Abstract
Single attached leaves of sunflower (Helianthus annus L. “Mennonite”) were supplied 14CO2 of constant specific radioactivity in gas mixtures containing various CO2 and O2 concentrations. The 14CO2 and CO2 fluxes were measured concurrently in an open system using an ionization chamber and infrared gas analyzer.
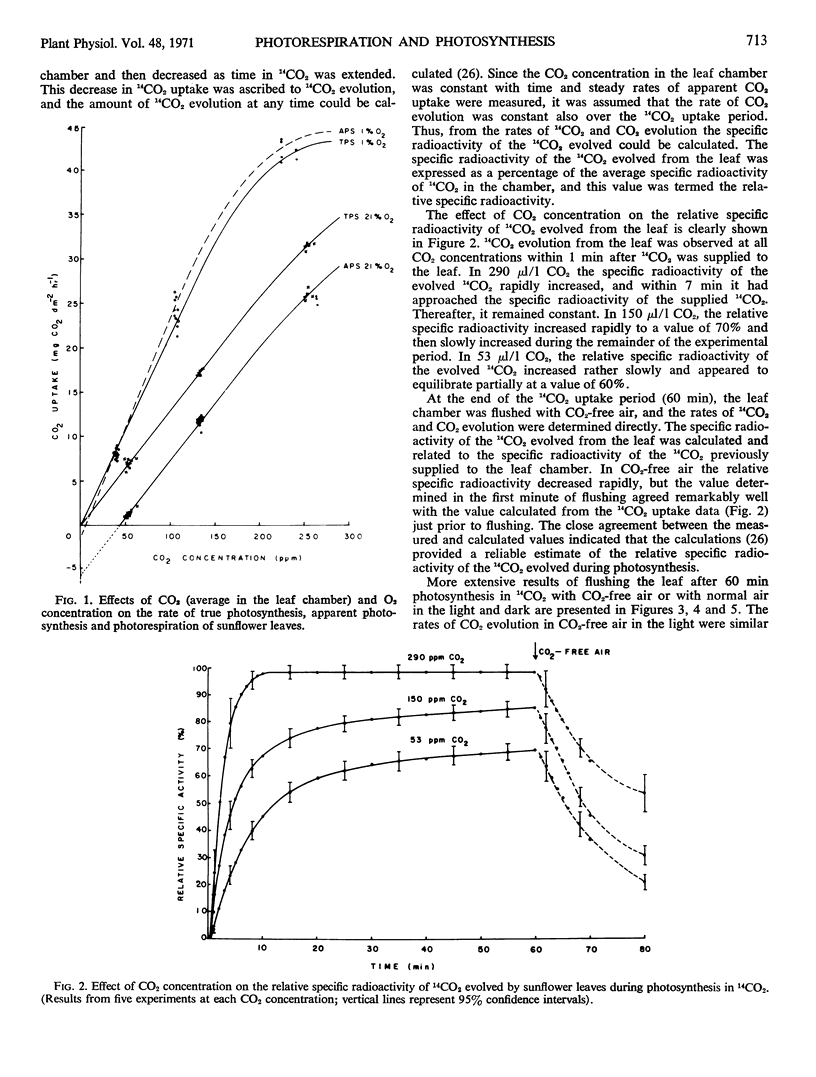
The rate of photorespiration (5.7 ± 0.3 mg CO2·dm−2·−1) during photosynthesis in 21% O2 at 25 C and 3,500 footcandles was over three times the rate of dark respiration and was independent of CO2 concentrations from 0 to 300 μl/l. The steady rate of CO2 evolution into CO2-free air was about 30% lower. Low oxygen (1%) inhibited both 14CO2 and CO2 evolution, both during photosynthesis and in CO2-free air in the light.
At 300 μl/l CO2 apparent photosynthesis was inhibited 41% by 21% O2. Two-thirds of the inhibition was due to the inhibition of true photosynthesis by oxygen and one-third due to the stimulation of photorespiration. At 50 μl/l CO2, where the percentage inhibition of apparent photosynthesis by 21% oxygen was 92%, photorespiration accounted for two-thirds of the total inhibition.
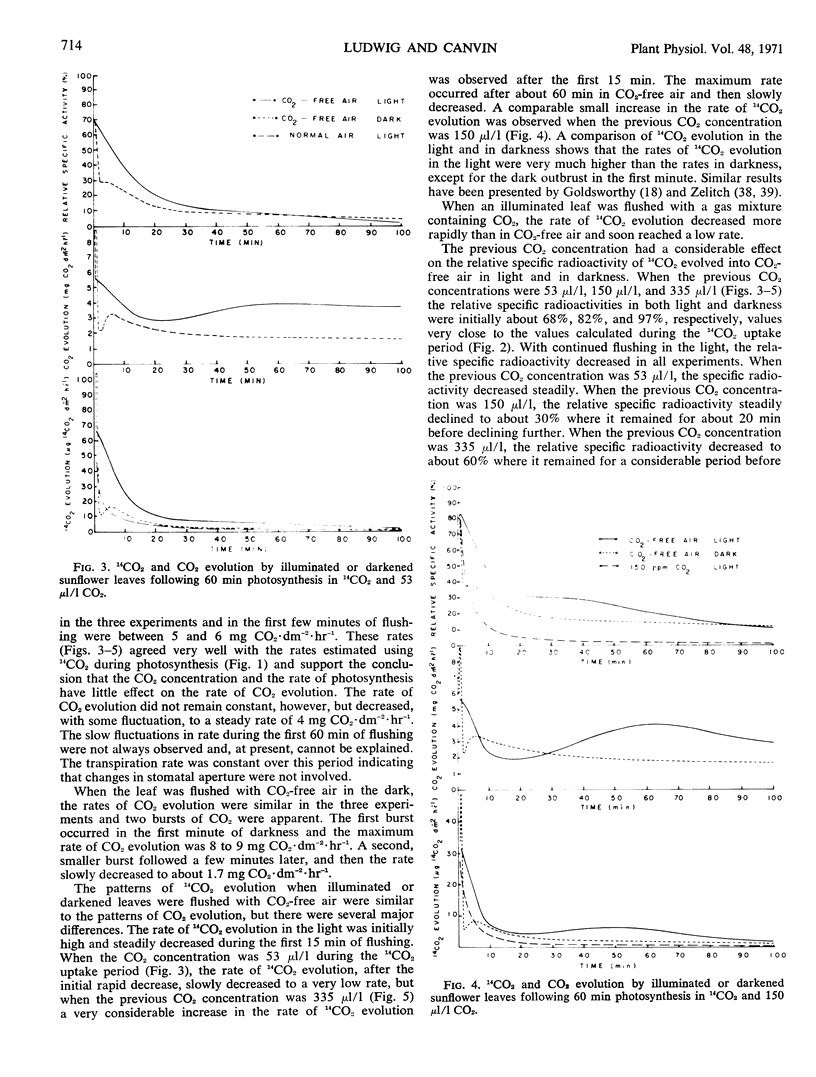
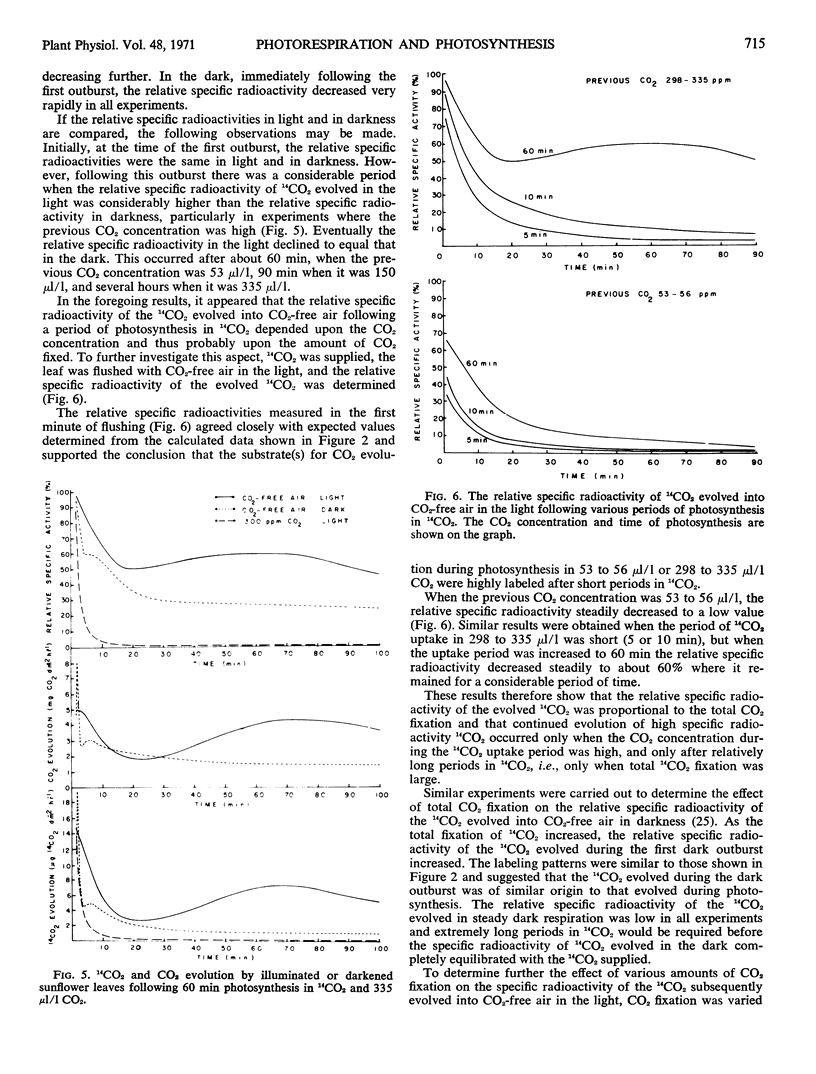
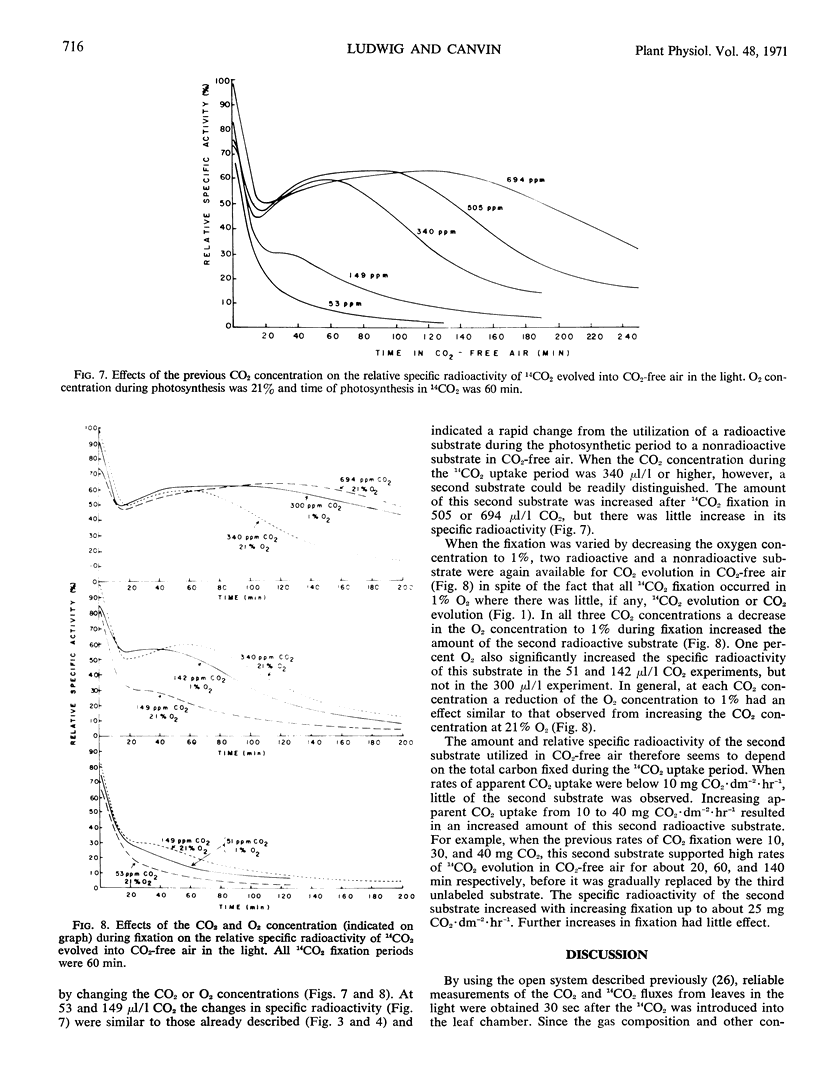
The rate of 14CO2 uptake by the leaf decreased about 30 seconds after the introduction of 14CO2, indicating that 14CO2 was rapidly evolved from the leaf. The rate of 14CO2 evolution increased rapidly with time, the kinetics depending on the CO2 concentration. The high specific radioactivity of the 14CO2 evolved during photosynthesis or in the early period of flushing in CO2-free air showed that the substrate for light respiration was an early product of photosynthesis. From the measurement of 14CO2 and CO2 evolution into CO2-free air over a longer time period it was apparent that at least three compounds, each of decreased 14C content, could supply the substrate for light respiration.
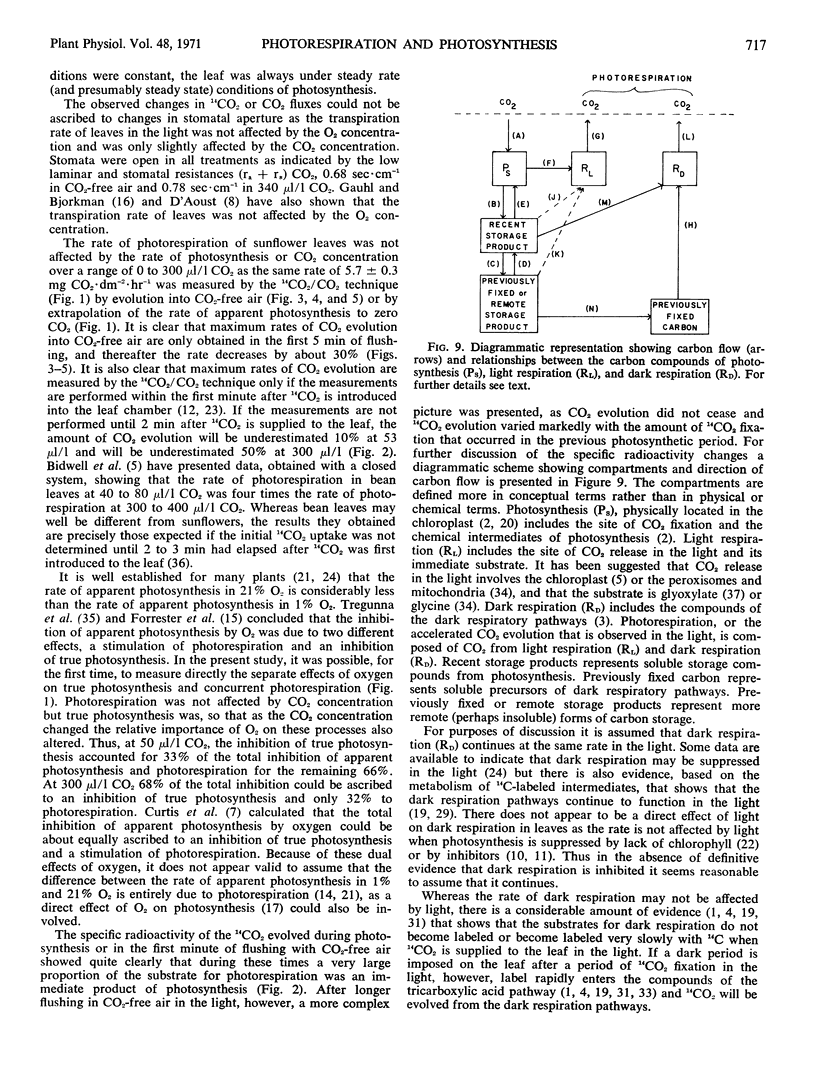
Based on a consideration of the specific radioactivity of 14CO2 evolved under a variety of conditions, it is suggested that total CO2 evolution in the light or photorespiration is composed of two processes, dark respiration and light respiration. Light respiration is a process that only occurs in the light, persists for some time on darkening, and metabolizes substrates that are quite different from those of dark respiration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidwell R. G., Levin W. B., Shephard D. C. Photosynthesis, Photorespiration and Respiration of Chloroplasts From Acetabularia mediterrania. Plant Physiol. 1969 Jul;44(7):946–954. doi: 10.1104/pp.44.7.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravdo B. A. Decrease in net photosynthesis caused by respiration. Plant Physiol. 1968 Apr;43(4):479–483. doi: 10.1104/pp.43.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downton W. J., Tregunna E. B. Photorespiration and Glycolate Metabolism: A Re-examination and Correlation of Some Previous Studies. Plant Physiol. 1968 Jun;43(6):923–929. doi: 10.1104/pp.43.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of oxygen on photosynthesis, photorespiration and respiration in detached leaves. I. Soybean. Plant Physiol. 1966 Mar;41(3):422–427. doi: 10.1104/pp.41.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM D., WALKER D. A. Some effects of light on the interconversion of metabolites in green leaves. Biochem J. 1962 Mar;82:554–560. doi: 10.1042/bj0820554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hew C. S., Krotkov G., Canvin D. T. Determination of the Rate of CO(2) Evolution by Green Leaves in Light. Plant Physiol. 1969 May;44(5):662–670. doi: 10.1104/pp.44.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hew C. S., Krotkov G. Effect of Oxygen on the Rates of CO(2) Evolution in Light and in Darkness by Photosynthesizing and Non-Photosynthesizing Leaves. Plant Physiol. 1968 Mar;43(3):464–466. doi: 10.1104/pp.43.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samish Y., Koller D. Photorespiration in Green Plants During Photosynthesis Estimated by Use of Isotopic CO(2). Plant Physiol. 1968 Jul;43(7):1129–1132. doi: 10.1104/pp.43.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K. Leaf peroxisomes and their relation to photorespiration and photosynthesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):325–341. doi: 10.1111/j.1749-6632.1969.tb43119.x. [DOI] [PubMed] [Google Scholar]
- Yemm E. W., Bidwell R. G. Carbon Dioxide Exchanges in Leaves. I. Discrimination Between CO(2) and CO(2) in Photosynthesis. Plant Physiol. 1969 Sep;44(9):1328–1334. doi: 10.1104/pp.44.9.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I., Day P. R. Variation in photorespiration. The effect of genetic differences in photorespiration on net photosynthesis in tobacco. Plant Physiol. 1968 Nov;43(11):1838–1844. doi: 10.1104/pp.43.11.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Investigation on photorespiration with a sensitive C-assay. Plant Physiol. 1968 Nov;43(11):1829–1837. doi: 10.1104/pp.43.11.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]



