Abstract
Twenty-six orthologs of the rice blast resistance gene Pid3 from cultivated varieties and wild rice accessions distributed in different areas were cloned by allele mining. Sequence analysis showed that while each of the orthologous genes from indica varieties and most wild accessions encodes a complete NBS-LRR protein, each of the proteins encoded by those from japonica varieties and few wild rice accessions presents a premature termination. Eleven of the 26 orthologs were selected for blast resistance testing by transforming into the blast susceptible rice variety TP309, respectively. Inoculation of 23 M. oryzae strains collected from diverse regions of China to the respective transgenic plants revealed that 6 Pid3 orthologs showed susceptible to all the tested strains, while the other 5 orthologs showed differential resistance spectra in a gradually spectrum-widen order as Pid3-W3, Pid3-W4, Pid3-I3, Pid3-W5 and Pid3-I1. Amino acid sequences alignment of these orthologs indicated that the sequence diversities between the blast resistance orthologs were mostly located in the LRR domain such as the substitutions of Q694H,D856H,Q896R,D899E etc. However, the differences between the resistance orthologs and the susceptible ones were mostly located in the NBS domain. The present experiments provide an example of that the ortholog evaluation of plant R genes could be an efficient way to expand the rice blast resistance and some other plant disease resistance as well for breeding.
Introduction
Rice blast, caused by the filamentous ascomycete Mangnaporthe oryzae, is the most devastating fungal diseases affecting rice production [1]. Up to now, at least 78 major blast resistance (R) genes have been identified and mapped genetically on 11 rice chromosomes in exception of chromosome 3. Among the mapped blast R genes, 24 have been cloned [2]–[5] and functionally analyzed. With these known and cloned blast R genes, rice blast resistance breeding has become much more effective than before by using molecule markers linked to the known blast R genes [6].
Most of rice blast resistance genes conduct their reactions against a specific part of Mangnaporthe oryzae strains, thus pyramiding different rice blast R genes would facilitate rice breeding towards more durable and broader resistance to rice blast. For example, the plants pyramided with Pi1, Piz-5 and Pi-ta showed enhanced resistance as compared to those carrying one or two of them, and the combination of these three blast R genes could be then deployed into superior rice varieties by marker-aided selection (MAS) [7]. By crossing and backcrossing rice lines C101LAC, C101A51 and Jin 23B, which contain Pi1, Pi2 and Pi33 respectively, Chen et al. introgressed the three blast R genes into a receptor parent Jin 23B. The pyramided lines showed a wide blast resistance spectrum covering 96.7% of the tested blast strains, which was much higher than that of the initial single R gene lines respectively [8]. In 2010, Koide combined two major rice blast resistance genes, Pish and Pib, from two near isogenic lines, the resulted pyramided line expanded the resistance spectrum by 64.3% [9].
However, in the practice of resistance breeding, using a single R gene which has a broad resistance spectrum is more effective. Of the known rice blast R genes, Pi1 [10], Pi2 [11], Pib [12], Pi9 [13], Pi5 [14], Pi-ta [15], Pik [16] and Pik-p [17] have been characterised as R genes with a relatively broad blast resistance spectum, respectively. Though the most other blast R genes may have a relatively narrow resistance spectrum, several experiments have confirmed that their alleles or orhtologs showed varied blast resistance spectra. These allelic or ortholog genes would be born with abundant allelic variations in rice resources due to the co-evolution of rice and blast pathogen in different rice growing environments, and could be utilized as more efficient and important gene resources in the improvement of rice blast resistance [18]. For example, Pi9,Pi2 and Piz-t are alleles from different rice resources while physically at the same gene locus on rice chromosome 6 with the sequence similarity up to 98.84%, but their resistance spectra are quite different, covering 93.7%, 92.2% and 54.5% of tested blast isolates, respectively [4], [19], [20]. The similar situation was also observed in Pik, Pik-m, Pik-p, Pi1, Pi54 and Pi54rh [3], [5], [16], [17], [21], [22], which were all from the same chromosome locus but cloned independently from different varieties and showed differential resistance spectra [23].
Pid3 (GenBank accession no.: FJ745364.1), was initially identified in an indica variety Digu by performing a genome-wide comparison of ‘9311’ (indica) and ‘Nipponbare’ (japonica) on the premise of the verification of obvious different resistance of indica and japonica varieties to M. oryzae strains collected from south and north China [24], and the Pid3 functional orthologs were found widely present in most tested indica varieties and wild rice species [25]. Later, Lv et al [2] isolated an ortholog of Pid3 from a common wild rice accession A4 (O. rufipogon) by sequencing-based allele mining, which confers a different resistance spectrum to a set of M. oryzae strains and was named Pid3-A4. The purpose here is to isolate more ortholog genes of Pid3 from cultivated varieties and wild rice accessions, and to evaluate their respective blast resistance by gene transformation and blast inoculation. Based on the respective blast resistance spectra of the cloned orthologs, comparative analysis were conducted between the amino acid polymorphic sites of the identified NBS-LRR proteins and their respective blast strain-specific resistances.
Materials and Methods
Plant materials and rice blast strains
There are 10 cultivated rice varieties including 5 indica varieties and 5 japonica varieties used in this study, collected from south or north of China and other countries. The 10 common cultivated rice varieties are kept in our lab, which are used and planted in China widely. Seventeen wild rice accessions were presented by the professor Zhukuan Cheng's lab, Institute of Genetics and Developmental Biology and Lili Hao's lab, Beijing Institute of Genomics (Table S1 in File S1). The species were cultivated in an experimental field of the Institute of Genetics and Developmental Biology in Beijing under normal growing conditions.
Twenty-three M. oryzae isolates used in this study are listed in Table 1. Of them, 20 isolates were collected from south of China and other 3 isolates from north of China. Zhong-10-8-14 was the initial isolate for the determination of the Pid3 gene [25].
Table 1. Rice blast resistance spectra of Pid3 orthologs.
| M. oryzae strains | Transgenetic lines | |||||||||||
| Pid3-I1 | Pid3-I3 | Pid3-W3 | Pid3-W4 | Pid3-W5 | Pid3-W8 | Pid3-W9 | Pid3-W10 | Pid3-W14 | Pid3-W16 | Pid3-J1 | J1 | |
| Zhong-10-8-14 | R | R | R | R | R | S | S | S | S | S | S | S |
| 07-31-1-2 | R | R | R | mR | R | S | S | S | S | S | S | S |
| 03-10-76-3 | R | R | S | mR | R | S | S | S | S | S | S | S |
| 04-8-2-1 | mR | S | S | mR | S | S | S | S | S | S | S | S |
| 10-25-1-1 | R | S | S | mS | R | S | S | S | S | S | S | S |
| 10-32-2-1 | R | S | S | S | mR | S | S | S | S | S | S | S |
| 10-128-2-1 | R | S | S | S | NA | S | S | S | S | S | S | S |
| 10-62-3-1 | mR | S | S | S | mR | S | S | S | S | S | S | S |
| 10-117-17-1 | S | S | S | S | S | S | S | S | S | S | S | S |
| 03-11-37-1 | R | S | S | mS | R | S | S | S | S | S | S | S |
| 07-55-1-1 | R | R | mS | mR | R | S | S | S | S | S | S | S |
| 07-26-2-2 | NA | R | mR | S | R | S | S | S | S | S | S | S |
| 10-120-21-2 | R | S | mS | NA | R | S | S | S | S | S | S | S |
| 07-21-1-1 | NA | R | mR | R | R | S | S | S | S | S | S | S |
| 99-20-2 | R | R | S | S | R | S | S | S | S | S | S | S |
| 99-26-2 | R | mR | mR | S | mR | S | S | S | S | S | S | S |
| 03-10-66-1 | R | R | S | S | R | S | S | S | S | S | S | S |
| Chuang ZB15 | R | S | S | NA | S | S | S | S | S | S | S | S |
| 97-27-2 | R | R | R | R | R | S | S | S | S | S | S | S |
| Y34 | R | R | mS | R | NA | S | S | S | S | S | S | S |
| B04 | R | R | S | R | R | S | S | S | S | S | S | S |
| B16 | R | R | S | R | R | S | S | S | S | S | S | S |
| B23 | R | R | S | R | R | S | S | S | S | S | S | S |
(S:susceptible; mS:medium susceptible; R:resistant; mR:medium resistant; NA:no result).
Gene cloning
DNA was extracted from the fresh leaves of 27 cultivated and wild rice varieties. Forward primer Pid3F: 5′ - TTTCTAGAAGTAACACCCAAGGATAGGATAG - 3′ and reverse primer Pid3R: 5′ - CTGTCGACGAACGACAAGTGCGACATGATTG - 3′ were designed and used to amplify the full coding sequence of Pid3 based on the published dada [2]. An XbaI and a SalI recognition site (underlined) with two protecting bases (TT and CT) were added to their 5′ ends, respectively. PCR amplification was carried out using the following profile: initial DNA denaturation, 95°C for 4 min; followed by 30 cycles of denaturation, 95°C for 30 s; annealing, 58°C for 30 s; extension, 72°C for 3 min; and final extension at 72°C for 5 min. The PCR products were purified and sequenced respectively.
Rice transformation
No intron is present in the Pid3 gene. Therefore, the cloned gene fragments above were inserted into the binary vector pZH01 [26] through the XbaI and SalI cloning sites. After sequence verification, the final construct was introduced into Agrobacterium tumefaciens LBA4404. Agrobacterium-mediated transformation was performed using calli derived from mature embryos of susceptible rice variety TP309 according to Hiei et al [27].
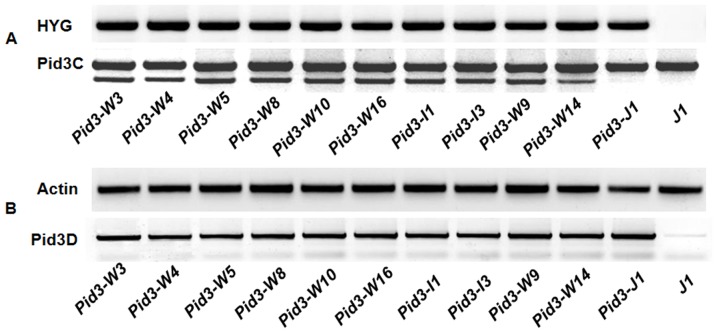
Positive transgenic plants were detected through the amplification of the marker gene hygromycin (HYG) in vector using forward primer HYG-F: 5′ - TGCGCCCAAGCTGCATCAT - 3′ and reverse primer HYG-R: 5′ - TGAACTCACCGCGACGTCTGT - 3′. PCR amplification was carried out using the following profile: initial DNA denaturation, 95°C for 4 min; followed by 35 cycles of denaturation, 95°C for 30 s; annealing, 58°C for 30 s; extension, 72°C for 30 s; and final extension at 72°C for 5 min. To confirm the positive transformants precisely, one cleaved amplified polymorphic sequence (CAPS) marker was designed based on the nonsense mutation locus of Pid3 in TP309 variety. A 658-bp fragment was amplified using the primer Pid3C (Pid3C-F: 5′ - TACTACTCATGGAAGCTAGTTCTC - 3′ and Pid3C-R: 5′ - ACGTCACAAATCATTCGCTC - 3′) and then digested with BamHI. The digested PCR products was resolved on 2% agarose gels as one band in TP309 variety and three premature termination Pid3 orthologs of Pid3-W9, Pid3-W14, and Pid3-J1 transformants while two bands in the other positive transgenic plants.
Expression analysis of Pid3 orthologs
RNA were isolated from leaf sheath tissue with the TRIzol reagent (Invitrogen, Carlsbad, CA) and cDNA was synthesized from poly(A)+ RNA using a cDNA synthesis kit (Transgen, Beijing). Semi-quantitative reverse-transcription (RT)-PCR was performed with the specific primer pair Pid3C for 30 cycles of amplification. Transcription of the Actin gene was used to normalize the cDNA levels with the primer pair 5′ - AGCAACTGGGATGATATGGA - 3′ and 5′ -CAGGGCGATGTAGGAAAGC - 3′. Amplification of the Actin gene was conducted for 27 cycles. And the over-expression of Pid3 gene was detected by the primer pair Pid3D (Pid3D-F: 5′- GAATGCAAATGTTTGGTTCG-3′ and reverse primer Pid3D-R: 5′-CGCCACATCATAATTCCTTG-3′). RT-PCR was initiated with one cycle at 95°C for 2 min followed by 30 or 27 cycles at 95°C for 30 s, 58°C for 30 s, and 72°C for 45 s, and the reaction was terminated with a final extension at 72°C for 5 min. The PCR products were resolved on 1% agarose gels.
Fungal inoculation
Rice seedlings at the tillering stage were inoculated by injection of 0.1–0.2 ml of a 2.5×105 conidia ml−1 spore suspension in the field. The disease reaction was evaluated seven days after inoculation with the susceptible transgenic line, Pid3-J1, as a control. Leaves lesion types were observed and scored as resistance (R), medium resistance (mR), medium susceptibility (mS), and susceptibility (S), respectively.
Computational analysis of DNA and protein sequences
Sequences were aligned using DNAMAN (http://www.lynnon.com/), Clustal X version 2.0 [28], DNAStar (https://www.dnastar.com/products/lasergene.php) and manually edited using BioEdit version 7.0.1 [29]. Protein motif search was performed using the SMART program (http://smart.embl-heidelberg.de/) and the RCM program (http://144.92.198.58/main/main.php). MEGA 5 [30] was used to build phylogenetic tree of Pid3 orthologs. Sliding-window analysis, polymorphism and neutral tests of different regions of the Pid3 orthologs were conducted using DnaSP version 4.0 [31].
Results
Sequence characteristics of the Pid3 orthologs
In addition to previously reported Pid3 (hereafter renamed as Pid3-I3) from the indica variety Digu [25] and Pid3-A4 (hereafter renamed as Pid3-W5) from the wild rice accession A4 (O. rufipogon) [2], a total of 28 Pid3 orthologs were cloned, including 19 from 16 wild rice accessions (the additional three orthologs were from the wild rice accession W1, W11 and W13, respectively), 4 from indica and 5 from japonica varieties by allele mining. The details for the varieties are listed in Table S1 in File S1. They are distinguished in geographic distributions.
The open reading frame (ORF) analysis revealed that 26 Pid3 orthologs each consist of 2775 nucleotides while the other two from W1-2 and W16 each consist of 2778 nucleotides. The pairwise alignment of all 30 Pid3 orthologs showed high homologous with an average identity from 99.0% to 100% at the DNA level. Of note, the ortholog sequences from W12 and I2 are identical, and those from J3 and J5 and from W11-1, J2 and J1 are the same too. Therefore, we have actually obtained 26 different Pid3 orthologs.
Nucleotide polymorphism of Pid3 orthologs
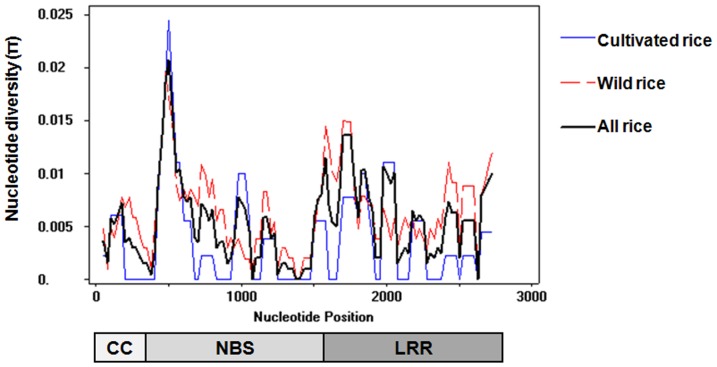
Pid3 genes encode a typical CC-NBS-LRR type protein with the CC, NBS and LRR domains coded in positions of 1-300, 481-1560 and 1621-2670, respectively, along the DNA sequence. By sliding window analysis of the 26 Pid3 orthologs, the nucleotide diversity distribution in the whole encoding sequences was revealed in Figure 1, showing two obvious characteristics: firstly, a variation peak exists in the NBS domain; secondly, the nucleotide diversities of Pid3 orthologs among the wild rice accessions are obviously greater than those among the cultivated rice varieties.
Figure 1. Sliding-window analysis of diversities in Pid3 coding region.
The nucleotide diversity (π; Y-axis) was generated by DNAsp5.0, and the X-axis represents the positions of nucleotides. The blue line stands for the result of cultivated rice varieties; the red line stands for the wild rice, and the black line stands for all rice. The map below the sliding window is the encoding structure of the Pid3 gene, the shaded box denotes the exon region, and CC, NBS, LRR region are marked on its corresponding region.
Further analysis was conducted in terms of different regions of Pid3 orthologs by the neutral tests (Table 2). Tajima's D values were mostly negative but not significant, suggesting that Pid3 orthologs may experience a little preference to positive selection but not balancing selection. The smaller π values (less than 0.5%) also showed the less variations for Pid3 orthologs, while the nucleotide diversity in LRR domain of Pid3 gene was larger than that in the CC and NBS domain. In Pid3 orthologs of wild rice accessions, the non-synonymous substitution rate (Ks) of the LRR domain (−2.04091) and synonymous substitution rate (Ka) in the NBS domain (−2.10354) were statistically significant, but the K values in those of the cultivated rice varieties were not-significant.
Table 2. Polymorphism and neutral test of Pid3 orthologs.
| Pid3 orthologs | Total sites | S | π | Tajima's D | Ka | Ks | Ka/Ks | |
| All rice | Total | 2778 | 110 | 0.585% | −1.63952 | −1.58613 | −1.63824 | 0.96819 |
| CC | 300 | 12 | 0.473% | −1.76079 | −1.84601 | −0.88986 | 2.07449 | |
| NBS | 1080 | 37 | 0.481% | −1.71188 | −2.06212* | −1.12712 | 1.82954 | |
| LRR | 1050 | 39 | 0.601% | −1.31948 | −0.97568 | −2.14213* | 0.45547 | |
| Cultivated rice | Total | 2778 | 27 | 0.372% | 0.20057 | 0.04541 | 0.51546 | 0.08810 |
| CC | 300 | 3 | 0.252% | −1.03446 | −0.69098 | −1.1173 | 0.62153 | |
| NBS | 1080 | 9 | 0.342% | 0.69853 | 0.26384 | 0.93097 | 0.28341 | |
| LRR | 1050 | 10 | 0.351% | 0.19314 | 0.19314 | 0 | 0 | |
| Wild rice | Total | 2778 | 101 | 0.651% | −1.52933 | −1.48135 | −1.53105 | 0.96754 |
| CC | 300 | 10 | 0.551% | −1.44664 | −1.52506 | −0.79238 | 1.92465 | |
| NBS | 1080 | 34 | 0.519% | −1.70834 | −2.10354* | −1.11112 | 1.89318 | |
| LRR | 1050 | 37 | 0.666% | −1.30873 | −0.99243 | −2.04091* | 0.48627 |
Note: S, number of segregating sites; π, nucleotide diversity; Statistical significance: * P<0.05; Not significant: 0.10>P>0.05; Ka: the rate of non-synonymous substitution, Ks: the rate of synonymous substitution.
Amino acid sequence analysis of Pid3 orthologs
Although the nucleotide sequences of Pid3 orthologs showed highly homologous, their encoded amino acids display a distinct characteristics among indica, japonica varieties and wild rice accessions. The gene sequences from all the indica varieties and most of the wild rice accessions (15 of 19) encode the complete CC-NBS-LRR proteins with 924 amino acids but the gene sequences from all the japonica varieties and few wild rice accessions (4 of 19) showed premature transcription termination, which occurred at the position of 737 for individual wild rice accessions W11 and W17 and all japonica varieties, and at the position of 770 and 635 respectively for W14 and W9.
Amino acid sequences alignment of Pid3 proteins revealed the homology rate, ranging from 97.9% to 100%. Several conserved motifs could be identified in the three functional domains, such as EDVVD in the CC domain (Figure 2A); Kinase 1a, Kinase 2, Kinase 3a, GLPL, RNBS-D and MHD in the NBS domain (Figure 2B); and 14 imperfect LxxLxLxx repeat units in the LRR domain (‘x’ indicates any amino acid residue) (Figure 2C). In the alignment, only few amino acid substitutions were observed in the motifs in addition to the positions of 204 (Kinase 1a), 311(Kinase 3a), 439 and 441 (RNBS-D) in the NBS domain, and the x loci of the third, fourth, eighth, tenth, thirteenth repeat unit in the LRR domain.
Figure 2. Conservatism analysis of amino acid sequences among Pid3 orthologs.
The conservatism analysis of amino acid sequences was produced by the software WebLogo on line. The piled height of amino acid loci showed the degree of conservatism, and the piled height of different amino acids in single locus reflected the degree of correlation with this site. (A): CC Domain. EDVVD was indicated in this domain; (B): NBS Domain. Several conserved motifs: Kinase 1a, Kinase 2, Kinase 3a, GLPL, RNBS-D and MHD were indicated in this domain; (C): LRR Domain. 14 imperfect LxxLxLxx repeat units were indicated in this domain.
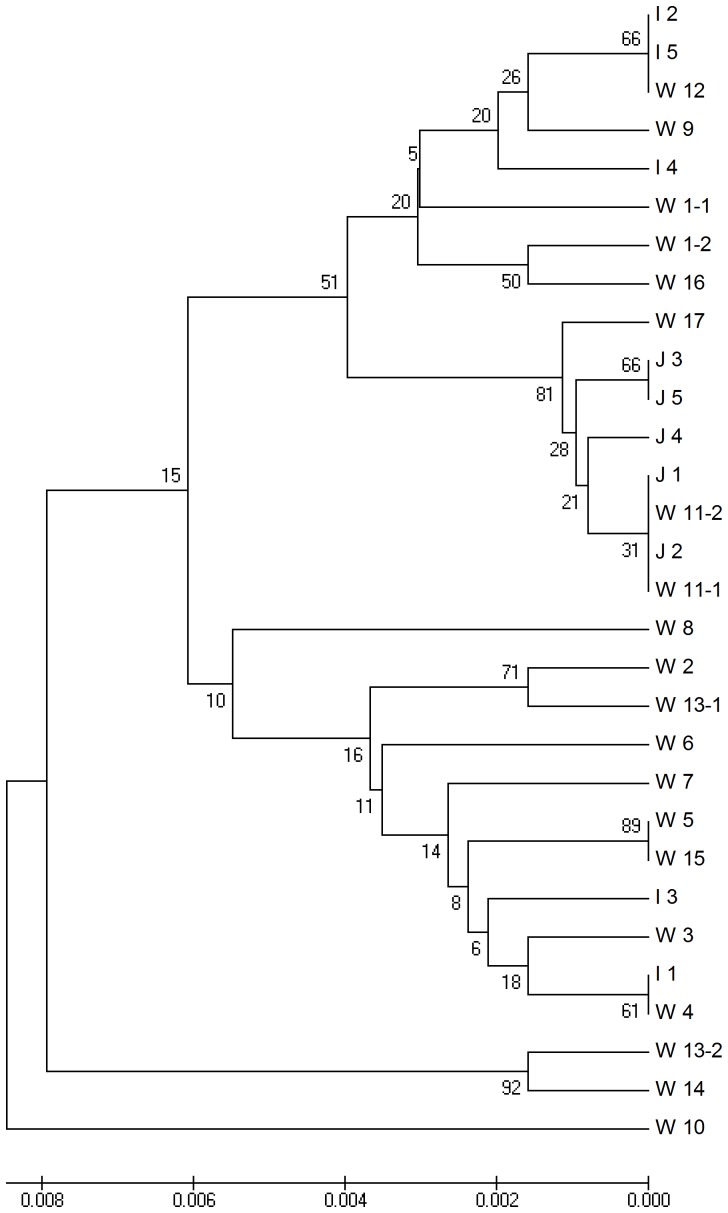
A neighbor-joining tree was constructed with the encoded amino acid sequences to evaluate the phylogenetic relationships of Pid3 orthologs (Figure 3). Indica, japonica and wild rice varieties were relatively clustered in groups separately. The orthologs of most wild rice accessions were far from those of the cultivated varieties, but few ones were present between indica and japonica groups, which might reveal their possible genetic correlation in evolution.
Figure 3. Phylogenetic analysis of Pid3 orthologs.
W: wild rice; I: indica; J: japonica.
Blast resistance of Pid3 orthologs
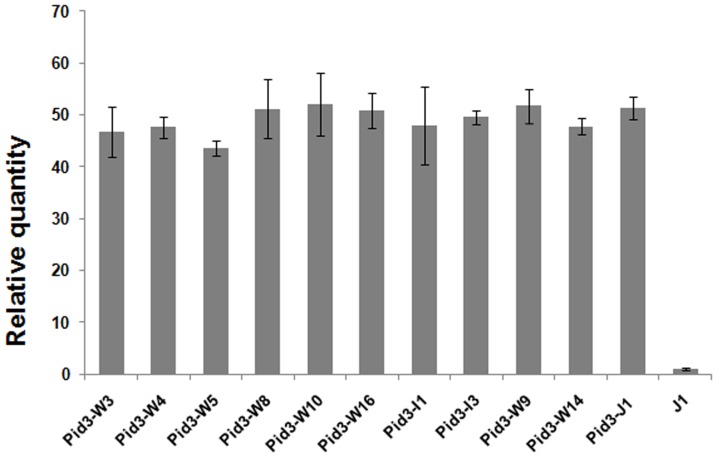
Eleven Pid3 orthologs, including 3 prematurely terminated Pid3 orthologs from wild rice accessions of W9, W14 and japonica variety of J1 and 8 complete Pid3 orthologs from wild rice accessions of W3, W4, W5, W8, W10, W16 and indica varieties I1 and I3, were selected for evaluation of their respective resistance to a group of rice blast strains based on their geographic distribution. To ensure the consistency, each of the Pid3 orthologs were inserted into the binary vector pZH01 under Cauliflower mosaic virus (CAMV)35S promoter control and transformed into the susceptible rice variety TP309, which was the same recipient used for pCaMV35S::Pid3 [25] and pCaMV35S::Pid3-A4 [2] in our previous study. The independent primary transgenic lines (T0) were obtained and determined by the transgene CAPS marker (Figure 4A) and the transgene transcripts (Figure 4B). The results showed that all the candidate transformants were transgene-positive and over-expressed. The number of the positive transgenic plants in T0 generations was shown in Table S2 in File S1. Further, the transgenic lines, in which the respective transgenes were expressed at the approximately same levels (Figure 5), were selected for co-segregation analysis in their respective T1 (the selfed progeny of T0 line) generations (there was an example in Figure S1 in File S1) and then homozygous transgnic T2 (the selfed progeny of T1 line) lines were identified. The homozygous T2 lines from each of the 11 Pid3 orthologs were applied to subsequent blast inoculation assays. As we expected, all of the Pid3 orthologs transgenic plants did not present any observable side effects on phenotypes.
Figure 4. Detection of the DNA and RNA to the transformants.
(A) HYG marker was used in the DNA detection. In addition, CAPS marker was used to confirm the positive transformants precisely and the restriction enzyme cutting site (BamHI) lied in the premature stopped position of Pid3-J1; (B) Pid3D marker was used in the RNA detection, and Actin is as the referring primer marker.
Figure 5. Quantitative reverse-transcription polymerase chain reaction analysis of the transcript levels of Pid3 orthologs.
Relative quantity charted the expression levels of Pid3 orthologs in TP309 (J1). TP309 (J1) was used as the control.
Next, all Pid3 orthologs homozygous were inoculated with 23 M. oryzae strains respectively, which were collected from south and north of China. These blast inoculation experiments repeated 3 times on average and the results were listed in Table 1. Five completely encoded Pid3 orthologs, Pid3-W3, Pid3-W4, Pid3-W5, Pid3-I1 and Pid3-I3, were resistant to Zhong-10-8-14, the evaluating strain of the Pid3 gene [2], while the other three completely encoded Pid3 orthologs (Pid3-W8, Pid3-W10 and Pid3-W16) and three premature terminated Pid3 orthologs (Pid3-W9, Pid3-W14 and Pid3-J1) were susceptible (Figure 6). In the further inoculation test, the 6 Zhong-10-8-14 susceptible Pid3 orthologs also showed susceptibility to the other 22 M. oryzae strains, whereas the 5 Zhong-10-8-14 resistant Pid3 orthologs displayed differential blast resistance spectra of the remaining strains: Pid3-I1, Pid3-W5, Pid3-I3, Pid3-W4 and Pid3-W3 were resistance to 19, 17, 13, 10 and 5 M. oryzae strains, respectively.
Figure 6. Pid3 orthologs confer resistant and susceptible to the blast strain Zhong-10-8-14.

Functional polymorphisms of Pid3 orthologs
Of the 11 Pid3 orthologs, Pid3-W9, Pid3-J1 and Pid3-W14 were premature terminated at the peptide position of 635, 737 and 770, respectively, resulting in deletions of 289, 187 and 154 amino acids, respectively, at the C-terminal of the Pid3 proteins, which may affect the function of the LRR domain.
Of the 8 completely encoded Pid3 orthologs, 5 showed resistance to rice blast but with different spectra while the other 3 ones showed completely susceptible to all strains tested. In comparison of the protein sequences encoded by the two-group genes (resistance and susceptibility), the polymorphic loci were found mostly located in the NBS domain (Table 3), such as the G204C substitution in the Kinase1a motif of Pid3-W16 and the P441L substitution in the RNBS-D motif of Pid3-W10, indicating that the varied amino acids are important to the NBS domain's function.
Table 3. Comparison of Pid3 proteins.
| Domain | CC | NBS | LRR | |||||||||||||||||||||||||||||||||
| Amino | 2 | 2 | 4 | 6 | 1 | 1 | 2 | 2 | 2 | 2 | 4 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | 6 | 7 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 9 | 9 |
| acid | 2 | 4 | 6 | 5 | 9 | 0 | 0 | 5 | 9 | 2 | 4 | 1 | 1 | 3 | 3 | 3 | 5 | 5 | 6 | 7 | 7 | 7 | 8 | 2 | 9 | 9 | 1 | 2 | 5 | 5 | 9 | 9 | 9 | 0 | 0 | |
| position | 3 | 1 | 3 | 4 | 9 | 7 | 3 | 1 | 1 | 8 | 2 | 6 | 7 | 1 | 3 | 9 | 1 | 3 | 7 | 9 | 5 | 4 | 9 | 5 | 4 | 1 | 6 | 4 | 6 | 9 | 0 | 6 | ||||
| Pid3-I1 | - | V | R | A | T | E | M | G | I | D | L | P | L | N | I | R | R | H | P | S | H | L | V | S | N | Q | N | Y | E | V | D | I | Q | E | R | V |
| Pid3-W5 | - | V | R | V | T | E | M | G | I | D | L | P | L | N | I | R | R | H | P | S | H | L | V | L | N | Q | S | F | E | M | D | V | Q | E | Q | V |
| Pid3-I3 | - | V | G | A | T | E | M | G | V | D | L | P | L | N | I | R | R | H | P | S | H | L | V | S | N | Q | N | F | E | V | H | V | R | E | R | V |
| Pid3-W4 | - | V | R | A | T | E | M | G | I | D | L | P | L | N | I | R | R | H | P | S | H | L | V | S | N | Q | N | Y | E | V | D | I | Q | D | R | V |
| Pid3-W3 | - | A | R | A | T | V | M | G | I | D | L | P | L | N | I | R | R | H | P | S | H | L | V | S | N | H | N | F | E | V | H | V | Q | E | R | V |
| Pid3-W8 | - | V | R | A | T | E | V | G | I | D | L | P | L | S | T | R | R | H | L | S | H | S | V | S | N | Q | N | F | E | V | H | V | Q | E | R | V |
| Pid3-W10 | - | V | R | A | M | E | M | G | I | E | F | L | P | N | I | R | C | H | P | L | H | L | V | S | N | Q | N | F | E | V | H | V | Q | E | R | A |
| Pid3-W16 | A | V | R | A | T | E | M | C | I | D | L | P | L | N | I | H | R | Y | P | S | Q | L | M | S | I | Q | N | Y | K | V | D | I | Q | E | R | V |
Note: The bold markers were the diverse loci compared to Pid3-I1, which had the broadest resistant spectrum.
Among the 5 resistance Pid3 orthologs with different resistance spectra, the polymorphic loci were mostly present in the LRR domain, implying that the region may function in recognizing the special blast strains. By comparison of the LRR domain sequences between the resistance orthologs, some amino acids could be recognized as important to the blast resistance spectra. For example, Q694H substitution that was only present in Pid3-W3, which showed the narrowest resistance spectrum; the D899E substitution between Pid3-I1 and Pid3-W4 led to a decrease of the resistance spectrum from 86.95% (Pid3-I1) to 47.82% (Pid3-W4); the substitutions, Q694H of Pid3-I3 and Q896R of Pid3-W3, altered their resistance spectrum from 60.86% (Pid3-I3) to 26.08% (Pid3-W3); and notably, the D856H substitution in either of Pid3-I3 and Pid3-W3, could result in a relatively narrow resistance spectrum.
Discussion
Allele mining is an important way to expand the rice blast resistance
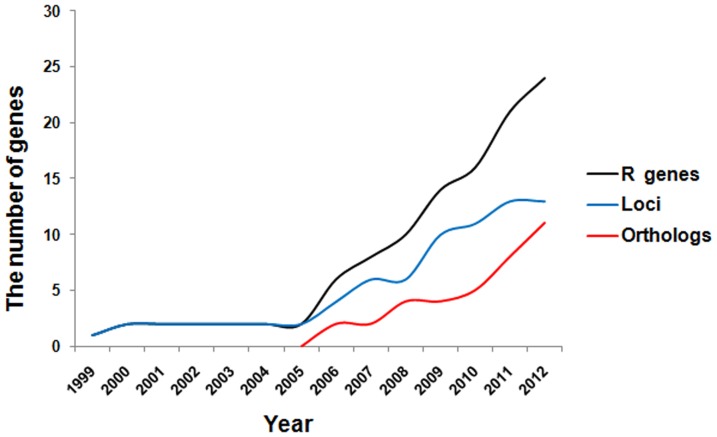
With the proceeding of the rice genome sequencing projects and the map-based cloning technology, over 20 rice blast resistance genes have been identified, but, in recent years, the number of new blast resistance loci have not increased as much (Figure 7). Some of the newly found genes were finally determined as an allele or an ortholog to one of the previously cloned genes [3]–[5], [32]. The blast resistance alleles or orthologs showing different resistance spectra from each other have thus greatly expanded the rice blast resistance resource for breading. This fact indicates that sequencing-based allele mining, as initially termed by Kumar et al [33], should be an efficient and economical way to expand the roles of each blast resistance loci in rice genome.
Figure 7. Cloned rice blast resistant genes and loci in proceeding.
The black, blue and red line separately represents the total number of the R genes, R gene loci, and orthologs cloned year by year.
Some studies involving the silencing or over-expression of plant R genes have shown that disease resistance is correlated with R-gene expression, thus we have to admit that the acquisition of promoter sequences may play a crucial role to the functions of the involved R-genes in evolution. For example, a recent reported rice blast resistance gene, Pit, may be a result of the refunctionalization of some ‘sleeping’ rice blast R genes by transposon-mediated transcriptional activation in rice genome [34]. Here in current study, our research purpose was to evaluate and compare differential resistance spectra of Pid3 ortholog sequences cloned directly by allele mining. Since the cloned ortholog sequences only involved their respective ortholog coding sequences, all the Pid3 ortholog coding sequences were driven by the same CaMV35S promoter rather than their respective native promoters and were respectively expressed in transgenic rice plants of TP309 with much the same expression levels. This approach is an easy and effective way to explore possible available Pid3 orthologs in spite of that some of the orthologs might be not functional in their own rice sources. Of course, we are unable to exclude the possibility that stronger expression of a Pid3 ortholog in other rice plants might change its inherent blast resistance spectrum though such unusual situation did not occur in our previous studies [2], [25].
Thus, by this way, a total of 26 Pid3 orthologs were cloned respectively from wild rice accessions and cultivated rice varieties distributed in different geographical areas, respectively. Eleven of them were tested for the rice blast resistance and showed different blast resistance spectra to these Chinese M. oryzae strains. The blast resistance spectra of Pid3-I1 from Kasalath (indica) and Pid3-W5 from O. rufipogon (A4) increased up to 86.95% and 78.26% respectively, as compared with the corresponding spectrum (60.86%) of Pid3-I3, the original Pid3 gene from Digu. Noteworthily, Pid3-I1, which is from an Indian variety Kasalath, showed the best resistance to Chinese M. oryzae strains.
Wild rice is an important resource for blast resistance
Sun et al [35], [36] reported that during rice domestication the number of alleles of cultivated rice was only 60% that of wild rice, leading to lower genetic diversity of the cultivated rice [37]. The two well-known rice R genes, Xa21 for resistance to Xanthomonas oryzae and Pi9 for resistance to Mangnaporthe oryzae were originated from the wild rice O. longistaminat [38] and O. minuta [39], respectively. They both showed broader-spectrum resistances to pathogens and have been widely applied to rice breeding. Thus the two R genes have set good examples for exploring more disease resistance genes in wild rice resources.
In plant, R genes were classified into four types according to their polymorphism level (π value): conserved (type I; π<0.5%), intermediate-diversified (type II; 0.5%<π<5%), highly diversified (type III; π>5%), and present/absent genes (type IV; P/A) [40], [41]. The higher π value means the evolution of the gene responding to the pathogen more rapidly. R locus with a low level of polymorphism usually has a relatively slow evolutionary rate. Pid3 orthologs widely exist in the genomes of either cultivated rice subspecies or wild rice species. The present study showed that the π value, the nucleotide diversity, of Pid3 orthologs in wild rice accessions is 0.651% (intermediate-diversified class) while 0.372% (conserved type) in cultivated rice varieties. Obviously, the diversity of wild rice is larger than that in cultivated rice, which supports that Pid3 gene encoding sequences gradually tend to unity during the domestication of rice species. It also indicates that wild rice accessions are worthwhile for further allele mining of broad-spectrum Pid3 gene orthologs.
In this study, we compared the blast resistance spectra of 5 Pid3 orthologs, of which 3 were from wild rice accessions. As mentioned above, Pid3-W5 had a blast resistance spectrum 17% wider than the original Pid3-I3 from Digu. And Pid3-W4 showed a resistance spectrum which is complement to that of Pid3-I3. Therefore, allelic mining from the wild rice is an effective way to expand rice blast resistance spectra in breeding.
The amino acids variations in Pid3 protein domains relative to blast resistance and resistance spectra
In the neighbor-joining tree constructed with the amino acid sequences of all Pid3 proteins (Figure 3), the identified 5 resistant Pid3 orthologs (Pid3-W3, Pid3-W4, Pid3-W5, Pid3-I1, Pid3-I3) are aligned in a branch, suggesting that some conservation in Pid3 orthologs sequences is necessary for their resistance function. Based on such conservation, we may predict that in this group (Figure 3) the other two members, Pid3-W7 and Pid3-W15, would have a blast resistance function too, though this prediction need to be further confirmed.
Many evidences showed that NBS domain in the resistance NBS-LRR proteins functions as a molecular switch in processing the resistance reaction, which decides the resistance level of the R genes [42]. In this study, of the 8 Pid3 orthologs, each encoding a complete NBS-LRR protein, five were tested to be blast resistant while the other three were blast susceptible. When the susceptible protein sequences were compared with the resistance ones, the altered amino acids were found mainly in the NBS domain (as shown in Table 3, of total 13 substituted amino acids in the susceptible gene group, 11 were in NBS domain). Notably, only two substitution sites, G204 and, P441L are located in the conserved Kinase1a motif and RNBS-D motif, respectively, which suggest that amino acid substitution in other region of the NBS domain, besides the two conserved motifs, may also have the loss-of-function effects on the blast resistance of NBS-LRR proteins.
Many studies suggested that the LRR domain of R proteins is involved in avirulence (AVR) recognition or in physical interaction with AVR proteins, such as the recognition between barley Mla1 and powdery mildew AvrMla1 [43], potato Rx and PVX [44]; Pita and blast AVR-Pita [45]; flax L and rust AVR-L567 [46]. However, in some cases, the CC domain was also found to play a major role in the interactions between R and AVR proteins. For example, in rice the Pik and AVR-Pik interaction involves the CC domain, but not the LRR domain, of the R protein [47]. Besides, Chen et al. [48] reported that the potato RB (also known as Rpi-blb1) gene encoded NBS-LRR protein binds to Phytophthora infestans AVR protein IPI-O through its CC domain.
In this study, we found that the amino acid variation in the LRR domain of the allelic Pid3 proteins greatly affects their resistance spectra. Within the 5 resistance Pid3 orthologs, a total of 10 amino acid variations exist in the LRR domain, and accordantly their resistance spectra range from 86.95% to 26.08% of 23 tested blast isolations. Compared with the LRR domain of the original Pid3 allele from Digu (Pid3-I3), which showed a resistance spectrum of 60.86% (14/23), two substitutions of Q694H and R896Q in Pid3-W3 decreased the resistance spectrum to 26.08% (6/23); the substitutions of F815Y, H856D, V894I, R896Q, and E899D in Pid3-W4 decreased the resistance spectrum to 47.82% (11/23). Meanwhile, some mutation sites increased the recognition to M. oryzae avirulence (AVR) elements and broadened the resistance spectrum, such as the substitutions of F815Y, H856D, V894I, and R896Q in Pid3-I1 increased the resistance spectrum to 86.95% (20/23), the substitutions of S589L, N799S, V851M, H856D, R896Q, and R900Q in Pid3-W5 increased the resistance spectrum to 78.26% (18/23). The data here may provide important clues for further exploring the core AVR recognition elements in the Pid3 proteins and understanding the interaction mechanism. Nevertheless, our study is preliminary, and the present data cannot exclude the possible role of the CC domain in the AVR recognition by Pid3 proteins.
Up to now, researches on the defense mechanism of rice blast caused by Magnaporthe oryzae have made great progress, resulting in identification and cloning of many defense related genes, which had been expected to facilitate the rice disease resistance breeding program via marker assisted selection and transgenic approaches [49]. Nevertheless, the actual progress in application of these defense related genes to breeding is rather limited. After evaluating the modification of the expression of more than 60 defense-related genes, Delteil et al [50] concluded that altered expression of genes involved in resistance signal transduction and transcription could lead to many unwanted side effects, like lesion mimic phenotypes, in contrast to R genes which could confer broad spectrum and high levels of resistance without obvious side effects. In this research, all Pid3 orthologs transgenic plants though with the character of an over-expressed transgene did not present any observable side effects (data not shown). So far most of rice blast R genes have been cloned by map-based cloning approach, but this approach is labor-cost and time-consuming. The study here demonstrates that sequence-based allele mining (or coding sequence-based mining) is a more effective way to expand the rice blast R gene resource for controlling the devastating blast disease. This approach can be applied to control of other crop diseases too.
Supporting Information
Supporting information file containing Tables S1, S2 and Figure S1. Table S1. Rice varieties and wild rice accessions used in this experiment. (DOC). Table S2. The number of the positive transgenic plants in T0 generations. (DOC). Figure S1. Co-segregation of the Pid3-I1 gene with the resistant phenotype. The T1 generations (the selfed progeny of T0 line) carrying Pid3-I1 were inoculated with M. oryzae Zhong-10-8-14, and the genotypes were analyzed using the HYG gene and marker Pid3C. Susceptible TP309 (J1) was used as the control. (TIF).
(RAR)
Acknowledgments
We thank Zhukuan Cheng, Lili Hao and Yunliang Peng (Sichuan Academy of Agricultural Sciences), Cailin Lei (Chinese academy of agricultural sciences) for kindly providing wild rice accessions and M. oryzae isolates.
Funding Statement
This work was supported by grants from the National Basic Research Program of China (2012CB114005 and 2011CB100706), the National Transgenic Research Project (2013ZX08009-001, 2013ZX08009-003), the National High Technology Research and Development Program of China (2012AA101101), Beijing Natural Science Foundation (5122019), Bill and Melinda Gates Foundation (51587-24), Special Fund for Agroscientific Research in the Public Interest (201303007). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, et al. (2012) The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology 13: 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lv Q, Xu X, Shang J, Jiang G, Pang Z, et al. (2013) Functional analysis of Pid3-A4, an ortholog of rice blast resistance gene Pid3 revealed by allele mining in common wild rice. Phytopathology 103: 594–599. [DOI] [PubMed] [Google Scholar]
- 3. Hua L, Wu J, Chen C, Wu W, He X, et al. (2012) The isolation of Pi1, an allele at the Pik locus which confers broad spectrum resistance to rice blast. Theoretical And Applied Genetics 125: 1047–1055. [DOI] [PubMed] [Google Scholar]
- 4. Zhu X, Chen S, Yang J, Zhou S, Zeng L, et al. (2012) The identification of Pi50(t), a new member of the rice blast resistance Pi2/Pi9 multigene family. Theoretical and Applied Genetics 124: 1295–1304. [DOI] [PubMed] [Google Scholar]
- 5. Das A, Soubam D, Singh PK, Thakur S, Singh NK, et al. (2012) A novel blast resistance gene, Pi54rh cloned from wild species of rice, Oryza rhizomatis confers broad spectrum resistance to Magnaporthe oryzae . Functional & Integrative Genomics 12: 215–228. [DOI] [PubMed] [Google Scholar]
- 6. Skamnioti P, Gurr SJ (2009) Against the grain: safeguarding rice from rice blast disease. Trends in Biotechnology 27: 141–150. [DOI] [PubMed] [Google Scholar]
- 7. Hittalmani S, Parco A, Mew TV, Zeigler RS, Huang N (2000) Fine mapping and DNA marker-assisted pyramiding of the three major genes for blast resistance in rice. Theoretical And Applied Genetics 100: 1121–1128. [Google Scholar]
- 8. Chen H, Chen Z, Ni S, Zuo S, Pan X, et al. (2008) Pyramiding three genes with resistance to blast by marker-assisted selection to improve rice blast resistance of jin 23B. Chinese Journal of Rice Science 22: 23–27. [Google Scholar]
- 9. Koide Y, Kawasaki A, Telebanco-Yanoria MJ, Hairmansis A, Nguyet NTM, et al. (2010) Development of pyramided lines with two resistance genes, Pish and Pib, for blast disease (Magnaporthe oryzae B. Couch) in rice (Oryza sativa L.). Plant Breeding 129: 670–675. [Google Scholar]
- 10. Yu ZH, Mackill DJ, Bonman JM, Tanksley SD (1991) Tagging genes for blast resistance in rice via linkage to RFLP markers. Theoretical and Applied Genetics 81: 471–476. [DOI] [PubMed] [Google Scholar]
- 11. Chen D, Zeigler R, Ahn S, Nelson R (1996) Phenotypic characterization of the rice blast resistance gene Pi2(t) . Plant Disease 80: 52–56. [Google Scholar]
- 12. Wang Z-X, Yano M, Yamanouchi U, Iwamoto M, Monna L, et al. (1999) The Pib gene for rice blast resistance belongs to the nucleotide binging and leucine-rich repeat class of plant disease resistance gene. The Plant Journal 19: 55–64. [DOI] [PubMed] [Google Scholar]
- 13. Liu G, Lu G, Zeng L, Wang G-L (2002) Two broad-spectrum blast resistance genes, Pi9(t) and Pi2(t), are physically linked on rice chromosome 6. Molecular Genetics and Genomics 267: 472–480. [DOI] [PubMed] [Google Scholar]
- 14. Jeon J-S, Chen D, Yi G-H, Wang GL, Ronald PC (2003) Genetic and physical mapping of Pi5(t), a locus associated with broad-spectrum resistance to rice blast. Molecular Genetics and Genomics 269: 280–289. [DOI] [PubMed] [Google Scholar]
- 15. Yoshida K, Miyashita NT (2009) DNA polymorphism in the blast disease resistance gene Pi-ta of the wild rice Oryza rufipogon and its related species. Genes & Genetic Systems 84: 121–136. [DOI] [PubMed] [Google Scholar]
- 16. Zhai C, Lin F, Dong Z, He X, Yuan B, et al. (2011) The isolation and characterization of Pik, a rice blast resistance gene which emerged after rice domestication. New Phytologist 189: 321–334. [DOI] [PubMed] [Google Scholar]
- 17. Yuan B, Zhai C, Wang W, Zeng X, Xu X, et al. (2011) The Pik-p resistance to Magnaporthe oryzae in rice is mediated by a pair of closely linked CC-NBS-LRR genes. Theoretical And Applied Genetics 122: 1017–1028. [DOI] [PubMed] [Google Scholar]
- 18. Hulbert SH, Webb CA, Smith SM, Sun Q (2001) RESISTANCE GENE COMPLEXES: Evolution and Utilization. Annual Review of Phytopathology 39: 285–312. [DOI] [PubMed] [Google Scholar]
- 19. Zhou B, Qu S, Liu G, Dolan M, Sakai H, et al. (2006) The Eight Amino-Acid Differences Within Three Leucine-Rich Repeats Between Pi2 and Piz-t Resistance Proteins Determine the Resistance Specificity to Magnaporthe grisea . Molecular Plant-Microbe Interactions 19: 1216–1228. [DOI] [PubMed] [Google Scholar]
- 20. Nan J, Suhua W, Zhiqiang L (2012) Analysis of the antimicrobial spectrum of three rice blast resistance genes at Pi2/9 locus and genetic diversity of rice blast strains. Journal of Hunan Agricultural University 38: 506–510. [Google Scholar]
- 21. Ashikawa I, Hayashi N, Yamane H, Kanamori H, Wu J, et al. (2008) Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer Pikm-specific rice blast resistance. Genetics 180: 2267–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rai AK, Kumar SP, Gupta SK, Gautam N, Singh NK, et al. (2011) Functional complementation of rice blast resistance gene Pi-kh (Pi54) conferring resistance to diverse strains of Magnaporthe oryzae . Journal of Plant Biochemistry and Biotechnology 20: 55–65. [Google Scholar]
- 23. Wang L, Xu X, Lin F, Pan Q (2009) Characterization of rice blast resistance genes in the Pik cluster and fine mapping of the Pik-p locus. Phytopathology 99: 900–905. [DOI] [PubMed] [Google Scholar]
- 24.Shang J (2007) Analysis of the rice NBS-LRR gene family and isolation of a new rice blast resistance gene, Pid3. PhD dissertation, Institute of Genetics and Developmental Biology Chinese Academy of Sciences, Beijing City, China.
- 25. Shang J, Tao Y, Chen X, Zou Y, Lei C, et al. (2009) Identification of a new rice blast resistance gene, Pid3, by genomewide comparison of paired nucleotide-binding site-leucine-rich repeat genes and their pseudogene alleles between the two sequenced rice genomes. Genetics 182: 1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiao H, Wang Y, Liu D, Wang W, Li X, et al. (2003) Functional analysis of the rice AP3 homologue OsMADS16 by RNA interference. Plant Molecular Biology 52: 957–966. [DOI] [PubMed] [Google Scholar]
- 27. Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. The Plant Journal 6: 271–282. [DOI] [PubMed] [Google Scholar]
- 28. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 29. Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- 30. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximumparsimony methods. Molecular Biology and Evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rozas J, Sánchez-DelBarrio JC, Messeguer X, Rozas R (2003) DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19: 2496–2497. [DOI] [PubMed] [Google Scholar]
- 32. Chen J, Shi Y, Liu W, Chai R, Fu Y, et al. (2011) A Pid3 allele from rice cultivar Gumei2 confers resistance to Magnaporthe oryzae . Journal of Genetics and Genomics 38: 209–216. [DOI] [PubMed] [Google Scholar]
- 33. Kumar GR, Sakthivel K, Sundaram RM, Neeraja CN, Balachandran SM, et al. (2010) Allele mining in crops: prospects and potentials. Biotechnology Advances 28: 451–461. [DOI] [PubMed] [Google Scholar]
- 34. Hayashi K, Yoshida H (2009) Refunctionalization of the ancient rice blast disease resistance gene Pit by the recruitment of a retrotransposon as a promoter. The Plant Journal 57: 413–425. [DOI] [PubMed] [Google Scholar]
- 35. Sun CQ, Wang XK, Li ZC, Yoshimura A, Iwata N (2001) Comparison of the genetic diversity of common wild rice (Oryza rufipogon Griff.) and cultivated rice (O. sativa L.) using RFLP markers. Theoretical And Applied Genetics 102: 157–162. [Google Scholar]
- 36. Sun CQ, Wang XK, Yoshimura A, Doi K (2002) Genetic differentiation for nuclear, mitochondrial and chloroplast genomes in common wild rice (Oryza rufipogon Griff.) and cultivated rice (Oryza sativa L.). Theoretical And Applied Genetics 104: 1335–1345. [DOI] [PubMed] [Google Scholar]
- 37. Tian F, Li D, Fu Q, Zhu Z, Fu Y, et al. (2006) Construction of introgression lines carrying wild rice (Oryza rufipogon Griff.) segments in cultivated rice (Oryza sativa L.) background and characterization of introgressed segments associated with yield-related traits. Theoretical And Applied Genetics 112: 570–580. [DOI] [PubMed] [Google Scholar]
- 38. Ronald PC, Albano B, Tabien R, Abenes L, Wu KS, et al. (1992) Genetic and physical analysis of the rice bacterial blight disease resistance locus, Xa21 . Molecular & General Genetics 236: 113–120. [DOI] [PubMed] [Google Scholar]
- 39. Qu S, Liu G, Dai L, Han B, Zhou B, et al. (2006) The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 172: 1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shen J, Araki H, Chen L, Chen J-Q, Tian D (2006) Unique evolutionary mechanism in R-genes under the presence/absence polymorphism in Arabidopsis thaliana . Genetics 172: 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang S, Gu T, Pan C, Feng Z, Ding J, et al. (2008) Genetic variation of NBS-LRR class resistance genes in rice lines. Theoretical And Applied Genetics 116: 165–177. [DOI] [PubMed] [Google Scholar]
- 42. McHale L, Tan X, Koehl P, Michelmore RW (2006) Plant NBS-LRR proteins: adaptable guards. Genome Biology 7: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shen Q-H, Zhou F, Bieri S, Haizel T, Shirasu K, et al. (2003) Recognition Specificity and RAR1/SGT1 Dependence in Barley Mla Disease Resistance Genes to the Powdery Mildew Fungus. The Plant Cell 15: 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rairdan GJ, Moffett P (2006) Distinct domains in the ARC region of the potato resistance protein Rx mediate LRR binding and inhibition of activation. The Plant Cell 18: 2082–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. The EMBO Journal 19: 4004–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ravensdale M, Nemri A, Thrall PH, Ellis JG, Dodds PN (2011) Co-evolutionary interactions between host resistance and pathogen effector genes in flax rust disease. Molecular Plant Pathology 12: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kanzaki H, Yoshida K, Saitoh H, Fujisaki K, Hirabuchi A, et al. (2012) Arms race co-evolution of Magnaporthe oryzae AVR-Pik and rice Pik genes driven by their physical interactions. The Plant Journal 72: 894–907. [DOI] [PubMed] [Google Scholar]
- 48. Chen Y, Liu Z, Halterman DA (2012) Molecular determinants of resistance activation and suppression by Phytophthora infestans effector IPI-O. PLoS Pathogens 8: e1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen XW, Ronald PC (2011) Innate immunity in rice. Trends in Plant Science 16: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Delteil A, Zhang J, Lessard P, Morel J-B (2010) Potential candidate genes for improving rice disease resistance. Rice 3: 56–71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information file containing Tables S1, S2 and Figure S1. Table S1. Rice varieties and wild rice accessions used in this experiment. (DOC). Table S2. The number of the positive transgenic plants in T0 generations. (DOC). Figure S1. Co-segregation of the Pid3-I1 gene with the resistant phenotype. The T1 generations (the selfed progeny of T0 line) carrying Pid3-I1 were inoculated with M. oryzae Zhong-10-8-14, and the genotypes were analyzed using the HYG gene and marker Pid3C. Susceptible TP309 (J1) was used as the control. (TIF).
(RAR)