Abstract
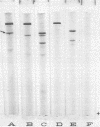
The relationship between a large molecular weight (9S) and a small molecular weight (4.5S, 60,000 molecular weight) species of phytochrome was examined to determine if the larger species was an aggregate of the smaller. Alterations of pH, salt concentration, or phytochrome concentration did not cause any observable formation of the large form from the small form. However, in partially purified phytochrome extracts from Secale cereale L. and Avena sativa L., the large form was converted to the small form over time at 4 C in the dark. This breakdown was inhibitable by the protease inhibitor phenylmethanesulfonyl fluoride. When highly purified large molecular weight rye phytochrome was incubated with a neutral protease isolated from etiolated oat shoots, the large phytochrome was converted to the small form without qualitative visible absorbancy changes. The effect of the oat protease could be mimicked by a wide variety of commercial endopeptidases, including trypsin. Examination of the trypsin-induced breakdown on sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed that as the size of the photoreversible unit changes from large to small, the size of its constituent polypeptide chains is reduced from 120,000 to 62,000 molecular weight. These experiments provide evidence that the endogenous breakdown observed in extracts is a result of contaminant protease and, consequently, that the small molecular weight species of phytochrome is an artifact due to proteolysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. R., Jenner E. L., Mumford F. E. Optical rotatory dispersion and circular dichroism spectra of phytochrome. Biochim Biophys Acta. 1970 Oct 20;221(1):69–73. doi: 10.1016/0005-2795(70)90198-4. [DOI] [PubMed] [Google Scholar]
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Briggs W. R. Long-lived Intermediates in Phytochrome Transformation I: In Vitro Studies. Plant Physiol. 1969 Aug;44(8):1081–1088. doi: 10.1104/pp.44.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W. R. Long-lived Intermediates in Phytochrome Transformation II: In Vitro and In Vivo Studies. Plant Physiol. 1969 Aug;44(8):1089–1094. doi: 10.1104/pp.44.8.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W. R., Siegelman H. W. Distribution of Phytochrome in Etiolated Seedlings. Plant Physiol. 1965 Sep;40(5):934–941. doi: 10.1104/pp.40.5.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W. R., Zollinger W. D., Platz B. B. Some Properties of Phytochrome Isolated From Dark-grown Oat Seedlings (Avena sativa L.). Plant Physiol. 1968 Aug;43(8):1239–1243. doi: 10.1104/pp.43.8.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C., Siegelman H. W. Nonphotochemical Transformations of Phytochrome in Vivo. Plant Physiol. 1963 Sep;38(5):514–519. doi: 10.1104/pp.38.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll D. L., Edwards J. L., Klein W. H., Shropshire W., Jr Phytochrome in etiolated annual rye. 3. Isolation of photoreversible phytochrome. Biochim Biophys Acta. 1968 Sep 10;168(1):36–45. doi: 10.1016/0005-2795(68)90231-6. [DOI] [PubMed] [Google Scholar]
- Correll D. L., Edwards J. L. The aggregation States of phytochrome from etiolated rye and oat seedings. Plant Physiol. 1970 Jan;45(1):81–85. doi: 10.1104/pp.45.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll D. L., Steers E., Jr, Towe K. M., Shropshire W., Jr Phytochrome in etiolated annual rye. IV. Physical and chemical characterization of phytochrome. Biochim Biophys Acta. 1968 Sep 10;168(1):46–57. doi: 10.1016/0005-2795(68)90232-8. [DOI] [PubMed] [Google Scholar]
- Cross D. R., Linschitz H., Kasche V., Tenenbaum J. Low-temperature studies on phytochrome: light and dark reactions in the red to far-red transformation and new intermediate forms of phytochrome. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1095–1101. doi: 10.1073/pnas.61.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett M. S., Briggs W. R. Some spectral properties of pea phytochrome in vivo and in vitro. Plant Physiol. 1970 Jun;45(6):679–683. doi: 10.1104/pp.45.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D. W., Butler W. L. Immunochemical and spectroscopic evidence for protein conformational changes in phytochrome transformations. Plant Physiol. 1970 May;45(5):567–570. doi: 10.1104/pp.45.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Tartakoff A. M., Law J. H. Cocoonase. I. Preliminary characterization of a proteolytic enzyme from silk moths. J Biol Chem. 1967 Apr 10;242(7):1477–1487. [PubMed] [Google Scholar]
- Kroes H. H. Reversible changes in the circular dichroism of phytochrome during photoisomerisation of the pigment. Biochem Biophys Res Commun. 1968 Jun 28;31(6):877–883. doi: 10.1016/0006-291x(68)90533-0. [DOI] [PubMed] [Google Scholar]
- Lam T. H., Shaw M. Removal of phenolics from plant extracts by grinding with anion exchange resin. Biochem Biophys Res Commun. 1970 Jun 5;39(5):965–968. doi: 10.1016/0006-291x(70)90418-3. [DOI] [PubMed] [Google Scholar]
- Linschitz H., Kasche V. KINETICS OF PHYTOCHROME CONVERSION: MULTIPLE PATHWAYS IN THE P(r) TO P(fr) REACTION, AS STUDIED BY DOUBLE-FLASH TECHNIQUE. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1059–1064. doi: 10.1073/pnas.58.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linschitz H., Kasche V. The kinetics of phytochrome conversion. J Biol Chem. 1966 Jul 25;241(14):3395–3403. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Moore G. L. Use of azo-dye-bound collagen to measure reaction velocities of proteolytic enzymes. Anal Biochem. 1969 Oct 15;32(1):122–127. doi: 10.1016/0003-2697(69)90111-0. [DOI] [PubMed] [Google Scholar]
- Mumford F. E., Jenner E. L. Catalysis of the phytochrome dark reaction by reducing agents. Biochemistry. 1971 Jan 5;10(1):98–101. doi: 10.1021/bi00777a015. [DOI] [PubMed] [Google Scholar]
- Mumford F. E., Jenner E. L. Purification and characterization of phytochrome from oat seedlings. Biochemistry. 1966 Nov;5(11):3657–3662. doi: 10.1021/bi00875a039. [DOI] [PubMed] [Google Scholar]
- Mumford F. E. Studies on the phytochrome dark reaction in vitro. Biochemistry. 1966 Feb;5(2):522–524. doi: 10.1021/bi00866a018. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Butler W. L. Phytochrome conversion by ultraviolet light. Photochem Photobiol. 1970 Jun;11(6):503–509. doi: 10.1111/j.1751-1097.1970.tb06021.x. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Butler W. L. Stabilization of phytochrome intermediates by low temperature. Photochem Photobiol. 1968 Nov;8(5):477–485. doi: 10.1111/j.1751-1097.1968.tb05891.x. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Butler W. L. The temperature dependence of phytochrome transformations. Photochem Photobiol. 1970 May;11(5):361–369. doi: 10.1111/j.1751-1097.1970.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Pringle J. R. The molecular weight of the undegraded polypeptide chain of yeast hexokinase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):46–52. doi: 10.1016/0006-291x(70)90755-2. [DOI] [PubMed] [Google Scholar]
- SIEGELMAN H. W., FIRER E. M. PURIFICATION OF PHYTOCHROME FROM OAT SEEDLINGS. Biochemistry. 1964 Mar;3:418–423. doi: 10.1021/bi00891a019. [DOI] [PubMed] [Google Scholar]
- Saeed S. A., Chadwick G. R., Mill P. J. Action of proteases on human plasma cholinesterase isoenzymes. Biochim Biophys Acta. 1971 Jan 19;229(1):186–192. doi: 10.1016/0005-2795(71)90332-1. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shaw E. Selective chemical modification of proteins. Physiol Rev. 1970 Apr;50(2):244–296. doi: 10.1152/physrev.1970.50.2.244. [DOI] [PubMed] [Google Scholar]
- Siegelman H. W., Wieczorek G. A., Turner B. C. Preparation of calcium phosphate for protein chromatography. Anal Biochem. 1965 Dec;13(3):402–404. doi: 10.1016/0003-2697(65)90332-5. [DOI] [PubMed] [Google Scholar]
- Walker T. S., Bailey J. L. Studies on phytochrome. Some properties of electrophoretically pure phytochrome. Biochem J. 1970 Dec;120(3):613–622. doi: 10.1042/bj1200613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Bailey J. L. Studies on phytochrome. Two photoreversible chromoproteins from etiolated oat seedlings. Biochem J. 1970 Dec;120(3):607–612. doi: 10.1042/bj1200607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whitaker J. R., Perez-Villase ñor J. Chemical modification of papain. I. Reaction with the chloromethyl ketones of phenylalanine and lysine and with phenylmethyl-sulfonyl fluoride. Arch Biochem Biophys. 1968 Mar 20;124(1):70–78. doi: 10.1016/0003-9861(68)90304-4. [DOI] [PubMed] [Google Scholar]