Highlights
-
•
This is the first meta-analysis of sex differences in the typical human brain.
-
•
Regional sex differences overlap with areas implicated in psychiatric conditions.
-
•
The amygdala, hippocampus, planum temporale and insula display sex differences.
-
•
On average, males have larger brain volumes than females.
-
•
Most articles providing sex differences in volume are in the ‘mature’ category.
Keywords: Brain, Sex differences, Meta-analysis, Gaussian-process regression (GPR), Voxel-based morphometry, Volume
Abstract
The prevalence, age of onset, and symptomatology of many neuropsychiatric conditions differ between males and females. To understand the causes and consequences of sex differences it is important to establish where they occur in the human brain. We report the first meta-analysis of typical sex differences on global brain volume, a descriptive account of the breakdown of studies of each compartmental volume by six age categories, and whole-brain voxel-wise meta-analyses on brain volume and density. Gaussian-process regression coordinate-based meta-analysis was used to examine sex differences in voxel-based regional volume and density. On average, males have larger total brain volumes than females. Examination of the breakdown of studies providing total volumes by age categories indicated a bias towards the 18–59 year-old category. Regional sex differences in volume and tissue density include the amygdala, hippocampus and insula, areas known to be implicated in sex-biased neuropsychiatric conditions. Together, these results suggest candidate regions for investigating the asymmetric effect that sex has on the developing brain, and for understanding sex-biased neurological and psychiatric conditions.
1. Introduction
The prevalence, age of onset, and symptomatology of many neurological and psychiatric conditions differ substantially between males and females (Bao and Swaab, 2010; Baron-Cohen et al., 2011; Central Brain Tumour Registry of the United States, 2012; Paus et al., 2008; Rutter et al., 2003). Examples of male-biased conditions include autism, attention deficit/hyperactivity disorder, conduct disorder, specific language impairment, Tourette syndrome, and dyslexia, and examples of female-biased conditions include depression, anxiety disorder, and anorexia nervosa (Bao and Swaab, 2010; Baron-Cohen et al., 2011; Rutter et al., 2003). Factors influencing the asymmetric effect that sex has on brain development may help us understand how and why male and female brains differ in their predisposition for risk for or resilience to such conditions. Identifying where and in what way male and female brains differ will help illuminate these factors and associated mechanisms. Previous whole-brain and region-of-interest studies on sex differences in typically developing human brains show contradictory results, which may be due to small sample sizes and/or variability in age range of the sample in individual studies, leading to opposing or non-significant findings. To summarize the evidence, we report the first meta-analysis of overall and voxel-wise regional brain structure of sex differences in the typically developing human brain and provide a descriptive account of the breakdown of studies providing overall volumes by age category.
Understanding the influence of sex on the developing brain can provide insight into what is happening during the development of psychopathological conditions that are asymmetrically affected between sexes. Sex differences in brain structure are a product of the interaction of biological and environmental influences on brain development (McCarthy and Arnold, 2011). Animal studies have shown that (prenatal) hormones (Arnold and Breedlove, 1985; Phoenix et al., 1959), sex chromosomes (Arnold and Chen, 2009; De Vries et al., 2002), and the immune system (Lenz et al., 2013) all have early roles in the development of neural sexual differentiation. In addition, brain development is also influenced by factors such as sex-biased gene expression (Kang et al., 2011), steroid hormones (Giedd et al., 2012), early life programming such as prenatal nutrition/starvation (DeLong, 1993; Heijmans et al., 2008), stress and maternal infections (Bale et al., 2010), and postnatal factors such as early child care (Center on the Developing Child, 2012; Cicchetti, 2013; Rutter et al., 2003).
Meta-analysis is a statistical framework summarizing themes from the existent literature. Within this framework bias and variability is characterized and quantified leading to a reliable consensus. Recent extension of meta-analysis to brain imaging datasets has identified key regions of structure and function that are consistently detected in a wide range of psychiatric disorders (Etkin and Wager, 2007; Menzies et al., 2008; Valera et al., 2007). However, although a variety of phenomena differ in many psychiatric conditions as a function of sex (Bao and Swaab, 2010; Baron-Cohen et al., 2011; Paus et al., 2008; Rutter et al., 2003), and sex differences in brain function have been systematically reviewed in the typically developing population (Giedd et al., 2012; Sacher et al., 2013; Stevens and Hamann, 2012), to our knowledge no meta-analysis has been conducted on overall or regional voxel-based structural brain differences between human males and females.
In the current study, we carried out two types of meta-analysis. First, we examined sex differences in overall brain volumes. As development and ageing have a large influence on total brain volume, we also investigated if different age categories were well represented in the literature by providing a description of the number of articles, number of total participants and weighted mean volume of each compartmental volume for each of the six age categories. Next, we conducted foci-based meta-analyses on regional differences between males and females, one with voxel-based studies of volume and one with voxel-based studies of tissue density. Gaussian-process regression coordinate-based meta-analysis (GPR-CBMA) was used for the voxel-based meta-analyses, as this new technique allows for relatively more accurate results by incorporating effect-size estimates from source data (Salimi-Khorshidi et al., 2011). Furthermore, GPR-CBMA is also advantageous because its output includes meta-analytic effects in both positive and negative directions as well as an estimate of magnitude models censoring within the source data (i.e., reporting significant foci only), infers the smoothness of meta-analytic statistic images, and provides an effect-size map (i.e., T- and/or Z-stat) across the entire intra-cranial space.
2. Methods
2.1. Systematic literature search
The literature search was conducted according to PRISMA guidelines (Moher et al., 2009) for reporting meta-analyses and systematic reviews. The search, conducted in PubMed, Web of Knowledge and Scopus, included articles published between 1990 and January 2013. Search terms used were “brain” AND (sex OR gender OR sex difference OR gender difference) AND (voxel* OR morphometry OR diffusion tensor imaging OR magnetic resonance imaging OR DTI or MRI OR VBM). MeSH terms for “brain” and “sex differences” were also included in the PubMed search.
Authors were contacted if articles were not available online and/or if there was a question about the data presented in the article (e.g., when parameters needed for the meta-analysis, such as effect size information or standard deviations, were not reported in the article). Only articles written in English were included in this analysis. Unpublished materials were not explored and publications performing region-of-interest analysis were excluded. Publications were first selected based on title and then imported into EndNote X4 for abstract selection. After abstract selection, publications were checked for inclusion criteria and reference lists of included articles were crosschecked for potential articles.
2.2. Selection criteria
Articles were included in the overall volumes analyses if they explicitly provided (1) any of the following raw (not corrected for age, body size, etc.) mean brain volumes for typically developing males and females: intracranial volume (ICV), total brain volume (TBV), cerebrum (Cb), grey matter (GM), white matter (WM), cerebrospinal fluid (CSF), or cerebellum (Cbl) and (2) standard deviations for these volumes. Articles were included in the regional voxel-based meta-analyses if they provided (1) an explicit whole-brain voxel-based analysis of brain volume or tissue density between typically developing males and females, (2) spatial coordinates for key results, and (3) statistics or effect sizes of key results (p, r, F, T, or Z-statistics), either present in the publication itself or provided by authors. Cross-sex/gender comparisons in studies performing a patient vs. control analysis were only included if the results of the cross-sex/gender comparison did not spatially overlap with regions showing a sex/gender-by-disorder interaction. All studies included in the analyses were double checked for inclusion criteria by A.N.V.R. and either J.S. or M-C.L.
2.3. Data analysis
2.3.1. Overall volumes meta-analysis
In a meta-analysis differences between studies and the omission of studies can bias results. For example, overlooking studies with a negative or non-significant result, perhaps due to publication bias, will tend to overestimate effect sizes. Studies are also likely to have differences in sample populations and study design. This leads to heterogeneity between the studies in the meta-analysis and increases sampling error. Our meta-analyses were therefore tested for bias and heterogeneity of the sample, and based on those outcomes either a random effect model (RFX) or a fixed effect model (FFX) was performed (Higgins et al., 2009). In an FFX it is assumed that there is one true effect size and differences between studies are due to sampling error, whereas in an RFX it is assumed that the true effect may vary from study to study due to differences in their design.
Cochran's Q test is the standard test to measure the presence of heterogeneity between studies (Huedo-Medina et al., 2006; Tsoi, 2011). However, the Q-statistic does not provide information on the significance of the heterogeneity unlike the I2 statistic (Huedo-Medina et al., 2006; Tsoi, 2011), which explains how much of the variation between studies in the analysis is due to significant heterogeneity rather than random chance; a meta-analysis with an I2 of zero means all variability of study effect size estimates is explained by sampling error within studies (Tsoi, 2011). If a significant heterogeneity was found the RFX model was used, otherwise the FFX model was applied.
In order to provide as much detail as possible about the pool of source data that our meta-analysis is based on, forest plots and funnel plots were generated (Salimi-Khorshidi et al., 2009a; Wager et al., 2009). A forest plot reports a summary of the information of individual studies that went into the overall volumes meta-analysis. It is essentially a number of bars with a square in the middle representing the mean effect-size and the length of the bar representing the 95% confidence interval for the mean. They show the amount of variation between the studies and an estimate of the overall result. A funnel plot, on the other hand, is a useful visual aid designed to examine the existence of publication bias (as well as heterogeneity) in systematic reviews and meta-analyses. When plotting the effect-size against its standard error, a symmetric funnel plot implies a ‘well-behaving’ dataset, in which publication bias is unlikely. An asymmetric funnel plot indicates a relationship between effect-size and study size, which may be due to publication bias or small-study effects (i.e., a systematic difference between smaller and larger studies).
2.3.2. Regional coordinate-based meta-analysis
We used Gaussian-process regression coordinate-based meta-analysis (GPR-CBMA), a newly developed tool (Salimi-Khorshidi et al., 2011), to investigate regional sex differences in voxel-based studies of tissue density and volume. In neuroimaging meta-analysis, image-based meta-analysis (IBMA) refers to methods that use full statistic images and allows for the use of hierarchical mixed effects models (accounting for differing intra-study variance and modelling of random inter-study variation). Although IBMA has been shown to be more accurate (Salimi-Khorshidi et al., 2009b), in the absence of full study-level images, CBMA methods have become the standard approach (Eickhoff et al., 2009; Salimi-Khorshidi et al., 2009a). In CBMA, each study included in the meta-analysis is summarized using only the reported (x, y, z) coordinates of peak activations (either with or without activation magnitude). Suppose the full-image study-level data were available, then for study s at voxel k, the contrast estimates can be modelled as
| (1) |
where μk denotes the overall population mean (i.e., what a meta-analysis is expected to estimate), σs,k is within-study standard deviation, τk is inter-study standard deviation and is the observation/reporting error.
Typically CBMA does not have access to study-level y and σ at every voxel; instead it has access to sparsely sampled standardized effect sizes (i.e., z = y/σ). This changes the model to
| (2) |
where . If we assume that every study has the same σ image (i.e., studies are similarly reliable in their effect-size estimates), then the model can be rewritten as
| (3) |
where
Even though CBMA only has access to n sparsely-located samples of Z-stat image (z = (z1, z2, …, zn)) with their corresponding voxel coordinates V = {v1, v2, …, vn}, we can employ GPR to model those voxels’ (unobserved) standardized mean effect size m. Under GPR, m is assumed to be a sample from a Gaussian process, i.e., m ∼ GP(0, C) with C denoting the covariance matrix of the GP. We employ a squared exponential (SE) covariance function whose shape can be described with two hyperparameters σf (describing m's variance) and λ (describing m's smoothness). Assuming that z is sampled from m with an additive Gaussian noise of distribution, results in
| (4) |
in which σn estimates
In the first step of this solution (inference), the model's hyperparameters (σn,σf, and λ) are estimated using evidence optimization. These estimates are used in the second step (prediction) to predict the full m map. We incorporate our prior knowledge about the smoothness of statistic images by employing a Gamma prior on λ in order to minimize the likelihood of an extremely high or low smoothness. This Gamma prior has a shape parameter of 7.7 and a scale parameter of 0.3 (i.e., 90% chance of image's smoothness in FWHM being between 0 and 8 mm).
2.3.2.1. False discovery rate control
Finding the appropriate threshold for voxel-wise meta-analytic statistics can be a challenge. With one test performed for every voxel in the resulting image, some correction of the thresholds is needed to control the overall error rates. Standard procedures for multiple hypotheses testing (e.g., Bonferroni), however, tend to not be sensitive enough to be useful in this context, as they tend to control the chance of any false positives (Genovese et al., 2002).
False discovery rate (FDR) controlling procedures, on the other hand, operate simultaneously on all voxel-wise statistics to determine which tests should be considered statistically significant by controlling the expected proportion of the rejected hypotheses that are falsely rejected. FDR controlling procedures exert a less stringent control over false discovery compared to family-wise error rate (FWER) procedures, which increases power at the cost of increasing the rate of type I errors. Note that, as the FDR threshold is determined from the observed P-value distribution, it is adaptive to the amount of signal in the data (Nichols and Hayasaka, 2003). The q-value is defined to be the FDR analogue of the P-value. The q-value of an individual hypothesis test is the minimum FDR at which the test may be called significant. In this study, q-values are estimated for both activation and deactivation images and thresholded in order to control the FDR at voxel level, e.g., at 5%.
3. Results
3.1. Literature search
The initial search identified 5600 possible articles after duplicates were removed. 5095 articles were excluded after abstract selection because they did not report a sex comparison between typical individuals. An additional 25 articles were found after inspection of the reference lists of the included articles. In total, 167 articles were identified after full-text selection. A total of 126 studies provided total volumes and were included in the overall volumes analysis (see Table 1 for study information and Supplementary Table 1 for an overview of imaging parameters).
Table 1.
Studies included in the overall volumes meta-analyses.
| ID | Study | Year | N (F) | Age |
Volumes | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Category | |||||
| 1 | Abbs | 2011 | 48 (21) | 40.5a (10.8a) | – | 4 | Cb |
| 2 | Acer | 2008 | 53 (27) | – | 20–25 | 4 | Cbl |
| 3 | Allen | 1991 | 24 (12) | 9.1a (3.8a) | 2–15 | 6 | Cb |
| 122 (61) | 42a (13.8a) | 16–79 | 6 | ||||
| 4 | Allen | 2002 | 46 (23) | 32.4a (8.2a) | 22–49 | 4 | Cbl |
| 5 | Allen | 2003 | 46 (23) | 32.4a (8.2a) | 22–49 | 4 | Cb, GM, WM |
| 6 | Andreasen | 1994 | 90 (42) | 27.4 (10.3) | – | 4 | TBV, CSF, Cbl |
| 7 | Arango | 2008 | 66 (32) | 34.1a (7.3a) | 23–51 | 4 | ICV, TBV, CSF |
| 8 | Baibakov | 2010 | 60 (30) | 1a (0.08a) | 0–1 | 1 | TBV |
| 9 | Barnes | 2010 | 82 (34) | – | 33–35 | 4 | GM |
| 10 | Barrick | 2005 | 30 (15) | 28.8a | – | 4 | Cb, GM, WM |
| 11 | Barta | 2003 | 30 (15) | 39.1a (10.5a) | – | 4 | TBV |
| 12 | BDCG | 2012 | 325 (173) | 10.9a (3.8a) | 4–18 | 3 | TBV, GM, WM, Cbl |
| 13 | Beacher | 2012 | 30 (15) | 30a (8a) | – | 4 | WM |
| 14 | Blanton | 2004 | 46 (25) | 10.9a (2.9a) | 6–17 | 3 | ICV, GM, WM |
| 15 | Blatter | 1995 | 44 (20) | 22.3a (2.2a) | 16–25 | 4 | ICV, TBV, GM |
| 43 (24) | 30.8a (3.2a) | 26–35 | 4 | WM, CSF | |||
| 38 (22) | 40.9a (2.9a) | 36–45 | 4 | ||||
| 39 (24) | 50.6a (2.7a) | 46–55 | 4 | ||||
| 30 (15) | 60.2a (2.6a) | 56–65 | 4 | ||||
| 16 | Bloss | 2007 | 27 (14) | 3.7a (1.1a) | 1–5 | 2 | ICV, TBV, GM, WM, Cbl |
| 17 | Boes | 2008 | 117 (56) | 12.3a (2.8a) | 7–17 | 3 | GM |
| 18 | de Bruin | 2005 | 91 (44) | 49.7a (7.9a) | – | 4 | ICV, Cb, GM, WM, CSF, Cbl |
| 19 | Bryant | 1999 | 37 (18) | 33.8a (7.3a) | 21–45 | 4 | ICV |
| 20 | Buckner | 2004 | 168 (88) | 29.1a (11.7a) | – | 4 | ICV |
| 77 (57) | 76.8a (8.8a) | – | 5 | ||||
| 21 | Carne | 2006 | 97 (49) | 33.7 (13.6) | 15–69 | 6 | TBV, Cb, Cbl |
| 22 | Caviness | 1996 | 30 (15) | 9.2a | 7–11 | 3 | ICV, Cb, GM, WM, Cbl |
| 23 | Chen | 2007 | 411 (227) | 46.7a (1.4a) | 44–48 | 4 | ICV, GM, WM, CSF |
| 24 | Chen | 2012 | 27 (15) | 22.6a (1.8a) | – | 4 | TBV |
| 25 | Cheng | 2009 | 50 (25) | 27.1a (9.4a) | 19–50 | 4 | TBV, GM, WM |
| 26 | Choi | 2010 | 43 (21) | 24.5a (4a) | – | 4 | ICV |
| 27 | Chung | 2005 | 118 (60) | 23 (2.6) | – | 4 | Cbl |
| 100 (59) | 47.5 (3.7) | – | 4 | ||||
| 28 | Clayden | 2011 | 59 (34) | 11.5 (2.1) | 8–16 | 3 | ICV, WM |
| 29 | Coffey | 1998 | 330 (201) | 75 (5.1) | 66–96 | 5 | Cb |
| 30 | Collinson | 2003 | 30 (12) | 16.4 (1.7) | – | 3 | Cb |
| 31 | Courchesne | 2000 | 116 (37) | 21.4 (20) | 1–80 | 6 | ICV |
| 32 | Cowell | 2007 | S, 18 (8) | 35.3a (3.3a) | 20–49 | 4 | ICV |
| S, 16 (10) | 57.8a (2.5a) | 50–72 | 5 | ||||
| L, 19 (8) | 35.3a (3.1a) | 20–49 | 4 | ||||
| L, 15 (10) | 58.5a (2.5a) | 50–72 | 5 | ||||
| 33 | Crespo-Facorro | 2011 | 76 (31) | 28.1a (7.6a) | 15–51 | 4 | ICV, GM |
| 34 | De Bellis | 2001 | 118 (57) | 11.9a (2.4a) | 6–17 | 3 | ICV, Cb, GM, WM |
| 35 | DeCarli | 2005 | 1181 (233) | – | 34–54 | 4 | ICV |
| 517 (288) | – | 55–61 | 4 | ||||
| 546 (275) | – | 62–70 | 5 | ||||
| 487 (272) | – | 71–96 | 5 | ||||
| 36 | Duggal | 2005 | 30 (15) | 30a (9.5a) | – | 4 | ICV |
| 37 | Edland | 2002 | 184 (121) | 79a (6.5a) | – | 5 | ICV |
| 38 | Eliez | 2001 | 85 (64) | 10.6a (2.9a) | – | 3 | Cb, GM, WM, CSF |
| 40 | Fahim | 2012 | 38 (20) | 8.4a (0.1a) | – | 3 | GM, WM, CSF |
| 41 | Filipek | 1994 | 20 (10) | 27.2a (5.2a) | 17–37 | 4 | TBV, Cb, GM, WM, Cbl |
| 42 | Frederikse | 1999 | 30 (15) | 39.1a | 23–58 | 4 | TBV |
| 43 | Garcia-Falgueras | 2006 | 91 (51) | 20.1a (2.8a) | 18–33 | 4 | ICV, GM, WM, CSF |
| 44 | Ge | 2002 | 54 (22) | 46.8 (19.3) | 20–86 | 6 | ICV, GM, WM |
| 45 | Gilmore | 2007 | 74 (34) | 0.8a (0.03a) | 0.7–0.9 | 1 | ICV, Cbl |
| 46 | Goldstein | 2001 | 48 (21) | 39.8 (11.4a) | – | 4 | Cb, GM, WM |
| 47 | Good | 2001a | 465 (200) | 32a (12.2a) | 17–79 | 6 | GM, WM, CSF |
| 48 | Guo | 2008 | 158 (80) | 15 (4.7) | 7–22 | 6 | ICV, GM, WM |
| 49 | Gur | 1991 | 69 (35) | 42.9a (20.4a) | 18–80 | 6 | TBV, CSF |
| 50 | Gur | 1999 | 80 (40) | 26a (5.5a) | 18–45 | 4 | ICV, TBV, GM, WM, CSF |
| 51 | Gur | 2002 | 116 (59) | 26a (5.5a) | 18–49 | 4 | ICV, GM, WM, CSF |
| 52 | Hänggi | 2010 | 43 (25) | 22.3 (2.5) | 19–32 | 4 | ICV, GM, WM, CSF |
| 53 | Hogan | 2011 | 228 (107) | 68.7a (0.7a) | 68–69 | 5 | ICV |
| 54 | Hommer | 2001 | 39 (19) | 40.8a (7.7a) | – | 6 | ICV, GM, WM |
| 55 | Hoogendam | 2012 | 3962 (2156) | 60.1 (8.5) | >45 | 6 | ICV |
| 56 | Hutchinson | 2003 | 120 (60) | 25.1a (4.6a) | – | 4 | TBV, Cbl |
| 57 | Jenkins | 2000 | 52 (28) | 56.1 (12.1) | 36–85 | 6 | ICV |
| 58 | Knaus | 2004 | 24 (12) | 31.4 (8.9) | 21–53 | 4 | ICV |
| 59 | Koscik | 2009 | 76 (38) | 26.7a (7.2a) | 18–47 | 4 | Cb |
| 60 | Kruggel | 2006 | 290 (145) | 24.2a (2.8a) | 18–32 | 4 | ICV, TBV, GM, WM, CSF |
| 61 | Lai | – | 60 (30) | 27.9a (6.1a) | 18–49 | 4 | ICV, TBV, GM, WM, CSF |
| 62 | Lavretsky | 2004 | 41 (20) | 72.2 (7.3) | – | 5 | ICV |
| 63 | Lee | 2004 | 62 (28) | – | 18–77 | 6 | ICV, GM, WM |
| 64 | Lee | 2009a | 57 (29) | 24.4a (2.1a) | 20–28 | 4 | ICV, Cbl |
| 58 (26) | 67.8a (3.1a) | 62–74 | 5 | ||||
| 65 | Lee | 2009b | 68 (39) | 21.7 (2.3) | 20–29 | 4 | ICV |
| 66 | Lemaître | 2005 | 662 (331) | 69.5a (3a) | 63–75 | 5 | ICV, GM, WM, CSF |
| 67 | Lentini | 2012 | 86 (45) | 35.1a (7.1a) | 26–51 | 4 | ICV, TBV, GM, WM, CSF |
| 68 | Leonard | 2008 | 200 (100) | 21.6a | – | 4 | ICV, GM, WM, CSF, Cbl |
| 69 | Li | 2012 | 76 (38) | 43a (15.9a) | 19–70 | 6 | TBV, GM |
| 70 | Luders | 2002 | 100 (50) | 24.7a (4.4a) | – | 4 | ICV, GM, WM, CSF |
| 71 | Luders | 2003 | 59 (29) | 24.3a (5a) | – | 4 | ICV |
| 72 | Luders | 2005 | 60 (30) | 24.8a (4.5a) | – | 4 | ICV, GM, WM, CSF |
| 73 | Maller | 2006 | 150 (74) | 62.3a (1.4a) | 60–64 | 5 | TBV |
| 74 | Mann | 2011 | 70 (35) | 54.5a (20.3a) | 20–87 | 6 | Cb |
| 75 | Matsumae | 1996 | 12 (6) | – | 24–40 | 4 | ICV, TBV, |
| 15 (5) | – | 41–60 | 4 | CSF | |||
| 22 (12) | – | 61–80 | 5 | ||||
| 76 | Mitchell | 2003 | 100 (56) | 32.6 (12.3) | 14–68 | 6 | Cb |
| 77 | Mortamet | 2005 | 10 (5) | – | 20–29 | 4 | ICV, GM, |
| 10 (5) | – | 30–39 | 4 | WM, CSF | |||
| 10 (5) | – | 40–49 | 4 | ||||
| 10 (5) | – | 50–59 | 4 | ||||
| 10 (5) | – | 60–69 | 5 | ||||
| 78 | Narr | 2007 | 65 (35) | 28.2a (7.3a) | – | 4 | ICV, GM, WM, CSF |
| 79 | Neufang | 2009 | 46 (23) | 11.3a (2.2a) | 8–15 | 3 | TBV |
| 80 | Nopoulos | 1997 | 80 (40) | 28.1a (7.4a) | – | 4 | TBV, CSF |
| 81 | Nopoulos | 2000 | 84 (42) | 23.3a (3.3a) | 19–31 | 4 | ICV, Cb, CSF, Cbl |
| 82 | Nunnemann | 2009 | 133 (73) | – | 29–80 | 6 | ICV, GM |
| 83 | Passe | 1997 | 43 (30) | 53.7a (17.9a) | 24–82 | 6 | TBV, GM, WM |
| 84 | Paus | 2010 | 419 (215) | 15.3a (1.92a) | – | 3 | ICV, GM, WM |
| 85 | Peper | 2009 | 78 (41) | 11.9a (1.1a) | 10–14 | 3 | ICV, GM, WM, Cbl |
| 86 | Hulshoff Pol | 2006 | 15 (6) | 24.2a (7.3a) | 16–50 | 4 | ICV, TBV, GM, WM |
| 87 | Ponseti | 2007 | 49 (25) | 25.1a (3.3a) | – | 4 | GM, WM, CSF |
| 88 | Qiu | 2013 | 161 (74) | 0.03a (0.02a) | – | 1 | ICV |
| 89 | Rametti | 2011 | 38 (19) | 32.5a (7.2a) | – | 4 | ICV, GM, WM, CSF |
| 90 | Reig | 2011 | 94 (33) | 15.4 (1.4) | 9–19 | 3 | ICV, GM, WM, CSF |
| 91 | Reiss | 1996 | 85 (64) | 10.6a (2.9a) | 5–17 | 3 | ICV, GM, WM, CSF |
| 92 | Reiss | 2004 | 31 (17) | 8.5 (0.7) | 7–11 | 3 | GM, WM, CSF |
| 93 | Resnick | 2000 | 116 (48) | 70.4 (7.5) | 59–85 | 5 | TBV, GM, WM |
| 94 | Rhyu | 1999 | 20 (11) | – | 20–29 | 4 | Cbl |
| 19 (9) | – | 30–39 | 4 | ||||
| 23 (13) | – | 40–49 | 4 | ||||
| 20 (10) | – | 50–59 | 4 | ||||
| 23 (12) | – | 60–69 | 5 | ||||
| 19 (12) | – | 70–79 | 5 | ||||
| 95 | Riello | 2005 | 133 (95) | – | ≤60 | 4 | ICV, GM, |
| 96 (63) | – | >60 | 5 | WM | |||
| 96 | Rijpkema | 2012 | 1004 (591) | 22.8a (3.4a) | 18–36 | 4 | GM, WM |
| 97 | Sachdev | 2008 | 383 (172) | 62.7a (1.4a) | 60–64 | 5 | ICV |
| 98 | Salinas | 2012 | 108 (54) | 12.3a (2.9a) | 7–17 | 3 | Cb |
| 99 | Sandu | 2008 | 18 (10) | 13.8 (0.5) | – | 3 | ICV |
| 100 | Savic | 2011 | 48 (24) | 34a (6a) | 26–48 | 4 | ICV, TBV, GM, WM |
| 101 | Schlaepfer | 1995 | 60 (17) | 31.6a (7.9a) | – | 4 | ICV |
| 102 | Sgouros | 1999 | 33 (15) | 2.1a (1.6a) | 0–4 | 2 | ICV |
| 11 (3) | 8.1a (1.5a) | 5–9 | 3 | ICV | |||
| 24 (8) | 12.9a (1.5a) | 10–15 | 3 | ICV | |||
| 103 | Shan | 2005 | 13 (8) | 27.4a (4.9a) | – | 4 | TBV |
| 13 (8) | 69.8a (1.9a) | – | 5 | ||||
| 104 | Shin | 2005 | 30 (15) | 27.2a (6.3a) | – | 4 | ICV |
| 105 | Soloff | 2008 | 30 (19) | 25.6 (7.7) | – | 4 | ICV |
| 106 | Sowell | 2007 | 176 (86) | 32.4a (21.8a) | 7–87 | 6 | ICV, GM, WM, CSF |
| 107 | Sparks | 2002 | 26 (8) | 4 (0.5) | 3–4 | 2 | Cb, Cbl |
| 108 | Sullivan | 2001 | 92 (41) | 46.9a (15.1a) | 22–71 | 6 | ICV |
| 109 | Sullivan | 2004 | 143 (48) | 49.3a (16.9a) | 20–85 | 6 | ICV |
| 110 | Sullivan | 2005 | 128 (44) | – | 20–85 | 6 | ICV |
| 111 | Takahashi | 2004 | 61 (31) | 24.5 (5.5) | 18–38 | 4 | ICV |
| 112 | Takao | 2010 | 109 (58) | 26.3a (2.1a) | 21–29 | 4 | GM, WM |
| 113 | Tepest | 2010 | 29 (11) | 33 (9.1) | 20–55 | 4 | TBV |
| 114 | Uematsu | 2012 | 27 (9) | – | 0–2 | 1 | TBV |
| 34 (17) | – | 2–10 | 2 | ||||
| 48 (26) | – | 10–25 | 6 | ||||
| 115 | Wang | 2012 | 140 (70) | 20.9a (1.8a) | 18–26 | 4 | ICV, GM, WM, CSF |
| 116 | Whitwell | 2001 | 55 (31) | – | 23–83 | 6 | ICV |
| 117 | Wilke | 2007 | 67 (34) | 7.6a (1.2a) | – | 3 | GM, WM |
| 66 (34) | 11.1a (1a) | – | 3 | ||||
| 67 (34) | 15.6a (1.7a) | – | 3 | ||||
| 118 | Witte | 2010 | 34 (17) | 26.6 (5) | 21–47 | 4 | ICV, GM, WM, CSF |
| 119 | Wood | 2008a | 60 (30) | 28.9a (8.4a) | 18–50 | 4 | ICV, GM |
| 120 | Wood | 2008b | 74 (37) | 12.5a (2.9a) | 7–17 | 3 | ICV, Cb |
| 121 | Yamasue | 2008 | 155 (66) | 28.5a (4.2a) | 21–40 | 4 | ICV, GM, WM, CSF |
| 122 | Yang | 2008 | 57 (23) | 11.7 | 7–17 | 3 | ICV, GM, WM, CSF |
| 123 | Yoshii | 1988 | 58 (29) | 56.3 (17.5) | 21–81 | 6 | ICV |
| 124 | Ystad | 2009 | O, 84 (60) | 65.1 | 47–75 | 5 | ICV, Cb |
| B, 86 (60) | 59.3 | 46–77 | 5 | ||||
| 125 | Yurgelun-Todd | 2002 | 30 (20) | 14.7 (1.5) | 13–17 | 3 | ICV, GM, WM |
| 126 | Zhang | 2007 | 24 (12) | 27.7 (4.4) | 17–35 | 4 | WM |
| 12 (7) | 74.8 (2.6) | 72–80 | 5 | ||||
Abbreviations: B, Bergen; BDCG, brain development cooperative group; Cb, cerebrum (excluding CSF and Cbl); Cbl, cerebellum; CSF, cerebrospinal fluid; F, female; GM, grey matter; ICV, intracranial volume (including CSF); ID, identification number; L, Liverpool; N, number of participants; O, Oslo; S, Sheffield; SD, standard deviation; TBV, total brain volume (including Cbl but excluding CSF); WM, white matter.
Weighted mean and standard deviations.
Sample overlap in the final study sample was solved by including the studies according to the following weighted criteria: (1) the study with the largest sample (i.e. excluding studies with smaller samples sizes that were part of the same study); (2) the first study using that specific sample (unless a later study included that sample in a larger overall sample); (3) a study that reported a different compartmental volume than a study with the same sample. For example the Sachdev et al. (2008) sample includes the Maller et al. (2006) sample and both are included in the meta-analysis because they report different compartmental volumes. Sachdev et al. (2008) only reports ICV, whereas Maller et al. (2006) reports both ICV and TBV. However, because the Sachdev et al. (2008) sample is larger, the ICV measures of Sachdev et al. (2008) are used in the meta-analysis and the ICV measures of Maller et al. (2006) are excluded. Because Sachdev et al. (2008) does not report TBV, this measure is included from the Maller et al. (2006) paper.
Articles that performed voxel- or tensor-based morphometry were included in a brain tissue density (9 articles) or brain volume (15 articles) meta-analysis. Another article providing results to a brain volume voxel-based morphometry analysis by our group (Lai et al., 2013) was also included in the voxel-based volume meta-analysis, bringing the total included articles for the volume analysis to 16 (see Table 2 for study demographics and Supplementary Table 2 for an overview of the analysis and imaging parameters). For a complete overview of the data selection, see Fig. 1.
Table 2.
Studies included in the regional meta-analyses.
| ID | Study | Year | N (F) | Age |
Volume | No. peak voxels | ||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Category | ||||||
| Regional volume meta-analysis | ||||||||
| 127 | Almeida | 2009 | 55 (32) | ±31 | – | 4 | GM | 9 |
| 23 | Chen | 2007 | 411 (227) | 46.7a (1.4a) | 44–48 | 4 | GM | 11 |
| 25 | Cheng | 2009 | 50 (25) | 27.1a (9.4a) | 19–50 | 4 | GM | 14 |
| 128 | Fan | 2010 | 112 (46) | 24.7a (2.8a) | 18–33 | 4 | Cbl GM | 14 |
| 47 | Good | 2001a | 465 (200) | 32a (12.2a) | 17–79 | 6 | GM; WM | 18; 7 |
| 48 | Guo | 2008 | 158 (80) | 15.3 (4.7) | 7–22 | 6 | GM | 14 |
| 61 | Lai | – | 60 (30) | 27.9a (6.1a) | 18–49 | 4 | GM; WM | 42; 18 |
| 67 | Lentini | 2012 | 86 (45) | 35.1a (7.1a) | 26–51 | 4 | GM; WM | 8; 2 |
| 129 | Lombardo | 2012 | 217 (116) | 9.5a (1.1a) | 8–11 | 3 | GM | 31 |
| 82 | Nunnemann | 2009 | 133 (73) | – | 29–80 | 6 | GM | 32 |
| 130 | Pletzer | 2010 | 28 (14) | 25.6a (4.7a) | – | 4 | GM | 25 |
| 100 | Savic | 2011 | 48 (24) | 34a (6a) | 26–48 | 4 | GM; WM | 7; 3 |
| 131 | Welborn | 2009 | 117 (58) | 22.8 | 18–40 | 4 | GM & WM | 7 |
| 118 | Witte | 2010 | 34 (17) | 26.6 (5) | 21–47 | 4 | GM | 18 |
| 121 | Yamasue | 2008 | 155 (66) | 28.5a (4.2a) | 21–40 | 4 | GM | 12 |
| 122 | Yang | 2008 | 57 (23) | 11.7 | 7–17 | 3 | GM | 2 |
| Regional density meta-analysis | ||||||||
| 132 | Dong | 2010 | 40 (20) | – | 20–25 | 4 | GM; WM | 3; 3 |
| 43 | Garcia-Falgueras | 2006 | 91 (51) | 20.1a (2.8a) | 18–33 | 4 | GM | 14 |
| 47 | Good | 2001a | 465 (200) | 32a (12.2a) | 17–79 | 6 | WM | 9 |
| 40 | Fahim | 2012 | 38 (20) | 8.4a (0.1a) | – | 3 | GM; WM | 3; 1 |
| 133 | Lord | 2010 | 46 (31) | – | 50–74 | 5 | GM | 11 |
| 85 | Peper | 2009 | 78 (41) | 11.9a (1.1a) | 10–15 | 3 | GM | 17 |
| 134 | Takahashi | 2011 | 91 (35) | 31.4a (8a) | 20–79 | 6 | GM | 15 |
| 136 (81) | 65.7a (8.4a) | 5 | 4 | |||||
| 135 | Van Laere | 2001 | 81 (41) | 44.2 | 20–81 | 6 | GM | 3 |
| 115 | Wang | 2012 | 140 (70) | 20.9a (1.8a) | 18–26 | 4 | GM | 16 |
Notes: Study ID count corresponds to and continues on from Table 1.
Abbreviations: F, female; GM, Grey matter; ID, identification number; N, number of participants; No., number; SD, standard deviation; WM, white matter.
Weighted mean and standard deviations.
Fig. 1.
Flow diagram based on PRISMA statement (www.prisma-statement.org).
3.2. Sex differences in overall volumes
The compartmental brain volumes most often reported in articles include Cbl, CSF, GM, WM, Cb, TBV, and ICV. Separate meta-analyses were conducted for each measurement. Some studies provided total volumes of more than one age- or scanner-matched group, leading to a difference in the number of studies and the number of subject groups in the analyses (see Table 3).
Table 3.
Results of the overall volumes meta-analyses.
| Volume | aStudies | bGroups | N (Fc) | Analysis type | Q | I2 | Outcome | Cohen's d | Percentage difference | dMean difference | CI 95% | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICV | 77 | 100 | 14,957 (48%) | FFX | 62.3 | 0 | M > F | 3.03 | 12% | 135.3 | 117.8–152.8 | <10−6 |
| TBV | 31 | 40 | 2532 (50%) | RFX | 92.8 | 57.9 | M > F | 2.1 | 10.8% | 131 | 92.1–170.0 | <10−6 |
| Cb | 22 | 24 | 1851 (54%) | FFX | 12.5 | 0 | M > F | 3.35 | 9.8% | 51.06 | 38.7–63.5 | <10−6 |
| GM | 60 | 71 | 7934 (52%) | FFX | 26.5 | 0 | M > F | 2.13 | 9.4% | 56.51 | 44.2–68.9 | <10−6 |
| WM | 57 | 69 | 7515 (52%) | FFX | 39.9 | 0 | M > F | 2.06 | 12.9% | 44.4 | 34.2–54.6 | <10−6 |
| CSF | 35 | 45 | 4484 (50%) | FFX | 15.5 | 0 | M > F | 1.21 | 11.5% | 18.72 | 9.6–27.8 | 3.2 × 10−5* |
| Cbl | 19 | 26 | 1842 (51%) | FFX | 9.7 | 0 | M > F | 1.68 | 8.6% | 7.78 | 4.2–11.4 | 1.4 × 10−5* |
Abbreviations: Cb, cerebrum; Cbl, cerebellum; CSF, cerebrospinal fluid; F, females; FFX, fixed effects model; GM, grey matter; I2, I2 index; ICV, intracranial volume; N, number of subjects; Q, Cochran's Q test; RFX, random effects model; TBV, total brain volume; WM, white matter.
Number of studies reporting the respective volumes.
Number of age-matched gender groups in the analysis (some studies provide more than one sample).
Number of females in percentages.
Values reported in mL.
The FFX model was found to be appropriate and hence used for all overall volume meta-analyses except for the TBV analysis, where an RFX model was used. For forest plots of the GM volume meta-analysis, see Fig. 2 and for ICV, TBV, Cb, WM, CSF, and Cbl forest plots see Supplementary Figures 1–6 respectively; for funnel plots see Supplementary Figures 7–13 and Supplementary Methods in the Supplementary Information.
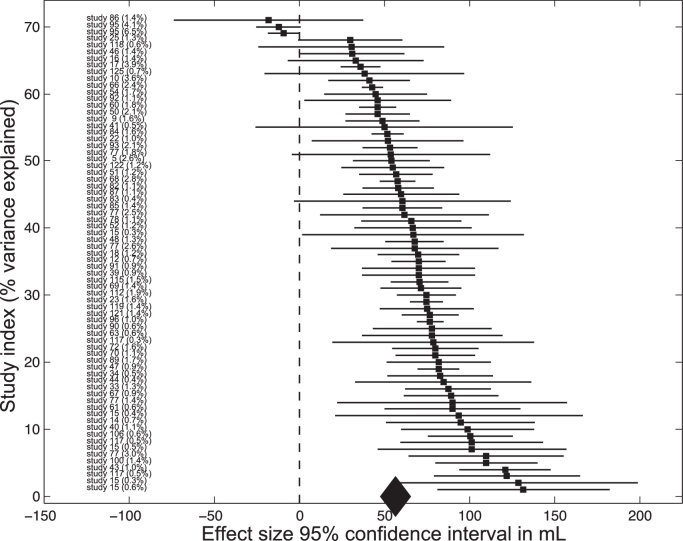
Fig. 2.
Forest plot for the grey matter volume meta-analysis. Overview of all studies included in the grey matter volume meta-analysis. The square indicates the effect size in mL of each study (i.e. the difference in mL volume between males and females) and the bars indicate the 95% confidence interval of each study. The studies corresponding to the effect size can be found on the left. Study IDs correspond to the study IDs in Table 1. The diamond at the bottom of the figure indicates the meta-analytic effect size and its variance.
Males have on average larger overall absolute volumes (i.e. not corrected for body size) in each volume category (see Table 3), ranging from 8% to 13% larger volume in males. Sex differences are on average most pronounced in the ICV and Cb volumes. Large effects are also found for TBV, GM, WM, CSF and Cbl volumes.
3.2.1. Breakdown of studies looking at overall volume
Sex differences in total brain volumes vary substantially by chronological age (Brain Development Cooperative Group, 2012; Koolschijn and Crone, 2013; Lenroot et al., 2007; Li et al., 2014; Pfefferbaum et al., 2013). Many study samples in the present meta-analysis cover a large age range: some span from birth to 18 years old or 18–60 years old, whilst others include ages from 1 to 80 years old. Only some report sex differences in compartmental volumes separately for different age groups. Unfortunately, not all studies included here reported information on sex-by-age interactions so this could not be meta-analytically investigated. As an alternative, we wanted to examine the average compartmental volumes change across age ranges as a descriptive report of any chronological age effect. However, when the studies were broken down into different age categories, some categories were more represented than others, depending on the compartmental volume. A statistical comparison between age categories was thus not possible. We therefore instead present a descriptive overview of the current state of the literature with regard to the representation of the examination of sex differences across different age categories.
Data were split into six categories. The first – infant – includes data from newborns to 1 year-olds, the second – early childhood – covers 2–6 year-olds, the third – late childhood – includes 7–17 year-olds, the fourth – mature – is made up of 18–59 year-olds, the fifth – senior – included individuals over 60 years old, and lastly a six category – lifespan – encompasses studies with wide age ranges (encompassing more than 2 of the above age categories), e.g. spanning from infancy, mid-teens or early twenties up to the seventh or eighth decade of life (e.g. Courchesne et al., 2000; Good et al., 2001a; Hoogendam et al., 2012).
Fig. 3 gives a descriptive overview of the articles providing ICV (Fig. 3a–c) and GM (Fig. 3d–f) and Supplementary Figures 14–18 give an overview of TBV, Cb, WM, CSF and Cbl respectively. As can be seen from Fig. 3a,d and Supplementary Figures 14a–18a, the ‘mature’ age category is best represented with by far the largest number of studies across all volumes. In addition, the ‘infant’ and ‘early childhood’ categories are sometimes empty, showing that these age groups and others are underrepresented in this meta-analysis.
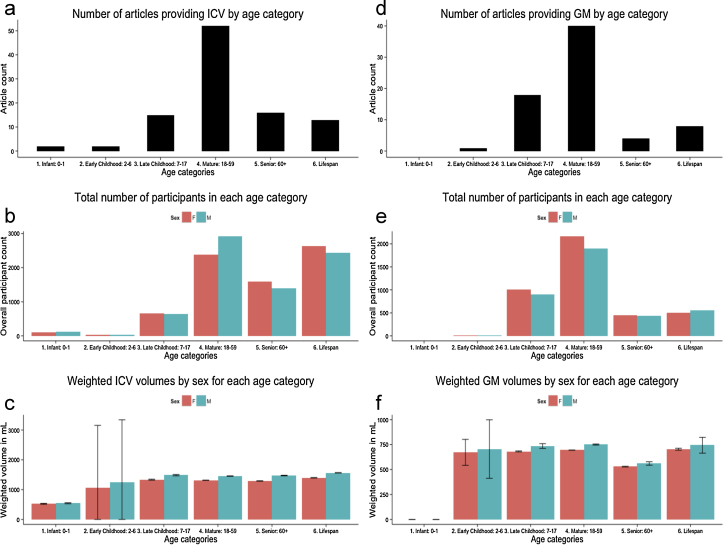
Fig. 3.
Breakdown by age categories for reports providing intracranial volume and grey matter volume. Three plots display the breakdown of studies examining intracranial volume (ICV) and grey matter volume (GM) in the current literature across six age categories: ‘infant’ (0–1 years), ‘early childhood’ (2–6 years), ‘late childhood’ (7–17 years), ‘mature’ (18–59 years), ‘senior’ (60+ years), and ‘lifespan’ (any study covering more than 2 age ranges): (a) the total number of articles providing ICV in each age category; (b) the sum of the total number of male and female participants included in those age categories; and (c) displays the weighted mean volumes of ICV and weighted error bars for males and females across all age categories. (d) The total number of articles providing GM in each age category; (e) the sum of the total number of male and female participants included in those age categories; and (f) displays the weighted mean volumes of GM and weighted error bars for males and females across all age categories.
Fig. 3b,e and Supplementary Figures 14b–18b display the sum of the total number of male and female participants across all the studies in each age category. From this it is again apparent that the ‘mature’ category is best represented, and depending on the volume, the next best representations are in the ‘late childhood’, ‘senior’ and ‘lifespan’ categories. However, since the number of studies in those categories are still much lower than in the ‘mature’ age category but the number of participants are still quite high, this may suggest larger sample sizes in studies examining sex differences in ‘late childhood’, ‘senior’ and ‘lifespan’ categories.
Lastly, Fig. 3c,e and Supplementary Figures 14c–18c show the weighted volume and weighted error bars for each compartmental volume per sex. From these graphs it is apparent that the size of the error bars significantly depends on the number of studies and subjects in each age category. When taking into account the widely various number of articles and subjects in each age bin it would not be statistically valid to compare volumes across the different age categories. In addition, these graphs indicate that the meta-analytic overall volume results may be skewed towards sex differences present in the 18–45 years old ‘mature’ age-range.
3.3. Regional sex differences in volume and tissue density
Table 2 and Supplementary Tables 2 and 3 show that studies included in the meta-analyses are substantially different with respect to sample size, age range, image acquisition parameters, statistical models and thresholds. The GPR-CMBA estimates the extent/variance of such an inconsistency/heterogeneity in our study pool, similar to RFX variance in hierarchical models.
Group difference information for location (x, y, z coordinates in either Montreal Neurological Institute (MNI) or Talairach anatomical spaces) and effect size information (P-values, Cohen's d, Pearson's r, f2-, T-, or Z-statistics) were gathered for all reported data points (or foci) of source studies. Reported statistics were converted into Z-statistics and coordinates were transformed to MNI space when necessary. Meta-analyses results were all reported in MNI space on a Z-map thresholded at their respective FDR-corrected Z-value, see Table 4 for results of GM volume and tissue density. For uncorrected meta-analytic summary images and FDR-corrected images of the key results, see Fig. 4 for volume and Fig. 5 for density.
Table 4.
Sex differences in GM volume and density.
| Region | Sizea | Z statisticb | x | y | z |
|---|---|---|---|---|---|
| GM volume | |||||
| Males > Females | |||||
| Right amygdala, anterior parahippocampal gyrus, hippocampus, putamen and temporal pole | 824 | 7.29 | 28 | −2 | −34 |
| 6.89 | 26 | −6 | −20 | ||
| 5.71 | 26 | 10 | −6 | ||
| 5.2 | 26 | −14 | −26 | ||
| 4.83 | 22 | 6 | −12 | ||
| Left amygdala, anterior parahippocampal gyrus, hippocampus, putamen, temporal pole and orbitofrontal cortex | 611 | 8.76 | −16 | −4 | −26 |
| 5.17 | −36 | 4 | −18 | ||
| 5.67 | −28 | −12 | −18 | ||
| 5.07 | −20 | 10 | −16 | ||
| Left and right posterior cingulate gyrus and precuneus | 225 | 6.26 | −4 | −48 | 30 |
| 5.35 | 8 | −50 | 24 | ||
| 4.94 | 12 | −56 | 32 | ||
| Left cerebellum, VI, VIIb, VIIIa and Crus I | 210 | 7.82 | −28 | −60 | −32 |
| 4.45 | −28 | −48 | −36 | ||
| 4.3 | −20 | −66 | −46 | ||
| Left anterior and posterior cingulate gyrus and precentral gyrus | 117 | 5.93 | −2 | −6 | 44 |
| 5.74 | −2 | −18 | 46 | ||
| Left temporal pole | 123 | 5.68 | −46 | 10 | −42 |
| 4.69 | −50 | 16 | −30 | ||
| 3.91 | −44 | 24 | −30 | ||
| Right cerebellum, VIIb, VIIIa and Crus I and II | 100 | 5.12 | 40 | −52 | −56 |
| 4.37 | 32 | −64 | −42 | ||
| 4.14 | 28 | −56 | −46 | ||
| Left cerebellum VIIIa and VIIb | 71 | 5.17 | −34 | −48 | −54 |
| Right frontal pole | 66 | 5.33 | 10 | 60 | 34 |
| Left temporal pole | 60 | 5.29 | −48 | −70 | −20 |
| Males < Females | |||||
| Right inferior frontal gyrus, pars triangularis and pars opercularis, middle frontal gryus, frontal pole | 238 | 7.28 | 54 | 34 | 18 |
| 5.79 | 46 | 36 | 18 | ||
| 5.34 | 52 | 28 | 28 | ||
| 4.59 | 56 | 20 | 22 | ||
| Left and right thalami | 182 | 6.01 | −10 | −8 | 16 |
| 5.63 | −4 | −4 | 10 | ||
| 5.41 | 6 | −4 | 8 | ||
| Right parietal operculum cortex and planum temporale | 115 | 6.66 | 54 | −28 | 18 |
| 5.89 | 54 | −28 | 18 | ||
| Right anterior cingulate gyrus | 85 | 5.85 | 8 | 26 | 22 |
| Left and right precuneus | 79 | 5.14 | −2 | −60 | 60 |
| 4.87 | 4 | −52 | 64 | ||
| Right frontal orbital cortex | 67 | 5.07 | 22 | 24 | −20 |
| 5 | 20 | 28 | −24 | ||
| 4.9 | 28 | 26 | −20 | ||
| Left posterior parahippocampal gyrus | 66 | 6.92 | −14 | −36 | −6 |
| Left lateral occipital cortex, superior division | 64 | 5.92 | −38 | −78 | 32 |
| Right insular cortex and Heschl's gyrus | 63 | 5.78 | 32 | −22 | 6 |
| 5.27 | 38 | −22 | 8 | ||
| GM density | |||||
| Males > Females | |||||
| Left amygdala, hippocampus, insula, pallidum, putamen, claustrum | 475 | 6.42 | −20 | −12 | −22 |
| 6.26 | −36 | −6 | 12 | ||
| 5.78 | −26 | −10 | 2 | ||
| 5.29 | −22 | −14 | −6 | ||
| 4.56 | −30 | −18 | 12 | ||
| 4.08 | −34 | −6 | −24 | ||
| Right cerebellum VI | 81 | 6.86 | 32 | −52 | −30 |
| Males < Females | |||||
| Left frontal pole | 64 | 7.28 | −14 | 38 | −24 |
Abbreviations: GM, grey matter; MNI, Montreal Neurological Institute.
Size in 2 mm voxels on MNI152_T1_2mmbrain mask.
Values were thresholded at qFDR < 0.01 for GM volume, which is equal to Z > 3.44 for M > F and Z > 3.62 for F > M, and at qFDR < 0.05 for GM density, which is equal to Z > 3.25 for M > F and Z > 3.44 for F > M. With a minimum cluster size of 60 voxels. The MNI coordinates indicate the value of the peak voxel and local minima of the cluster and the size the span of the cluster.
Fig. 4.
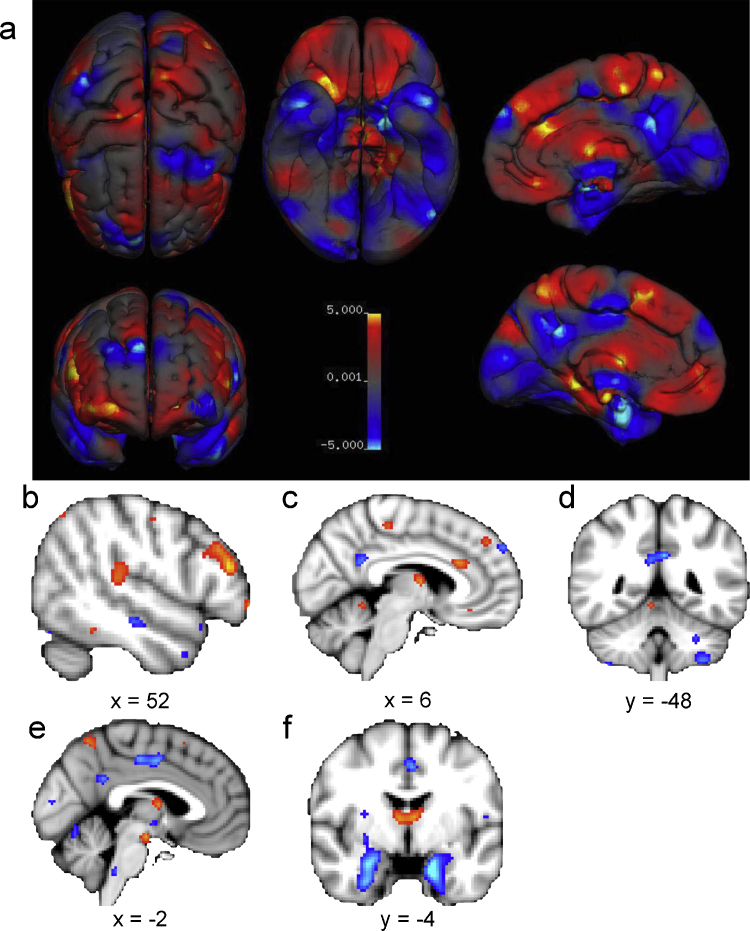
Voxel-based regional sex differences in grey matter volume. Female > Male in red, and Male > Female is in blue. Panel a, rendered overview of uncorrected regional sex differences in grey matter volume. All other panels are thresholded at FDR q < 0.01. Panels b–f display areas of larger volume in females (red) including (b) the right inferior and middle frontal gyri, pars triangularis and planum temporale; (c) thalamus and right anterior cingulate gyrus; and (f) left and right thalamus; and areas of larger volume in males (blue), including (c) the anterior cingulate gyrus; (d) bilateral posterior cingulate gyrus and precuneus and left cerebellum; (e) anterior and posterior cingulate gyri; and (f) left and right amygdalae, hippocampi and parahippocampal gyri.
Fig. 5.
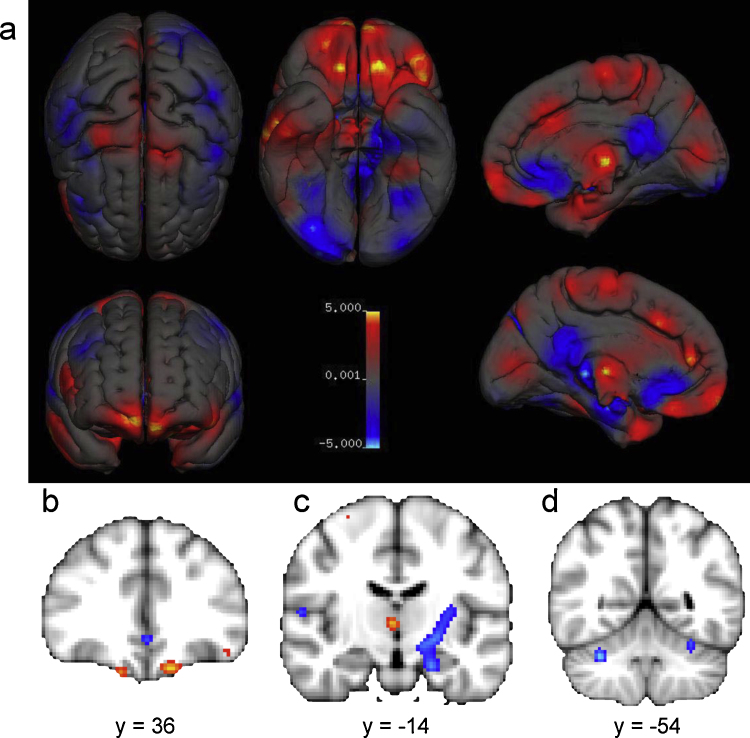
Voxel-based regional sex differences in grey matter density. Female > Male in red, and Male > Female is in blue. Panel a, rendered overview of uncorrected regional sex differences in grey matter concentration. All other panels are thresholded at FDR q < 0.05. Panels b–c display areas of larger volume in females (red) in (b) frontal pole and (c) right thalamus; and in males (blue) including (c) left amygdala, hippocampus, insular cortex and putamen; (d) right and left cerebellum VI lobe.
3.3.1. Regional volume meta-analysis
All 16 studies included in the volume voxel-based meta-analysis included a between-group comparison of GM volume, leading to a total of 264 reported GM foci. Only 4 studies performed a WM volume comparison, with a total of 30 WM foci. Since 30 data points are insufficiently spatially dense to perform a meta-analysis, only a coordinate-based meta-analysis on GM volume is currently possible. The 16 studies provided a total of 2186 brains (49% female) aged between 7 and 80 years old. Because an FDR-correction at voxel-level q = 0.05 gave diffuse spatial results, we opted for a more stringent correction to capture the most reliable group differences. The (FDR q = 0.01) thresholded Z-value was 3.428 for the male > female contrast and 3.616 for the female > male contrast, and results are reported in Table 4 using an extent threshold of 60 continuous voxels.
On average, males have larger grey matter volume in bilateral amygdalae, hippocampi, anterior parahippocampal gyri, posterior cingulate gyri, precuneus, putamen and temporal poles, areas in the left posterior and anterior cingulate gyri, and areas in the cerebellum bilateral VIIb, VIIIa and Crus I lobes, left VI and right Crus II lobes. Females on average have larger volume at the right frontal pole, inferior and middle frontal gyri, pars triangularis, planum temporale/parietal operculum, anterior cingulate gyrus, insular cortex, and Heschl's gyrus; bilateral thalami and precuneus; the left parahippocampal gyrus and lateral occipital cortex (superior division).
3.3.2. Regional tissue density meta-analysis
Eight of the nine studies (eight of the ten age-matched groups) investigating voxel-based sex differences in brain tissue density performed a GM analysis, with a total of 86 reported foci. Only three performed a WM density analysis with a total of 13 foci again discouraging a meta-analysis. The eight studies provided a total number of 741 brains (53% female), aged between 10 and 81 years. Results are reported (with FDR q = 0.05). Z-values were 3.247 for the male > female contrast and 3.445 for the female > male contrast, reported in Table 4 with an extent threshold of 60 continuous voxels. Areas of higher GM density in males compared to females included the left amygdala, hippocampus, insular cortex, pallidum, putamen, claustrum, and an area in the right VI lobe of the cerebellum. The left frontal pole has significantly higher GM tissue density in females compared to males.
4. Discussion
This meta-analysis collated and quantified current literature regarding sex differences in human brain morphology. Our first aim was to examine in what way and where typically developing male and female brains differ. Furthermore we explored the question that if male and female brains differ, do such areas of differences overlap with areas commonly implicated in psychiatric conditions? We found that across a wide age range, from newborns to individuals over 80 years old, differences in overall brain volumes are sustained between males and females. On average males have larger ICV (12%), TBV (11%), Cb (10%), GM (9%), WM (13%), CSF (11.5%) and Cbl (9%) absolute volumes than females. In addition, the ‘mature’ (18–59 years old) age category is best represented with by far the largest number of studies across all volumes and may thus have skewed the meta-analytic results.
At a regional level, males on average have larger volumes and higher tissue densities in the left amygdala, hippocampus, insular cortex, putamen; higher densities in the right VI lobe of the cerebellum and in the left claustrum; and larger volumes in the bilateral anterior parahippocampal gyri, posterior cingulate gyri, precuneus, temporal poles, and cerebellum, areas in the left posterior and anterior cingulate gyri, and in right amygdala, hippocampus, and putamen. Females have on average higher density in the left frontal pole, and larger volumes in the right frontal pole, inferior and middle frontal gyri, pars triangularis, planum temporale/parietal operculum, anterior cingulate gyrus, insular cortex, and Heschl's gyrus; bilateral thalami and precuneus; the left parahippocampal gyrus and lateral occipital cortex (superior division).
The results from the regional volume and density analyses mostly include areas that are part of the limbic and language systems. Additionally, they also indicate a potential lateral asymmetry in sex differences. Volume increases in males are mostly in bilateral limbic areas and left posterior cingulate gyrus, whereas higher densities are mostly limited to the left side of the limbic system. On the other hand, larger volumes in females were most pronounced in areas in the right hemisphere related to language in addition to several limbic structures such as the right insular cortex and anterior cingulate gyrus. Despite this seeming sex difference in patterns of lateralization, it was unfortunately not possible to statistically, directly examine sex differences in asymmetry in this meta-analysis due to the limited number of articles performing a voxel-wise asymmetry analysis. Existing literature employing region-of-interest analyses (Chiarello et al., 2009; Sommer et al., 2008) have provided further exploration to this issue. Given the rich evolutionary and neurobiological implications in sex differences and brain lateralization, future studies on sex differences in human neuroanatomy should investigate patterns of asymmetry in a whole-brain framework (Crow et al., 2013; Good et al., 2001b; Fan et al., 2010).
4.1. Brain development
Recent studies have shown different developmental trajectories for regional volumes as well as for compartmental volumes (Brain Development Cooperative Group, 2012; Good et al., 2001b; Koolschijn and Crone, 2013; Lenroot et al., 2007; Li et al., 2014; Pfefferbaum et al., 2013;). Longitudinal studies on specific neuroanatomical structures usually show sex and age effects, but not necessarily sex by age interaction effects, on trajectories for most of the structures we found to be different between males and females in this meta-analyses (e.g., the amygdala, hippocampus, putamen, precuneus, and thalamus) in adulthood (Li et al., 2014) and during adolescence (Brain Development Cooperative Group, 2012; Koolschijn and Crone, 2013; Lenroot et al., 2007).
We recognize the limitations of the existing literature in our study in providing a descriptive account across six age categories for overall volumes. We were not able to perform statistical tests comparing volume differences between age groups due to heterogeneous sample sizes: Fig. 3 and Supplementary Figures 14–18 show a bias in the number of studies examining sex differences in the 18–45 year-old ‘mature’ age categories. Future research should explore sex differences in other age categories separately, and more importantly, across time using longitudinal designs to provide a better understanding of the development of total brain volumes across the lifespan.
4.2. Potential implications for understanding neuropsychiatric conditions
The findings in this study may serve as a foundation for future studies by providing sex-differential norms of brain volume and density information. Studying sex differences in regional and overall brain volumes could also provide clues about how biological, environmental and gene-environment interaction mechanisms associated with sexual differentiation shape brain development. Previous studies found significant correlations of hormones on regional and overall sex differences in brain volume in children (Lombardo et al., 2012), adolescents (Herting et al., 2012; Paus et al., 2010; Witte et al., 2010) and adults (Lentini et al., 2013; Pletzer et al., 2010). Genetic influences, such as variation in the number of CAG repeats in the androgen receptor gene (Raznahan et al., 2010) and sex-biased gene expression (Hawrylycz et al., 2012; Kang et al., 2011), also have an impact on (cortical) brain development. In addition, environmental influences such as birth weight (Raznahan et al., 2012) and effects of prenatal nutrition, which can influence DNA methylation of insulin-like growth factors (Heijmans et al., 2008), affect general (brain) development as well (Hansen-Pupp et al., 2011).
The majority of the regions displaying sex differences in this meta-analysis also show structural differences between typically developing individuals and individuals with neuropsychiatric conditions (areas of the limbic system, e.g., amygdala, hippocampus and insula) such as autism (Beacher et al., 2012; Cauda et al., 2011; Lai et al., 2013), depression (Bora et al., 2012), schizophrenia (Shepherd et al., 2012) and attention deficit hyperactivity disorder (Etkin and Wager, 2007), providing some bases for the hypothetical view that factors driving the development of typical sex differences might also play a role in the emergence of these neuropsychiatric conditions. Most of these conditions are neurodevelopmental and their prevalence may change over developmental periods. For example, autism has a male bias from childhood onwards, higher prevalence and earlier age of onset for schizophrenia are reported for males, whereas for depression and anxiety disorder the prevalence doubles in girls during adolescence (Rutter et al., 2003). From these we could speculate that sexually differentiating mechanisms may be involved in the neurodevelopment of individuals who develop these psychiatric conditions. Therefore, research investigating differences in brain structure in psychiatric conditions that are asymmetrically affected by sex should stratify samples by sex and perform within sex case–control comparisons.
On a different note, stratifying by sex may also be important for studies measuring for regional cerebral blood flow (rCBF), such as in positron emission topography (PET) studies, since the size distribution volume of the area of interest may differ for males and females. Although sex differences in brain function have previously been reported and reviewed (Sacher et al., 2013; Stevens and Hamann, 2012), the link between function and structure is still under-explored; no predictions as to how structure may influence physiology or behaviour are possible from these meta-analyses.
4.3. Limitations
Several limitations regarding the sample size of the meta-analyses and individual study parameters should be acknowledged. First, the total volume analyses were all performed on absolute brain volumes. Most studies report absolute volumes rather than values adjusted for weight and/or height. The overall volume analyses are thus a reflection of the existing literature. As it could still be debated what the implications are by brain size ‘adjusted’ for body weight and/or height, and whether body weight and/or height (males are on average taller than females) influence brain size, the present results should be interpreted in light of the conventional ways of report in the literature. Future studies also investigate brain volumes adjusted for weight and height in addition to absolute volumes.
Second, we recognize that no definite statistical inference can be made from the analyses by age categories due to (1) too small sample sizes to perform volume meta-analyses in each age category and (2) heterogeneous age-range of the categories (e.g. the ‘mature’ category spans over 42 years). This reflects the limitation of the current literature. Longitudinal follow-up design is the only way to adequately address lifespan brain development, including how sex differences play a role.
Third, in the extant literature it is not always made clear if individual studies include the cerebellum and/or brainstem in their analysis. Although significant sex differences were found in cerebellum volume and thus sex differences may still be present, this inconsistency in the literature could affect results on total white or grey matter volumes.
Lastly, even though GPR-CMBA takes into account heterogeneity between studies, the variation in smoothing, sample size, and covariates in statistical models can all influence voxel-based morphometry analyses (Barnes et al., 2010; Shen and Sterr, 2013) and act as important sources of statistical noise.
4.4. Conclusion and future research
In summary, this study provides the first meta-analysis of sex differences in overall and regional brain volumes and regional brain tissue densities. Future research should test whether sex differences in brain structure underlies skewed sex ratios of neurological and psychiatric conditions and whether brain areas affected in such conditions are caused by physiological mechanisms associated with the development of typical sex differences. For example, recent studies show that sex differences in the adult (Hawrylycz et al., 2012) and developing (Kang et al., 2011) brain transcriptome could be analyzed in conjunction with neuroanatomy, to examine if sexually differentiated brain structures are driven by differences in the brain transcriptome, sex chromosomal and/or environmental effects.
Acknowledgements
We would like to thank all the authors of studies included in the meta-analyses who provided additional data. This study was supported by the Wellcome Trust and Medical Research Council (MRC) Behavioural and Clinical Neuroscience Institute. A.N.V.R. was funded by an UK MRC PhD Studentship grant number RNAG/261 Task 2, the Wellcome Trust, the Dr Hendrik Müller Vaderlandsch Fonds, and the Carolus Magnus Fonds under the Prins Bernard Cultuurfonds; M-C.L. was funded by the Waterloo Foundation grant number 921/1247; M.V.L. by the Wellcome Trust, Jesus College Cambridge, and the British Academy; and S.B-C. by the Wellcome Trust, the Autism Research Trust and the MRC.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Abbs B., Liang L., Makris N., Tsuang M., Seidman L.J., Goldstein J.M. Covariance modeling of MRI brain volumes in memory circuitry in schizophrenia: sex differences are critical. Neuroimage. 2011;56:1865–1874. doi: 10.1016/j.neuroimage.2011.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acer N., Sahin B., Usanmaz M., Tatoglu H., Irmak Z. Comparison of point counting and planimetry methods for the assessment of cerebellar volume in human using magnetic resonance imaging: a stereological study. Surg. Radiol. Anat. 2008;30:335–339. doi: 10.1007/s00276-008-0330-9. [DOI] [PubMed] [Google Scholar]
- Allen J.S., Damasio H., Grabowski T.J. Normal neuroanatomical variation in the human brain: an MRI-volumetric study. Am. J. Phys. Anthropol. 2002;118:341–358. doi: 10.1002/ajpa.10092. [DOI] [PubMed] [Google Scholar]
- Allen J.S., Damasio H., Grabowski T.J., Bruss J., Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage. 2003;18:880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Allen L.S., Richey M.F., Chai Y.M., Gorski R.A. Sex differences in the corpus callosum of the living human being. J. Neurosci. 1991;11:933–942. doi: 10.1523/JNEUROSCI.11-04-00933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J.R., Akkal D., Hassel S., Travis M.J., Banihashemi L., Kerr N., Kupfer D.J., Phillips M.L. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Res. 2009;171:54–68. doi: 10.1016/j.pscychresns.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C., Flashman L., Flaum M., Arndt S., Swayze V., 2nd, O’Leary D.S., Ehrhardt J.C., Yuh W.T. Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. J. Am. Med. Assoc. 1994;272:1763–1769. [PubMed] [Google Scholar]
- Arango C., McMahon R.P., Lefkowitz D.M., Pearlson G., Kirkpatrick B., Buchanan R.W. Patterns of cranial, brain and sulcal CSF volumes in male and female deficit and nondeficit patients with schizophrenia. Psychiatry Res. 2008;162:91–100. doi: 10.1016/j.pscychresns.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Arnold A.P., Breedlove S.M. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm. Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Arnold A.P., Chen X. What does the four core genotypes mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baibakov S.E., Fedorov V.P. Morphometric characteristics of the brain in children aged one year (magnetic resonance tomography data) Neurosci. Behav. Physiol. 2010;40:69–72. doi: 10.1007/s11055-009-9224-5. [DOI] [PubMed] [Google Scholar]
- Bale T.L., Baram T.Z., Brown A.S., Goldstein J.M., Insel T.R., McCarthy M.M., Nemeroff C.B., Reyes T.M., Simerly R.B., Susser E.S., Nestler E.J. Early life programming and neurodevelopmental disorders. Biol. Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao A.M., Swaab D.F. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist. 2010;16:550–565. doi: 10.1177/1073858410377005. [DOI] [PubMed] [Google Scholar]
- Barnes J., Ridgway G.R., Bartlett J., Henley S.M., Lehmann M., Hobbs N., Clarkson M.J., MacManus D.G., Ourselin S., Fox N.C. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53:1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Lombardo M.V., Auyeung B., Ashwin E., Chakrabarti B., Knickmeyer R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011;9:e1001081. doi: 10.1371/journal.pbio.1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrick T.R., Mackay C.E., Prima S., Maes F., Vandermeulen D., Crow T.J., Roberts N. Automatic analysis of cerebral asymmetry: an exploratory study of the relationship between brain torque and planum temporale asymmetry. Neuroimage. 2005;24:678–691. doi: 10.1016/j.neuroimage.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Barta P., Dazzan P. Hemispheric surface area: sex, laterality and age effects. Cereb. Cortex. 2003;13:364–370. doi: 10.1093/cercor/13.4.364. [DOI] [PubMed] [Google Scholar]
- Beacher F.D., Minati L., Baron-Cohen S., Lombardo M.V., Lai M.C., Gray M.A., Harrison N.A., Critchley H.D. Autism attenuates sex differences in brain structure: a combined voxel-based morphometry and diffusion tensor imaging study. Am. J. Neuroradiol. 2012;33:83–89. doi: 10.3174/ajnr.A2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton R.E., Levitt J.G., Peterson J.R., Fadale D., Sporty M.L., Lee M., To D., Mormino E.C., Thompson P.M., McCracken J.T., Toga A.W. Gender differences in the left inferior frontal gyrus in normal children. Neuroimage. 2004;22:626–636. doi: 10.1016/j.neuroimage.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Blatter D.D., Bigler E.D., Gale S.D., Johnson S.C., Anderson C.V., Burnett B.M., Parker N., Kurth S., Horn S.D. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. Am. J. Neuroradiol. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- Bloss C.S., Courchesne E. MRI neuroanatomy in young girls with autism: a preliminary study. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46:515–523. doi: 10.1097/chi.0b013e318030e28b. [DOI] [PubMed] [Google Scholar]
- Boes A.D., Tranel D., Anderson S.W., Nopoulos P. Right anterior cingulate: a neuroanatomical correlate of aggression and defiance in boys. Behav. Neurosci. 2008;122:677–684. doi: 10.1037/0735-7044.122.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E., Fornito A., Pantelis C., Yucel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb. Cortex. 2012;22:1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant N.L., Buchanan R.W., Vladar K., Breier A., Rothman M. Gender differences in temporal lobe structures of patients with schizophrenia: a volumetric MRI study. Am. J. Psychiatry. 1999;156:603–609. doi: 10.1176/ajp.156.4.603. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Head D., Parker J., Fotenos A.F., Marcus D., Morris J.C., Snyder A.Z. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Carne R.P., Vogrin S., Litewka L., Cook M.J. Cerebral cortex: an MRI-based study of volume and variance with age and sex. J. Clin. Neurosci. 2006;13:60–72. doi: 10.1016/j.jocn.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Cauda F., Geda E., Sacco K., D’Agata F., Duca S., Geminiani G., Keller R. Grey matter abnormality in autism spectrum disorder: an activation likelihood estimation meta-analysis study. J. Neurol. Neurosurg. Psychiatry. 2011;82:1304–1313. doi: 10.1136/jnnp.2010.239111. [DOI] [PubMed] [Google Scholar]
- Caviness V.S., Jr., Kennedy D.N., Richelme C., Rademacher J., Filipek P.A. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb. Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Center on the Developing Child . Harvard University; 2012. The Science of Neglect: The Persistent Absence of Responsive Care Disrupts the Developing Brain: Working Paper 12.www.developingchild.harvard.edu (accessed 21.05.13) [Google Scholar]
- Central Brain Tumor Registry of the United States . 2012. Statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2004–2008.http://www.cbtrus.org (accessed 21.05.13) [Google Scholar]
- Chen X., Sachdev P.S., Wen W., Anstey K.J. Sex differences in regional gray matter in healthy individuals aged 44–48 years: a voxel-based morphometric study. Neuroimage. 2007;36:691–699. doi: 10.1016/j.neuroimage.2007.03.063. [DOI] [PubMed] [Google Scholar]
- Chen X., Coles C.D., Lynch M.E., Hu X. Understanding specific effects of prenatal alcohol exposure on brain structure in young adults. Hum. Brain Mapp. 2012;33:1663–1676. doi: 10.1002/hbm.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Chou K.H., Decety J., Chen I.Y., Hung D., Tzeng O.J., Lin C.P. Sex differences in the neuroanatomy of human mirror-neuron system: a voxel-based morphometric investigation. Neuroscience. 2009;158:713–720. doi: 10.1016/j.neuroscience.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Chiarello C., Welcome S.E., Halderman L.K., Towler S., Julagay J., Otto R., Leonard C.M. A large-scale investigation of lateralization in cortical anatomy and word reading: are there sex differences? Neuropsychology. 2009;23:210–222. doi: 10.1037/a0014265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C.H., Lee J.M., Koo B.B., Park J.S., Kim D.S., Kwon J.S., Kim I.Y. Sex differences in the temporal lobe white matter and the corpus callosum: a diffusion tensor tractography study. Neuroreport. 2010;21:73–77. doi: 10.1097/WNR.0b013e3283345eb0. [DOI] [PubMed] [Google Scholar]
- Chung S.C., Lee B.Y., Tack G.R., Lee S.Y., Eom J.S., Sohn J.H. Effects of age, gender, and weight on the cerebellar volume of Korean people. Brain Res. 2005;1042:233–235. doi: 10.1016/j.brainres.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. Annual Research Review: Resilient functioning in maltreated children – past, present, and future perspectives. J. Child Psychol. Psychiatry. 2013;54:402–422. doi: 10.1111/j.1469-7610.2012.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayden J.D., Jentschke S., Munoz M., Cooper J.M., Chadwick M.J., Banks T., Clark C.A., Vargha-Khadem F. Normative development of white matter tracts: similarities and differences in relation to age, gender, and intelligence. Cereb. Cortex. 2012;22:1738–1747. doi: 10.1093/cercor/bhr243. [DOI] [PubMed] [Google Scholar]
- Coffey C.E., Lucke J.F., Saxton J.A., Ratcliff G., Unitas L.J., Billig B., Bryan R.N. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch. Neurol. 1998;55:169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- Collinson S.L., Mackay C.E., James A.C., Quested D.J., Phillips T., Roberts N., Crow T.J. Brain volume, asymmetry and intellectual impairment in relation to sex in early-onset schizophrenia. Br. J. Psychiatry. 2003;183:114–120. doi: 10.1192/bjp.183.2.114. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Chisum H.J., Townsend J., Cowles A., Covington J., Egaas B., Harwood M., Hinds S., Press G.A. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Cowell P.E., Sluming V.A., Wilkinson I.D., Cezayirli E., Romanowski C.A., Webb J.A., Keller S.S., Mayes A., Roberts N. Effects of sex and age on regional prefrontal brain volume in two human cohorts. Eur. J. Neurosci. 2007;25:307–318. doi: 10.1111/j.1460-9568.2006.05281.x. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B., Roiz-Santianez R., Perez-Iglesias R., Mata I., Rodriguez-Sanchez J.M., Tordesillas-Gutierrez D., Ortiz-Garcia de la Foz V., Tabares-Seisdedos R., Sanchez E., Andreasen N., Magnotta V., Vazquez Barquero J.L. Sex-specific variation of MRI-based cortical morphometry in adult healthy volunteers: the effect on cognitive functioning. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:616–623. doi: 10.1016/j.pnpbp.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T.J., Chance S.A., Priddle T.H., Radua J., James A.C. Laterality interacts with sex across the schizophrenia/bipolarity continuum: an interpretation of meta-analyses of structural MRI. Psychiatry Res. 2013;210:1232–1244. doi: 10.1016/j.psychres.2013.07.043. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Beers S.R., Hall J., Frustaci K., Masalehdan A., Noll J., Boring A.M. Sex differences in brain maturation during childhood and adolescence. Cereb. Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- de Bruin E.A., Hulshoff Pol H.E., Bijl S., Schnack H.G., Fluitman S., Bocker K.B., Kenemans J.L., Kahn R.S., Verbaten M.N. Associations between alcohol intake and brain volumes in male and female moderate drinkers. Alcohol. Clin. Exp. Res. 2005;29:656–663. doi: 10.1097/01.alc.0000159110.17351.c0. [DOI] [PubMed] [Google Scholar]
- DeCarli C., Massaro J., Harvey D., Hald J., Tullberg M., Au R., Beiser A., D’Agostino R., Wolf P.A. Measures of brain morphology and infarction in the Framingham heart study: establishing what is normal. Neurobiol. Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- De Vries G.J., Rissman E.F., Simerly R.B., Yang L.Y., Scordalakes E.M., Auger C.J., Swain A., Lovell-Badge R., Burgoyne P.S., Arnold A.P. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong G.R. Effects of nutrition on brain development in humans. Am. J. Clin. Nutr. 1993;57:286S–290S. doi: 10.1093/ajcn/57.2.286S. [DOI] [PubMed] [Google Scholar]
- Dong H.H., Guo M.X., Zhang Y.T., Fu Y., Shi H.L. 2010 3rd International Conference on Biomedical Engineering and Informatics (Bmei 2010), vols. 1–7. 2010. Sex differences in brain gray and white matter in healthy young adults: correlations with resting state ALFF; pp. 560–563. [Google Scholar]
- Duggal H.S., Muddasani S., Keshavan M.S. Insular volumes in first-episode schizophrenia: gender effect. Schizophr. Res. 2005;73:113–120. doi: 10.1016/j.schres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Edland S.D., Xu Y., Plevak M., O’Brien P., Tangalos E.G., Petersen R.C., Jack C.R. Total intracranial volume: normative values and lack of association with Alzheimer's disease. Neurology. 2002;59:272–274. doi: 10.1212/wnl.59.2.272. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Grefkes C., Wang L.E., Zilles K., Fox P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliez S., Blasey C.M., Freund L.S., Hastie T., Reiss A.L. Brain anatomy, gender and IQ in children and adolescents with fragile X syndrome. Brain. 2001;124:1610–1618. doi: 10.1093/brain/124.8.1610. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta- analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim C., Fiori M., Evans A.C., Perusse D. The relationship between social defiance, vindictiveness, anger, and brain morphology in eight-year-old boys and girls. Soc. Dev. 2012;21:592–609. [Google Scholar]
- Fan L., Tang Y., Sun B., Gong G., Chen Z.J., Lin X., Yu T., Li Z., Evans A.C., Liu S. Sexual dimorphism and asymmetry in human cerebellum: an MRI-based morphometric study. Brain Res. 2010;1353:60–73. doi: 10.1016/j.brainres.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Filipek P.A., Richelme C., Kennedy D.N., Caviness V.S., Jr. The young adult human brain: an MRI-based morphometric analysis. Cereb. Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Frederikse M.E., Lu A., Aylward E., Barta P., Pearlson G. Sex differences in the inferior parietal lobule. Cereb. Cortex. 1999;9:896–901. doi: 10.1093/cercor/9.8.896. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A., Junque C., Gimenez M., Caldu X., Segovia S., Guillamon A. Sex differences in the human olfactory system. Brain Res. 2006;1116:103–111. doi: 10.1016/j.brainres.2006.07.115. [DOI] [PubMed] [Google Scholar]
- Ge Y., Grossman R.I., Babb J.S., Rabin M.L., Mannon L.J., Kolson D.L. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. Am. J. Neuroradiol. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Raznahan A., Mills K.L., Lenroot R.K. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol. Sex Differ. 2012;3:19. doi: 10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Lin W., Prastawa M.W., Looney C.B., Vetsa Y.S., Knickmeyer R.C., Evans D.D., Smith J.K., Hamer R.M., Lieberman J.A., Gerig G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J. Neurosci. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.M., Seidman L.J., Horton N.J., Makris N., Kennedy D.N., Caviness V.S., Jr., Faraone S.V., Tsuang M.T. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb. Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hanggi J., Buchmann A., Mondadori C.R., Henke K., Jancke L., Hock C. Sexual dimorphism in the parietal substrate associated with visuospatial cognition independent of general intelligence. J. Cogn. Neurosci. 2010;22:139–155. doi: 10.1162/jocn.2008.21175. [DOI] [PubMed] [Google Scholar]
- Hansen-Pupp I., Hovel H., Hellstrom A., Hellstrom-Westas L., Lofqvist C., Larsson E.M., Lazeyras F., Fellman V., Huppi P.S., Ley D. Postnatal decrease in circulating insulin-like growth factor-I and low brain volumes in very preterm infants. J. Clin. Endocrinol. Metab. 2011;96:1129–1135. doi: 10.1210/jc.2010-2440. [DOI] [PubMed] [Google Scholar]
- Hawrylycz M.J., Lein E.S., Guillozet-Bongaarts A.L., Shen E.H., Ng L., Miller J.A., van de Lagemaat L.N., Smith K.A., Ebbert A., Riley Z.L., Abajian C., Beckmann C.F., Bernard A., Bertagnolli D., Boe A.F., Cartagena P.M., Chakravarty M.M., Chapin M., Chong J., Dalley R.A., Daly B.D., Dang C., Datta S., Dee N., Dolbeare T.A., Faber V., Feng D., Fowler D.R., Goldy J., Gregor B.W., Haradon Z., Haynor D.R., Hohmann J.G., Horvath S., Howard R.E., Jeromin A., Jochim J.M., Kinnunen M., Lau C., Lazarz E.T., Lee C., Lemon T.A., Li L., Li Y., Morris J.A., Overly C.C., Parker P.D., Parry S.E., Reding M., Royall J.J., Schulkin J., Sequeira P.A., Slaughterbeck C.R., Smith S.C., Sodt A.J., Sunkin S.M., Swanson B.E., Vawter M.P., Williams D., Wohnoutka P., Zielke H.R., Geschwind D.H., Hof P.R., Smith S.M., Koch C., Grant S.G., Jones A.R. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans B.T., Tobi E.W., Stein A.D., Putter H., Blauw G.J., Susser E.S., Slagboom P.E., Lumey L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Maxwell E.C., Irvine C., Nagel B.J. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb. Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G., Spiegelhalter D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M.J., Staff R.T., Bunting B.P., Murray A.D., Ahearn T.S., Deary I.J., Whalley L.J. Cerebellar brain volume accounts for variance in cognitive performance in older adults. Cortex. 2011;47:441–450. doi: 10.1016/j.cortex.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Hommer D., Momenan R., Kaiser E., Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. Am. J. Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Hoogendam Y.Y., van der Geest J.N., van der Lijn F., van der Lugt A., Niessen W.J., Krestin G.P., Hofman A., Vernooij M.W., Breteler M.M., Ikram M.A. Determinants of cerebellar and cerebral volume in the general elderly population. Neurobiol. Aging. 2012;33:2774–2781. doi: 10.1016/j.neurobiolaging.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Huedo-Medina T.B., Sanchez-Meca J., Marin-Martinez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol H.E., Cohen-Kettenis P.T., Van Haren N.E.M., Peper J.S., Brans R.G.H., Cahn W., Schnack H.G., Gooren L.J.G., Kahn R.S. Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure. Eur. Feder. Endocr. Soc.Eur. J. Endocrinol. 2006;155:S107–S114. [Google Scholar]
- Hutchinson S., Lee L.H., Gaab N., Schlaug G. Cerebellar volume of musicians. Cereb. Cortex. 2003;13:943–949. doi: 10.1093/cercor/13.9.943. [DOI] [PubMed] [Google Scholar]
- Jenkins R., Fox N.C., Rossor A.M., Harvey R.J., Rossor M.N. Intracranial volume and Alzheimer disease: evidence against the cerebral reserve hypothesis. Arch. Neurol. 2000;57:220–224. doi: 10.1001/archneur.57.2.220. [DOI] [PubMed] [Google Scholar]
- Kang H.J., Kawasawa Y.I., Cheng F., Zhu Y., Xu X., Li M., Sousa A.M., Pletikos M., Meyer K.A., Sedmak G., Guennel T., Shin Y., Johnson M.B., Krsnik Z., Mayer S., Fertuzinhos S., Umlauf S., Lisgo S.N., Vortmeyer A., Weinberger D.R., Mane S., Hyde T.M., Huttner A., Reimers M., Kleinman J.E., Sestan N. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn P.C., Crone E.A. Sex differences and structural brain maturation from childhood to early adulthood. Dev. Cogn. Neurosci. 2013;5:106–118. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus T.A., Bollich A.M., Corey D.M., Lemen L.C., Foundas A.L. Sex-linked differences in the anatomy of the perisylvian language cortex: a volumetric MRI study of gray matter volumes. Neuropsychology. 2004;18:738–747. doi: 10.1037/0894-4105.18.4.738. [DOI] [PubMed] [Google Scholar]
- Koscik T., O’Leary D., Moser D.J., Andreasen N.C., Nopoulos P. Sex differences in parietal lobe morphology: relationship to mental rotation performance. Brain Cogn. 2009;69:451–459. doi: 10.1016/j.bandc.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruggel F. MRI-based volumetry of head compartments: normative values of healthy adults. Neuroimage. 2006;30:1–11. doi: 10.1016/j.neuroimage.2005.09.063. [DOI] [PubMed] [Google Scholar]
- Lai M.C., Lombardo M.V., Suckling J., Ruigrok A.N.V., Chakrabarti B., Ecker C., Deoni S.C.L., Craig M.C., Murphy D.G.M., Bullmore E.T., MRC AIMS Consortium, Baron-Cohen S. Biological sex affects the neurobiology of autism. Brain. 2013;136:2799–2815. doi: 10.1093/brain/awt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H., Kurbanyan K., Ballmaier M., Mintz J., Toga A., Kumar A. Sex differences in brain structure in geriatric depression. Am. J. Geriatr. Psychiatry. 2004;12:653–657. doi: 10.1176/appi.ajgp.12.6.653. [DOI] [PubMed] [Google Scholar]
- Lee B.Y., Sohn J.H., Choi M.H., Lee S.J., Kim H.S., Yang J.W., Choi J.S., Kim H.S., Yi J.H., Tack G.R., Chung S.C. A volumetric study of the corpus callosum in 20s and 40s Korean people. Brain Struct. Funct. 2009;213:463–467. doi: 10.1007/s00429-009-0209-5. [DOI] [PubMed] [Google Scholar]
- Lee J.M., Yoon U., Kim J.J., Kim I.Y., Lee D.S., Kwon J.S., Kim S.I. Analysis of the hemispheric asymmetry using fractal dimension of a skeletonized cerebral surface. IEEE Trans. Biomed. Eng. 2004;51:1494–1498. doi: 10.1109/TBME.2004.831543. [DOI] [PubMed] [Google Scholar]
- Lee N.J., Park I.S., Koh I., Jung T.W., Rhyu I.J. No volume difference of medulla oblongata between young and old Korean people. Brain Res. 2009;1276:77–82. doi: 10.1016/j.brainres.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Lemaitre H., Crivello F., Grassiot B., Alperovitch A., Tzourio C., Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage. 2005;26:900–911. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S., Blumenthal J.D., Lerch J., Zijdenbos A.P., Evans A.C., Thompson P.M., Giedd J.N. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini E., Kasahara M., Arver S., Savic I. Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cereb. Cortex. 2013;23:2322–2336. doi: 10.1093/cercor/bhs222. [DOI] [PubMed] [Google Scholar]
- Lenz K.M., Nugent B.M., Haliyur R., McCarthy M.M. Microglia are essential to masculinization of brain and behavior. J. Neurosci. 2013;33:2761–2772. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C.M., Towler S., Welcome S., Halderman L.K., Otto R., Eckert M.A., Chiarello C. Size matters: cerebral volume influences sex differences in neuroanatomy. Cereb. Cortex. 2008;18:2920–2931. doi: 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., van Tol M.J., Li M., Miao W., Jiao Y., Heinze H.J., Bogerts B., He H., Walter M. Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging. Hum. Brain Mapp. 2014;35:238–247. doi: 10.1002/hbm.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M.V., Ashwin E., Auyeung B., Chakrabarti B., Taylor K., Hackett G., Bullmore E.T., Baron-Cohen S. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J. Neurosci. 2012;32:674–680. doi: 10.1523/JNEUROSCI.4389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Engert V., Lupien S.J., Pruessner J.C. Effect of sex and estrogen therapy on the aging brain: a voxel-based morphometry study. Menopause. 2010;17:846–851. doi: 10.1097/gme.0b013e3181e06b83. [DOI] [PubMed] [Google Scholar]
- Luders E., Narr K.L., Thompson P.M., Woods R.P., Rex D.E., Jancke L., Steinmetz H., Toga A.W. Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage. 2005;26:493–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Luders E., Rex D.E., Narr K.L., Woods R.P., Jancke L., Thompson P.M., Mazziotta J.C., Toga A.W. Relationships between sulcal asymmetries and corpus callosum size: gender and handedness effects. Cereb. Cortex. 2003;13:1084–1093. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- Luders E., Steinmetz H., Jancke L. Brain size and grey matter volume in the healthy human brain. Neuroreport. 2002;13:2371–2374. [PubMed] [Google Scholar]
- Maller J.J., Reglade-Meslin C., Anstey K.J., Sachdev P. Sex and symmetry differences in hippocampal volumetrics: before and beyond the opening of the crus of the fornix. Hippocampus. 2006;16:80–90. doi: 10.1002/hipo.20133. [DOI] [PubMed] [Google Scholar]
- Mann S.L., Hazlett E.A., Byne W., Hof P.R., Buchsbaum M.S., Cohen B.H., Goldstein K.E., Haznedar M.M., Mitsis E.M., Siever L.J., Chu K.W. Anterior and posterior cingulate cortex volume in healthy adults: effects of aging and gender differences. Brain Res. 2011;1401:18–29. doi: 10.1016/j.brainres.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumae M., Kikinis R., Morocz I.A., Lorenzo A.V., Sandor T., Albert M.S., Black P.M., Jolesz F.A. Age-related changes in intracranial compartment volumes in normal adults assessed by magnetic resonance imaging. J. Neurosurg. 1996;84:982–991. doi: 10.3171/jns.1996.84.6.0982. [DOI] [PubMed] [Google Scholar]
- McCarthy M.M., Arnold A.P. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L., Chamberlain S.R., Laird A.R., Thelen S.M., Sahakian B.J., Bullmore E.T. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci. Biobehav. Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T.N., Free S.L., Merschhemke M., Lemieux L., Sisodiya S.M., Shorvon S.D. Reliable callosal measurement: population normative data confirm sex-related differences. Am. J. Neuroradiol. 2003;24:410–418. [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br. Med. J. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamet B., Zeng D., Gerig G., Prastawa M., Bullitt E. Effects of healthy aging measured by intracranial compartment volumes using a designed MR brain database. Med. Image Comput. Comput. Assist. Interv. 2005;8:383–391. doi: 10.1007/11566465_48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr K.L., Woods R.P., Thompson P.M., Szeszko P., Robinson D., Dimtcheva T., Gurbani M., Toga A.W., Bilder R.M. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb. Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Neufang S., Specht K., Hausmann M., Gunturkun O., Herpertz-Dahlmann B., Fink G.R., Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb. Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Nichols T., Hayasaka S. Controlling the family wise error rate in functional neuroimaging: a comparative review. Stat. Methods Med. Res. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Nopoulos P., Flaum M., Andreasen N.C. Sex differences in brain morphology in schizophrenia. Am. J. Psychiatry. 1997;154:1648–1654. doi: 10.1176/ajp.154.12.1648. [DOI] [PubMed] [Google Scholar]
- Nopoulos P., Flaum M., O’Leary D., Andreasen N.C. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98:1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Nunnemann S., Wohlschlager A.M., Ilg R., Gaser C., Etgen T., Conrad B., Zimmer C., Muhlau M. Accelerated aging of the putamen in men but not in women. Neurobiol. Aging. 2009;30:147–151. doi: 10.1016/j.neurobiolaging.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Passe T.J., Rajagopalan P., Tupler L.A., Byrum C.E., MacFall J.R., Krishnan K.R. Age and sex effects on brain morphology. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1997;21:1231–1237. doi: 10.1016/s0278-5846(97)00160-7. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Nawaz-Khan I., Leonard G., Perron M., Pike G.B., Pitiot A., Richer L., Susman E., Veillette S., Pausova Z. Sexual dimorphism in the adolescent brain: role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm. Behav. 2010;57:63–75. doi: 10.1016/j.yhbeh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Peper J.S., Brouwer R.M., Schnack H.G., van Baal G.C., van Leeuwen M., van den Berg S.M., Delemarre-Van de Waal H.A., Boomsma D.I., Kahn R.S., Hulshoff Pol H.E. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34:332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Rohlfing T., Rosenbloom M.J., Chu W., Colrain I.M., Sullivan E.V. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage. 2013;65:176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix C.H., Goy R.W., Gerall A.A., Young W.C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pletzer B., Kronbichler M., Aichhorn M., Bergmann J., Ladurner G., Kerschbaum H.H. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res. 2010;1348:55–62. doi: 10.1016/j.brainres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Ponseti J., Siebner H.R., Kloppel S., Wolff S., Granert O., Jansen O., Mehdorn H.M., Bosinski H.A. Homosexual women have less grey matter in perirhinal cortex than heterosexual women. PLoS One. 2007;2:e762. doi: 10.1371/journal.pone.0000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A., Fortier M.V., Bai J., Zhang X., Chong Y.S., Kwek K., Saw S.M., Godfrey K.M., Gluckman P.D., Meaney M.J. Morphology and microstructure of subcortical structures at birth: a large-scale Asian neonatal neuroimaging study. Neuroimage. 2013;65:315–323. doi: 10.1016/j.neuroimage.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Rametti G., Carrillo B., Gomez-Gil E., Junque C., Zubiarre-Elorza L., Segovia S., Gomez A., Guillamon A. The microstructure of white matter in male to female transsexuals before cross-sex hormonal treatment. A DTI study. J. Psychiatr. Res. 2011;45:949–954. doi: 10.1016/j.jpsychires.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Lee Y., Stidd R., Long R., Greenstein D., Clasen L., Addington A., Gogtay N., Rapoport J.L., Giedd J.N. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Greenstein D., F N.R., Clasen L.S., Giedd J.N. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc. Natl. Acad. Sci. U. S. A. 2012;109:11366–11371. doi: 10.1073/pnas.1203350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig S., Parellada M., Castro-Fornieles J., Janssen J., Moreno D., Baeza I., Bargallo N., Gonzalez-Pinto A., Graell M., Ortuno F., Otero S., Arango C., Desco M. Multicenter study of brain volume abnormalities in children and adolescent-onset psychosis. Schizophr. Bull. 2011;37:1270–1280. doi: 10.1093/schbul/sbq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss A.L., Abrams M.T., Singer H.S., Ross J.L., Denckla M.B. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Reiss A.L., Kesler S.R., Vohr B., Duncan C.C., Katz K.H., Pajot S., Schneider K.C., Makuch R.W., Ment L.R. Sex differences in cerebral volumes of 8-year-olds born preterm. J. Pediatr. 2004;145:242–249. doi: 10.1016/j.jpeds.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Resnick S.M., Goldszal A.F., Davatzikos C., Golski S., Kraut M.A., Metter E.J., Bryan R.N., Zonderman A.B. One-year age changes in MRI brain volumes in older adults. Cereb. Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Rhyu I.J., Cho T.H., Lee N.J., Uhm C.S., Kim H., Suh Y.S. Magnetic resonance image-based cerebellar volumetry in healthy Korean adults. Neurosci. Lett. 1999;270:149–152. doi: 10.1016/s0304-3940(99)00487-5. [DOI] [PubMed] [Google Scholar]
- Riello R., Sabattoli F., Beltramello A., Bonetti M., Bono G., Falini A., Magnani G., Minonzio G., Piovan E., Alaimo G., Ettori M., Galluzzi S., Locatelli E., Noiszewska M., Testa C., Frisoni G.B. Brain volumes in healthy adults aged 40 years and over: a voxel-based morphometry study. Aging Clin. Exp. Res. 2005;17:329–336. doi: 10.1007/BF03324618. [DOI] [PubMed] [Google Scholar]
- Rijpkema M., Everaerd D., van der Pol C., Franke B., Tendolkar I., Fernandez G. Normal sexual dimorphism in the human basal ganglia. Hum. Brain Mapp. 2012;33:1246–1252. doi: 10.1002/hbm.21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M., Caspi A., Moffitt T.E. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J. Child Psychol. Psychiatry. 2003;44:1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Sachdev P.S., Chen X., Wen W., Anstey K.J. Light to moderate alcohol use is associated with increased cortical gray matter in middle- aged men: a voxel-based morphometric study. Psychiatry Res. 2008;163:61–69. doi: 10.1016/j.pscychresns.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Sacher J., Neumann J., Okon-Singer H., Gotowiec S., Villringer A. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn. Reson. Imaging. 2013;31:366–375. doi: 10.1016/j.mri.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Smith S.M., Nichols T.E. Organization for Human Brain Mapping; San Fransisco: 2009. Assessing the Bias and Heterogeneity in Image-Based Meta-Analysis. [Google Scholar]
- Salimi-Khorshidi G., Smith S.M., Keltner J.R., Wager T.D., Nichols T.E. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009;45:810–823. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Nichols T.E., Smith S.M., Woolrich M.W. Using Gaussian-process regression for meta-analytic neuroimaging inference based on sparse observations. IEEE Trans. Med. Imaging. 2011;30:1401–1416. doi: 10.1109/TMI.2011.2122341. [DOI] [PubMed] [Google Scholar]
- Salinas J., Mills E.D., Conrad A.L., Koscik T., Andreasen N.C., Nopoulos P. Sex differences in parietal lobe structure and development. Gend. Med. 2012;9:44–55. doi: 10.1016/j.genm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandu A.L., Specht K., Beneventi H., Lundervold A., Hugdahl K. Sex-differences in grey-white matter structure in normal-reading and dyslexic adolescents. Neurosci. Lett. 2008;438:80–84. doi: 10.1016/j.neulet.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Savic I., Arver S. Sex dimorphism of the brain in male-to-female transsexuals. Cereb. Cortex. 2011;21:2525–2533. doi: 10.1093/cercor/bhr032. [DOI] [PubMed] [Google Scholar]
- Schlaepfer T.E., Harris G.J., Tien A.Y., Peng L., Lee S., Pearlson G.D. Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatry Res. 1995;61:129–135. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- Sgouros S., Goldin J.H., Hockley A.D., Wake M.J., Natarajan K. Intracranial volume change in childhood. J. Neurosurg. 1999;91:610–616. doi: 10.3171/jns.1999.91.4.0610. [DOI] [PubMed] [Google Scholar]
- Shan Z.Y., Liu J.Z., Sahgal V., Wang B., Yue G.H. Selective atrophy of left hemisphere and frontal lobe of the brain in old men. J. Gerontol. A. Biol. Sci. Med. Sci. 2005;60:165–174. doi: 10.1093/gerona/60.2.165. [DOI] [PubMed] [Google Scholar]
- Shen S., Sterr A. Is DARTEL-based voxel-based morphometry affected by width of smoothing kernel and group size? A study using simulated atrophy. J. Magn. Reson. Imaging. 2013;37:1468–1475. doi: 10.1002/jmri.23927. [DOI] [PubMed] [Google Scholar]
- Shepherd A.M., Laurens K.R., Matheson S.L., Carr V.J., Green M.J. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci. Biobehav. Rev. 2012;36:1342–1356. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Shin Y.W., Kim D.J., Ha T.H., Park H.J., Moon W.J., Chung E.C., Lee J.M., Kim I.Y., Kim S.I., Kwon J.S. Sex differences in the human corpus callosum: diffusion tensor imaging study. Neuroreport. 2005;16:795–798. doi: 10.1097/00001756-200505310-00003. [DOI] [PubMed] [Google Scholar]
- Soloff P., Nutche J., Goradia D., Diwadkar V. Structural brain abnormalities in borderline personality disorder: a voxel-based morphometry study. Psychiatry Res. 2008;164:223–236. doi: 10.1016/j.pscychresns.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I.E., Aleman A., Somers M., Boks M.P., Kahn R.S. Sex differences in handedness, asymmetry of the planum temporale and functional language lateralization. Brain Res. 2008;1206:76–88. doi: 10.1016/j.brainres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Kan E., Woods R.P., Yoshii J., Bansal R., Xu D., Zhu H., Thompson P.M., Toga A.W. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb. Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks B.F., Friedman S.D., Shaw D.W., Aylward E.H., Echelard D., Artru A.A., Maravilla K.R., Giedd J.N., Munson J., Dawson G., Dager S.R. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Stevens J.S., Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Rosenbloom M.J., Desmond J.E., Pfefferbaum A. Sex differences in corpus callosum size: relationship to age and intracranial size. Neurobiol. Aging. 2001;22:603–611. doi: 10.1016/s0197-4580(01)00232-9. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Rosenbloom M., Serventi K.L., Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol. Aging. 2004;25:185–192. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Marsh L., Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol. Aging. 2005;26:1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Takahashi R., Ishii K., Kakigi T., Yokoyama K. Gender and age differences in normal adult human brain: voxel-based morphometric study. Hum. Brain Mapp. 2011;32:1050–1058. doi: 10.1002/hbm.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Suzuki M., Zhou S.Y., Hagino H., Kawasaki Y., Yamashita I., Nohara S., Nakamura K., Seto H., Kurachi M. Lack of normal gender differences of the perigenual cingulate gyrus in schizophrenia spectrum disorders. A magnetic resonance imaging study. Eur. Arch. Psychiatry Clin. Neurosci. 2004;254:273–280. doi: 10.1007/s00406-004-0491-4. [DOI] [PubMed] [Google Scholar]
- Takao H., Abe O., Yamasue H., Aoki S., Kasai K., Sasaki H., Ohtomo K. Aging effects on cerebral asymmetry: a voxel-based morphometry and diffusion tensor imaging study. Magn. Reson. Imaging. 2010;28:65–69. doi: 10.1016/j.mri.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Tepest R., Jacobi E., Gawronski A., Krug B., Moller-Hartmann W., Lehnhardt F.G., Vogeley K. Corpus callosum size in adults with high-functioning autism and the relevance of gender. Psychiatry Res. 2010;183:38–43. doi: 10.1016/j.pscychresns.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Tsoi K. The Hong Kong Brach of the Chinese Cochrane Centre; 2011. Heterogeneity and bias. http://www.hkcochrane.cuhk.edu.hk/hkcochrane/images/3rd_3_Heterogen eity_Bias_final%20[Compatibility%20Mode].pdf (accessed 21.05.13) [Google Scholar]
- Uematsu A., Matsui M., Tanaka C., Takahashi T., Noguchi K., Suzuki M., Nishijo H. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7:e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E.M., Faraone S.V., Murray K.E., Seidman L.J. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Van Laere K.J., Dierckx R.A. Brain perfusion SPECT: age- and sex-related effects correlated with voxel-based morphometric findings in healthy adults. Radiology. 2001;221:810–817. doi: 10.1148/radiol.2213010295. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Lindquist M.A., Nichols T.E., Kober H., Van Snellenberg J.X. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage. 2009;45:S210–S221. doi: 10.1016/j.neuroimage.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Shen H., Tang F., Zang Y., Hu D. Combined structural and resting-state functional MRI analysis of sexual dimorphism in the young adult human brain: an MVPA approach. Neuroimage. 2012;61:931–940. doi: 10.1016/j.neuroimage.2012.03.080. [DOI] [PubMed] [Google Scholar]
- Welborn B.L., Papademetris X., Reis D.L., Rajeevan N., Bloise S.M., Gray J.R. Variation in orbitofrontal cortex volume: relation to sex, emotion regulation and affect. Soc. Cogn. Affect. Neurosci. 2009;4:328–339. doi: 10.1093/scan/nsp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Crum W.R., Watt H.C., Fox N.C. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. Am. J. Neuroradiol. 2001;22:1483–1489. [PMC free article] [PubMed] [Google Scholar]
- Wilke M., Krageloh-Mann I., Holland S.K. Global and local development of gray and white matter volume in normal children and adolescents. Exp. Brain Res. 2007;178:296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte A.V., Savli M., Holik A., Kasper S., Lanzenberger R. Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. Neuroimage. 2010;49:1205–1212. doi: 10.1016/j.neuroimage.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Wood J.L., Heitmiller D., Andreasen N.C., Nopoulos P. Morphology of the ventral frontal cortex: relationship to femininity and social cognition. Cereb. Cortex. 2008;18:534–540. doi: 10.1093/cercor/bhm079. [DOI] [PubMed] [Google Scholar]
- Wood J.L., Murko V., Nopoulos P. Ventral frontal cortex in children: morphology, social cognition and femininity/masculinity. Soc. Cogn. Affect. Neurosci. 2008;3:168–176. doi: 10.1093/scan/nsn010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H., Abe O., Suga M., Yamada H., Rogers M.A., Aoki S., Kato N., Kasai K. Sex-linked neuroanatomical basis of human altruistic cooperativeness. Cereb. Cortex. 2008;18:2331–2340. doi: 10.1093/cercor/bhm254. [DOI] [PubMed] [Google Scholar]
- Yang P., Wang P.N., Chuang K.H., Jong Y.J., Chao T.C., Wu M.T. Absence of gender effect on children with attention-deficit/hyperactivity disorder as assessed by optimized voxel-based morphometry. Psychiatry Res. 2008;164:245–253. doi: 10.1016/j.pscychresns.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Yoshii F., Barker W.W., Chang J.Y., Loewenstein D., Apicella A., Smith D., Boothe T., Ginsberg M.D., Pascal S., Duara R. Sensitivity of cerebral glucose metabolism to age, gender, brain volume, brain atrophy, and cerebrovascular risk factors. J. Cereb. Blood Flow Metab. 1988;8:654–661. doi: 10.1038/jcbfm.1988.112. [DOI] [PubMed] [Google Scholar]
- Ystad M.A., Lundervold A.J., Wehling E., Espeseth T., Rootwelt H., Westlye L.T., Andersson M., Adolfsdottir S., Geitung J.T., Fjell A.M., Reinvang I., Lundervold A. Hippocampal volumes are important predictors for memory function in elderly women. BMC Med. Imaging. 2009;9:17. doi: 10.1186/1471-2342-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd D.A., Killgore W.D., Young A.D. Sex differences in cerebral tissue volume and cognitive performance during adolescence. Psychol. Rep. 2002;91:743–757. doi: 10.2466/pr0.2002.91.3.743. [DOI] [PubMed] [Google Scholar]
- Zhang L., Dean D., Liu J.Z., Sahgal V., Wang X., Yue G.H. Quantifying degeneration of white matter in normal aging using fractal dimension. Neurobiol. Aging. 2007;28:1543–1555. doi: 10.1016/j.neurobiolaging.2006.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.