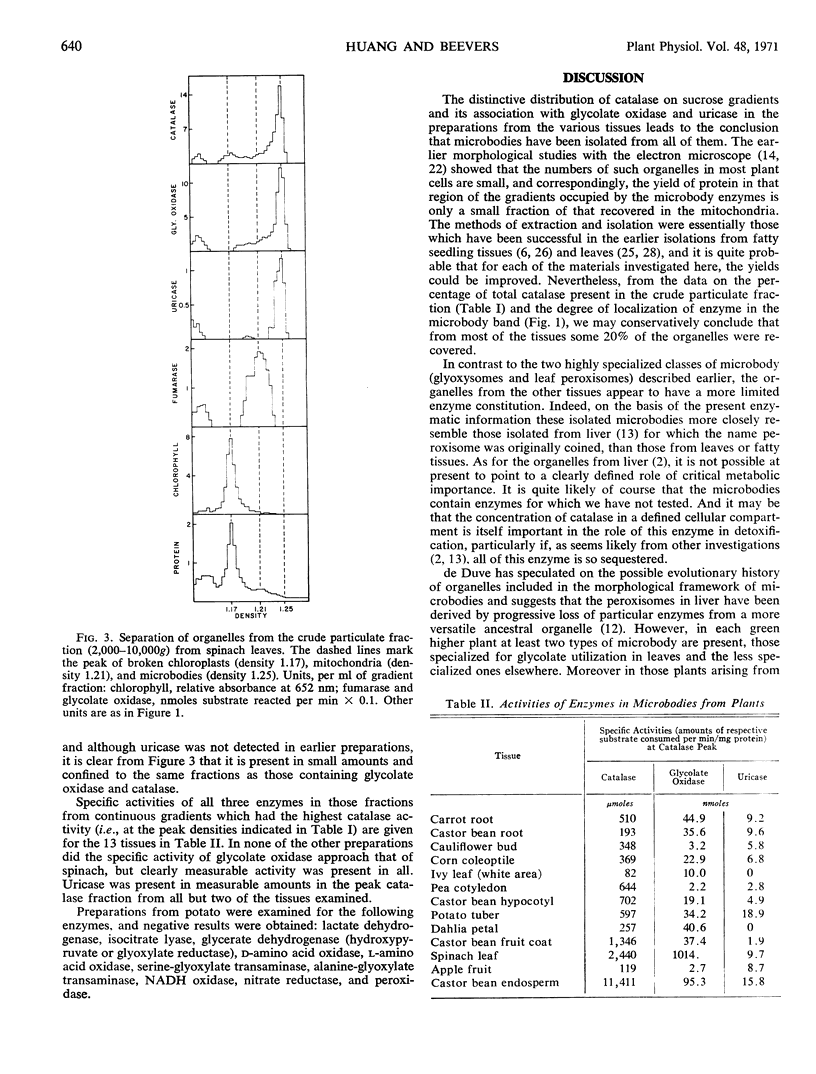
Specialized microbodies have previously been isolated and characterized from fatty seedling tissues (glyoxysomes) and leaves (leaf peroxisomes). We have now examined 11 other plant tissues, including tubers, fruits, roots, shoots, and petals, and find that all contain particulate catalase, a distinctive common enzyme component of microbodies. On linear sucrose gradients the catalase activity peaks sharply at a higher equilibrium density (1.20 to 1.25 gram per cm3 in the various tissues) than the mitochondria (1.17 to 1.20). Only small amounts of protein are recovered in the fractions containing catalase, although a definite band is visible in preparations from some tissues, e.g., potato. As in the preparations from castor bean endosperm and spinach leaves for which comparable data are provided, the distribution of glycolate oxidase and uricase follows closely that of catalase on the gradients. The preparations from potato lack glyoxylate reductase and the transaminases, typical enzymes of leaf peroxisomes, and the distinctive enzymes of glyoxysomes are missing. Nonspecialized microbodies with limited enzyme composition can thus be isolated from a variety of plant tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P. Liver peroxisomes, cytology and function. Ann N Y Acad Sci. 1969 Dec 19;168(2):214–228. doi: 10.1111/j.1749-6632.1969.tb43111.x. [DOI] [PubMed] [Google Scholar]
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M. Glyoxysomes in megagamethophyte of germinating ponderosa pine seeds. Plant Physiol. 1970 Sep;46(3):475–482. doi: 10.1104/pp.46.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- De Duve C. Evolution of the peroxisome. Ann N Y Acad Sci. 1969 Dec 19;168(2):369–381. doi: 10.1111/j.1749-6632.1969.tb43124.x. [DOI] [PubMed] [Google Scholar]
- Frederick S. E., Newcomb E. H. Cytochemical localization of catalase in leaf microbodies (peroxisomes). J Cell Biol. 1969 Nov;43(2):343–353. doi: 10.1083/jcb.43.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat Metabolism in Higher Plants. XXXVII. Characterization of the beta-Oxidation Systems From Maturing and Germinating Castor Bean Seeds. Plant Physiol. 1969 Apr;44(4):508–516. doi: 10.1104/pp.44.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisaki T., Tolbert N. E. Glycolate and glyoxylate metabolism by isolated peroxisomes or chloroplasts. Plant Physiol. 1969 Feb;44(2):242–250. doi: 10.1104/pp.44.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Longo C. P., Longo G. P. The development of glyoxysomes in peanut cotyledons and maize scutella. Plant Physiol. 1970 Mar;45(3):249–254. doi: 10.1104/pp.45.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Rocha V., Ting I. P. Preparation of cellular plant organelles from spinach leaves. Arch Biochem Biophys. 1970 Oct;140(2):398–407. doi: 10.1016/0003-9861(70)90081-0. [DOI] [PubMed] [Google Scholar]
- Theimer R. R., Beevers H. Uricase and allantoinase in glyoxysomes. Plant Physiol. 1971 Feb;47(2):246–251. doi: 10.1104/pp.47.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Enzymic characterization of leaf peroxisomes. J Biol Chem. 1970 Oct 10;245(19):5137–5144. [PubMed] [Google Scholar]