Highlights
-
•
We explored Cervarix® HPV vaccine cross-reactive antibody specificity.
-
•
L1 VLP binding was a poor surrogate for L1L2 pseudovirus neutralization specificity.
-
•
Cross-neutralizing antibodies comprise a small proportion of total antibody.
-
•
Multiple, overlapping cross-neutralizing antibody specificities exist.
Keywords: HPV, Vaccine, Antibody
Abstract
The highly efficacious human papillomavirus (HPV) vaccines contain virus-like particles (VLP) representing genotypes HPV16 and HPV18, which together account for approximately 70% of cervical cancer cases. Vaccine-type protection is thought to be mediated by high titer, type-specific neutralizing antibodies. The vaccines also confer a degree of cross-protection against some genetically-related types from the Alpha-9 (HPV16-like: HPV31, HPV33, HPV35, HPV52, HPV58) and Alpha-7 (HPV18-like: HPV39, HPV45, HPV59, HPV68) species groups. Cross-protection is coincident with the detection of low titer serum responses against non-vaccine types by vaccinees. Such antibodies may be the effectors of cross-protection or their detection may be useful as a correlate or surrogate.
This study evaluated whether cross-neutralization of HPV types from the Alpha-9 species group is mediated by antibodies with a predominantly type-restricted specificity for HPV16 that nevertheless exhibit low affinity interactions with non-vaccine types, or by antibody specificities that demonstrate similar recognition of vaccine and non-vaccine types but are present at very low levels.
Antibodies generated following Cervarix® vaccination of 13–14 year old girls were evaluated by pseudovirus neutralization, VLP ELISA and by enrichment of target antigen specificity using VLP-immobilized beads. Two-dimensional hierarchical clustering of serology data demonstrated that the antibody specificity profile generated by VLP ELISA was both quantitatively and qualitatively different from the neutralizing antibody specificity profile. Target-specific antibody enrichment demonstrated that cross-neutralization of non-vaccine types was due to a minority of antibodies rather than by the weak interactions of a predominantly type-restricted HPV16 antibody specificity. Furthermore, cross-neutralization of non-vaccine types appeared to be mediated by multiple antibody specificities, recognizing single and multiple non-vaccine types, and whose specificities were not predictable from examination of the serum neutralizing antibody profile. These data contribute to our understanding of the antibody specificities elicited following HPV vaccination and have potential implications for vaccine-induced cross-protection.
1. Introduction
The human papillomavirus (HPV) vaccines, Cervarix® and Gardasil®, comprise virus-like particles (VLP) based upon the major capsid protein, L1, of HPV16 and HPV18. Both vaccines are highly efficacious at preventing persistent infection and more progressive disease associated with HPV16 and HPV18 [1,2]. Antibodies capable of neutralizing pseudoviruses representing HPV16 and HPV18 can be detected in the serum and cervicovaginal secretions of vaccinees [3–5]. Together with passive transfer studies demonstrating that immune sera, purified IgG or monoclonal antibodies (MAbs) can protect animals against papillomavirus challenge [6–8], has led to the reasonable assumption that vaccine-induced type-specific protection is mediated by neutralizing antibodies [9,10].
A degree of cross-protection has also been demonstrated against some closely-related types within the Alpha-papillomavirus species groups, Alpha-9 (HPV16-like: HPV31, HPV33, HPV35, HPV52, HPV58) and Alpha-7 (HPV18-like: HPV39, HPV45, HPV59, HPV68) [1,2]. Cross-protection is coincident with the detection of cross-neutralizing antibodies against these types in the serum and cervicovaginal secretions of vaccinees [4,11–13]. Whether such antibodies are effectors, or their detection has some utility as a correlate or surrogate of vaccine-induced cross-protection is uncertain.
The antibody response following VLP immunization has been measured using a VLP enzyme-linked immunosorbent assay (ELISA) [14], a pseudovirus-based neutralization assay [15] and a competitive Luminex® immunoassay (cLIA) [16]. Different antibody specificities are measured by each of these assays but the nature of any potential discrepancies are not fully understood [9,11]. The cLIA assay uses the type-restricted murine MAb H16.V5 [17], whose human homologue appears to be the majority specificity generated during natural infection [18] and is assumed to constitute a high proportion of the antibodies elicited during vaccination.
The magnitude and breadth of the vaccine-induced serum neutralizing antibody response against non-vaccine types generally increases with the vaccine-type response [4,12,13]. It is unclear whether cross-neutralization within the Alpha-9 group is facilitated by antibodies other than the H16.V5-like human homologue or that this antibody exhibits some degree of cross-recognition not present in the murine version.
In this study we attempted to dissect the serum antibody response generated against non-vaccine types from the Alpha-9 group following Cervarix® vaccination in order to further describe the antibody specificities responsible for cross-neutralization.
2. Material and methods
2.1. Study samples
Serum samples (n = 69) were collected from 13 to 14 year old girls a median 5.9 months following their third dose of Cervarix® [12].
2.2. L1L2 pseudovirus neutralization assay
L1L2 pseudoviruses representing vaccine-relevant Alpha-9 types (HPV16, HPV31, HPV33, HPV35, HPV52 and HPV58) and carrying a luciferase reporter were expressed from transiently transfected 293TT cells, purified and characterized as previously described [12]. The equivalent of a Tissue Culture Infectious Dose 50% (TCID50) was estimated using the Spearman–Karber equation and a standardized input of 300 TCID50 was used for all pseudoviruses [12,15]. Serum samples were subjected to 4-5 serial dilutions and the 80% reciprocal neutralization titer estimated by interpolation. A panel of six serum samples were retested against the six pseudoviruses (n = 36; Pearson's r = 0.976; p < 0.001) and demonstrated good inter-assay reproducibility.
2.3. L1 VLP ELISA
L1 VLP were expressed using the Bac-to-Bac® Baculovirus System (Life Technologies), as previously described [20], wherein the L1 genes shared 100% amino acid sequence identity with the L1 genes of the Alpha-9 pseudovirus clones [12]. The L1 VLP were used as target antigens in a ELISA, as previously described [4]. Serum samples were subjected to 4–5 serial dilutions and the 50% reciprocal binding titer estimated by interpolation. Good inter-assay reproducibility was demonstrated by retesting a panel of six serum samples against the six L1 VLP (n = 36; Pearson's r = 0.947; p < 0.001).
2.4. Hierarchical clustering of serology data
Serological and viral dendrograms were generated by calculating the pairwise Euclidean distances for the Log10-transformed pseudovirus neutralization assay and VLP ELISA data, generating distance matrices that were then clustered using a neighbor-joining algorithm (http://evolution.genetics.washington.edu/phylip.html). The resulting viral dendrograms were bootstrapped by resampling the sera data to generate 500 pseudoreplicates. Dendrograms were viewed using FigTree 1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/). The serological data were then represented by a heat map ordered according to the resulting serological and viral dendrograms.
2.5. Antibody adsorption and elution from L1 VLP
VLP (HPV16 10 μg; non-vaccine type 5 μg) were coupled to magnetic sepharose beads (GE Healthcare) overnight at 4 °C. Antibody adsorption and elution were performed as described elsewhere [21,22] with minor modifications. Sera for adsorption were diluted five-fold in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) (Life Technologies) and incubated with beads for 1 h at room temperature. The post-adsorption serum fraction was separated from the beads using a magnetic rack before being subjected to a second round of adsorption using a freshly coupled bead set. Both bead sets were then washed three times in DMEM containing 10% FBS. No residual antibody activity was detectable in the final washes. Antibodies were eluted using 0.1 M glycine–HCI (pH 2.9–1.9) and neutralized with 1 M Tris–HCI, pH 9 (GE Healthcare). The pooled eluted antibody fractions were concentrated using Vivaspin 500 columns (GE Healthcare). Each serum was also subjected to two rounds of adsorption on, and elution from, beads coupled with 10 μg BSA which was used as a control for non-specific activity; when eluted fractions were tested against the HPV16 pseudovirus they were found to have levels of neutralizing antibody below the detection threshold.
2.6. Statistical methods
Pearson's correlation was used to evaluate the relationship between HPV16 antibody titers. Fisher's exact test was used to determine whether the proportion of sera reactive against a particular non-vaccine type differed between the two assay systems. Tests were 2-tailed where appropriate and performed using Stata 12.1 (Statacorp, College Station, TX).
3. Results
Sixty nine serum samples from Cervarix® vaccinees, previously tested in the pseudovirus neutralization assay against vaccine-relevant Alpha-9 types [12] were tested against VLP representing the same HPV types by ELISA.
3.1. Antibody titers measured in pseudovirus neutralization assay and VLP ELISA
As in the pseudovirus neutralization assay [12], all sera (n = 69, 100%) tested positive for HPV16 antibodies by VLP ELISA. A significant correlation was observed between the antibody titers generated by the pseudovirus neutralization assay (median 19,258 [inter-quartile range, IQR, 11,730–28,132]) and VLP ELISA (9279 [7290–44,719]) (Pearson's r = 0.833; p < 0.001).
For non-vaccine types, there were differences between antibody titers generated in the VLP ELISA and the pseudovirus neutralization assay. While the number of samples positive for HPV31 antibodies in the VLP ELISA (n = 58; 84%) and pseudovirus neutralization assay (n = 60; 87%) were similar (p = 0.810), antibody titers of sera positive in both assays were higher in the VLP ELISA (median 651 [IQR 576–771]) than in the pseudovirus neutralization assay (96 [50–203]) (p < 0.001). More serum samples were positive for HPV33 antibodies by VLP ELISA (n = 47; 68%) than by the pseudovirus neutralization assay (n = 29; 42%; p = 0.003) with dual positive titers higher in the VLP ELISA (600 [374–735]) than in the pseudovirus neutralization assay (29 [25–54]) (p < 0.001).
These data suggest that there were quantitative differences between the pseudovirus neutralization assay and VLP ELISA and/or target antigens, particularly for non-vaccine types. We next sought to evaluate whether these data also reflected qualitative differences.
3.2. Hierarchical clustering of serological data
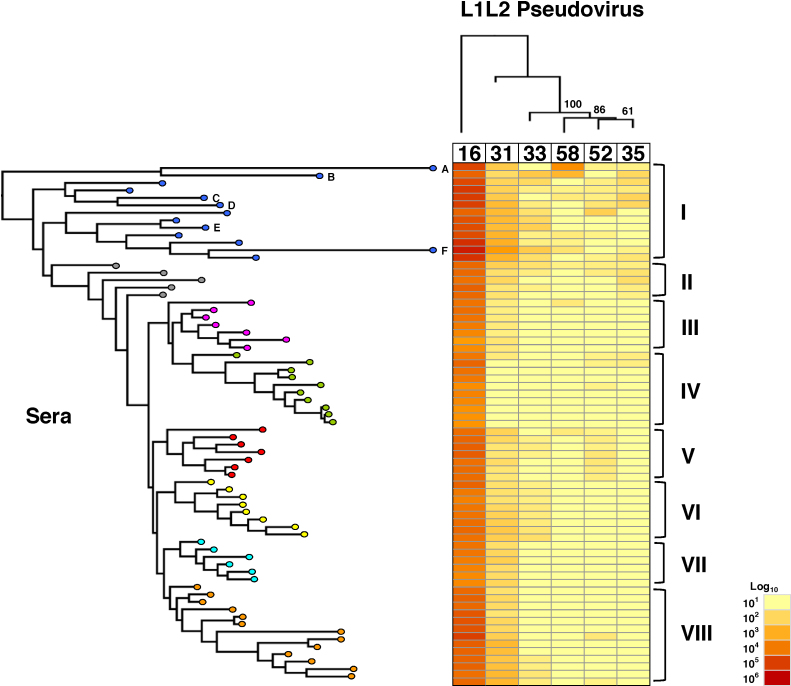
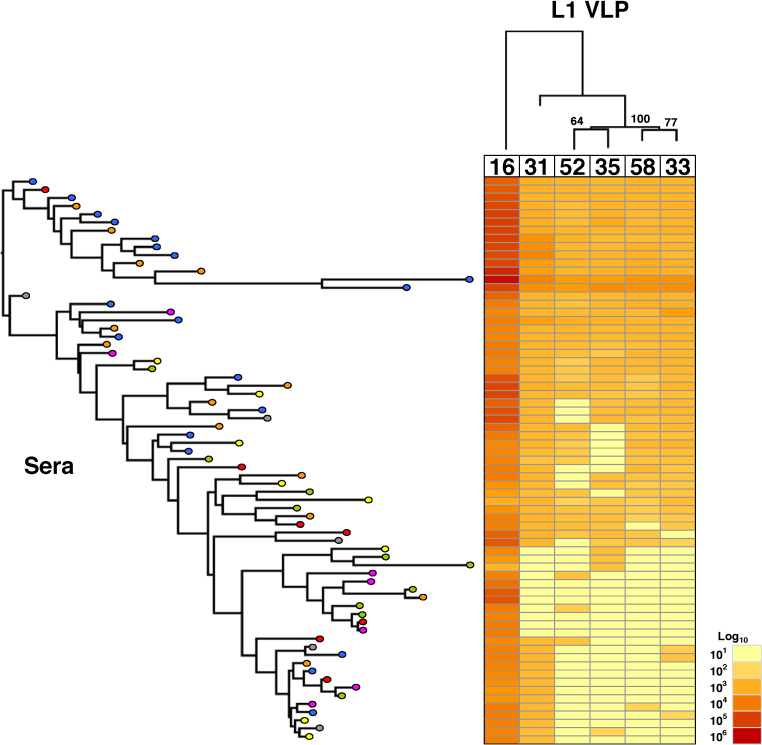
Two-dimensional antigenic dendrograms were constructed by hierarchical clustering of the pseudovirus neutralization assay (Fig. 1) and VLP ELISA (Fig. 2) data. The target antigens (L1L2 pseudovirus or L1 VLP) were clustered horizontally while the sera were clustered vertically against a heat map representing the Log10-transformed antibody titer data. This approach allowed us to sort the pseudovirus neutralization and VLP ELISA data into clusters of sera displaying similar antigenic profiles.
Fig. 1.
Hierarchical clustering of L1L2 pseudovirus neutralization data.
Log10-transformed pseudovirus neutralization data (centre, heat map) were subjected to two-dimensional hierarchical clustering and re-ordered according to serological (left) and pseudovirus (top) dendrograms constructed from the resulting distance matrices. The serological dendrogram is labeled I-VIII based upon intuitive clustering of serological data whereas the pseudovirus target dendrogram clusters are supported by bootstrapping of 500 pseudoreplicates.
Fig. 2.
Hierarchical clustering of L1 VLP binding data.
Log10-transformed VLP binding data (centre, heat map) were subjected to two-dimensional hierarchical clustering and re-ordered according to serological (left) and pseudovirus (top) dendrograms constructed from the resulting distance matrices. VLP target dendrogram is supported by bootstrapping of 500 pseudoreplicates.
The magnitude and breadth of the individual serum neutralizing antibody responses against vaccine and non-vaccine types permitted intuitive clustering (Fig. 1). Serum samples in Cluster I displayed the highest HPV16 neutralization titers and the broadest coverage of non-vaccine types, while Cluster VI included samples that had intermediate HPV16 neutralization titers and whose breadth of reactivity extended to HPV31 and HPV33 (Table 1). These data support a generally quantitative relationship between the level of antibodies in vaccinee sera against HPV16 and an ability to recognize non-vaccine types. However, there also appeared to be a number of antibody specificities displayed. Samples within Clusters II, V and VI for example exhibited differential neutralization of HPV33, HPV35 or HPV52, in addition to HPV31 despite similar HPV16 antibody titers.
Table 1.
Differential pseudovirus neutralization responses informed by hierarchical clustering.
| Cluster | n | Median (IQR) serum neutralization titers against indicated HPV pseudovirusa |
|||||
|---|---|---|---|---|---|---|---|
| HPV16 | HPV31 | HPV33 | HPV35 | HPV52 | HPV58 | ||
| I | 13 | 74,295 (55,880–122,896) | 482 (195–665) | 54 (24–87) | 22 (10–68) | 21 (10–25) | 20 (10–32) |
| II | 5 | 20,556 (20,032–20,559) | 58 (51–98) | 23 (10–27) | 27 (25–49) | 10 (10–25) | 10 (10–10) |
| III | 7 | 9721 (5959–12,954) | 31 (27–33) | 10 (10–10) | 10 (10–10) | 10 (10–10) | 10 (10–10) |
| IV | 10 | 6953 (4366–11,584) | 10 (10–10) | 10 (10–10) | 10 (10–10) | 10 (10–18) | 10 (10–10) |
| V | 7 | 18,351 (17,026–25,055) | 45 (42–84) | 10 (10–21) | 10 (10–10) | 28 (25–35) | 10 (10–10) |
| VI | 8 | 13,302 (11,612–17,578) | 108 (57–166) | 30 (25–37) | 10 (10–10) | 10 (10–10) | 10 (10–10) |
| VII | 6 | 8275 (6386–11,407) | 87 (70–107) | 10 (10–10) | 10 (10–10) | 10 (10–10) | 10 (10–10) |
| VIII | 13 | 25,962 (21,195–40,113) | 152 (90–399) | 10 (10–26) | 10 (10–10) | 10 (10–10) | 10 (10–10) |
Median (IQR, interquartile range) neutralizing antibody titers of sera within indicated intuitive clusters against indicated HPV pseudoviruses.
The serological dendrogram based upon VLP ELISA binding titers (Fig. 2) permitted the formation of branches but the ordering of individual sera bore little relation to the arrangement in the serological dendrogram based upon the pseudovirus neutralization data.
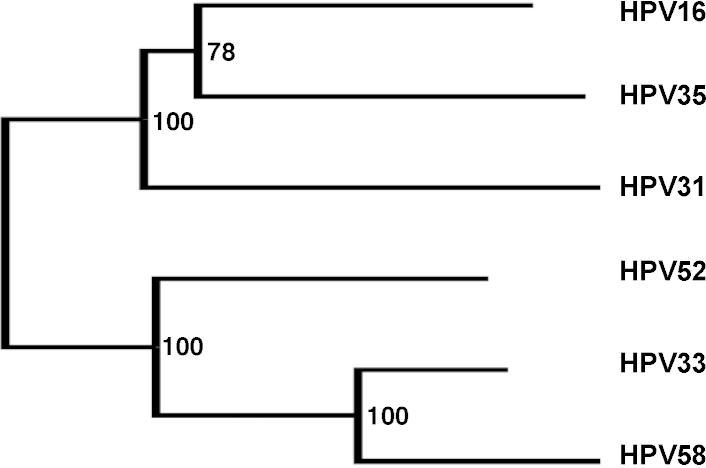
The hierarchical clustering of antibody responses also permitted the ranking of the target antigens. Pseudoviruses HPV31 and HPV33 were the nearest antigenic relatives to HPV16 followed by HPV58 (Fig. 1). HPV52 and HPV35 pseudoviruses clustered together suggesting a close antigenic relationship between these types. The antigenic dendrogram based upon VLP ELISA data (Fig. 2) was broadly similar such that the nearest antigenic relative to HPV16 was HPV31, followed by two separate clusters of HPV33 and HPV58, and HPV35 and HPV52. These inter-type antigenic relationships had good bootstrap support and differed somewhat from the inter-type genetic distances based upon L1 amino sequence (Fig. 3).
Fig. 3.
Distance matrix based upon L1 amino acid sequence used for both Alpha-9 pseudoviruses and VLP, generated using a neighbor-joining algorithm and supported by bootstrap values.
3.3. Enrichment of vaccine and non-vaccine antibody specificities
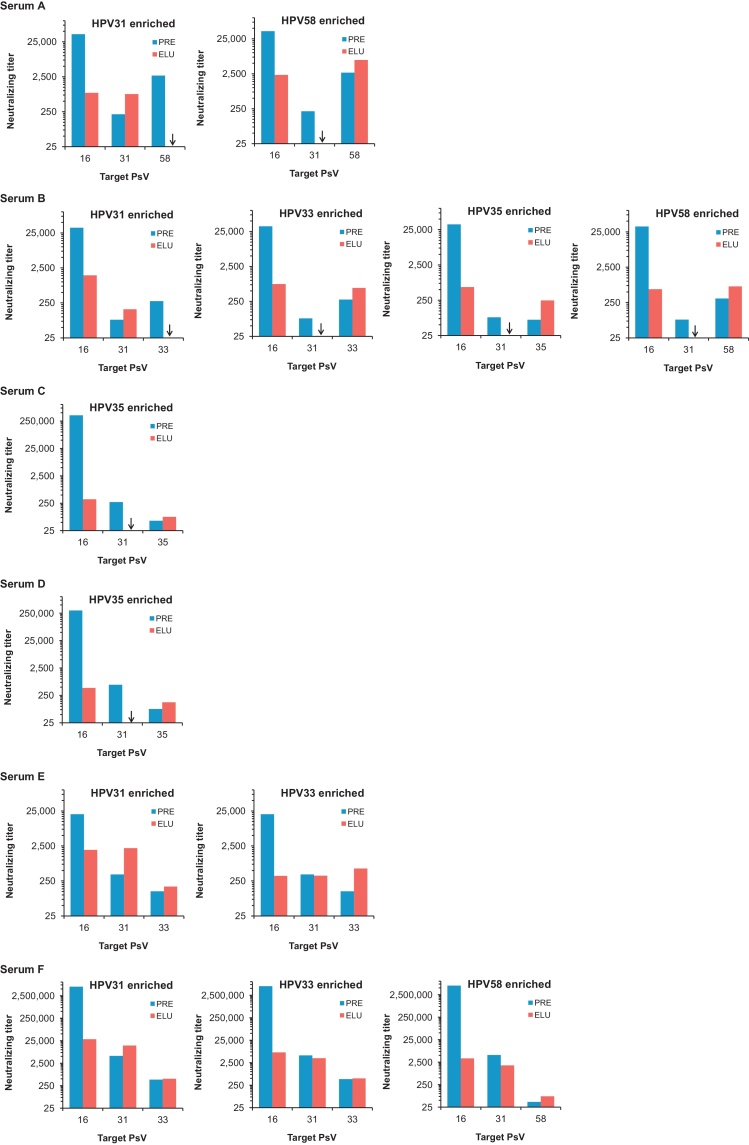
Potential differences in cross-neutralizing antibody specificity were addressed by adsorption on, and elution from, individual non-vaccine type VLP. We reasoned that if cross-neutralization was due to antibodies that constitute a minor fraction of the total vaccine antibody repertoire, such an approach should enrich for these specificities in preference to type-specific HPV16 antibodies. Six serum samples (A–F) were selected from Cluster I (Fig. 1) for enrichment and the neutralization titers against pseudoviruses HPV16, HPV31 and another relevant type were determined prior to and post enrichment. Antibodies enriched on non-vaccine type VLP displayed a range of different cross-neutralizing specificities (Fig. 4).
Fig. 4.
L1L2 pseudovirus neutralization titers prior to and post antibody enrichment on non-vaccine L1 VLP.
Serum samples (A–F) were enriched on VLP representing non-vaccine A9 types. The neutralization titer against pseudoviruses (PsV) representing HPV16, HPV31 and another relevant type were determined for the serum samples prior to and post enrichment.
The enrichment of sera A–D on a particular non-vaccine type VLP did not also enrich for antibodies against another non-vaccine type. Enrichment of serum A on HPV31 or HPV58 VLP yielded antibodies capable of recognizing HPV16 and only the type used for enrichment. For example, the pre-treatment titers against HPV31 and HPV58 were 211 and 2696, respectively. Enrichment on HPV58 VLP increased the titer against HPV58 to 6188 but no HPV31 antibody reactivity was detectable. Serum B which demonstrated post-enrichment neutralization activity against HPV31, HPV33, HPV35 and HPV58 appeared to comprise multiple antibody specificities that recognized HPV16 and only the indicated non-vaccine type. Enrichment of sera C and D on HPV35 VLP yielded antibodies capable of recognising HPV16 and HPV35, but not HPV31.
Antibodies enriched from serum E and F exhibited cross-recognition of more than one non-vaccine type. The enrichment of serum E on HPV31 or HPV33 VLP yielded antibodies capable of recognizing HPV16, HPV31 and HPV33 pseudoviruses. Serum F when enriched on HPV31, HPV33 and HPV58 demonstrated neutralization of HPV31 pseudovirus to a comparable level, and serum F antibodies enriched on HPV31 or HPV33 VLP had similar titers against HPV33.
The HPV16 titer dropped by a median 1.8 Log10 (IQR 1.7–2.8; n = 13) fold following enrichment on non-vaccine VLP. Enriched antibody titers against HPV16 were similar to the titers observed against the type used for enrichment, for example antibodies in serum A when enriched on HPV31 VLP neutralized HPV16 and HPV31 at titers of 861 and 795, respectively.
Antibodies enriched from serum samples A–F, were also tested against L1 VLP representing the same HPV types (Supplementary material S1). Antibody binding titers further confirmed the observations that non-vaccine type antibodies are a minority species which display similar reactivity against HPV16 and non-vaccine types and again highlighted discrepancies between binding and neutralizing antibody specificity.
4. Discussion
We undertook a proof of concept study to investigate the cross-neutralizing antibody specificities generate in response to HPV vaccination. Cross-neutralizing antibodies are elicited in response to both licensed vaccines, Cervarix® and Gardasil® [4,11–13] and this is coincident with differential degrees of vaccine-induced cross-protection [1,2], although a direct link between the two observations has not been established. The characterisation of the cross-neutralizing response beyond antibody titer has been limited to studies of avidity [23] and the vaccine-type specificity of cross-neutralizing antibodies [24]. Sera from Cervarix® vaccinees were chosen since it is this vaccine that appears to elicit the broadest cross-neutralization of non-vaccine types [4].
In the present study, sera from Cervarix® vaccinees were shown to have high antibody titers with broad reactivity against L1 VLP with homologous L1 sequences to those of the pseudoviruses. HPV16 neutralizing antibody titers were similar to those generated in the VLP ELISA corroborating observations in other studies [5,25]. Agreement between antibody reactivity against L1L2 pseudoviruses and L1 VLP representing non-vaccine HPV types was weaker with VLP ELISA antibody titers generally an order of magnitude higher than the corresponding pseudovirus neutralizing titers [4,26].
To examine the discrepancy between cross-reactive antibody profiles, both sets of serological data were subjected to hierarchical clustering. This approach has been used for the evaluation of HIV [27–30], foot and mouth disease virus [31] and H5N1 avian Influenza virus [32] antibody specificities, but we believe this is the first time that this approach has been used to examine HPV vaccine antibody specificity. Differences between pseudovirus neutralizing and VLP binding antibody profiles were stark. There are likely several confounding factors that contribute to this outcome including technical differences between the assays and differences between the range of binding and neutralizing antibody specificities generated. Thus, while L1 VLP binding may be a useful surrogate for type-specific vaccine antibody responses [25] they may not be a similarly useful surrogate for neutralizing antibody reactivity against non-vaccine types.
A number of murine MAbs are capable of binding L1 VLP but lack the ability to neutralize the homologous L1L2 pseudovirus [17,33–35]. For example, MAb H16.J4 cross-reacts with L1 VLP representing various HPV types by ELISA [17], cross-neutralizes HPV31, HPV33 and HPV58 in an L1-based reporter transduction assay [36], but poorly recognizes its epitope on HPV16 L1L2 pseudoviruses [34,35]. Conversely, the neutralizing type-specific MAb H16.V5 appears to recognize its epitope on L1 VLP and L1L2 pseudoviruses to a similar extent [35]. It is reasonable to assume, therefore, that the majority of non-neutralizing antibodies in vaccine sera that recognize VLP representing non-vaccine types, bind to portions of the L1 protein not involved in (pseudo)virus entry or to domains that become altered when L2 is incorporated into the capsid.
There was some agreement in the antigenic inter-type ranking of target HPV types. For both L1 VLP and L1L2 pseudovirus antigens, HPV31 was ranked as the nearest relative to HPV16, and both HPV33/HPV58 and HPV35/HPV52 appeared to share some antigenic similarity, at least based upon reactivity of antibodies generated against the archetypal Alpha-9 group type, HPV16. Some of these antigenic similarities could have been predicted from the distance matrix based upon the L1 amino acid sequence (HPV33 and HPV58), while some could not (HPV35 and HPV52).
Hierarchical clustering of the pseudovirus neutralization data also suggested that Cervarix® vaccination elicits multiple cross-reactive antibody specificities. The underlying basis for individuals generating such a range of cross-reactive antibody specificities is unclear. There may be a genetic component [37] that could impact on an individual's ability to process certain immunogenic epitopes displayed on the vaccine antigens but identifying such contributing factors is challenging. In an attempt to examine the multiplicity of this cross-neutralizing response, we performed antibody enrichment of sera using L1 VLP immobilized onto beads and then tested the eluted fractions against relevant pseudoviruses. The enrichment of antibody specificities using this approach appears to suggest that cross-reactive antibodies formed a distinct, minority specificity within the vaccine-induced antibody repertoire and were not a consequence of a low affinity interaction of an otherwise predominantly type-specific antibody.
The enriched fractions displayed a range of cross-neutralizing antibody specificities including those that recognize multiple non-vaccine types and those that recognize only single non-vaccine types. The cross-neutralizing specificities of the enriched antibody fractions could not have been predicted from the neutralization profile of the source serum. These data suggest that there are multiple immunogenic sites on the surface-exposed domains of the HPV16 L1 protein that share sequence and/or structural homology with other Alpha-9 types. These regions may include the variable loops DE, FG and HI that appear to be common target domains of antibodies generated by natural HPV16 infection [38].
There are several potential shortcomings to this work. Only six sera were evaluated from individuals given Cervarix® vaccine. Caution should therefore be employed when attempting to extrapolate these findings to the majority of HPV vaccinees. Extending this work to include sera from both Cervarix® and Gardasil® vaccinees will support a more robust evaluation. The target antigens for the enriched antibodies were L1L2 pseudoviruses whereas the antigens used for the enrichment were L1 VLP which may have introduced some bias in the antibody specificities being measured. This approach was used for two reasons. First, in our hands, the expression and purification of L1 VLP generates purer populations of antigen than the corresponding purification of L1L2 pseudoviruses. Second, the immunogens used in the HPV vaccines are L1 VLP and so the use of L1 VLP as the immobilized antigen should have allowed capture of the majority of L1-specific antibodies able to recognize a particular HPV type. The recovery of high titer cross-neutralizing antibodies following enrichment on non-vaccine VLP appears to support the maintenance of some VLP conformational integrity following bead immobilisation.
If cross-neutralizing antibodies form a tiny minority of the antibodies elicited following HPV vaccination it is possible that their generation and maintenance is more precarious than those of vaccine type antibodies. HPV31 cross-neutralizing antibody can be detected at 18 months after the third Cervarix® vaccine dose suggesting some degree of stability in this regard [26]. A two-dose schedule may also be an issue for the generation and maintenance of a sizeable cross-neutralizing antibody fraction. While HPV16 antibody titers following a two dose schedule appear to be non-inferior to those following a three dose schedule [19], the impact on the generation of antibodies to non-vaccine types is unclear. Understanding the potential impact of prior infection on vaccine antibody responses [23] and differences between the specificities of antibodies generated following vaccination and during natural infection will also be important.
Overall, these data support the notion that antibody neutralization of non-vaccine types by Cervarix® vaccine sera is due to a small fraction of antibodies exhibiting different but overlapping specificities, rather than a predominantly type-specific antibody specificity that nevertheless exhibits a small degree of cross-recognition of non-vaccine types. Identifying the HPV16 L1 domains responsible for their generation and perhaps improving HPV16 VLP immunogenicity toward the generation of such antibodies will be important if the development of high titer neutralizing antibodies targeting non-vaccine types is considered to be a desirable outcome of HPV vaccination.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was in part supported by the UK Medical Research Council (grant number G0701217). We are indebted to Prof. John T. Schiller and Dr. Chris Buck (National Cancer Institute, Bethesda, U.S.A.) for providing the HPV16, HPV31, HPV52 and HPV58 pseudovirus clones and Dr. H Faust and Prof. J Dillner (Malmö University Hospital, Malmö, Sweden) for providing the HPV33 pseudovirus clone.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Lu B., Kumar A., Castellsague X., Giuliano A.R. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011;11:13. doi: 10.1186/1471-2334-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romanowski B. Long term protection against cervical infection with the human papillomavirus: review of currently available vaccines. Hum Vaccin. 2011;7(2):161–169. doi: 10.4161/hv.7.2.13690. [DOI] [PubMed] [Google Scholar]
- 3.Einstein M.H., Baron M., Levin M.J., Chatterjee A., Edwards R.P., Zepp F. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009;5(10):705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 4.Draper E., Bissett S.L., Howell-Jones R., Waight P., Soldan K., Jit M. A randomized, observer-blinded immunogenicity trial of Cervarix((R)) and Gardasil((R)) human papillomavirus vaccines in 12–15 year old girls. PLoS One. 2013;8(5):e61825. doi: 10.1371/journal.pone.0061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemp T.J., Garcia-Pineres A., Falk R.T., Poncelet S., Dessy F., Giannini S.L. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008;26(29-30):3608–3616. doi: 10.1016/j.vaccine.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzich J.A., Ghim S.J., Palmer-Hill F.J., White W.I., Tamura J.K., Bell J.A. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. PNAS. 1995;92(25):11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitburd F., Kirnbauer R., Hubbert N.L., Nonnenmacher B., Trin-Dinh-Desmarquet C., Orth G. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69(6):3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longet S., Schiller J.T., Bobst M., Jichlinski P., Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2012;85(24):13253–13259. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiller J.T., Lowy D.R. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J Infect Dis. 2009;200(2):166–171. doi: 10.1086/599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanley M. Potential mechanisms for HPV vaccine-induced long-term protection. Gynecol Oncol. 2010;118(1 Suppl):S2–S7. doi: 10.1016/j.ygyno.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Smith J.F., Brownlow M., Brown M., Kowalski R., Esser M.T., Ruiz W. Antibodies from women immunized with Gardasil ((R)) cross-neutralize HPV 45 pseudovirions. Hum Vaccin. 2007;3(4):109–116. doi: 10.4161/hv.3.4.4058. [DOI] [PubMed] [Google Scholar]
- 12.Draper E., Bissett S.L., Howell-Jones R., Edwards D., Munslow G., Soldan K. Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera. Vaccine. 2011;29(47):8585–8590. doi: 10.1016/j.vaccine.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp T.J., Hildesheim A., Safaeian M., Dauner J.G., Pan Y., Porras C. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011;29(11):2011–2014. doi: 10.1016/j.vaccine.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannini S.L., Hanon E., Moris P., Van Mechelen M., Morel S., Dessy F. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24(33-34):5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Pastrana D.V., Buck C.B., Pang Y.Y., Thompson C.D., Castle P.E., FitzGerald P.C. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321(2):205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Opalka D., Lachman C.E., MacMullen S.A., Jansen K.U., Smith J.F., Chirmule N. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003;10(1):108–115. doi: 10.1128/CDLI.10.1.108-115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen N.D., Dillner J., Eklund C., Carter J.J., Wipf G.C., Reed C.A. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology. 1996;223(1):174–184. doi: 10.1006/viro.1996.0466. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z., Christensen N., Schiller J.T., Dillner J. A monoclonal antibody against intact human papillomavirus type 16 capsids blocks the serological reactivity of most human sera. J Gen Virol. 1997;78:2209–2215. doi: 10.1099/0022-1317-78-9-2209. [DOI] [PubMed] [Google Scholar]
- 19.Dobson S.R., McNeil S., Dionne M., Dawar M., Ogilvie G., Krajden M. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793–1802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 20.Huo Z, Bissett SL, Giemza R, Beddows S, Oeser C, Lewis DJ. Systemic and mucosal immune responses to sublingual or intramuscular Human Papilloma Virus antigens in healthy female volunteers. PLoS One;7(3):e33736. [DOI] [PMC free article] [PubMed]
- 21.Li Y., Migueles S.A., Welcher B., Svehla K., Phogat A., Louder M.K. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med. 2007;13(9):1032–1034. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y., Svehla K., Louder M.K., Wycuff D., Phogat S., Tang M. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J Virol. 2009;83(2):1045–1059. doi: 10.1128/JVI.01992-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemp T.J., Safaeian M., Hildesheim A., Pan Y., Penrose K.J., Porras C. Kinetic and HPV infection effects on cross-type neutralizing antibody and avidity responses induced by Cervarix((R)) Vaccine. 2012;31(1):165–170. doi: 10.1016/j.vaccine.2012.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scherpenisse M, Schepp RM, Mollers M, Meijer CJ, Berbers GA, van der Klis FR. Characteristics of HPV-specific antibody responses induced by infection and vaccination: cross-reactivity, neutralizing activity, avidity and IgG subclasses. PLoS One;8(9):e74797. [DOI] [PMC free article] [PubMed]
- 25.Dessy F.J., Giannini S.L., Bougelet C.A., Kemp T.J., David M.P., Poncelet S.M. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4(6):425–434. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 26.Einstein M.H., Baron M., Levin M.J., Chatterjee A., Fox B., Scholar S. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18–45 years. Hum Vaccin. 2011;7(12):1359–1373. doi: 10.4161/hv.7.12.18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binley J.M., Wrin T., Korber B., Zwick M.B., Wang M., Chappey C. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78(23):13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray E.S., Taylor N., Wycuff D., Moore P.L., Tomaras G.D., Wibmer C.K. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J Virol. 2009;83(17):8925–8937. doi: 10.1128/JVI.00758-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seaman M.S., Janes H., Hawkins N., Grandpre L.E., Devoy C., Giri A. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol. 2010;84(3):1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shang H., Han X., Shi X., Zuo T., Goldin M., Chen D. Genetic and neutralization sensitivity of diverse HIV-1 env clones from chronically infected patients in China. J Biol Chem. 2011;286(16):14531–14541. doi: 10.1074/jbc.M111.224527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeve R., Blignaut B., Esterhuysen J.J., Opperman P., Matthews L., Fry E.E. Sequence-based prediction for vaccine strain selection and identification of antigenic variability in foot-and-mouth disease virus. PLoS Comput Biol. 2010;6(12):e1001027. doi: 10.1371/journal.pcbi.1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai A.C., Wu W.L., Lau S.Y., Guan Y., Chen H. Two-dimensional antigenic dendrogram and phylogenetic tree of avian influenza virus H5N1. FEMS Immunol Med Microbiol. 2012;64(2):205–211. doi: 10.1111/j.1574-695X.2011.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen N.D., Reed C.A., Cladel N.M., Hall K., Leiserowitz G.S. Monoclonal antibodies to HPV-6 L1 virus-like particles identify conformational and linear neutralizing epitopes on HPV-11 in addition to type-specific epitopes on HPV-6. Virology. 1996;224(2):477–486. doi: 10.1006/viro.1996.0554. [DOI] [PubMed] [Google Scholar]
- 34.Rizk R.Z., Christensen N.D., Michael K.M., Muller M., Sehr P., Waterboer T. Reactivity pattern of 92 monoclonal antibodies with 15 human papillomavirus types. J Gen Virol. 2008;89(Pt 1):117–129. doi: 10.1099/vir.0.83145-0. [DOI] [PubMed] [Google Scholar]
- 35.Culp T.D., Spatz C.M., Reed C.A., Christensen N.D. Binding and neutralization efficiencies of monoclonal antibodies, Fab fragments, and scFv specific for L1 epitopes on the capsid of infectious HPV particles. Virology. 2007;361(2):435–446. doi: 10.1016/j.virol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Combita A.L., Touze A., Bousarghin L., Christensen N.D., Coursaget P. Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses. J Virol. 2002;76(13):6480–6486. doi: 10.1128/JVI.76.13.6480-6486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newport M.J., Goetghebuer T., Weiss H.A., Whittle H., Siegrist C.A., Marchant A. Genetic regulation of immune responses to vaccines in early life. Genes Immun. 2004;5(2):122–129. doi: 10.1038/sj.gene.6364051. [DOI] [PubMed] [Google Scholar]
- 38.Carter J.J., Wipf G.C., Madeleine M.M., Schwartz S.M., Koutsky L.A., Galloway D.A. Identification of human papillomavirus type 16 L1 surface loops required for neutralization by human sera. J Virol. 2006;80(10):4664–4672. doi: 10.1128/JVI.80.10.4664-4672.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.