Abstract
Commensal bacteria regulate the homeostasis of host effector immune cell subsets. The mechanisms involved in this commensal–host crosstalk are not well understood. Intestinal epithelial cells (IECs) not only create a physical barrier between the commensals and immune cells in host tissues, but also facilitate interactions between them. Perturbations of epithelial homeostasis or function lead to the development of intestinal disorders such as inflammatory bowel diseases (IBD) and intestinal cancer. IECs receive signals from commensals and produce effector immune molecules. IECs also affect the function of immune cells in the lamina propria. Here we discuss some of these properties of IECs that define them as innate immune cells. We focus on how IECs may integrate and transmit signals from individual commensal bacteria to mucosal innate and adaptive immune cells for the establishment of the unique mucosal immunological equilibrium.
Keywords: Intestinal epithelial cells, IEC, commensal bacteria, SFB, Th17, Treg
Mucous membranes (mucosae) line body cavities and passages. Together with the skin, mucosae form a contiguous barrier that separates the body’s internal organs from the outside environment. Mucosal surfaces represent a first line of defense against invading pathogens. They provide physical protection, but also an interface, through which cells of the host immune system detect foreign substances and initiate appropriate immune responses. The mucosa of the gastrointestinal (GI) tract is particularly enriched in environment–host interactions. Owing to its function as a nutrient portal, the gut is constantly ‘flooded’ with non-self-derived antigenic substances, which include, in addition to food antigens, potential hazards, such as invasive and non-invasive pathogens or environmental toxins. In addition, the intestinal mucosa is a permanent home to ~1014 bacteria, called commensal microbiota, that peacefully colonize the human GI tract.1 All this accounts for an astronomic antigenic load in the lumen of the intestine, which in turn leads to accumulation of immune cells in the mucosa. Despite the panoply of antigenic substances in the lumen, the various immune cell subsets in the mucosa normally exist in a controlled equilibrium without causing any overt pathology. Perturbations of the mechanisms controlling this immune homeostasis lead to loss of protective immunity and result in disease. Multiple factors help establish and regulate mucosal immune homeostasis, among which, commensal bacteria have a dominant role. Moreover, individual bacterial species or groups have now been shown to specifically modulate different aspects of host immunity, including anatomical location, abundance or function of various immune subsets. Commensals are physically separated from immune cells in the lamina propria (LP) by the intestinal epithelial cell (IEC) layer. Therefore, how they exercise immunomodulatory effects on LP immune cells is an area of intense investigation.
In the GI tract, the crosstalk between microbiota and immune cells occurs across a single layer of IECs. IECs have an important role in separating the two sides of this ‘discussion’. Formation of tight junctions and secretion of mucus or antimicrobial peptides (AMPs) are examples of the barrier function of IECs. At the same time, because of these mechanisms, IECs also represent the main cell type that is in direct contact with both luminal microbiota (or their products) and LP immune cells. Therefore, IECs must be crucial participants in commensal–host interactions. Indeed it is well accepted that proper IEC barrier function is essential for sustaining a healthy immune status. However, IECs are more than just a barrier. IECs communicate constantly with commensal bacteria and a lot is known about how commensals affect IEC function and, at the same time, how IEC activity affects and regulates bacterial populations in the lumen. Relatively less is known about how IEC function controls the immune homeostasis of LP effector cells. Here, we review the role of IECs as important innate immune cells that interact and influence the activity of both commensal bacteria and host immune cells. We discuss specifically how IECs may participate in mediating commensal immunomodulatory effects by integrating signals from lumenal bacteria and affecting immune homeostasis in the LP.
IMMUNOMODULATORY FUNCTIONS OF COMMENSAL BACTERIA
The presence of commensal bacteria affects multiple facets of host immunity, and several comprehensive reviews have recently examined this question in detail.2,3 Here we briefly outline some of these effects in order to demonstrate the diversity of commensal immune functions.
Development of organized GALT
In the gut, mucosal immune cells are either organized in gut-associated lymphoid tissues (GALT), where they carry out antigen-specific adaptive immune responses, or accumulate in the LP as a network of innate and adaptive effector cells. Commensal bacteria control the general cellularity and organization of both organized GALT and the LP immune network. Organized GALT consists of anatomical structures of various sizes that contain lymphoid follicles. These include mesenteric lymph nodes, Peyer’s patches (PPs), isolated lymphoid follicles (ILFs) and cryptopatches. Organogenesis of these lymphoid organs initiates during embryonic life when commensals are not present. Nevertheless, commensal bacteria are required for their maturation and maintenance after birth. Indeed, germ-free (GF) mice have hypoplastic PPs and lack most ILFs.4 Colonization with commensal bacteria induces both the generation of immature ILFs (iILFs), presumably from cryptopatches, and their maturation to mature ILFs (mILFs), which contain a fully organized B-cell follicle. Interestingly, these two steps of ILF development seem to be controlled by different types of commensals. Thus, Gram-negative bacteria induce iILF development through the nucleotide-binding oligomerization domain containing 1 (NOD1) receptor and, accordingly, iILFs are absent in NOD1-deficient mice. At the same time, generation of mILFs from iILFs in the SI, but not in the colon, is blocked in mice with perturbed TLR signaling due to absence of the adapter proteins MyD88 or TRIF,4 suggesting that different bacterial signals are required for this step.
Mucosal immunoglobulin A (IgA) production
IgA production and secretion into the lumen are characteristic features of gut immunity. IgA is produced by GALT and LP B-cells, and transported through IECs into the gut lumen as secretory IgA (SIgA). Most SIgA recognizes and opsonizes bacteria in the lumen, thus preventing their access to the LP. IgA is induced by two general mechanisms in the gut—T-cell-dependent and T-cell-independent. Organized GALT is important in both cases. T-cell-dependent IgA induction occurs in the context of the germinal center reaction in PPs. T-cell-independent IgA induction mostly occurs in mILFs.5 As maturation of GALT, as discussed above, requires commensal bacteria, IgA production could be expected to also depend on the presence of microbiota. An alternative T-cell-independent pathway of IgA induction relies on B-cell-activating cytokines produced by IECs in response to commensal signals, as discussed in more detail below. In agreement with these mechanisms, IgA production is impaired in GF mice, even though B-cells are present in the LP. The ability of different commensal bacteria to induce IgA has not been studied systematically. There are, nevertheless, reports that not all commensal bacteria induce IgA. In gnotobiotic experiments, segmented filamentous bacteria (SFB) induced IgA, but a mixture of 46 Clostridia did not.6 The IgA induction may also depend on the anatomical location. For example, in monocolonized GF mice Bacteroides acidifaciens induces IgA in the colon, but not in the ileum.7 The mechanisms of commensal-mediated IgA induction are also unclear. In accordance with the NOD1-dependent mILF induction by Gram-negative bacteria, B. acidifaciens (G−), but not Lactobacillus johnsonii (G+), induced germinal centers and IgA production in organized GALT.7 At the same time, mucosa-associated bacteria, such as SFB, may modify IEC function to induce IgA. The secretion of opsonizing SIgA in response to commensals can be considered a mechanism of mucosal protection from pathogens, but also a mechanism to establish a long-term commensal–host mutualism by sequestering commensals in the gut lumen (Figure 1).
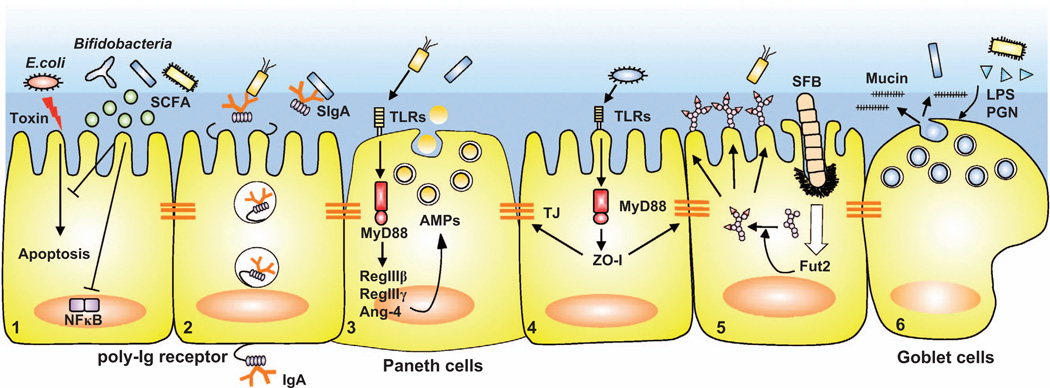
Figure 1.
Commensal bacteria regulate IEC barrier functions. Microbiota induce epithelial barrier mechanisms: (1) Commensal bacteria, such as Bifidobacteria, produce SCFAs, which protect IECs from epithelial apoptosis induced by enteropathogenic Escherichia coli and inhibit NF-κB activation. (2) Dimeric IgA produced by mucosal plasma cells binds to polymeric immunoglobulin receptor (poly-Ig receptor) expressed on basolateral side of IECs and is transcytosed to the apical surface, where it is released as SIgA. Both IgA class switching and poly-Ig receptor expression are regulated by commensal bacteria. (3) Commensal bacteria induce multiple AMPs such as RegIIIβ, RegIIIγ, Angiogenin-4 from Paneth cells in a TLR-dependent manner. (4) Commensal bacteria strengthen tight junctions (TJ). Epithelial tight junction protein, ZO-1, is upregulated by commensals in a TLR-dependent manner. (5) Commensal bacteria induce the expression of Fut2 and fucosylation of surface proteins on IECs. (6) Commensal bacterial products such as lipopolysaccharide (LPS) and peptidoglycan (PGN) induce mucus production from goblet cells.
Innate immune cells
Innate immune cells are functionally hard-wired to recognize microbes and their products. Commensal bacteria are therefore expected to affect the development or function of innate immune cells. Several reviews in this issue describe in detail the different subsets of innate immune cells and their interactions with the microbiota. In some cases, studies of gut innate immune subsets have been difficult to reconcile, possibly due to the difficulty in isolating these cells, differences in isolation procedures, and also due to the lack of reliable in vivo genetic ablation models to study their function. Nevertheless, it seems that commensal bacteria are not generally required for the development of dendritic cells (DCs), macrophages (Mf), natural killer (NK) cells, or innate lymphoid cells (ILCs). Even though some studies have reported differences in DC or ILC numbers in GF mice, in general, all major innate immune subsets are present in GF mice.8,9 What seems to be under the control of commensal-derived signals, are the effector functions of innate immune cells. Pro- and anti-inflammatory cytokine production, for example, IL-6, IL-23, TGF-β, IL-10, by DCs and Mfs can be induced by different types of commensals. IL-22 production by ILCs, which mediates many of their intestinal homeostatic functions, also seems to be controlled by commensal-derived signals, although the direction of this control is a matter of debate (see review by Philip et al.10 in this issue). Whether the effects of microbiota are direct or indirect is an important question in each case, as it provides crucial mechanistic insights. Direct effects result from the direct detection of commensal products by innate immune cell, for example by DCs in PPs or DCs sampling luminal contents.11 In contrast, commensals mediate NK cell function indirectly. Even though NK cell numbers are normal in GF mice, they are functionally deficient in antiviral activity.12 NK cell priming requires interferon, and it was shown that the NK cell priming deficiency is an indirect effect of the lack of type I interferon production by DCs in the absence of commensal bacteria.12 Similarly, commensal-induced epithelial expression of IL-25 indirectly controls IL-22 production by ILCs.13
T-cells and T-cell homeostasis
The balance between multiple effector T-cell subsets in the gut mucosa is referred to as T-cell homeostasis. The maintenance of this balance and the relative contribution of different effector T-cell subsets at any given moment direct the maintenance of a healthy immune state and the progression of intestinal diseases. Some of the best known examples of commensal immunomodulatory effects occur at the level of T-cell homeostasis, and the presence of commensals influences the development or function of almost all T-cell subsets in the gut.
Intraepithelial lymphocytes (IELs)
IELs are a unique subset of intestinal T-cells that is located in the epithelial layer and is therefore physically separated from LP lymphocytes. In contrast to LP T-cells, IELs are enriched in T-cell receptor (TCR) γδ cells, as well as in CD8+ cells. They also contain a unique population of CD8αα+ cells. IEL development and functions are not completely understood, but spatially they are well situated to receive signals from both commensals and IECs. IELs promote epithelial barrier functions, have cytotoxic activity to help clear infected or damaged IECs, and induce antimicrobial peptide production from IECs. Therefore, there is a constant IEL–IEC crosstalk. Indeed, TCRαβ+ IELs do not respond to conventional major histocompatibility complex (MHC)-peptide ligands, but to ligands expressed abundantly on IECs, such as the thymus leukemia antigen ligand for CD8αα+ IELs.14 Besides affecting IEC function, IELs also have regulatory functions and suppress inflammation in animal models.15 TCRγδ IELs have been shown to produce AMPs.16 The presence of commensal bacteria affects development and function of IELs. In GF mice, TCRαβ+ IELs are almost absent and TCRγδ IELs have impaired cytolitic function.17–19 Production of the AMP RegIIIγ by TCRγδ IELs also depends on the presence of commensal bacteria.16 Interestingly, commensal modulation of IEL homeostasis seems to depend on the type of commensal. For example, restoration of RegIIIγ production occurs after colonization with only certain commensals.16 SFB colonization restores TCRαβ IEL numbers preferentially in the small intestine (SI), whereas colonization with a mix of autochthonous Clostridia restores IEL numbers in the colon.6 Therefore, the composition of commensal bacteria may control the outlook of the IEL compartment by controlling the abundance and function of various IEL subsets. The molecular and cellular mechanisms of this control remain to be elucidated, but could involve transmission of signals through the IECs.
LP CD4 T-cell homeostasis
In the LP CD4 TCRαβ T-cells are the predominant T cell type. At steady state, in specific pathogen-free mouse colonies, the two most abundant effector CD4 T-cell types in the LP are IL-17-producing Th17 cells and regulatory T-cells (Treg). Both of these subsets are heterogeneous, but in general, Th17 cells promote inflammatory protective immune responses and Tregs suppress excessive or unwanted immune activation, and therefore have a general anti-inflammatory function. The balance between these two functionally antagonistic subsets establishes the immune status in the LP. It also represents one of the better-studied examples of control by the composition of commensal microbiota.
Th17 cells are abundant in the SI at steady state in specific pathogen-free mice, but are almost absent in GF mice.20 Moreover, GF mice are deficient in some systemic Th17 cell responses as well, such as generation of encephalitogenic brain-infiltrating Th17 cells in the EAE model of MS.21 Therefore, commensal bacteria are required for the induction of mucosal, and at least some non-mucosal, Th17 cells. Interestingly, when mice from different commercial colonies were examined, Th17 cell presence depended on the microbiota composition. C57BL/6 (B6) mice from the Jackson laboratory (Bar Harbor, ME, USA) had very low levels of mucosal Th17 cells compared to B6 mice from Taconic Farms (Germantown, NY, USA), and transfer of commensal microbiota from Jackson B6 mice induced much lower levels of Th17 cells in GF mice than did the transfer of Taconic B6 microbiota.20 Direct comparison between Taconic and Jackson B6 microbiota identified SFB as the most overrepresented commensal species in the SI lumen of Taconic B6 mice.22 SFB are Clostridia that colonize preferentially the terminal ileum of many animal species. Although they are present in the fecal material throughout the GI tract, in the terminal ileum, SFB grow as characteristic long filaments that establish tight contacts with the IECs. In gnotobiotic studies, out of ~80 different commensal isolates tested, SFB were the only commensals that could induce high levels of Th17 cells in the LP of GF mice.22 Induction of Th17 cells by SFB also did not require additional commensals.22 Thus SFB is a commensal species whose presence is sufficient to induce mucosal Th17 cells. The molecular and cellular mechanisms by which SFB induce Th17 cells are currently unknown. However, in contrast to most other commensals, SFB interact directly with IECs. Hence, modulations of IEC function are suspected to somehow be involved. Indeed, SFB have multiple epithelial effects as discussed in more detail below.
Tregs are present in all peripheral tissues where they provide immune suppression and downregulate excessive inflammatory responses. They are enriched throughout the GI tract, presumably due to the astronomic amount of antigens present and the enhanced requirement for immune tolerance. Tregs are especially enriched in the colon, where they may represent up to 40–50% of CD4 T-cells.23 Interestingly, Treg induction in the SI is not dependent on commensal signals, because Tregs are present in similar or even enriched numbers in the SI of GF mice and have unperturbed function.23 In contrast, GF mice contain three–fourfold less Foxp3+ Tregs in the colon, and colonization with fecal microbiota from non-GF mice restores Treg numbers.23 Therefore, colonic Tregs are controlled by commensal-derived signals. As in the case of Th17 cells, not all commensal bacteria are capable of inducing colonic Tregs. It was shown that colonization with a mixture of 46 autochthonous commensal species belonging to Clostridia clusters IV and XIVa is sufficient to completely restore Treg levels in the colon of GF mice.23 In specific pathogen-free mice, these Clostridia colonize preferentially the colon where they occupy the mucus layer, again, in close proximity to IECs. In human, members of the same groups of Clostridia have been associated with IL-10 induction and protection from colitis.24 Lumenal bacteria may also affect the function of Tregs. For example, B. fragilis induces IL-10 production, and increases suppressive and anti-inflammatory functions of Foxp3+ Tregs.25
The ability of different commensals to induce or modify the function of CD4 T-cell subsets has important functional consequences. It shows that the relative abundance of these immunomodulatory commensals can dictate the composition of the LP CD4 T-cell compartment. Moreover, the composition of the LP CD4 T-cell compartment directs the nature and intensity of the adaptive immune response. Indeed, SFB colonization is associated with protection from intestinal infections in mice, rats and rabbits,22,26,27 and colonization with Treg-inducing Clostridia increases resistance to colitis and systemic IgE responses.23 On the other hand, the Th17 cell-biased T-cell homeostasis in the presence of SFB renders mice with these bacteria more susceptible to autoimmunity upon external challenge, as demonstrated in increased pathology in animal models of multiple sclerosis and rheumatoid arthritis.21,28
Invariant NKT cells
Invariant NKT (iNKT) cells are innate-like T-cells that express invariant TCRs and respond to glycolipids presented by the non-classical MHC-I molecule, CD1d. iNKT cells have various functions in first-line responses to microbial infections and tumors, but, similarly to Th17 cells, may also contribute to autoimmune inflammation in colitis, rheumatoid arthritis and asthma. Development and function of iNKT cells is controlled by commensal microbiota. In GF mice, elevated numbers of iNKT cells are observed in both the colonic and lung mucosa.29 This leads to increased susceptibility and morbidity of GF mice in oxazolone-induced colitis and allergic asthma, which are mediated by CD1d-restricted iNKT cells. Interestingly, colonization of GF mice with conventional microbiota in the neonatal stage had preventive effects on autoimmunity by decreasing mucosal iNKT cell levels, but colonization of adult GF mice had no impact on iNKT cells and disease susceptibility.29 Moreover, the effects of commensal bacteria on mucosal iNKT accumulation were dependent on epithelial-derived signals.29 The role of microbiota composition on decreasing mucosal iNKT cell levels was not examined in this study. However, effects of different commensal bacteria on the levels and function of systemic iNKT cells have been reported. In contrast to mucosal iNKT cells, systemic iNKT cell function seems to require Sphingomonas spp., which carry antigenic glycosphingolipid products presented by CD1d.30 Further studies are needed to characterize the detailed molecular mechanisms and spatiotemporal regulation of iNKT cell development and maintenance by commensal bacteria.
IECs AND THE COMMENSAL-IMMUNE CROSSTALK
The epithelial barrier separates commensal bacteria from host immune cells. The separation is important for maintaining a healthy host as well as for the establishment of mutualistic environment for the microbiota. Therefore, one of the main functions of the single layer of IECs in the gut is to sustain this separation. At the same time, IECs can receive and integrate signals from both sides, and provide an important way of communication between bacteria and host. Therefore, IECs may serve as crucial mediators of immunomodulatory commensal effects.
INTESTINAL EPITHELIAL BARRIER SYSTEM
Several subsets of IECs compose the gut epithelial monolayer. These include entero-absorptive enterocytes, entero-endocrine cells, goblet cells and Paneth cells, all of which differentiate from epithelial stem cells residing in the villous crypt region. Tight junctions between IECs form a contiguous physical barrier that separates the gut lumen from the LP. At the same time tight junctions divide the IEC membrane into an apical and basolateral part, and help establish and maintain IEC polarity, which is crucial for IEC function. Goblet cells produce heavily glycosylated mucins, which form the gel-like layer of mucus that covers the luminal surface of the epithelium. In mouse colon, the mucus consists of two layers, an outer diffuse layer and an inner layer that is firmly associated with the epithelium. The vast majority of commensals are ‘trapped’ in the outer layer and the inner layer is virtually devoid of bacteria.31 The critical role of the mucus layer in microbiota sequestration is demonstrated by studies in mice that lack the main structural mucus protein, MUC2. MUC2-deficient mice lack mucus layer, allowing direct contact of commensal bacteria with IECs, which leads to spontaneous colitis and colorectal cancer.32,33 In addition to this physical separation, the mucus layer provides an environment in which secreted antimicrobial molecules accumulate to further facilitate bacterial sequestration. These molecules include IEC-derived substances, such as AMPs, and non-IEC derived substances, such as SIgA, which is transcytosed by the IEC. In addition, Paneth cell-derived microbicidal molecules such as defensins, lysozymes, cathelicidins, secretory phospholipase A2 and C-type lectins are secreted in the lumen and contribute to general bacterial sequestration as well as protection of stem cells in the crypt.34
IECs RECEIVE SIGNALS FROM COMMENSAL BACTERIA
Commensal bacteria control epithelial barrier functions
Even though IECs employ mechanisms to limit direct access of live bacteria to the epithelial surface, they are capable of detecting bacterial products. Similarly to conventional innate immune cells, IECs express pattern-recognition receptors (PRRs) to detect common microbial ligands. PRRs, include Toll-like receptors (TLRs), Nod-like receptors (NLRs) and Rig-I like receptors (for a recent review on mucosal PRRs see35). Moreover, almost all of the mechanisms promoting epithelial barrier function are influenced by the presence of microbiota. Commensal bacteria can upregulate tight junction molecules and control intestinal permeability,36 and microbial signals through TLR receptors are required for maintenance of the epithelial barrier. Indeed, ZO-1 expression, formation of tight junctions, and epithelial turnover are disrupted in TLR2- and MyD88-deficient mice, which leads to increased susceptibility to epithelial damage.37,38
Secretion of mucus and AMPs is also induced by commensal bacteria. The mucus layer is considerably reduced in GF mice, but recovers upon exposure to bacterial products, such as LPS or peptidoglycan.39 RegIIIγ is a member of the C-type lectin family and targets cell wall peptidoglycan of Gram-positive bacteria.40 RegIIIγ expression in ileal tissues is absent in GF mice and is induced by commensal bacteria.40 Production of RegIIIγ by IECs at steady state is directly induced by microbial signals in a TLR/MyD88-dependent manner, and Paneth cell-restricted MyD88 expression was sufficient to recover microbiota-induced RegIIIγ expression and to restore defects in epithelial barrier function in MyD88-deficient mice.41 The expression of angiogenin-4, a Paneth cell-secreted ribonuclease-like protein with microbicidal activity, is dramatically reduced in GF mice.42 Likewise, the expression of RegIIIβ, CRP-ductin and resistin-like molecule β is regulated by MyD88, suggesting that expression of multiple antimicrobial molecules occurs in response to commensal stimulation in a TLR-dependent manner41 (Figure 1). In contrast, the expression of several AMPs such as lysozyme, secretory phospholipase A2, human cathelicidin LL-37, α-defensins and certain β-defensins does not seem to be affected by the microbiota.43 Combined with the control of SIgA, these studies show that commensals are major regulators of the intestinal epithelial barrier system. How the composition of commensal bacteria affect barrier function is not completely clear. It is possible that a full complement of diverse microbiota is required for optimal induction of the complete array of barrier mechanisms. Combined, these mechanisms help sequester commensal bacteria, without ablating them. For example, the mucus restricts direct bacterial access to the LP, and at the same time facilitates the host–commensal crosstalk by providing a specific environment for the growth of the microbiota and the diffusion of commensal bioproducts. Therefore, the ability of commensals to induce these mechanisms may represent evolutionary adaptation for the establishment of mutualism.
Commensal bacteria control epithelial intracellular signaling and homeostasis
Even though sequestration mechanisms prevent most direct interaction with IECs, commensal signals are continuously reaching the epithelium, and are involved in maintaining immune tolerance and homeostasis. Indeed, loss of steady state commensal detection by TLRs in MyD88-KO mice leads to a deficiency in epithelial homeostasis and increased mortality in the DSS model of colitis.37 Through interaction with TLRs and NLRs, commensals or their products may activate NF-κB signaling in IECs. For example, B. vulgatus activates the NF-κB pathway through IkB degradation and RelA phosphorylation in IECs.44 NF-κB activation is crucial for maintaining epithelial homeostasis. Indeed IEC-specific ablation of the pathway activator NEMO (NF-kB-essential modifier) or of the two upstream NF-kB-activating kinases, IKK1 and IKK2 (though not individually), leads to severe chronic intestinal inflammation, accompanied by increased IEC apoptosis, loss of barrier function and bacterial translocation.45 The colitis is ameliorated in the absence of MyD88, suggesting that it requires commensal-derived signals.45 Similarly, epithelial cell-specific deletion of TAK1, a TLR-signaling molecule upstream of the IKK complex, elicits increased number of apoptotic cells and severe intestinal inflammation, which supports a role for NF-κB signaling in IEC maintenance.46
At the same time, excessive NF-κB activation in IECs may predispose to colitis, and commensals can control this by inhibiting NF-κB signaling and exerting anti-inflammatory effects. For example, commensal signals inhibit the pathway activator IRAK1 in neonatal mice to prevent epithelial damage in early life47 or may inhibit the degradation of the pathway inhibitor IκBα after contact with IECs.48 B. thetaiotaomicron can negatively regulate NF-κB function and exert anti-inflammatory effects by inducing peroxisome proliferator activated receptor-γ (PPAR-γ), which binds and diverts activated NF-κB from the nucleus to the cytoplasm.49 Mice deficient in single immunoglobulin IL-1R-related receptor, a negative regulator of TLR/IL-1R signaling (SIGIRR), show loss of epithelial homeostasis, hyperactivation of NF-κB and increased susceptibility to DSS-colitis and colitis-associated cancer.50
Microbial products are also detected by NLR receptors that induce the formation of inflammasomes. Inflammasomes are a group of large protein complexes that include PRR microbial sensors, such as NLRs or AIM2; the adapter protein Apoptotic Speck protein containing a Caspase-recruitment domain (ASC); and inflammatory caspases, the most important of which is caspase-1. Inflammasome activation, through activated caspase-1 and other caspases, regulates the production of active forms of important pro-inflammatory cytokines, such as IL-1β and IL-18, as well as other inflammatory processes. Although details remain to be investigated, inflammasome function in the gut mucosa seems crucial for the maintenance of immune, epithelial and microbiota homeostasis.51 A recent study reported that an NLRP6 inflammasome in IECs regulates the composition of commensal microbiota and epithelial homeostasis. This occurred through the induction of basolateral secretion of IL-18 in IECs.52 As a result, NLRP6-deficient mice acquire colitogenic microflora and have increased susceptibility to colonic inflammation.52 The NLRP3 inflammasome has also been implicated in commensal regulation and promotion of epithelial regeneration.51 Revealing the details on the expression and function of different inflammasomes in IECs, and hematopoietic immune cells and their role in immune homeostasis and the microbiota–IEC–immune cells crosstalk represent exciting future directions.
Combined, the above studies provide examples of how epithelial PRRs, through NF-κB signaling or inflammasome activation, maintain epithelial homeostasis under the control of commensal bacteria.
Commensal-derived metabolites regulate epithelial function
Commensals are permanent inhabitants of the intestinal lumen. Therefore, factors that are produced during the life cycle of these organisms may act directly on the neighboring epithelium or modify the luminal environment in order to generate metabolites that affect epithelial functions. One of the main functions of commensal bacteria is to process dietary or environmental substances. This generates vitamins or metabolites that, although not produced by bacteria per se, are nevertheless dependent on commensal activity. For example, commensal bacteria are required to break down complex dietary polysaccharides into short-chain fatty acids (SCFAs).53 SCFAs, mainly acetate, butyrate and propionate, are energy sources, but they also affect epithelial and immune host cell functions. There are two known mechanisms of SCFA action—direct inhibition of histone deacetylases and ligation of the G-protein-coupled receptors, GPR43 and GPR41.53 SCFAs are important for intestinal and epithelial homeostasis. Indeed, decreased SCFA levels are observed in IBD patients,54 and butyrate deficiency due to downregulation of expression of the transporter MCT-1 is associated with IBD pathogenesis.55 Butyrate reduces the expression of inflammatory cytokines such as tumor necrosis factor (TNF), IL-6 and IL-1β by LP cells in Crohn’s disease patients and ameliorates trinitrobenzene sulfonic acid-induced colitis through inhibition of NF-κB activation in IECs.56 In general, generation of SCFAs in the colon by microbiota has been associated with decreased incidence of colorectal cancer and IBD.54 Mice lacking GPR43 show increased susceptibility in several models of autoimmune inflammation, including DSS-colitis.57 SCFAs have been shown to affect epithelial barrier function. Butyrate and propionate generated by commensal microbiota upregulate cytoprotective heat-shock proteins in IECs.58 Acetate and butyrate stimulate the secretion of mucins both in vitro and in vivo.59 GPR109A, a low-affinity receptor for butyrate expressed on IECs and LP cells, is downregulated in colon cancer, and has been associated with tumor suppressive effects of butyrate.60
Commensals differ in their ability to process complex polysaccharides and generate SCFAs. Commensals from the Bacteroidetes group produce acetate and propionate, whereas butyrate, is mainly provided by Firmicutes.61 SCFA production could also be a major mechanism of protection by probiotic bacteria. Indeed, acetate produced by protective Bifidobacteria strains prevented IEC apoptosis induced by enteropathogenic Escherichia coli.62 Only protective Bifidobacteria contained ATP-binding cassette transporters that enabled production of acetate, which acted on IECs to increase barrier function and prevent lethal translocation of Shiga toxin from the gut lumen to the blood62 (Figure 1).
Combined, these data show mostly beneficial effects of commensal-derived SCFAs on epithelial integrity and modulation of epithelial cell function. However, in most cases the molecular mechanisms of IEC activation and the relative participation of individual commensals have not been studied in detail.
Other commensal effects on IECs
Commensal bacteria elicit expression of various genes from IECs that may potentially affect immune responses. For example, commensal bacteria upregulate MHCII expression by IECs.63 That IECs express MHCII, has been appreciated for some time, but the function of this expression is not known.64 In contrast to the conventional antigen presenting cells, IECs hardly express costimulatory molecules, which has led to a speculation that MHCII on IECs may elicit T-cell tolerance. Interestingly, MHCII expression is induced on IECs only by certain bacteria, most notably by immunomodulatory SFB, which raises interesting questions of whether it may somehow be involved in commensal effects on T-cell homeostasis.
Another important effect of commensals is the modification of IEC glycosylation patterns. The enzyme fucosyltransferase 2 (Fut2) and fucosyl asialo GM1 glycolipids on IECs are induced by commensal bacteria.63 Fut2 catalyzes the addition of α(1,2)-fucose to terminal galactose residues of secreted and membrane-bound IEC proteins (Figure 1). It is noteworthy that fucose moieties expressed on IECs and fucosylated carbohydrate chains secreted by goblet cells could be utilized as nutrients for Bacteroides spp.65,66 Therefore, epithelial fucose may help maintain homeostasis of the gut microbiota. In humans, non-functional Fut2 leads to alterations of the microbiota and specific loss of the diversity of Bifidobacteria.67 In genome-wide association studies, Fut2 mutations show strong association with several host disorders that have a known microbial component. Fut2 loss-of-function mutation W143X (G428A) is associated with susceptibility to type 1 diabetes and Crohn’s disease.68,69
IECs REGULATE IMMUNE FUNCTIONS OF THE HOST
In addition to receiving signals from commensal bacteria, and secreting mucus and antimicrobial substances in the lumen, IECs also modulate the function of host immune cells. These functions are mediated by the expression of immune receptors, and the secretion of cytokines and chemokines from the IEC basolateral side. In such way, IECs recruit and activate immune cells in the mucosa.
IEC effects on GALT development and function
Organized GALT structures, such as PPs, ILFs and cryptopatches are intimately associated with the epithelial layer. Indeed, PPs and ILFs are covered by a modified epithelium called follicle-associated epithelium (FAE). FAE has an important role in the structural organization and function of PPs and ILFs as sites for generation of intestinal antigen-specific immune responses, and in particular T-cell-dependent IgA production. PPs and ILFs are the sites of sampling of luminal content. Therefore, FAE is designed to both facilitate and control this process. IECs in the FAE show modified expression of TLRs, compared to the rest of the epithelium, which is thought to mediate these gatekeeping functions.70 One of the main features of the FAE is the presence of microfold or M cells. M cells are specialized antigen-sampling IECs, which have increased levels of endocytosis and can, in such way, acquire particulate antigens from the lumen. In contrast to phagocytic cells, M cells are devoid of lysosomes, and instead unidirectionally transport almost intact luminal antigens to antigen presenting cells situated underneath the FAE, in the so-called subepithelial dome area. These antigen presenting cells are mostly DCs and they take up the antigens and prime T-and B-cells in the context of a germinal center reaction to initiate Ag-specific immune responses. This requires structural organization that is controlled by chemokines expressed by the FAE, which include CCL20 and CCL9 in mice, and CCL20 and CCL23 in humans.71,72 Although the exact details are not completely worked out, certain combinations of FAE chemokines recruit DCs into the subepithelial dome area.72,73 CCL20–CCR6 interactions are also important for the recruitment of B-cells into ILF and PP follicles.74 At the same time, recognition of commensal signals through NOD1 on IECs has been shown to induce maturation of cryptopatches into ILFs.4 Therefore, IEC-derived signals are necessary for organized GALT development.
IEC effects on IEL function
IELs are spatially distributed within the epithelial layer and, therefore, closely interact with IECs. Indeed, signals from IECs control the recruitment, maturation and function of IELs. The chemokine CCL25 produced constitutively by IECs recruits CCR9+ IELs.75 The main adhesion molecule that localizes IELs to the epithelium is the heterodimeric integrin αEβ7 (the αE chain is also known as CD103). αEβ7 on IELs interacts with E-cadherin expressed on the basolateral side of IECs.76 The maintenance of the IEL compartment also depends on IEC-derived signals. IL-15 has an important role in IEL maintenance, and IL-15-deficient mice have severely reduced numbers of both TCRαβ and TCRγδ IELs.77 IL-15 and IL-15Rα are expressed by IECs, and IECs participate in IEL maintenance through trans-presentation of IL-15 bound to IL-15Rα.78 At the same time, epithelial expression of IL-7 restores TCRγδ+ IEL numbers in IL-7- deficient mice.79 Both IL-7 and IL-15 are induced following bacterial exposure. In addition, secretion of IL-15 by IECs is MyD88-dependent80 and MyD88-KO mice have reduced numbers of IELs, which can be restored upon transgenic expression of IL-15. This suggests that commensal signals regulate IEL numbers partially through the induction of IL-15 and/or IL-7 production by IECs. Finally, as discussed earlier, IEL function requires activation by non-classical MHCI-like ligands, such as the thymus leukemia antigen, which are expressed by IECs14,64 (Figure 2a).
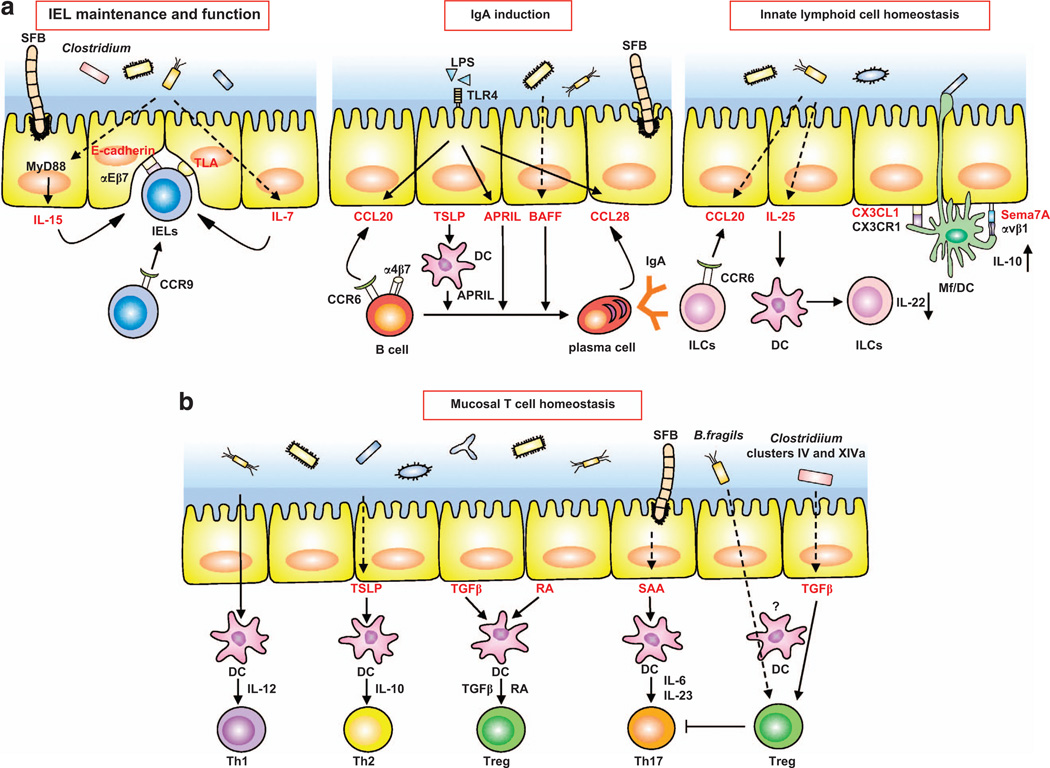
Figure 2.
IECs integrate signals from the commensal microbiota to regulate homeostasis of mucosal immune cells in the LP. (a) Commensal bacteria regulate the recruitment, maintenance and function of intraepithelial lymphocytes (IELs) (left panel), T-cell-independent IgA induction (middle panel), and innate lymphoid cell homeostasis (right panel). (Left panel) Recruitment of IELs is mediated by epithelial E-cadherin and αEβ7 on IELs. IL-7 and IL-15 secretion by IECs induced by commensal bacteria leads to expansion of IELs. IEL function is regulated by ligation of IEL TCRs by thymus leukemia antigen on IECs. (Middle panel) Commensal-derived lipopolysaccharide (LPS) recruits B-cells and plasma cells by inducing CCL20 and CCL28, respectively, from IECs. Commensal bacteria and commensal-derived LPS also induce BAFF and APRIL production from IECs. TSLP produced by IECs acts on LP DCs and induces APRIL secretion. Combined, these cytokines elicit IgA class switching in a T-cell-independent manner. (Right panel) Commensal bacteria induce CCL20 and IL-25 from IECs, which respectively regulate recruitment and IL-22 production of ILCs. Extension of dendrites between IECs by CX3CR1+ Mfs/DC are regulated by commensal bacteria and epithelial CX3CL1. Semaphorin 7A (Sema7A) on IECs induces IL-10 from CX3CR1+ Mf. Dashed arrows indicate IEC molecules induced by commensal bacteria. (b) Commensal bacteria induce production of various cytokines from IECs, which may help modulate mucosal T-cell differentiation. IEC-derived RA and TGF-β instruct DCs to induce Treg. DCs directly stimulated by commensal bacteria produce IL-12 and induce Th1 cells. IECs stimulated by commensal bacteria produce TSLP to upregulate IL-10 production by DCs, leading to Th2 cell induction. SFB are able to attach to IECs and induce serum amyloid A (SAA). DCs stimulated by SAA produce IL-6 and IL-23, and may drive Th17 cell differentiation. Clostridia clusters IV and XIVa induce TGF-β from IECs, which may promote differentiation of Treg.
IEC effects on LP immunity
Most currently known effects of IECs on processes in the LP probably occur through secretion of cytokines or cytokine-like molecules. Multiple studies have shown that IECs can secrete a number of cytokines both in vitro and in vivo. These include molecules that are known to be crucial for effector B- and T-cell differentiation or DC function, such as TGF-β, TGF-α, IL-6, IL-7, TNFα, IL-1 and so on. However, these cytokines can be produced by many other cell types as well, and in most cases the contribution of IEC-derived cytokine production to individual immune homeostatic mechanisms or responses is not completely clear. Nevertheless, IECs have the potential to modulate immune responses through cytokine production, and examples of this have accumulated.
As described above, IgA class switching and generation of IgA-producing B-cells occurs in the context of germinal centers in PPs in a T-cell dependent manner. However, T-cell-deficient mice have SIgA,81 and a T-cell-independent pathway of IgA production has been described to occur in ILFs and LP.5 IgA production in the LP seems to be directed by cytokine production from IECs. Class switching to IgA2, the main mucosal IgA class in humans, is induced by the production of the TNF-superfamily members, a proliferation-inducing ligand (APRIL) and B-cell-activating factor of the tumor necrosis factor family (BAFF) from IECs in a T-cell-independent matter.82 IECs secrete APRIL, as well as thymic stromal lymphopoietin (TSLP) that stimulates APRIL production by DCs, in response to TLR-mediated signals from commensal bacteria82 (Figure 2a). In mice, targeted overexpression of TLR4 in IECs leads to an increased IEC expression of CCL20, CCL28 and APRIL, which results in an increase in LP B-cell recruitment and IgA class switching.83 These data support a model in which commensal-derived signals induce cytokine expression from IECs to control steady-state T-cell-independent IgA production.
LP DCs and Mfs present antigens for initiation of Ag-specific T-cell responses, but also provide cytokines for T-cell differentiation or for activation of other immune cells. The intestinal LP contains several subsets of MHCII+CD11c+ cells that can be divided into CD103+ DC lineage cells and CX3CR1+ Mf lineage cells (for details see review by Farache et al.84 in this issue). LP DCs and Mfs can extend dendrites in-between IECs to sample luminal contents,11,85 and this requires TLR-dependent commensal signals.85 In the case of CX3CR1+ cells, the process is controlled by the expression of its ligand, CX3CL1, on IECs.86 Expression of Semaphorin 7A on the basolateral surface of IECs has been shown to engage CX3CR1+ Mfs through αvβ1 integrins, stimulate Mf IL-10 production and suppress inflammatory responses. Semaphorin 7A-deficient mice are susceptible to DSS-induced colitis accompanied by reduction of IL-10 expression from Mfs87 (Figure 2a).
The function and ability of LP DCs to prime and direct T-cell responses is also modulated by IEC-derived factors. IEC supernatants can condition DCs in vitro to produce IL-10 and direct differentiation of T-cells into more anti-inflammatory fates, such as Th2 or Treg.88 In this study, the anti-inflammatory DC modulation was a preferential property of supernatants from IECs treated with non-invasive commensal bacteria. Even more interestingly, if the bacteria were allowed to interact directly with DCs (bypassing the IEC), they induced pro-inflammatory IL-12 production and Th1 differentiation.88 In vitro, the IEC effects on DCs were shown to depend on the epithelial production of TSLP.88 TSLP derived from IECs prevents secretion of IL-12 from mucosal DCs stimulated by bacterial and TLR ligands. TSLP-conditioned DCs instead induce IL-10, leading to the induction of Th2 cells.88,89 Although the mechanisms of induction of TSLP at steady state are not clear, intrinsic IKKβ-mediated NF-κB signaling in IECs is important for the production of TSLP in an infection model with the helminth Trichuris muris.89 Successful clearance of Trichuris infection requires protective Th2 type immune response. IEC-specific deletion of IKKβ leads to decrease in TSLP production and consequent increase in proinflammatory cytokine production from LP DCs, increase in Th1/Th17 differentiation and severe intestinal inflammation following Trichuris infection.89 In agreement with TSLP effects on DCs, LP DCs from TSLPR-deficient mice have a propensity to produce IL-12, have defective Th2 responses and are susceptible to Trichuris infection.89
TGF-β is another immunoregulatory cytokine in the intestine. TGF-β generally has anti-inflammatory effects. It inhibits pro-inflammatory cytokine production by intestinal DCs and Mfs and controls T-cell homeostasis by participating in both Th17 and Treg differentiation, depending on the partnering cytokines.90 TGF-β is produced by IECs and IEC-derived TGF-β together with retinoic acid (RA) can convert intestinal DCs into a Treg-promoting tolerogenic phenotype.91 RA, a vitamin A metabolite, has pleiotropic effects in the mucosal immune system. RA is crucial for imprinting gut-homing capabilities on B- and T-cells and production of RA by CD103+ DCs is important for LP Treg induction (see review by Stock et al.92 in this issue). In addition to CD103+ DCs, IECs can also express RA and may, in such way, modulate mucosal T-cell homeostasis.93 Supporting this idea, human IECs produce RA that conditions DCs to induce Treg cells in in vitro co-culture system.94 Therefore, IEC cytokines can modulate the function of LP DCs, which in turn control T-cell homeostasis (Figure 2b).
IL-25 (IL-17E) is an IL-17 cytokine family member originally described as a Th2 cytokine. However, IL-25 expression is observed in IECs.95 IEC-derived IL-25 expression is defective in GF mice, suggesting that commensal bacteria induce IL-25 from IECs.13 Microbiota-induced IL-25 can repress the number and IL-22 expression of RORγt+ ILCs,13 however, the IL-25 effects seem indirect because they required the presence of DCs in vitro.13 IL-22 is a major effector ILC cytokine, which is required for their immunoprotective functions. Combined, the data suggest that IEC-derived IL-25, induced by commensal microbiota, controls ILC function, although whether IECs are the only IL-25-producing cell requires further study (Figure 2a).
CONNECTING THE DOTS
IECs are uniquely located at the border of two different and mutually exclusive environments. They form a physical separation, which is de facto necessary for the co-existence of these two environments. It has now been well established that signals from luminal commensal microbiota are transmitted and modulate immune responses in the LP of the host. Therefore, the epithelium has an important role in mediating these interactions. It may be that the barrier mechanisms ensure that only the right type of signals will be transmitted to the host, which is illustrated by the fact that a lot of these barrier mechanisms are induced by the commensals themselves.
It is clear that intact IEC function is required for healthy intestinal homeostasis. Perturbations of epithelial function are major contributors to disease pathogenesis in many intestinal immune diseases, including IBD and cancer, however relatively little is known about the exact mechanisms by which IECs control immune homeostasis and the initiating signals. In particular, whether and how IECs participate in immunomodulatory effects of commensal bacteria is not known.
Commensals in the lumen affect the homeostasis of effector immune cells in the LP. These effects are not simply a result of the presence of innocuous bacteria. They depend on the presence of microbiota that has been evolutionarily adapted to the host species.96 Moreover, they depend on the composition of the microbiota, and in the last few years several commensal species have been shown to induce specific changes in steady-state immune homeostasis.
As discussed above, there are two indirect lines of evidence that IECs can be crucial for the immunomodulatory effects of commensals. Firstly, commensal bacteria or their products are in constant interaction with IECs and modulate IEC function. Secondly, IECs produce cytokines and chemokines that are known to regulate immune cell accumulation, differentiation or function, and in many cases the production of IEC cytokines is induced by bacteria-derived signals. However, connecting these data into a definitive demonstration of the role of IECs has been difficult. One reason for this is that most IEC-derived cytokines are also produced by other cell types, and therefore the IEC involvement in vivo is inferred, but has not been proven. Even TSLP, which in the intestine is considered an epithelial or stromal cell-derived cytokine, is also produced by intestinal DCs in a MyD88-dependent fashion.97 Conditional ablation of effector molecules specifically in intestinal epithelium will help define the role of IECs in their immune effects. A lot of questions remain also about the role of commensals in induction of effectors from IECs. In most cases this has been inferred from studies in PRR-deficient mice, mostly MyD88- or TLR-deficient. However, effects on IECs in MyD88-deficient mice do not necessarily mean that the IEC is the microbe-recognizing cell. Indeed, elegant studies from the groups of Lora Hooper and Eric Pamer have shown that even for IEC-specific effectors, such as RegIIIγ, the mechanisms are complex. Steady-state RegIIIγ production is induced by direct engagement of TLRs on IECs.41 In contrast, in the context of opportunistic infections, increased RegIIIγ expression also depends on TLR-signaling, but the microbial product-recognizing cells are TLR5+ DCs, which produce IL-23 upon bacterial flagellin detection. IL-23 in turn induces IL-22 production, most likely from ILCs, which induces RegIIIγ production from IECs.98 Conditional ablation of TLR-signaling in IECs will help better define the roles of IECs as actual sensors of commensal bacterial signals.
Another complicating issue is the heterogeneity and redundancy in commensal effects. Different commensals may affect IEC function through different pathways or induce different responses from IECs. Therefore, investigation of the function of IECs in the context of the effects of individual commensals, rather than the vastly diverse complete microbiota, is necessary to define the contribution of individual mechanisms. Examples of immunomodulatory commensals, commensals that regulate immune cell homeostasis, have now been described. Investigation of the role of IECs in these interactions is particularly interesting. For example, as described above, two types of commensals have now been shown to affect Th17–Treg homeostasis in the LP. SFB induce preferentially Th17 cells and Clostridia from clusters IV and XIVa induce Tregs.22,23 Almost nothing is currently known about the underlying mechanisms. However, IECs are likely to be involved. Colonization with Treg-inducing Clostridia was shown to induce TGF-β production from IECs, and TGF-β induces Tregs.23 SFB can affect multiple aspects of IEC function. These effects include production of AMPs (for example, RegIIIγ and resistin-like molecule β) and enzymes involved in AMP activation (for example, MMP7), modification of IEC glycosylation patterns through induction of cellular glycosyl-transferases (for example Fut2) and induction of basolaterally secreted cytokines, such as an isoform of serum amyloid A.22,63 Serum amyloid A isoforms have been shown to stimulate IL-23 production from DCs, and IL-23 is necessary for Th17 cell maintenance.99 Therefore, IECs may mediate SFB Th17 cell-inducing effects through the production of serum amyloid A or other cytokines (Figure 2b). However, all this remains to be tested. How individual commensals activate IECs is also unclear. In the case of both SFB and Clostridia, the effects on T-cell homeostasis are specific for the particular commensal, and seem independent of TLR or NLR signaling.20,23,100 Therefore, bacteria-specific mechanisms must be invoked. Clostridia reside in the intestinal mucus, and therefore secreted bacterial products are likely to be involved. In contrast, SFB attachment to IECs results in a well-defined synapse with apparent actin cytoskeletal re-organization in the IEC. Therefore, recognition of surface receptors by unique bacterial ligands and activation of specific intracellular signaling pathways in the IEC may be required.
As discussed above, IECs respond to commensal signals, and modulate the tropism and function of various mucosal immune cells through the activation of antigen receptors and production of effector cytokines. IECs, therefore, function in a lot of ways as an innate immune cell subset, despite their non-hematopoietic origin. In particular, these properties make IECs ideal candidates for transmitting immunomodulatory signals from various commensal bacteria. Future studies will be required to define the specific role of IECs and the bacterial products and bacterial recognition systems involved in this fascinating crosstalk.
ACKNOWLEDGEMENTS
This work was supported by a grant to YG from the Japan Society for the Promotion of Science (JSPS) and by grants to III from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the Crohn’s and Colitis Foundation of America (CCFA).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun. 1999;67:3504–3511. doi: 10.1128/iai.67.7.3504-3511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanagibashi T, Hosono A, Oyama A, Tsuda M, Suzuki A, Hachimura S, et al. IgA production in the large intestine is modulated by a different mechanism than in the small intestine: Bacteroides acidifaciens promotes IgA production in the large intestine by inducing germinal center formation and increasing the number of IgA(+) B cells. Immunobiology. 2012 doi: 10.1016/j.imbio.2012.07.033. (in press). [DOI] [PubMed] [Google Scholar]
- 8.Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1 + dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184:2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- 9.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, et al. Lineage relationship analysis of RORgammat + innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 10.Philip NH, Artis D. New friendships and old feuds: relationships between innate lymphoid cells and microbial communities. Immunol Cell Biol. doi: 10.1038/icb.2013.2. (in press). [DOI] [PubMed] [Google Scholar]
- 11.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 12.Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37:171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, et al. RORgammat + innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 14.Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, et al. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 15.Das G, Augustine MM, Das J, Bottomly K, Ray P, Ray A. An important regulatory role for CD4 + CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Nat Acad Sci USA. 2003;100:5324–5329. doi: 10.1073/pnas.0831037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, et al. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Nat Acad Sci USA. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imaoka A, Matsumoto S, Setoyama H, Okada Y, Umesaki Y. Proliferative recruitment of intestinal intraepithelial lymphocytes after microbial colonization of germ-free mice. Eur J Immunol. 1996;26:945–948. doi: 10.1002/eji.1830260434. [DOI] [PubMed] [Google Scholar]
- 18.Bandeira A, Mota-Santos T, Itohara S, Degermann S, Heusser C, Tonegawa S, et al. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. J Exp Med. 1990;172:239–244. doi: 10.1084/jem.172.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawaguchi-Miyashita M, Shimizu K, Nanno M, Shimada S, Watanabe T, Koga Y, et al. Development and cytolytic function of intestinal intraepithelial T lymphocytes in antigen-minimized mice. Immunology. 1996;89:268–273. doi: 10.1046/j.1365-2567.1996.d01-740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Nat Acad Sci USA. 2011;108:4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garland CD, Lee A, Dickson MR. Segmented filamentous bacteria in the rodent small intestine: their colonization of growing animals and possible role in host resistance to Salmonella. Microb Ecol. 1982:181–190. doi: 10.1007/BF02010451. [DOI] [PubMed] [Google Scholar]
- 27.Heczko U, Abe A, Finlay BB. Segmented filamentous bacteria prevent colonization of enteropathogenic Escherichia coli O103 in rabbits. J Infect Dis. 2000;181:1027–1033. doi: 10.1086/315348. [DOI] [PubMed] [Google Scholar]
- 28.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei B, Wingender G, Fujiwara D, Chen DY, McPherson M, Brewer S, et al. Commensal microbiota and CD8 + T cells shape the formation of invariant NKT cells. J Immunol. 2010;184:1218–1226. doi: 10.4049/jimmunol.0902620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 34.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–964. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 35.Lavelle EC, Murphy C, O’Neill LA, Creagh EM. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. 2010;3:17–28. doi: 10.1038/mi.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 37.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 39.Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, Lundberg JO, et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G327–G333. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 43.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haller D, Russo MP, Sartor RB, Jobin C. IKK beta and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-kappa B activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277:38168–38178. doi: 10.1074/jbc.M205737200. [DOI] [PubMed] [Google Scholar]
- 45.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 46.Kajino-Sakamoto R, Inagaki M, Lippert E, Akira S, Robine S, Matsumoto K, et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol. 2008;181:1143–1152. doi: 10.4049/jimmunol.181.2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chassin C, Kocur M, Pott J, Duerr CU, Gutle D, Lotz M, et al. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe. 2010;8:358–368. doi: 10.1016/j.chom.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, et al. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007;26:4457–4466. doi: 10.1038/sj.emboj.7601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 50.Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 52.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KM, et al. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol Rev. 2012;245:164–176. doi: 10.1111/j.1600-065X.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 54.Galvez J, Rodriguez-Cabezas ME, Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Mol Nutr Food Res. 2005;49:601–608. doi: 10.1002/mnfr.200500013. [DOI] [PubMed] [Google Scholar]
- 55.Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C, et al. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology. 2007;133:1916–1927. doi: 10.1053/j.gastro.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 56.Segain JP, Raingeard de la Bletiere D, Bourreille A, Leray V, Gervois N, Rosales C, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren H, Musch MW, Kojima K, Boone D, Ma A, Chang EB. Short-chain fatty acids induce intestinal epithelial heat shock protein 25 expression in rats and IEC 18 cells. Gastroenterology. 2001;121:631–639. doi: 10.1053/gast.2001.27028. [DOI] [PubMed] [Google Scholar]
- 59.Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 62.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 63.Umesaki Y, Okada Y, Matsumoto S, Imaoka A, Setoyama H. Segmented filamentous bacteria are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol Immunol. 1995;39:555–562. doi: 10.1111/j.1348-0421.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- 64.Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells - polarity and complexity. Immunol Today. 2000;21:123–128. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 65.Hooper LV, Xu J, Falk PG, Midtvedt T, Gordon JI. A molecular sensor that allows a gut commensal to control its nutrient foundation in a competitive ecosystem. Proc Natl Acad Sci USA. 1999;96:9833–9838. doi: 10.1073/pnas.96.17.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coyne MJ, Reinap B, Lee MM, Comstock LE. Human symbionts use a host-like pathway for surface fucosylation. Science. 2005;307:1778–1781. doi: 10.1126/science.1106469. [DOI] [PubMed] [Google Scholar]
- 67.Wacklin P, Makivuokko H, Alakulppi N, Nikkila J, Tenkanen H, Rabina J, et al. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS One. 2011;6:e20113. doi: 10.1371/journal.pone.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smyth DJ, Cooper JD, Howson JM, Clarke P, Downes K, Mistry T, et al. FUT2 nonsecretor status links type 1 diabetes susceptibility and resistance to infection. Diabetes. 2011;60:3081–3084. doi: 10.2337/db11-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGovern DP, Jones MR, Taylor KD, Marciante K, Yan X, Dubinsky M, et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Hum Mol Genet. 2010;19:3468–3476. doi: 10.1093/hmg/ddq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chabot S, Wagner JS, Farrant S, Neutra MR. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol. 2006;176:4275–4283. doi: 10.4049/jimmunol.176.7.4275. [DOI] [PubMed] [Google Scholar]
- 71.Anderle P, Rumbo M, Sierro F, Mansourian R, Michetti P, Roberts MA, et al. Novel markers of the human follicle-associated epithelium identified by genomic profiling and microdissection. Gastroenterology. 2005;129:321–327. doi: 10.1053/j.gastro.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 72.Zhao X, Sato A, Dela Cruz CS, Linehan M, Luegering A, Kucharzik T, et al. CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer’s patch CD11b + dendritic cells. J Immunol. 2003;171:2797–2803. doi: 10.4049/jimmunol.171.6.2797. [DOI] [PubMed] [Google Scholar]
- 73.Iwasaki A, Kelsall BL. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–1394. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams IR. CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann N Y Acad Sci. 2006;1072:52–61. doi: 10.1196/annals.1326.036. [DOI] [PubMed] [Google Scholar]
- 75.Marsal J, Svensson M, Ericsson A, Iranpour AH, Carramolino L, Marquez G, et al. Involvement of CCL25 (TECK) in the generation of the murine small-intestinal CD8alpha alpha + CD3 + intraepithelial lymphocyte compartment. Eur J Immunol. 2002;32:3488–3497. doi: 10.1002/1521-4141(200212)32:12<3488::AID-IMMU3488>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 76.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 77.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma LJ, Acero LF, Zal T, Schluns KS. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs. J Immunol. 2009;183:1044–1054. doi: 10.4049/jimmunol.0900420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laky K, Lefrancois L, Lingenheld EG, Ishikawa H, Lewis JM, Olson S, et al. Enterocyte expression of interleukin 7 induces development of gammadelta T cells and Peyer’s patches. J Exp Med. 2000;191:1569–1580. doi: 10.1084/jem.191.9.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu Q, Tang C, Xun S, Yajima T, Takeda K, Yoshikai Y. MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8 alpha alpha TCR alpha beta and TCR gamma delta intestinal intraepithelial lymphocytes. J Immunol. 2006;176:6180–6185. doi: 10.4049/jimmunol.176.10.6180. [DOI] [PubMed] [Google Scholar]
- 81.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 82.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial- cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 83.Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, Maussang D, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farache J, Zigmond E, Shakhar G, Jung S. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunol Cell Biol. doi: 10.1038/icb.2012.79. (in press). [DOI] [PubMed] [Google Scholar]
- 85.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim KW, Vallon-Eberhard A, Zigmond E, Farache J, Shezen E, Shakhar G, et al. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood. 2011;118:e156–e167. doi: 10.1182/blood-2011-04-348946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang S, Okuno T, Takegahara N, Takamatsu H, Nojima S, Kimura T, et al. Intestinal epithelial cell-derived semaphorin 7A negatively regulates development of colitis via alphavbeta1 integrin. J Immunol. 2012;188:1108–1116. doi: 10.4049/jimmunol.1102084. [DOI] [PubMed] [Google Scholar]
- 88.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 89.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 90.Zhou L, Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol. 2009;21:146–152. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2:340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 92.Stock A, Napolitani G, Cerundolo V. Intestinal DC in migrational imprinting of immune cells. Immunol Cell Biol. doi: 10.1038/icb.2012.73. e-pub ahead of print 8 January 2013; [DOI] [PubMed] [Google Scholar]
- 93.Cassani B, Villablanca EJ, De Calisto J, Wang S, Mora JR. Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol Aspects Med. 2012;33:63–76. doi: 10.1016/j.mam.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 95.Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spadoni I, Iliev ID, Rossi G, Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 2012;5:184–193. doi: 10.1038/mi.2011.64. [DOI] [PubMed] [Google Scholar]
- 98.Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He R, Shepard LW, Chen J, Pan ZK, Ye RD. Serum amyloid A is an endogenous ligand that differentially induces IL-12 and IL-23. J Immunol. 2006;177:4072–4079. doi: 10.4049/jimmunol.177.6.4072. [DOI] [PubMed] [Google Scholar]
- 100.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]