Abstract
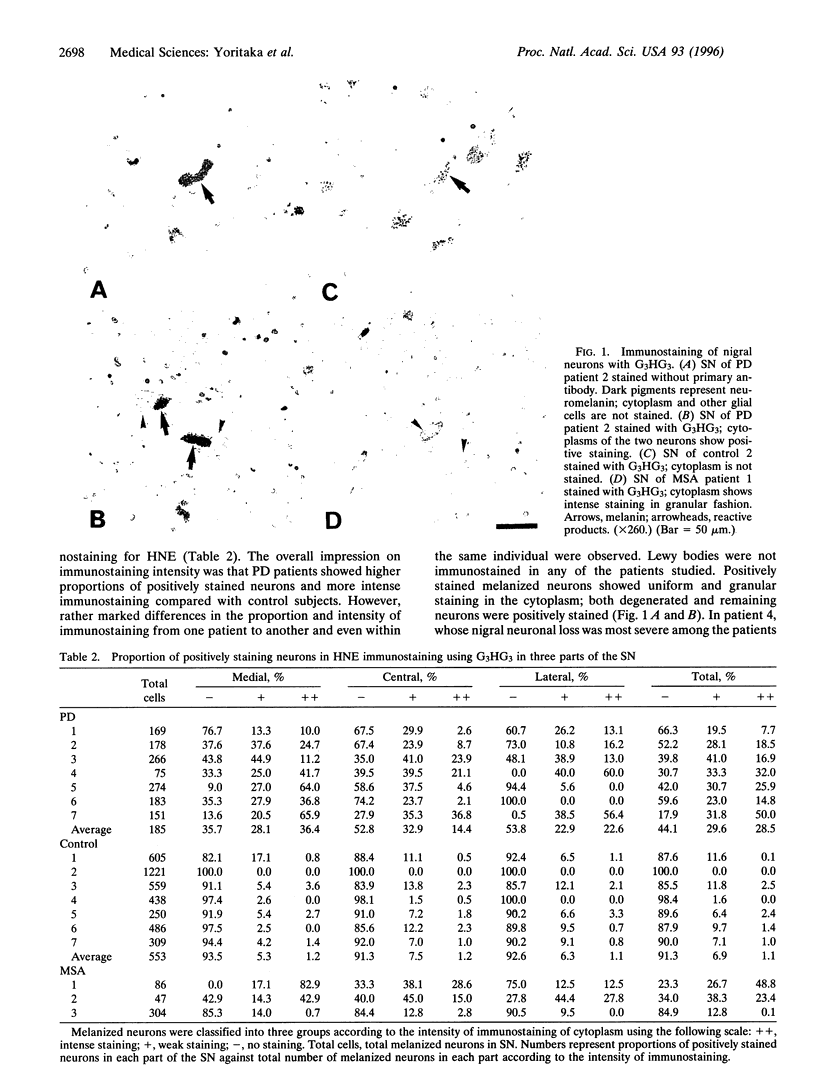
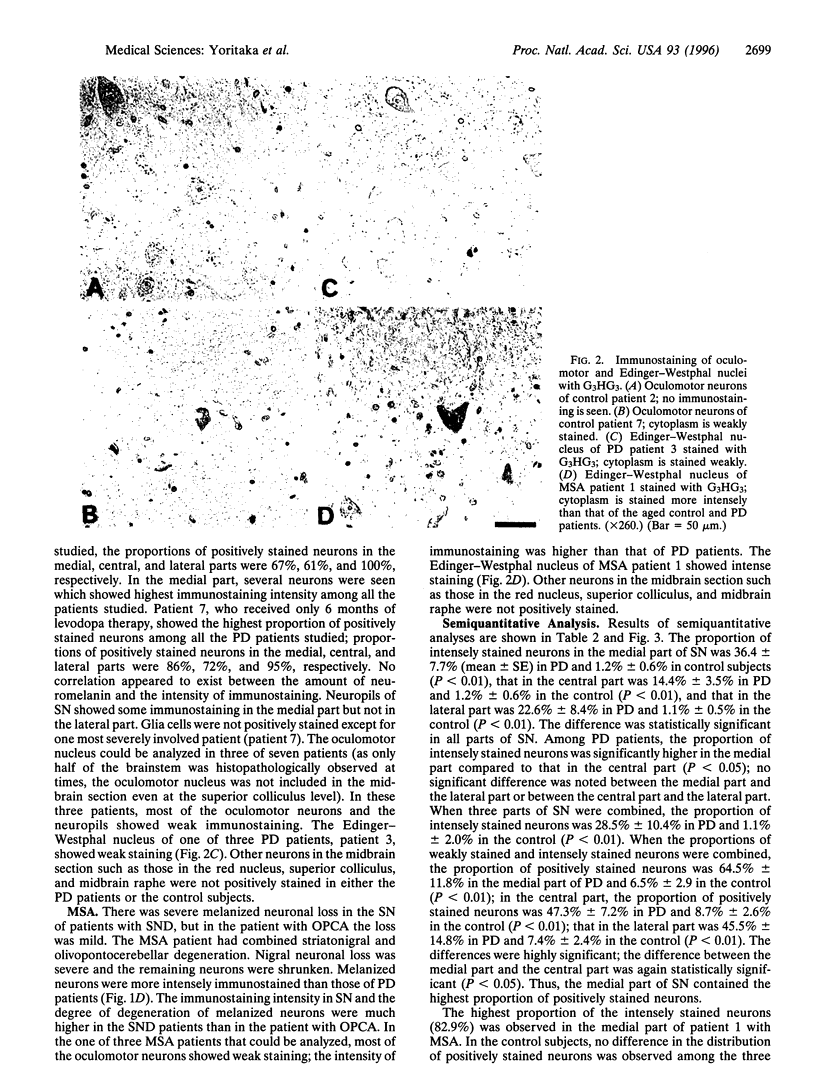
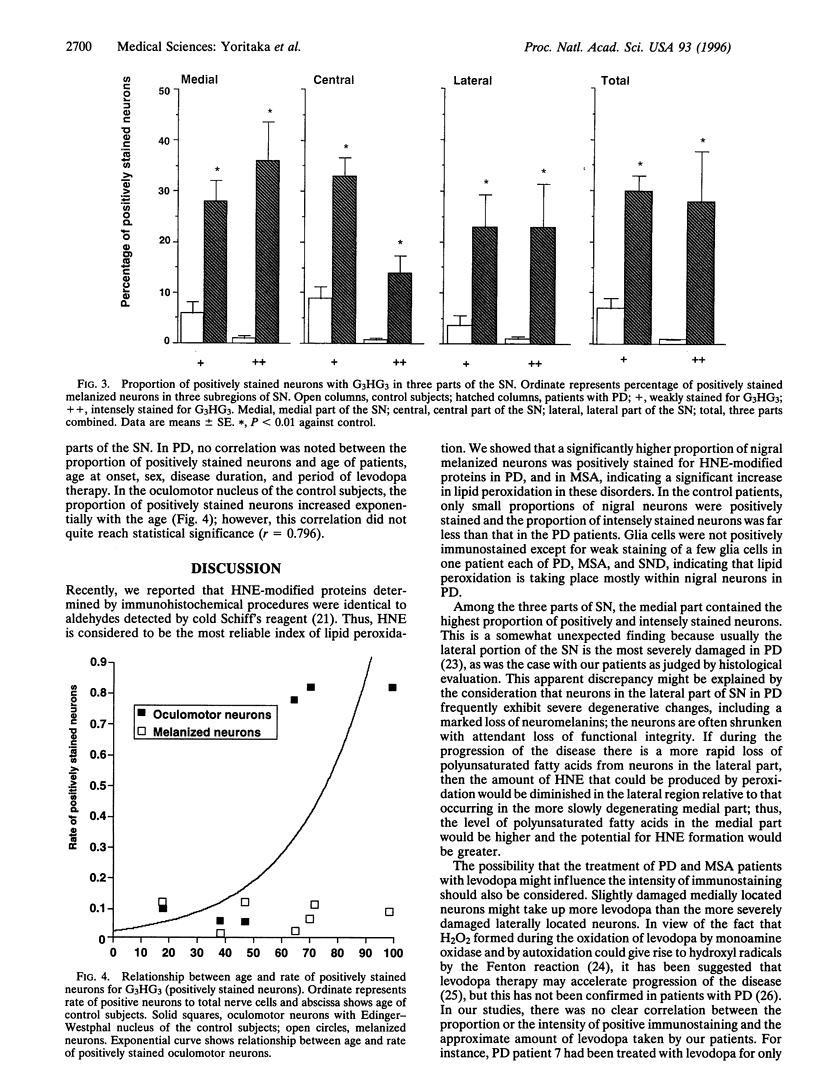
There is growing evidence that oxidative stress and mitochondrial respiratory failure with attendant decrease in energy output are implicated in nigral neuronal death in Parkinson disease (PD). It is not known, however, which cellular elements (neurons or glial cells) are major targets of oxygen-mediated damage. 4-Hydroxy-2-nonenal (HNE) was shown earlier to react with proteins to form stable adducts that can be used as markers of oxidative stress-induced cellular damage. We report here results of immunochemical studies using polyclonal antibodies directed against HNE-protein conjugates to label the site of oxidative damage in control subjects (ages 18-99 years) and seven patients that died of PD (ages 57-78 years). All the nigral melanized neurons in one of the midbrain sections were counted and classified into three groups according to the intensity of immunostaining for HNE-modified proteins--i.e., no staining, weak staining, and intensely positive staining. On average, 58% of nigral neurons were positively stained for HNE-modified proteins in PD; in contrast only 9% of nigral neurons were positive in the control subjects; the difference was statistically significant (Mann-Whitney U test; P < 0.01). In contrast to the substantia nigra, the oculomotor neurons in the same midbrain sections showed no or only weak staining for HNE-modified proteins in both PD and control subjects; young control subjects did not show any immunostaining; however, aged control subjects showed weak staining in the oculomotor nucleus, suggesting age-related accumulation of HNE-modified proteins in the neuron. Our results indicate the presence of oxidative stress within nigral neurons in PD, and this oxidative stress may contribute to nigral cell death.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Shachar D., Riederer P., Youdim M. B. Iron-melanin interaction and lipid peroxidation: implications for Parkinson's disease. J Neurochem. 1991 Nov;57(5):1609–1614. doi: 10.1111/j.1471-4159.1991.tb06358.x. [DOI] [PubMed] [Google Scholar]
- Dexter D. T., Carter C. J., Wells F. R., Javoy-Agid F., Agid Y., Lees A., Jenner P., Marsden C. D. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989 Feb;52(2):381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Dexter D. T., Wells F. R., Lees A. J., Agid F., Agid Y., Jenner P., Marsden C. D. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J Neurochem. 1989 Jun;52(6):1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Schaur R. J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Gibb W. R., Lees A. J. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1991 May;54(5):388–396. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good P. F., Olanow C. W., Perl D. P. Neuromelanin-containing neurons of the substantia nigra accumulate iron and aluminum in Parkinson's disease: a LAMMA study. Brain Res. 1992 Oct 16;593(2):343–346. doi: 10.1016/0006-8993(92)91334-b. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and the central nervous system: some fundamental questions. Is oxidant damage relevant to Parkinson's disease, Alzheimer's disease, traumatic injury or stroke? Acta Neurol Scand Suppl. 1989;126:23–33. doi: 10.1111/j.1600-0404.1989.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Hattori N., Tanaka M., Ozawa T., Mizuno Y. Immunohistochemical studies on complexes I, II, III, and IV of mitochondria in Parkinson's disease. Ann Neurol. 1991 Oct;30(4):563–571. doi: 10.1002/ana.410300409. [DOI] [PubMed] [Google Scholar]
- Hirsch E. C. Why are nigral catecholaminergic neurons more vulnerable than other cells in Parkinson's disease? Ann Neurol. 1992;32 (Suppl):S88–S93. doi: 10.1002/ana.410320715. [DOI] [PubMed] [Google Scholar]
- Jellinger K., Kienzl E., Rumpelmair G., Riederer P., Stachelberger H., Ben-Shachar D., Youdim M. B. Iron-melanin complex in substantia nigra of parkinsonian brains: an x-ray microanalysis. J Neurochem. 1992 Sep;59(3):1168–1171. doi: 10.1111/j.1471-4159.1992.tb08362.x. [DOI] [PubMed] [Google Scholar]
- Jenner P., Dexter D. T., Sian J., Schapira A. H., Marsden C. D. Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson's Disease Research Group. Ann Neurol. 1992;32 (Suppl):S82–S87. doi: 10.1002/ana.410320714. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Itagaki S., Akiyama H., McGeer E. G. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988 Oct;24(4):574–576. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Ohta S., Tanaka M., Takamiya S., Suzuki K., Sato T., Oya H., Ozawa T., Kagawa Y. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1450–1455. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- Mytilineou C., Han S. K., Cohen G. Toxic and protective effects of L-dopa on mesencephalic cell cultures. J Neurochem. 1993 Oct;61(4):1470–1478. doi: 10.1111/j.1471-4159.1993.tb13642.x. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Toyokuni S., Uchida K., Ogawa O., Takenewa J., Kakehi Y., Kinoshita H., Hattori-Nakakuki Y., Hiai H., Yoshida O. Formation of 8-hydroxy-2'-deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int J Cancer. 1994 Sep 15;58(6):825–829. doi: 10.1002/ijc.2910580613. [DOI] [PubMed] [Google Scholar]
- Perry T. L., Godin D. V., Hansen S. Parkinson's disease: a disorder due to nigral glutathione deficiency? Neurosci Lett. 1982 Dec 13;33(3):305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- Riederer P., Sofic E., Rausch W. D., Schmidt B., Reynolds G. P., Jellinger K., Youdim M. B. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem. 1989 Feb;52(2):515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Saggu H., Cooksey J., Dexter D., Wells F. R., Lees A., Jenner P., Marsden C. D. A selective increase in particulate superoxide dismutase activity in parkinsonian substantia nigra. J Neurochem. 1989 Sep;53(3):692–697. doi: 10.1111/j.1471-4159.1989.tb11759.x. [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Jenner P., Clark J. B., Marsden C. D. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989 Jun 3;1(8649):1269–1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Sian J., Dexter D. T., Lees A. J., Daniel S., Agid Y., Javoy-Agid F., Jenner P., Marsden C. D. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994 Sep;36(3):348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Carney J. M., Starke-Reed P. E., Oliver C. N., Stadtman E. R., Floyd R. A., Markesbery W. R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal R. S., Agarwal S., Dubey A., Orr W. C. Protein oxidative damage is associated with life expectancy of houseflies. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyokuni S., Uchida K., Okamoto K., Hattori-Nakakuki Y., Hiai H., Stadtman E. R. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2616–2620. doi: 10.1073/pnas.91.7.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K., Stadtman E. R. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4544–4548. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K., Toyokuni S., Nishikawa K., Kawakishi S., Oda H., Hiai H., Stadtman E. R. Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry. 1994 Oct 18;33(41):12487–12494. doi: 10.1021/bi00207a016. [DOI] [PubMed] [Google Scholar]
- Youdim M. B., Ben-Shachar D., Riederer P. Iron in brain function and dysfunction with emphasis on Parkinson's disease. Eur Neurol. 1991;31 (Suppl 1):34–40. doi: 10.1159/000116719. [DOI] [PubMed] [Google Scholar]





