Highlights
-
•
Effects on immunobiochemical pathways of TiO2 materials were investigated in vitro.
-
•
TiO2 bulk and nanomaterial stimulated neopterin production in human PBMC.
-
•
There was no stimulatory influence of particles on tryptophan breakdown.
-
•
At high particles concentrations, tryptophan breakdown was suppressed.
-
•
Results suggest that the total effect of particles is even stronger pro-inflammatory.
Keywords: Titanium dioxide; Nanoparticles; Immune system; Neopterin; Indoleamine 2,3-dioxygenase
Abstract
Nanomaterials are increasingly produced and used throughout recent years. Consequently the probability of exposure to nanoparticles has risen. Because of their small 1–100 nm size, the physicochemical properties of nanomaterials may differ from standard bulk materials and may pose a threat to human health. Only little is known about the effects of nanoparticles on the human immune system. In this study, we investigated the effects of TiO2 nanoparticles and bulk material in the in vitro model of human peripheral blood mononuclear cells (PBMC) and cytokine-induced neopterin formation and tryptophan breakdown was monitored. Both biochemical processes are closely related to the course of diseases like infections, atherogenesis and neurodegeneration. OCTi60 (25 nm diameter) TiO2 nanoparticles and bulk material increased neopterin production in unstimulated PBMC and stimulated cells significantly, the effects were stronger for OCTi60 compared to bulk material, while P25 TiO2 (25 nm diameter) nanoparticles had only little influence. No effect of TiO2 nanoparticles on tryptophan breakdown was detected in unstimulated cells, whereas in stimulated cells, IDO activity and IFN-γ production were suppressed but only at the highest concentrations tested. Because neopterin was stimulated and tryptophan breakdown was suppressed in parallel, data suggests that the total effect of particles would be strongly pro-inflammatory.
1. Introduction
The use of nanomaterials in consumer products, electronics, sporting goods and medicine has grown enormously in the past years (Kaida et al., 2004; Thomas et al., 2006). In parallel, the potential exposure of humans to nanoparticles has increased. However, aside from many positive properties of nanomaterials, which make them desirable for many applications, they represent an unknown risk factor for human health, which needs to be investigated in more detail. Because of the small 1–100 nm size, which causes a huge increase of total surface area, the physicochemical properties of nanoparticles may considerably differ from bulk materials and may pose a threat to human cells. However it is still important to investigate possible harmful effects and potential risks of these materials on biochemical processes especially those mediated by the immune system.
Titanium dioxide (TiO2) crystallizes in three major different structures: anatase, rutile and brookite. However, only rutile and anatase play any role in the applications of TiO2 (Diebold, 2003). Anatase is more chemically reactive than rutile, and it has been suggested that anatase has a greater toxic potential than rutile (Warheit et al., 2007b; Sayes et al., 2006; Xue et al., 2010; Petkovic et al., 2011). TiO2 normally presents as a mixture of anatase and rutile crystal forms. The principal parameters of the particles affecting their physicochemical properties are shape, size, surface characteristics and inner structures. TiO2 fine particles are poorly soluble and have only little toxic features. However, smaller sized nanoparticles may exert different physicochemical properties with increased bioactivity compared to fine particles. Based on this fact, toxicity can rise and exhibit harmful effects on human health, associated with the decreased size of particles (Shi et al., 2013; Andersson et al., 2011; Wang and Li, 2012; Iavicoli et al., 2011, 2012).
TiO2, the most widely used nanoparticle, is a white pigment and has a very high refractive index. Four million tons TiO2 account for 70% of total production volume of pigments worldwide (Ortlieb, 2010; Baan et al., 2006). TiO2 nanoparticles are a promising material for many applications and are used in a multitude of consumer products (Valdiglesias et al., 2013; Zhang et al., 2012; Akhavan et al., 2013; Shi et al., 2013). Because of its low production costs, the photostability in solution, general non-toxicity, anticorrosive properties, high stability and redox selectivity, this metal oxide continues to gain further interest for novel applications and industrial use (Gupta and Tripathi, 2011; Riu et al., 2006). Actually TiO2 is used in sunscreens for UV protection, as a white dye in food and toothpaste or in dental and surgical implants (Lomer et al., 2002; Gélis et al., 2003; Olmedo et al., 2009; Lin et al., 2013; Brown and Clark, 2013; Rossi et al., 2008; Chung et al., 2013). At the interface to the biological medium, TiO2 corrosion and biodegradation may occur, causing the release of ions to the microenvironment and the possible harmful effects have to be investigated (Olmedo et al., 2009).
There are several studies, which analyzed the different adsorption and uptake routes in the human body. Most of the literature focuses on the respiratory system, which is the primary uptake route of nanoparticles (Shi et al., 2013; Lee et al., 2011; Mühlfeld et al., 2007). After the initial absorption of TiO2, the particles can be distributed to all organs and tissues in the body. However, the translocation of nanoparticles within the body is not well approved. An explanation can be the interaction with plasma-proteins or coagulation factors, platelets and red or white blood cells (Deng et al., 2009). The gastrointestinal absorption may be also an important route since nanoparticles are present in drug carriers, food products and beverages (Hagens et al., 2007; Lomer et al., 2002). Senzui et al. analyzed skin penetration on intact and injured skin studies and concluded that TiO2 did not penetrate the human skin (Senzui et al., 2010). There are few in vivo studies which investigate the genotoxicity of TiO2, however, one group found a correlation between nanoparticles and inflammation, which is caused by interleukin-(IL)-1β (Yazdi et al., 2010). Ciu et al. identified that TiO2 can generate liver injury via activation of toll like receptors (TLR), nuclear factor kappa B (NF-κB) and inflammation outbreak in mice (Cui et al., 2011). Another animal study found an altered mRNA expression of inflammatory cytokines like IL-6, IL-1β, tumor necrosis factor-(TNF)-α or transcription factor NF-κB (Ma et al., 2009).
In human epidermal cells TiO2 nanoparticles were found to exert genotoxicity via the induction of reactive oxygen species (ROS) (Shukla et al., 2011). Similarly neurotoxicological effects caused by exposure to TiO2 nanomaterials were detected in mice (Hu et al., 2010) and in human neuronal cells (Valdiglesias et al., 2013). Obviously these compounds are able to gain entry into the body and could exert potential toxic effects at several levels. The US Food and Drug Administration (FDA) established a regulation for TiO2 nanoparticles as a color additive for food (FDA, 2002). However, thus far effects of nanomaterials to the human immune system were still only rarely described.
During immune activation different types of immune responses can be distinguished by distinct types of T-helper (Th) cells like Th1- and Th2-cells (Jin et al., 2012; Romagnani, 2006). Th1-type cells are characterized by IL-2 and IFN-γ secretion and are primarily observed in the course of viral and microbial infections, malignant tumor diseases and allograft rejections after transplantation. Th2-type cells are predominantly responsible for allergic diseases and asthma (Romagnani, 2004). There exist also other subsets like Th-17, which link innate and adaptive immune responses (Yu and Gaffen, 2008), or regulatory T cells (Tregs), which play a role in the development of immunological self-tolerance (Hori et al., 2003).
In the course of a cellular immune response the pro-inflammatory cytokine IFN-γ is released preferentially from T-helper cells type 1. Aside from its immunoregulatory relevance, IFN-γ represents an important trigger for ROS production in human macrophages as part of its cytocidal and antimicrobial repertoire (Nathan et al., 1983). In parallel, IFN-γ induces the expression of the enzymes GTP-cyclohydrolase I (GCH-I) giving raise to the formation of neopterin and of indoleamine 2,3-dioxygenase (IDO). IDO catalyses the initial step in the breakdown of the essential amino acid tryptophan via the kynurenine pathway. A high IDO activity, which is estimated by the kynurenine to tryptophan ratio (Kyn/Trp), results from a strong cellular immune activation (Fuchs et al., 1991). Further, the estimation of neopterin production and tryptophan breakdown have been shown earlier to be robust read outs to monitor and investigate Th1-type immune response in vitro (Jenny et al., 2011).
For testing the effects of chemicals and compounds on the cellular immune response, an in vitro assay based on freshly isolated human peripheral blood mononuclear cells (PBMC) turned out to be useful (Winkler et al., 2006; Maier et al., 2010; Jenny et al., 2011). In this study applying the PBMC assay, we examined the influence of two different preparations of TiO2 nanoparticles and of commercial bulk material. Two types of TiO2 nanoparticles (anatase + rutile) were tested in order to compare effects regarding their different crystalline phase. In culture supernatants the production of neopterin and IFN-γ as well as the breakdown of tryptophan were examined.
2. Materials and methods
2.1. Chemicals
TiO2 bulk material was purchased from Sigma–Aldrich (Paris, France) and P25 nanomaterial was from Degussa-Evonik (Germany). OCTi60 are laboratory samples from the Service des Photons (Saclay, Gif-sur Yvette, France). Phytohemagglutinin (PHA) was purchased from Sigma–Aldrich (Vienna, Austria) and dissolved in phosphate buffered saline (PBS) and stored at −20 °C until use.
2.2. PBMC isolation and culture
PBMC were isolated from whole blood obtained from healthy donors, which confirmed that their donated blood might be used for scientific purposes in case, when it was not selected for transfusion. Separation of blood cells was performed by density centrifugation (Lymphoprep, Nycomed Pharma AS, Oslo, Norway) as described in more detail earlier (Jenny et al., 2011). After isolation, PBMC were washed three times in phosphate buffered saline containing 1 μmol/L ethylenediamine diacetate (EDTA). Cells were cultivated in RPMI 1640 supplemented with 10% heat-inactivated foetal calf serum (Biochrom, Berlin, Germany), 2 mmol/L l-glutamine (Serva, Heidelberg, Germany) and 50 μg/ml gentamicin (Bio-Whittaker, Walkersville, MD) at 37 °C in a humidified atmosphere containing 5% CO2.
2.3. Preparation of nanoparticles
In this study, we used TiO2 nanopowders from commercial source (P25, Degussa-Evonik, Germany) as well as a laboratory sample (OCTi60, France). These samples are synthesized by gas phase methods, combustion (P25) or laser pyrolysis (OCTi60) (Pignon et al., 2008) and are composed of a major phase of anatase and a minor phase of rutile.
The preparation of suspensions was done using a several steps method detailed elsewhere (Carrière et al., 2013). A stock solution was prepared using a one hundred mg of TiO2 material manually mixed with 10 ml of sterile filtered water. The suspension was sonicated by a high intensity sonicator for a total time of 1 h at 5 °C (Sonicator Model 750 series autotune watts ref 75043; pulsed 0.1 s on, 0.1 s off). 1.25 ml of resulting suspension was diluted in 3.75 ml foetal calf serum (FCS, =2 mg/ml) and mixed for 1.5–2 h using a magnetic bar. Average hydrodynamic size, size distribution and zeta potential of particles in suspension were determined by Photon Correlation Spectroscopy (PCS) using a Zetasizer (Nano-3000 HS, Malvern Instruments Ltd., Malvern, UK). One ml of the intermediate preparation was diluted in 9 ml of culture medium RPMI 1640 containing 10% FCS and mixed for 1.5–2 h using a magnetic bar. In addition to PCS measurements, the stability of suspensions was explored by turbidimetry using a Turbiscan (Formulaction).
2.4. PBMC assay
Isolated PBMC in supplemented RPMI 1640 medium were seeded in 48 well plates at a density of 1.5 × 106 cells/ml. PBMC were exposed to increasing doses of P25 (85% anatase; Degussa-Evonik, Germany) and OCTi60 (90% anatase; Laboratory samples, Paris, France) TiO2 nanoparticles and commercially obtained TiO2 bulk material (Sigma–Aldrich) for 30 min. Afterwards PBMC where either stimulated with 10 μg/ml PHA or left untreated. After 48 h incubation time with nanoparticles, cell culture supernatants were collected and submitted to analytical measurements as described (Jenny et al., 2011). PBMC were prepared freshly from blood of in total six different healthy donors and each of the six single experiments was run in duplicates.
Cell viability was assessed by cell titer blue (CTB) assay (Promega, Madison, WI). PBMC showed no change in viability after nanoparticle exposure. These results are in accordance with earlier observations made by others using the same nanomaterials (Valdiglesias et al., 2013).
2.5. Determination of tryptophan, kynurenine, neopterin and interferon-γ concentrations
A reverse phase high performance liquid chromatography (HPLC) method was applied to measure concentrations of tryptophan and kynurenine (Pro-Star Varian, Palo Alto, CA) with 3-nitro-l-tyrosine as internal standard (Widner et al., 1997; Laich et al., 2002). 100 μl of supernatant, 100 μl of internal standard, 500 μmol/L 3-nitro-l-tyrosine and 25 μl of 2 mol/L trichloroacetic acid were vortexed and centrifuged to precipitate proteins. The external standard consisted of an albumin-based mixture of 50 μmol/L tryptophan and 10 μmol/L kynurenine and underwent the same procedures as supernatant specimens. The elution buffer contained 15 mmol/L acetic acid-sodium acetate (pH = 4.0) with 50 ml/L methanol. Kynurenine and 3-nitro-l-tyrosine concentrations were determined by means of their UV absorption at 360 nm wavelength (SPD-6A, Shimadzu, Korneuburg, Austria). Tryptophan was monitored by its fluorescence at 286 nm excitation and 366 nm emission wavelengths (ProStar 360, Varian, Palo Alto, CA). Kyn/Trp was calculated as an index of tryptophan breakdown and expressed as μmol/mmol that estimates IDO activity in PBMC (Widner et al., 1997).
All chemicals used for HPLC measurements were obtained from Sigma–Aldrich and were of the highest available purity grade. The sensitivity of the measurements is 0.5 μmol/L kynurenine and 0.1 μmol/L tryptophan.
2.6. ELISAs for measurement of neopterin and IFN-γ concentrations
Neopterin and IFN-γ concentrations were determined by ELISA (BRAHMS, Hennigsdorf, Germany, and R&D International, Biomedica, Vienna, Austria) following the manufacturers’ instructions. Sensitivity of the neopterin test was 2 nmol/L, and 8 pg/ml for IFN-γ. The culture supernatants of PHA-stimulated PBMC were diluted 1:10 before the IFN-γ ELISA.
2.7. Statistics
Results are expressed as percent of unstimulated and PHA-stimulated control and are shown as means ± standard error of the mean (S.E.M.). For statistical evaluation of the data, PASW Statistics 18.0 and SPSS version 19 software were-used. Because some of the data sets did not show normal distribution, group comparisons were performed using non parametric Friedman- and Mann–Whitney U-test. To calculate the strength and the direction of associations, Spearman Rank Oder Correlation was performed. P-values <0.05 were considered to indicate significances.
3. Results
3.1. Characterization of nanoparticles
The samples differ mainly by their average diameter as shown in Table 1. The hydrodynamic measurements in water suspension show a smaller average diameter of the laboratory sample compared to P25 TiO2. The z-average values in the range of 100 nm indicate the presence of a limited amount of agglomerates in the suspension. The hydrodynamic increased in both cases after transfer to the biologic medium with a decrease of the zeta potential. It is −9 mV just after transfer to the medium and still decreases to −12 mV after 48 h. On this time period, no significant change is seen in the signal of the turbidimeter which indicates good stability of both suspensions.
Table 1.
Main characteristics of TiO2 nanoparticles P25 and OCTi60.
| Anatase/rutile | Diameter (nm) (TEM) | Diameter (nm) (BET) | Diameter in water Z average | Z-potential in water (mV) | Diameter in medium Z average | Z-potential in medium (mV) | |
|---|---|---|---|---|---|---|---|
| P25 | 85/15 | 23 | 25 (60 m2/g) | 140 | +1 | 220 | −8 to −12 |
| OCTi60 | 90/10 | 10 | 16 (95 m2/g) | 70 | −6 | 170 | −9 to −12 |
3.2. Concentrations at baseline
Baseline concentrations of neopterin were 2.47 + 0.13 nmol/L, tryptophan and kynurenine concentrations were 28.3 + 1.18 and 0.84 + 0.05 μmol/L resulting in 30.2 + 3.07 μmol/mmol Kyn/Trp (all mean + S.E.M.). Upon stimulation of PBMC with PHA, neopterin formation and tryptophan breakdown increased significantly (neopterin: 7.06 + 0.96 nmol/L, Kyn/Trp: 2494 + 475 μmol/mmol, all p < 0.01).
3.3. Influence of TiO2 materials on neopterin production and tryptophan breakdown
In unstimulated cells, OCTi60 nanomaterial dose-dependently enhanced neopterin formation, bulk material had a similar but slightly smaller effect (Fig. 1). In PHA-stimulated cells similar results were obtained: bulk material and OCTi60 increased the neopterin production (Fig. 2, all p < 0.05). The OCTi60 TiO2 nanoparticles had a stronger effect compared to the bulk material. There was only a small but still significant effect on neopterin production of the P25 TiO2 nanoparticles.
Fig. 1.
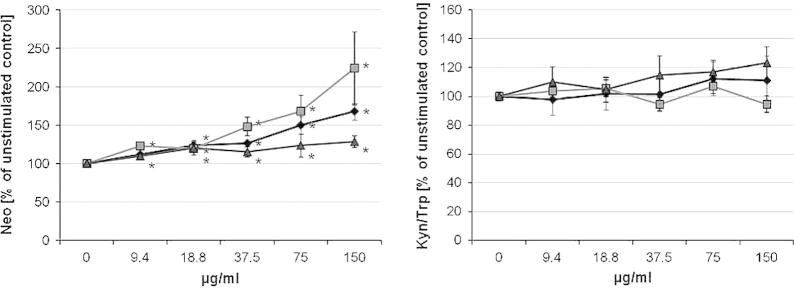
Influence of TiO2 nanoparticles (OCTi60, gray squares, and P25, gray triangles) and bulk material (filled diamonds) on neopterin production as well as tryptophan breakdown indicated as the kynurenine to tryptophan ratio (Kyn/Trp) in umstimulated peripheral blood mononuclear cells (PBMC). Results indicate mean values + S.E.M. of 3 separate experiments performed in duplicates using PBMC of 3 different donors.
Fig. 2.
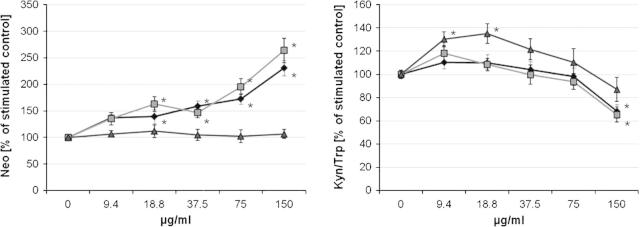
Influence of TiO2 nanoparticles (OCTi60, gray squares, and P25, gray triangles) and bulk material (filled diamonds) on neopterin production as well as tryptophan breakdown indicated as the kynurenine to tryptophan ratio (Kyn/Trp) in peripheral blood mononuclear cells (PBMC) stimulated with mitogen phytohaemagglutinin. Results indicate mean values + S.E.M. of 3 separate experiments performed in duplicates using PBMC of 3 different donors.
There was no significant influence of TiO2 preparations on tryptophan breakdown, e.g. Kyn/Trp, tryptophan and kynurenine concentrations remained unchanged in unstimulated cells (Fig. 1). However in stimulated cells all TiO2 preparations exerted a biphasic effect: at low concentrations (9.4 and 18.8 μg/ml) OCTi60 and bulk material stimulated the tryptophan breakdown significantly (data not shown). The increases of kynurenine and Kyn/Trp, which were observed with low concentrations of nanomaterials, were only rarely significant, but when PBMC were pre-exposed to the highest applied dose of 150 μg/ml TiO2 OCTi60 nanoparticles and bulk material, Kyn/Trp was significantly suppressed (Fig. 2). There was no significant effect with P25 TiO2 nanoparticles.
Accordingly, there existed inverse associations between neopterin and Kyn/Trp concentrations in cells exposed to OCTi60 (p < 0.05) and bulk TiO2 material (p < 0.01) in PHA-stimulated PBMC. This correlation was positive but not significant for the P25 TiO2 nanomaterial (p > 0.05).
3.4. Influence on IFN-γ production
PBMC stimulated with PHA released higher levels of IFN-γ, compared to unstimulated cells. Treatment with TiO2 nanoparticles resulted in a lower IFN-γ production compared to untreated cells (details not shown). However at low treatment concentrations with bulk material, P25 or OCTi60 TiO2 nanomaterials (9.4 and 18.8 μg/ml) a trend to higher IFN-γ secretion compared to PHA stimulation only was observed, although without statistical significance due to high interassay variability and low sample number.
There existed a positive correlation between IFN-γ and Kyn/Trp concentrations. Vice versa an inverse association between IFN-γ and neopterin was apparent for bulk material and p25 nanoparticles.
4. Discussion
The whitening and photocatalytic effects of TiO2 nanoparticles are used in a wide range of consumer products (Thomas et al., 2006). Because of their small size, TiO2 nanoparticles are able to move in the respiratory system after inhalation to the alveolar region or can be absorbed after food intake in the gastrointestinal tract, where they can diffuse to other tissues like lymphoid tissues and can get in contact to cells of the immune system. Geiser and Kreyling reported that particles in the alveolar region are able to cross the air-blood-barrier and migrate from the blood to the liver, the lymphatic system to other organs and tissues or are eliminated out of the body (Geiser and Kreyling, 2010; Shi et al., 2013). Also, particles are taken-up and accumulated in immune cells, which are located in epithelial tissues, e.g. in cells of Peyer’s patches (Becker et al., 2012). In addition, there are several in vitro studies that examined the toxicity of TiO2 in mammalian cells, for example oxidative DNA damage and apoptosis after exposure of TiO2 in human liver cells (Shukla et al., 2013) or induced single strand breaks, oxidative lesions to DNA and oxidative stress in A459 cells after exposure of TiO2 (Jugan et al., 2012). Kang et al. published the induction of apoptosis in human lymphocytes after exposure to TiO2 nanoparticles (Kang et al., 2009).
Due to the fact that nanoparticles can reach the blood, and get in close contact with the cells of the immune system, it is of utmost importance to investigate the particle-cell interaction and the influence on the immune system in more detail.
Potential immunomodulatory effects of particles might be involved in the development of severe pathological conditions such as hypersensitivities, allergies, inflammatory respiratory and gastrointestinal diseases, but also in processes such as failure of dental or surgical implants (Siddiqi et al., 2011).
In our study with freshly isolated PBMC, an effect on the human immune system was observed. The investigated TiO2 materials had a dose-dependent effect on the induction of neopterin release (Fig. 1). In contrast, tryptophan breakdown was not significantly influenced by TiO2 particles at any concentrations. The concentrations used in our experiments are comparable to the concentrations, which are used in sunscreens (Tan et al., 1996; Bennat and Müller-Goymann 2000) and to those from other studies for genotoxicity tests (Petkovic et al., 2011; Warheit et al., 2007a; Kang et al., 2008, 2009).
Results of our study showed that OCTi60 TiO2 nanoparticles and bulk material stimulated dose-dependently the formation of neopterin in PBMC, whereas P25 TiO2 nanoparticles had no significant effect on the neopterin formation. Donaldson et al. already have mentioned that ultrafine particles (<20 nm) like TiO2 had a deleterious effect on phagocytosis of macrophages, compared with larger particles (ca. 200 nm) (Donaldson et al., 2001). Like in our study, smaller ultrafine particles had a greater effect on PBMC. Basically a relationship seems to exist between the surface area of nanoparticles and the enhanced ROS generating capability and pro-inflammatory effects. Interestingly also the bulk material elicited a similar effect on the PBMC as the OCTi60 nanoparticles, but revealed different results as compared with the P25 nanomaterial. It is unclear whether different crystal structures of the nanomaterials were important for the different effects, or the differences in the specific surface area or hydrodynamic diameter or zeta potential were critical. For example the stimulation effect of OCTi60 vs. P25 material was associated with a much larger specific surface area of OCTi60 nanoparticles. Still it has to be kept in mind that also TiO2 bulk material had a stimulation effect albeit somewhat weaker than TiO2 OCTi60 nanoparticles. Still the stimulation effect of TiO2 nanomaterial can be of greater pathophysiological relevance, because of the easier penetration through cellular membranes and barriers as compared with larger TiO2 agglomerates (Shi et al., 2013; Oberdörster et al., 1994).
Culture medium may promote agglomeration of nanomaterials and in addition an increase in the hydrodynamic sizes was observed in an earlier study using the same TiO2 nanomaterial (Valdiglesias et al., 2013). However, in that study the graphs obtained for the TiO2 nanoparticles still indicated a good dispersion and are in line with the unchanged zeta potential in complete medium, which was found for the same nanoparticles in other previous studies (Wu et al., 2010; Shukla et al., 2011). Moreover, for the testing of compounds using PBMC in vitro, the first 30 min. of exposure seem to be most important, because effects on cytokine cascades can be detected within short periods of exposure (Jenny et al., 2011).
ROS generation and oxidative stress may partially explain toxic effects of nanomaterials (Nel et al., 2006; Wu et al., 2010; Shukla et al., 2011; Jin et al., 2011; Wan et al., 2012). Nanoparticles are considered to generate free radicals and to induce oxidative stress (Simko and Mattsson, 2010). Particularly, TiO2 nanoparticles were reported to induce oxidative stress in many cell types (Wu et al., 2010), among them human bronchial epithelial cells (Gurr et al., 2005) and hepatocytes (Petkovic et al., 2011), but also mouse microglia (Long et al., 2006, 2007) and human liver cells (Shukla et al., 2013). While in earlier studies the genotoxic effects induced by nanoparticles were quite similar, TiO2 OCTi60 nanoparticles appeared to be more effective in inducing cell cycle alterations, which corresponded to their higher cellular uptake (Valdiglesias et al., 2013).
In human body fluids, higher concentrations of neopterin indicate cellular immune activation, which are detected in diseases like infections and cancer but also parallel the course of atherogenesis and neurodegeneration (Schroecksnadel et al., 2005, 2006; Fuchs et al., 1992, 2009; De Rosa et al., 2011). Moreover higher levels predict adverse outcome in patients suffering from such diseases (Capuron et al., 2013). A recently performed pilot study performed in zinc-exposed galvanization workers an influence on neopterin levels and tryptophan breakdown has been described recently (Sarac et al., 2013).
The increase of neopterin production upon treatment of PBMC with TiO2 implies that the compounds induce pro-inflammatory immunoregulatory pathways in T-cells and macrophages. As a consequence, they can elicit the production of ROS and contribute to the development of oxidative stress with all its eventual negative consequences (Hoffmann et al., 2003). However, a stimulatory effect was only seen on the neopterin production rate whereas the rates of tryptophan breakdown and IFN-γ production rather declined with increasing exposure to TiO2 materials. Data suggests that there is no direct effect of materials on the T-cells, which would stimulate IFN-γ and neopterin production as well as parallel tryptophan breakdown in monocytes/macrophages. Rather TiO2 materials target macrophages directly. Basically, there seems to exist a relationship between the surface area of nanoparticles and the enhanced ROS generating capability and pro-inflammatory effects. However, Kyn/Trp data suggests a strong relation of the effects with local particle concentration. Of note, also low particle concentrations are able to elicit strong biological responses, and low dose responses may differ from high dose responses, as it was shown for Kyn/Trp that indicates IDO activity.
Mano et al. reported the involvement of toll like receptor 4 (TLR4) signaling in the inflammatory response elicited by TiO2 nanoparticles in human pulmonary epithelial cell lines (Mano et al., 2013). Accordingly, compounds may promote ROS production and thereby activate translocation of the signal transduction element nuclear factor-κB (NF-kB), which is associated with the induction of pro-inflammatory pathways that are also accompanied by neopterin formation (Hoffmann et al., 2003; Schroecksnadel et al., 2010). In agreement, free radical production was already demonstrated to be elicited by TiO2 nanoparticles in aqueous suspension (Hirakawa et al. 2004). It could be possible that PBMC are activated through ROS, but further investigations are still required to confirm this assumption.
The respiratory system, blood, central nervous system (CNS), gastrointestinal (GI) tract and skin have been shown to be targeted by nanoparticles (Medina et al. 2007). TiO2 nanomaterial was found to affect the ion balance and cause calcification in brain mouse models. The dystrophic calcification was a result of inflammation or damage to the site, and the imbalance of ion levels could be caused by TiO2 accumulation in several structures of the brain (Hu et al., 2010). It appears plausible that monocyte-derived macrophages activate inflammation processes in face of accumulating TiO2 nanoparticles or a disturbance of the ion balance – but further investigations are needed to resolve this relationship.
The inhibitory influence of TiO2 materials on IDO activity suggests that the net effect of the tested particles would be even stronger pro-inflammatory, when in parallel to the pro-oxidative insult on macrophages mediated by, e.g., neopterin, the immunosuppressive activity of IDO is absent. With this background, exposure to TiO2 bulk and nanomaterials potentially elicits immune responses and this may contribute to the development and progression of certain immunopathologies. However, our data can be considered only as a pilot study, and such an effect has to be confirmed in a living organism before such a conclusion can be taken. Clearly, further experiments are needed to explore effects of materials on monocytes-macrophages.
Earlier investigations using this PBMC in vitro system implied that antioxidant compounds down-regulate Th1-type immune response (Jenny et al., 2011). However, it has to be shown if the ability of TiO2 materials, promote Th1-type immune response, is due to a pro-oxidant property of compounds. The influence of TiO2 on neopterin production and tryptophan breakdown was quite different, while the pro-oxidative nature of TiO2 seems to be of greater importance for enhanced neopterin production. The consequences of the combined effects of particles to promote neopterin production and at the same time suppress IDO activity and its effect on the success of immune response still has to be elucidated in more detail.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Transparency document associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.fct.2013.12.018.
Acknowledgement
Support by the Austrian Research Funds (Project 25150-B13) and the European Commission (ERA NET–New INDIGO Program, NanoLINEN is gratefully acknowledged. The authors thank Mrs. Maria Pfurtscheller and Mr. Thomas Nuener for excellent technical assistance.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Akhavan Sadr F., Montazer M. In situ sonosynthesis of nano TiO2 on cotton fabric. Ultrason. Sonochem. 2013;S1350–4177:00229. doi: 10.1016/j.ultsonch.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Andersson P.O., Lejon C., Ekstrand-Hammarström B., Akfur C., Ahlinder L., Bucht A., Osterlund L. Polymorph- and size-dependent uptake and toxicity of TiO2 nanoparticles in living lung epithelial cells. Small. 2011;7:514–523. doi: 10.1002/smll.201001832. [DOI] [PubMed] [Google Scholar]
- Baan R., Straif K., Grosse Y., Secretan B., El Ghissassi F., Cogliano V. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol. 2006;7:295–296. doi: 10.1016/s1470-2045(06)70651-9. [DOI] [PubMed] [Google Scholar]
- Becker H.M., Bertschinger M.M., Rogler G. Microparticles and their impact on intestinal immunity. Dig. Dis. 2012;30(Suppl. 3):47–54. doi: 10.1159/000342602. [DOI] [PubMed] [Google Scholar]
- Bennat C., Müller-Goymann C.C. Skin penetration and stabilization of formulations containing microfine titanium dioxide as physical UV filter. Int. J. Cosmet. Sci. 2000;22:271–283. doi: 10.1046/j.1467-2494.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- Brown S., Clark R.J. Anatase: important industrial white pigment and date-marker for artwork. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013;110:78–80. doi: 10.1016/j.saa.2013.03.041. [DOI] [PubMed] [Google Scholar]
- Capuron, L., Geisler, S., Kurz, K., Leblhuber, F., Sperner-Unterweger, B., Fuchs, D., in press. Activated immune system and inflammation in healthy ageing: relevance for tryptophan and neopterin metabolism. Curr. Pharm. Des. [DOI] [PubMed]
- Carrière M., Pigeot-Rémy S., Casanova A., Dhawan A., Lazzaroni J.C., Guillard C., Herlin-Boime N. Impact of titanium dioxide nanoparticle dispersion state and dispersion method on their toxicity towards a549 lung cells and Escherichia coli bacteria. J. Translat. Toxicol. 2013;1:1–11. [Google Scholar]
- Chung C.J., Su R.T., Chu H.J., Chen H.T., Tsou H.K., He J.L. Plasma electrolytic oxidation of titanium and improvement in osseointegration. J. Biomed. Mater. Res. B Appl. Biomater. 2013;101:1023–1030. doi: 10.1002/jbm.b.32912. [DOI] [PubMed] [Google Scholar]
- Cui Y., Liu H., Zhou M., Duan Y., Li N., Gong X., Hu R., Hong M., Hong F. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J. Biomed. Mater. Res. A. 2011;96:221–229. doi: 10.1002/jbm.a.32976. [DOI] [PubMed] [Google Scholar]
- De Rosa S., Cirillo P., Pacileo M., Petrillo G., D’Ascoli G.L., Maresca F., Ziviello F., Chiariello M. Neopterin: from forgotten biomarker to leading actor in cardiovascular pathophysiology. Curr. Vasc. Pharmacol. 2011;9:188–199. doi: 10.2174/157016111794519372. [DOI] [PubMed] [Google Scholar]
- Deng Z.J., Mortimer G., Schiller T., Musumeci A., Martin D., Minchin R.F. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology. 2009;20:455101. doi: 10.1088/0957-4484/20/45/455101. [DOI] [PubMed] [Google Scholar]
- Diebold U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003;48:53–229. [Google Scholar]
- Donaldson K., Stone V., Clouter A., Renwick L., MacNee W. Ultrafine particles. Occup. Environ. Med. 2001;58:211–216. doi: 10.1136/oem.58.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA), 2002. Listing of color additives exempt from certification. In: 21-Food and Drugs. Code of Federal Regulations, 21 C.F.R. 73.2575.
- Fuchs D., Avanzas P., Arroyo-Espliguero R., Jenny M., Consuegra-Sanchez L., Kaski J.C. The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr. Med. Chem. 2009;16:4644–4653. doi: 10.2174/092986709789878247. [DOI] [PubMed] [Google Scholar]
- Fuchs D., Weiss G., Reibnegger G., Wachter H. The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious, and malignant diseases. Crit. Rev. Clin. Lab. Sci. 1992;29:307–341. doi: 10.3109/10408369209114604. [DOI] [PubMed] [Google Scholar]
- Fuchs D., Möller A.A., Reibnegger G., Werner E.R., Werner-Felmayer G., Dierich M.P., Wachter H. Increased endogenous interferon-gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol. Lett. 1991;28:207–211. doi: 10.1016/0165-2478(91)90005-u. [DOI] [PubMed] [Google Scholar]
- Geiser M., Kreyling W.G. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 2010;7:2. doi: 10.1186/1743-8977-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélis C., Girard S., Mavon A., Delverdier M., Paillous N., Vicendo P. Assessment of the skin photoprotective capacities of an organo-mineral broad-spectrum sunblock on two ex vivo skin models. Photodermatol. Photoimmunol. Photomed. 2003;19:242–253. doi: 10.1034/j.1600-0781.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- Gupta S.M., Tripathi M. A review of TiO2 nanoparticles. Phys. Chem. 2011;56:1639–1657. [Google Scholar]
- Gurr J.R., Wang A.S., Chen C.H., Jan K.Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. 2005;213:66–73. doi: 10.1016/j.tox.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Hagens W.I., Oomen A.G., de Jong W.H., Cassee F.R., Sips A.J. What do we (need to) know about the kinetic properties of nanoparticles in the body? Regul. Toxicol. Pharmacol. 2007;49:217–229. doi: 10.1016/j.yrtph.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Hirakawa K., Mori M., Yoshida M., Oikawa S., Kawanishi S. Photo-irradiated titanium dioxide catalyzes site specific DNA damage via generation of hydrogen peroxide. Free Radic. Res. 2004;38:439–447. doi: 10.1080/1071576042000206487. [DOI] [PubMed] [Google Scholar]
- Hoffmann G., Wirleitner B., Fuchs D. Potential role of immune system activation associated production of neopterin derivatives in humans. Inflamm. Res. 2003;52:313–321. doi: 10.1007/s00011-003-1181-9. [DOI] [PubMed] [Google Scholar]
- Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Hu R., Gong X., Duan Y., Li N., Che Y., Cui Y., Zhou M., Liu C., Wang H., Hong F. Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials. 2010;31:8043–8050. doi: 10.1016/j.biomaterials.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Iavicoli I., Leso V., Fontana L., Bergamaschi A. Toxicological effects of titanium dioxide nanoparticles: a review of in vitro mammalian studies. Eur. Rev. Med. Pharmacol. Sci. 2011;15:481–508. [PubMed] [Google Scholar]
- Iavicoli, I., Leso, V., Bergamaschi, A., 2012. Toxicological Effects of Titanium Dioxide Nanoparticles: A Review of In Vivo Studies. J. Nanomater. Article ID 964381. [PubMed]
- Jenny M., Klieber M., Zaknun D., Schroecksnadel S., Kurz K., Ledochowski M., Schennach H., Fuchs D. In vitro testing for anti-inflammatory properties of compounds employing peripheral blood mononuclear cells freshly isolated from healthy donors. Inflamm. Res. 2011;60:127–135. doi: 10.1007/s00011-010-0244-y. [DOI] [PubMed] [Google Scholar]
- Jin B., Sun T., Yu X.H., Yang Y.X., Yeo A.E. The effects of TLR activation on T-cell development and differentiation. Clin. Dev. Immunol. 2012;2012:836485. doi: 10.1155/2012/836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Tang Y., Yang F.G., Li X.L., Xu S., Fan X.Y., Huang Y.Y., Yang Y.J. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biol. Trace Elem. Res. 2011;141:3–15. doi: 10.1007/s12011-010-8707-0. [DOI] [PubMed] [Google Scholar]
- Jugan M.L., Barillet S., Simon-Deckers A., Herlin-Boime N., Sauvaigo S., Douki T., Carriere M. Titanium dioxide nanoparticles exhibit genotoxicity and impair DNA repair activity in A549 cells. Nanotoxicology. 2012;6:501–513. doi: 10.3109/17435390.2011.587903. [DOI] [PubMed] [Google Scholar]
- Kaida T., Kobayashi K., Adachi M., Suzuki F. Optical characteristics of titanium oxide interference film and the film laminated with oxides and their applications for cosmetics. J. Cosmet. Sci. 2004;55:219e20. [PubMed] [Google Scholar]
- Kang S.J., Kim B.M., Lee Y.J., Chung H.W. Titanium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ. Mol. Mutagen. 2008;49:399–405. doi: 10.1002/em.20399. [DOI] [PubMed] [Google Scholar]
- Kang S.J., Kim B.M., Lee Y.J., Hong S.H., Chung H.W. Titanium dioxide nanoparticles induce apoptosis through the JNK/p38-caspase-8-Bid pathway in phytohemagglutinin-stimulated human lymphocytes. Biochem. Biophys. Res. Commun. 2009;386:682–687. doi: 10.1016/j.bbrc.2009.06.097. [DOI] [PubMed] [Google Scholar]
- Laich A., Neurauter G., Widner B., Fuchs D. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin. Chem. 2002;48:579–581. [PubMed] [Google Scholar]
- Lee J.H., Kwon M., Ji J.H., Kang C.S., Ahn K.H., Han J.H., Yu I.J. Exposure assessment of workplaces manufacturing nanosized TiO2 and silver. Inhal. Toxicol. 2011;23:226–236. doi: 10.3109/08958378.2011.562567. [DOI] [PubMed] [Google Scholar]
- Lin D., Wang H., Wang D., Hu L., Loy D.A. Computational and experimental determinations of the UV adsorption of polyvinylsilsesquioxane-silica and titanium dioxide hybrids. Biomed. Mater. Eng. 2013;23:S671–677. doi: 10.3233/BME-130853. [DOI] [PubMed] [Google Scholar]
- Lomer M.C., Thompson R.P., Powell J.J. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn’s disease. Proc. Nutr. Soc. 2002;61:123–130. doi: 10.1079/pns2001134. [DOI] [PubMed] [Google Scholar]
- Long T.C., Tajuba J., Sama P., Saleh N., Swartz C., Parker J., Hester S., Lowry G.V., Veronesi B. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ. Health Perspect. 2007;115:1631–1637. doi: 10.1289/ehp.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long T.C., Saleh N., Tilton R.D., Lowry G., Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006;40:4346e52. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- Ma L., Zhao J., Wang J., Liu J., Duan Y., Liu H., Li N., Yan J., Ruan J., Wang H., Hong F. The acute liver injury in mice caused by nano-anatase TiO2. Nanoscale Res. Lett. 2009;4:1275–1285. doi: 10.1007/s11671-009-9393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier E., Kurz K., Jenny M., Schennach H., Ueberall F., Fuchs D. Food preservatives sodium benzoate and propionic acid and colorant curcumin suppress Th1-type immune response in vitro. Food Chem. Toxicol. 2010;48:1950–1956. doi: 10.1016/j.fct.2010.04.042. [DOI] [PubMed] [Google Scholar]
- Mano S.S., Kanehira K., Taniguchi A. Comparison of cellular uptake and inflammatory response via toll-like receptor 4 to lipopolysaccharide and titanium dioxide nanoparticles. Int. J. Mol. Sci. 2013;14:13154–13170. doi: 10.3390/ijms140713154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina C., Santos-Martinez M.J., Radomski A., Corrigan O.I., Radomski M.W. Nanoparticles: pharmacological and toxicological significance. Brit. J. Pharmacol. 2007;150:552–558. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlfeld C., Geiser M., Kapp N., Gehr P., Rothen-Rutishauser B. Re-evaluation of pulmonary titanium dioxide nanoparticle distribution using the “relative deposition index”: Evidence for clearance through microvasculature. Part Fibre Toxicol. 2007;29:4–7. doi: 10.1186/1743-8977-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C.F., Murray H.W., Wiebe M.E., Rubin B.Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A., Xia T., Mädler L., Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Oberdörster G., Ferin J., Lehnert B.E. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994;102(Suppl. 5):173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo D.G., Tasat D.R., Duffó G., Guglielmotti M.B., Cabrini R.L. The issue of corrosion in dental implants: a review. Acta Odontol. Latinoam. 2009;22:3–9. [PubMed] [Google Scholar]
- Ortlieb M. White giant or white Dwarf? Particle size distribution measurements of TiO2. GITLab J. Eur. 2010;14:42–43. [Google Scholar]
- Petkovic J., Zegura B., Stevanovic M., Drnovšek N., Uskokovic D., Novak S., Filipic M. DNA damage and alterations in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells. Nanotoxicology. 2011;5:341–353. doi: 10.3109/17435390.2010.507316. [DOI] [PubMed] [Google Scholar]
- Pignon B., Maskrot H., Leconte Y., Reynaud C., Herlin-Boime N., Pouget T., Tranchant J.F., Gervais M., Coste S. Versatility of laser pyrolysis applied to the synthesis of TiO2 nanoparticles, application to UV attenuation. Eur. J. Inorg. Chem. 2008;208:883–889. [Google Scholar]
- Riu J., Maroto A., Rius F.X. Nanosensors in environmental analysis. Talanta. 2006;69:288–301. doi: 10.1016/j.talanta.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J. Allerg. Clin. Immunol. 2004;113:395–400. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Regulation of the T cell response. Clin. Exp. Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- Rossi S., Tirri T., Paldan H., Kuntsi-Vaattovaara H., Tulamo R., Närhi T. Peri-implant tissue response to TiO2 surface modified implants. Clin. Oral Implants Res. 2008;19:348–355. doi: 10.1111/j.1600-0501.2007.01478.x. [DOI] [PubMed] [Google Scholar]
- Sarac E.S., Girgin G., Palabiyik S.S., Charehsaz M., Aydin A., Sahin G., Baydar T. A pilot study on neopterin levels and tryptophan degradation in zinc-exposed galvanization workers. Biol. Trace Elem. Res. 2013;151:330–334. doi: 10.1007/s12011-012-9569-4. [DOI] [PubMed] [Google Scholar]
- Sayes C.M., Wahi R., Kurian P.A., Liu Y., West J.L., Ausman K.D., Warheit D.B., Colvin V.L. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol. Sci. 2006;92:174–185. doi: 10.1093/toxsci/kfj197. [DOI] [PubMed] [Google Scholar]
- Schroecksnadel K., Winkler C., Fuith L.C., Fuchs D. Tryptophan degradation in patients with gynecological cancer correlates with immune activation. Cancer Lett. 2005;223:323–329. doi: 10.1016/j.canlet.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Schroecksnadel K., Wirleitner B., Winkler C., Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Schroecksnadel S., Jenny M., Kurz K., Klein A., Ledochowski M., Ueberall F., Fuchs D. LPS-induced NF-kappaB expression in THP-1Blue cells correlates with neopterin production and activity of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2010;399:642–646. doi: 10.1016/j.bbrc.2010.07.134. [DOI] [PubMed] [Google Scholar]
- Senzui M., Tamura T., Miura K., Ikarashi Y., Watanabe Y., Fujii M. Study on penetration of titanium dioxide (TiO2) nanoparticles into intact and damaged skin in vitro. J. Toxicol. Sci. 2010;35:107–113. doi: 10.2131/jts.35.107. [DOI] [PubMed] [Google Scholar]
- Shi H., Magaye R., Castranova V., Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part. Fibre Toxicol. 2013;15:10–15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla R.K., Kumar A., Gurbani D., Pandey A.K., Singh S., Dhawan A. TiO(2) nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology. 2013:748–760. doi: 10.3109/17435390.2011.629747. [DOI] [PubMed] [Google Scholar]
- Shukla R.K., Sharma V., Pandey A.K., Singh S., Sultana S., Dhawan A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol. In Vitro. 2011;25:231–241. doi: 10.1016/j.tiv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Siddiqi A., Payne A.G., De Silva R.K., Duncan W.J. Titanium allergy: could it affect dental implant integration? Clin. Oral. Implants Res. 2011;22:673–680. doi: 10.1111/j.1600-0501.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- Simko M., Mattsson M.O. Risks from accidental exposures to engineered nanoparticles and neurological health effects: a critical review. Part. Fibre Toxicol. 2010;7:42. doi: 10.1186/1743-8977-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M.H., Commens C.A., Burnett L., Snitch P.J. A pilot study on the percutaneous absorption of microfine titanium dioxide from sunscreens. Australas. J. Dermatol. 1996;37:185–187. doi: 10.1111/j.1440-0960.1996.tb01050.x. [DOI] [PubMed] [Google Scholar]
- Thomas T., Thomas K., Sadrieh N., Savage N., Adair P., Bronaugh R. Research strategies for safety evaluation of nanomaterials, part VII: evaluating consumer exposure to nanoscale materials. Toxicol. Sci. 2006;91:14–19. doi: 10.1093/toxsci/kfj129. [DOI] [PubMed] [Google Scholar]
- Valdiglesias V., Costa C., Sharma V., Kiliç G., Pásaro E., Teixeira J.P., Dhawan A., Laffon B. Comparative study on effects of two different types of titanium dioxide nanoparticles on human neuronal cells. Food Chem. Toxicol. 2013;57:352–361. doi: 10.1016/j.fct.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Wan R., Mo Y., Feng L., Chien S., Tollerud D.J., Zhang Q. DNA damage caused by metal nanoparticles: involvement of oxidative stress and activation of ATM. Chem. Res. Toxicol. 2012;25:1402–1411. doi: 10.1021/tx200513t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Li Y. Interaction and nanotoxic effect of TiO2 nanoparticle on fibrinogen by multi-spectroscopic method. Sci. Total Environ. 2012;429:156–160. doi: 10.1016/j.scitotenv.2012.03.048. [DOI] [PubMed] [Google Scholar]
- Warheit D.B., Hoke R.A., Finlay C., Donner E.M., Reed K.L., Sayes C.M. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol. Lett. 2007;171:99–110. doi: 10.1016/j.toxlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Warheit D.B., Webb T.R., Reed K.L. Pulmonary toxicity screening studies in male rats with M5 respirable fibers and particulates. Inhal. Toxicol. 2007;19:951–963. doi: 10.1080/08958370701515852. [DOI] [PubMed] [Google Scholar]
- Widner B., Werner E.R., Schennach H., Wachter H., Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin. Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- Winkler C., Frick B., Schroecksnadel K., Schennach H., Fuchs D. Food preservatives sodium sulfite and sorbic acid suppress mitogen-stimulated peripheral blood mononuclear cells. Food Chem. Toxicol. 2006;44:2003–2007. doi: 10.1016/j.fct.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Wu J., Sun J., Xue Y. Involvement of JNK and P53 activation in G2/M cell cycle arrest and apoptosis induced by titanium dioxide nanoparticles in neuron cells. Toxicol. Lett. 2010;199:269–276. doi: 10.1016/j.toxlet.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Xue C., Wu J., Lan F., Liu W., Yang X., Zeng F., Xu H. Nano titanium dioxide induces the generation of ROS and potential damage in HaCaT cells under UVA irradiation. J. Nanosci. Nanotechnol. 2010;10:8500–8507. doi: 10.1166/jnn.2010.2682. [DOI] [PubMed] [Google Scholar]
- Yazdi A.S., Guarda G., Riteau N., Drexler S.K., Tardivel A., Couillin I., Tschopp J. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1α and IL-1β. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19449–19454. doi: 10.1073/pnas.1008155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.J., Gaffen S.L. Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front. Biosci. 2008;13:170–177. doi: 10.2741/2667. [DOI] [PubMed] [Google Scholar]
- Zhang R., Bai Y., Zhang B., Chen L., Yan B. The potential health risk of titania nanoparticles. J. Hazard. Mater. 2012;211–212:404–413. doi: 10.1016/j.jhazmat.2011.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


