Abstract
Local recurrence represents a significant challenge in the management of patients with glioblastoma multiforme. Salvage treatment options are limited by lack of clinical efficacy. Recent studies have demonstrated a significant response rate and acceptable toxicity with the use of fractionated stereotactic radiosurgery in this patient population. Our primary objective was to determine the efficacy and toxicity of fractionated stereotactic radiosurgery combined with concurrent temozolomide chemotherapy as a salvage treatment for recurrent glioblastoma multiforme. We prospectively collected treatment and outcome data for patients having fractionated stereotactic radiosurgery for locally recurrent glioblastoma multiforme after radical radiotherapy. Eligible patients had a maximum recurrence diameter of 60 mm without causing significant mass effect. The gross tumor volume was defined as the enhancing lesion on an enhanced fine-slice T1 (spin–lattice) magnetic resonance imaging, and a circumferential setup margin of 1 mm was used to define the planning target volume. All patients were treated using robotic radiosurgery with three dose/fractionation schedules ranging from 25 to 35 Gy in five fractions, depending on the maximum tumor diameter. Concurrent temozolomide 75 mg/m2 was prescribed to all patients. Tumor response was judged using the Macdonald criteria, and toxicity was assessed using the CTCAE (Common Terminology Criteria for Adverse Events). A total of 31 patients were enrolled in this study. The median overall survival was 9 months, and progression-free survival was 7 months. The 6-month progression-free survival was 60% with a 95% confidence interval of 43%–77%. The a priori stratification factor of small tumor diameter was shown to predict overall survival, while time to recurrence was not predictive of progression-free or overall survival. Three patients experienced grade 3 acute toxicity that responded to increased steroid dosing. One patient experienced a grade 4 acute toxicity that did not respond to increased steroids but did respond to anti-angiogenic therapy. Fractionated stereotactic radiosurgery with concurrent temozolomide has shown good short-term clinical and radiologic control with manageable acute toxicity. This regimen appears to provide superior efficacy to either temozolomide or fractionated radiosurgery alone. The results of this study support the continued evaluation of this regimen.
Keywords: GBM, re-treatment, brain tumor, anti-angiogenic therapy
Background
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults. In North America, the age-adjusted incidence of GBM is three per 100,000 population.1 GBM is a locally aggressive malignancy, with a median survival of 12–18 months and a 5-year survival of 10% with maximum therapy.2,3 One of the largest advances in the management of GBM is the recent randomized Phase III evidence showing the survival benefit of concurrent and adjuvant temozolomide (TMZ) chemotherapy combined with radiation therapy, as compared with radiation therapy alone.2,3 The mechanism of action of TMZ is thought to be both the direct antitumor effect of an alkylating agent, as well as synergy with the radiation therapy as a radiation sensitizer. This synergy with radiation therapy has likely accounted for the survival benefit seen with TMZ in the management of GBM.4,5 Unfortunately, despite these promising advances in the initial management of GBM, the majority of tumors recur. It has been reported that up to 85% of recurrences are within the previous radiation treatment field.6
Recurrent GBM (rGBM) poses a therapeutic challenge for oncologists. rGBM has a very poor prognosis with a median survival typically reported between 6 and 8 months and a poor response rate to salvage therapies.1 Therefore, the goal of any therapeutic intervention in this population is to improve quality of life by controlling the local disease, while minimizing the risk of significant toxicity.1 The local site of recurrence limits the ability to reuse conventional radiation therapy, due to the re-exposure of surrounding normal brain tissue to both full-dose radiation and re-irradiation.7 Combined with the fact that rGBM is more radioresistant than its initial presentation, the increased likelihood of radiation toxicity with repeat conventional radiation therapy substantially decreases its therapeutic ratio.1,7 Surgery offers an alternative aggressive local option; however, even a radical resection is unlikely to fully remove the recurrent tumor, leading to the need for further planned salvage therapy.1,8 The higher surgical morbidity in this population of patients, with previous surgery and radiation exposure, can further limit the quality of time remaining. This minimizes the efficacy of repeat surgical resection, making it a reasonable option in patients who present with progressive mass effect.1,8 Another common treatment for patients with rGBM is salvage systemic therapy.1,9–12 There are many options for salvage systemic therapy; however, TMZ and bevacizumab are commonly used. Six-month progression-free survival (PFS) rates between 30% and 50% are commonly reported with these approaches.9–12
Recently, many investigators have evaluated radiosurgery and fractionated stereotactic radiosurgery (FSRS) as a treatment option for rGBM.13–18 Preliminary single-institution and retrospective results have shown FSRS alone or combined with systemic therapy to be well tolerated, and efficacy results appear promising.13–18 FSRS allows the precise targeting of a tumor within a previous irradiation field, while simultaneously limiting the dose to the surrounding previously treated normal tissue, by utilizing techniques that target a lesion with sub-millimeter accuracy. Therefore, FSRS allows a radiation oncologist to maximize rGBM control by treating with locally ablative doses of radiation while minimizing the risk of radiation necrosis in the surrounding normal brain tissue. TMZ is the only systemic agent used in GBM that has been shown to have synergistic effects with concurrent radiation therapy.2,3 We therefore hypothesize that the combination of FSRS with TMZ will provide the most efficacious approach in the management of rGBM. We have prospectively evaluated the toxicity and efficacy of FSRS with concurrent TMZ in the management of rGBM.
Methods
The study comprises a prospective cohort of rGBM patients treated concurrently with FSRS and TMZ between January 1, 2011 and December 31, 2012 at the Jurvavinski Cancer Centre. Eligibility criteria were initial radical radiotherapy and one of the following: 1) rGBM (new or progressive enhancement) ≥6 months after radical therapy, 2) new enhancement outside of the radiation field, or 3) progressive enhancement ≥3 months after radical therapy with radiological or pathological evidence of tumor recurrence. Additional criteria were: 1) Karnofsky performance status ≥60, 2) maximum tumor diameter on magnetic resonance imaging (MRI) (gadolinium-enhanced T1 sequence) ≤60 mm with no significant mass effect. Significant mass effect was defined as >1 cm midline shift or rapidly progressing neurologic symptoms.
The gross tumor volume (GTV) included the enhancing abnormality on MRI obtained within 14 days of commencing FSRS. The planning target volume (PTV) included the GTV, with a 1 mm expansion to account for setup error. Plan was optimized such that at least 95% of the PTV received 100% of the prescription dose. Prescription doses were 25–30 Gy FSRS (January 1, 2011–February 1, 2012) or 30–35 Gy (February 2, 2012–December 31, 2012), depending on the size of the PTV. Concurrent daily TMZ (75 mg/m2) was started on day 1 of FSRS. Chemotherapy was continued indefinitely at the discretion of the treating neuro-oncologist. All patients were evaluated clinically and radiographically at least every 3 months until progression. Patients with symptoms of progression were clinically and radiographically evaluated on an urgent basis. Response was evaluated using the Macdonald criteria.19 Toxicity was evaluated using the National Cancer Institute CTCAE (Common Terminology Criteria for Adverse Events www.cancer.gov) version 4.0 toxicity scoring system. Among a group of patients with progressive brain tumors, it is difficult to differentiate tumor progression versus treatment-related toxicity. As FSRS with concurrent TMZ is a new treatment requiring evaluation, we elected to use a conservative approach. All toxicity not clearly due to tumor progression was deemed treatment-related toxicity. Date of death was recorded in all patients after progression. The Research Ethics Board at the Hamilton Health Sciences approved the study.
Statistical analyses
Continuous data were summarized as mean ± standard deviation, and dichotomous data as absolute values and percentages. Overall survival (OS) and PFS were graphically examined using Kaplan–Meier curves stratified a priori by 1) time-to-recurrence and 2) GTV diameter. Time-to- recurrence was dichotomized to 0 if <12 months and 1 otherwise; GTV was dichotomized to 0 if <30 mm, and 1 otherwise. Log-rank test was used to compare strata. All analyses were carried out using SAS 9.3 (SAS Institute, Cary, NC, USA). A P-value <0.05 was considered statistically significant.
Results
A total of 31 patients were enrolled in the cohort study. The average age of the cohort was 53, with a median Karnofsky performance status of 80. One patient had a recurrent lesion in the posterior fossa. The median GTV diameter was 32 mm, and the median irradiated volume was 12 cm3 (Table 1).
Table 1.
Patient characteristics
| Characteristic | Median (range) |
|---|---|
| Age, years | 53 (36–75) |
| KPS | 80 (60–90) |
| MMSE | 25 (22–30) |
| GTV mm | 32 (4–60) |
| Volume, cm3 | 12.1 (4.9–19.7) |
Abbreviations: GTV, gross tumor volume; KPS, Karnofsky performance status; MMSE, Mini-Mental State Examination.
Among the entire cohort, the median OS was 9 months, with an interquartile range of 7–15 months, and the median PFS was 7 months, with an interquartile range of 4–12 months (Figures 1 and 2). The 6-month PFS was 60%, with a 95% confidence interval of 43%–77%.
Figure 1.
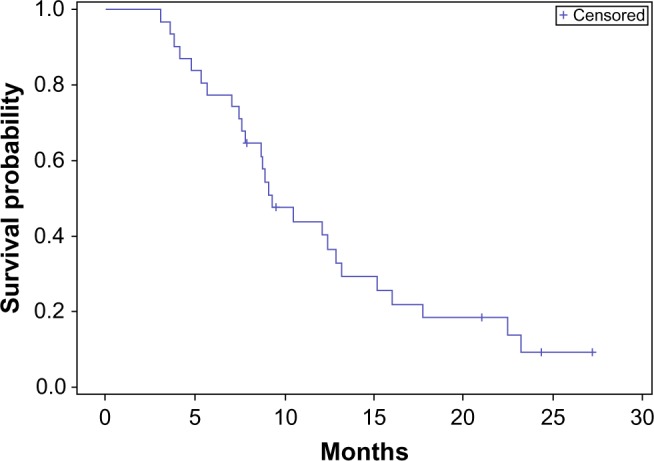
Overall survival – entire cohort.
Figure 2.
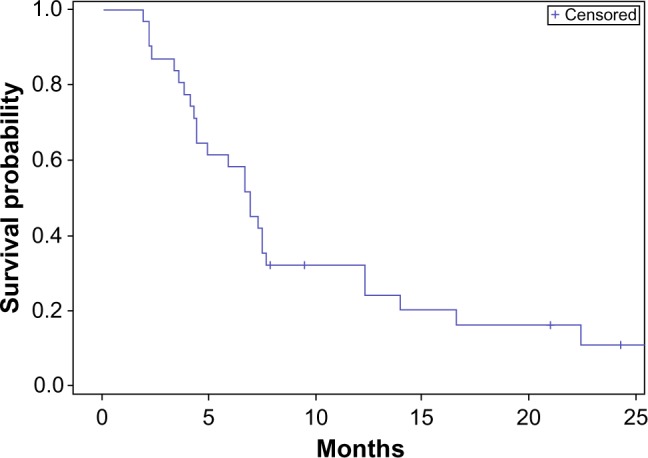
Progression-free survival – entire cohort.
A priori subgroup analyses were undertaken with two separate stratification factors: 1) time to recurrence short (<12 months) as opposed to long (>12 months), and 2) size small (GTV <30 mm) as opposed to large (GTV >30 mm). In our first subgroup, no statistical difference was found in OS or PFS when stratifying by time to recurrence (Figures 3 and 4).
Figure 3.
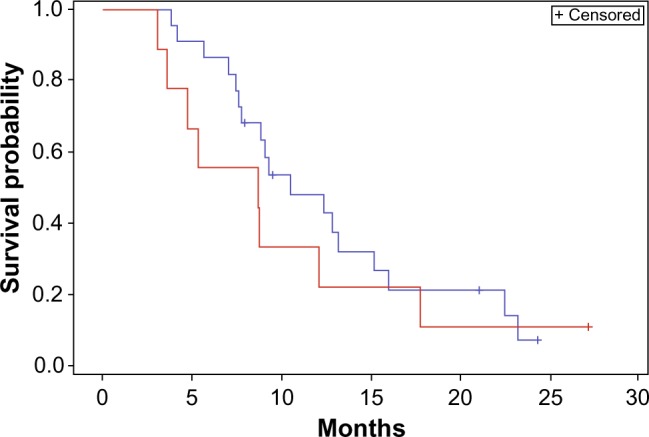
Overall survival stratified by time to recurrence.
Notes: Red <12 months; blue >12 months. Log-rank comparison P>0.05.
Figure 4.
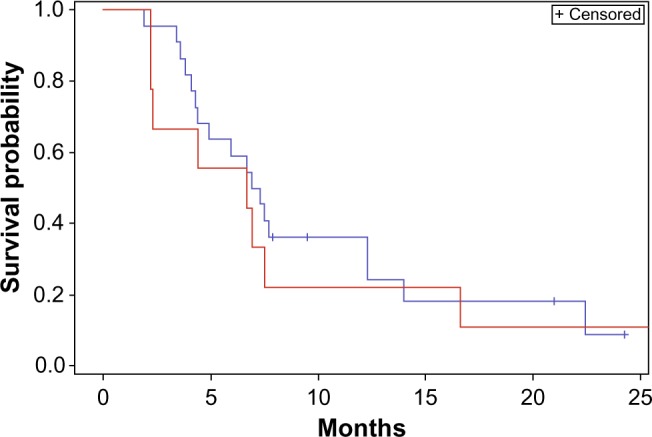
Progression-free survival stratified by time to recurrence.
Notes: Red <12 months; blue <12 months. Log-rank comparison P>0.05.
In the second subgroup analysis, a statistically significant improvement in survival was seen in the small GTV subgroup as compared with the large GTV subgroup (median survival 10.5 months versus 8.7 months P<0.05) (Figure 5). Although not statistically significant, a strong trend towards improvement in PFS was also seen in small GTV tumors as opposed to large GTV tumors (Figure 6).
Figure 5.
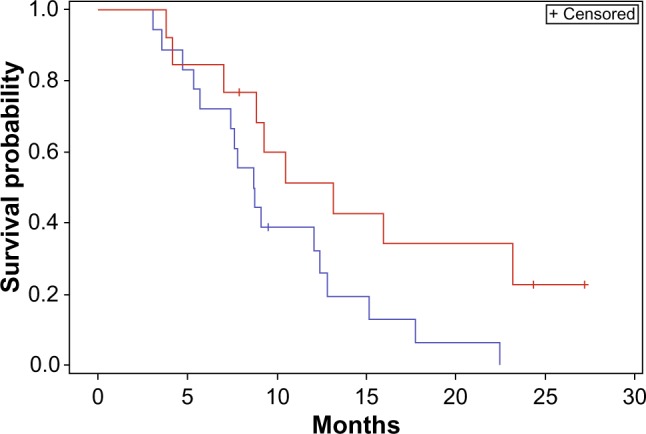
Overall survival stratified by gross tumor volume.
Notes: Red <30 mm; blue >30 mm. Log-rank comparison P=0.04. Median overall survival: red =10.5 months; blue =8.7 months.
Figure 6.
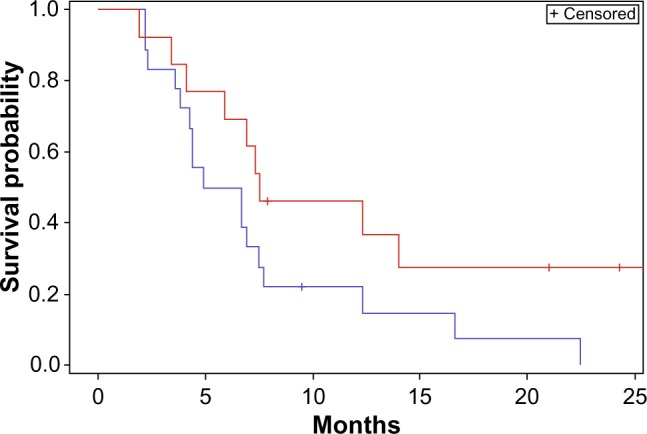
Progression-free survival stratified by gross tumor volume.
Notes: Red <30 mm; blue >30 mm. Log-rank comparison P=0.08.
Acute grade 3 central nervous system necrosis was seen in three patients. These patients required at least a doubling of their steroid dose within 3 months of FSRS to prevent progressive neurologic symptoms. One patient experienced an acute grade 4 central nervous system necrosis requiring urgent anti-angiogenic therapy, despite increased steroid dosing, and admission to hospital for progressive increased intracranial pressure. The patient responded well to anti-angiogenic therapy and was discharged from hospital on a tapering dose of steroids. No late toxicity was observed in this cohort.
Discussion
rGBM is a highly invasive disease, with tumor deposits frequently found multiple centimeters away from the imaged tumor mass.6 This has limited the theoretical benefit of FSRS for the treatment of rGBM. FSRS uses advanced imaging techniques to target a tumor to sub-millimeter accuracy. Although this approach, similar to surgery, will undertreat some distant tumor cells, it has been shown in prospective randomized trials of brain metastases and single institution experiences in rGBM that FSRS provides good local control of the treated lesion. However, unlike surgery, FSRS can be delivered without sedation and as an outpatient, which would help maximize the quality of life in patients with a limited life expectancy.
TMZ has been used with some success in the setting of rGBM. One of the drawbacks of salvage systemic therapy is the difficulty in achieving control of the dominant mass.9,10,12 Due to poor blood circulation into large necrotic lesions, TMZ alone may theoretically lack efficacy in optimally controlling larger volume rGBM. The previous randomized evidence that supports the synergistic effect between TMZ and radiation therapy2,3 coupled with the knowledge that both FSRS and TMZ alone have significant theoretical advantages and limitations for the treatment of rGBM, we hypothesized that the combination of TMZ + FSRS would show good efficacy and be well tolerated for the treatment of rGBM. We hypothesized also that FSRS would lead to control of the dominant mass, while TMZ would control the microscopic disease. The combination of TMZ and radiation has led in previous studies to enhanced acute toxicity.2,3 By limiting the exposure of normal tissues to re-irradiation using FSRS techniques, we hypothesized that FSRS + TMZ would be well tolerated, with minimal acute grade 3 or more toxicity.
Our study demonstrated that FSRS + TMZ has efficacy in the management of rGBM. With a 6-month PFS rate of 60% and a 95% confidence interval of 43%–77%, our results are similar to or superior to many published retrospective series looking at the combination of FSRS + TMZ. Although a single-arm prospective cohort is not a true head-to-head comparison, it appears that the combination of FSRS + TMZ is superior in the salvage setting to either FSRS alone or TMZ alone. In retrospective series, the 6-month PFS with either FSRS or TMZ alone is reported at approximately 30%.
Interestingly, our study showed no difference in survival or PFS if progression was early (<12 months) or late (>12 months) after initial radical therapy. Although one would expect patients who respond well to initial therapy (>12 months) without a recurrence to respond well when they receive salvage therapy, this study has demonstrated that salvage TMZ + FSRS may work on a different mechanism of tumor control than initial concurrent chemoradiotherapy. FSRS, by using large dose per fraction, likely causes local tumor ablation, which is in contrast to the accumulation of sublethal damage and potentially lethal damage seen in conventional fractionated radiation therapy.20 This difference in tumor cell kill mechanism may account for the lack of benefit seen in patients who recur late as opposed to early.
Our study did however demonstrate a significant difference in survival among small tumors (<3 cm) as opposed to larger (>3 cm) tumors. The median survival was shown to be 10.5 versus 8.7 months (P<0.05) for small as compared with large lesions. Although there was not a statistically significant difference in PFS, there was a strong trend to benefit for smaller lesions. This prognostic benefit of small size recurrence was likely multifactorial. First, patients with small volume recurrence were treated with higher dose FSRS as compared with patients with larger volume recurrence. The higher dose FSRS may contribute to more long-term tumor control and survival. Second, patients with smaller recurrences would likely receive a higher concentration of TMZ within the main tumor volume. This increased concentration of TMZ would directly contribute to tumor control, and this elevated concentration of TMZ within the FSRS volume would increase the synergistic effect of TMZ and FSRS. Third, patients with smaller tumor recurrences may respond better than those with larger recurrences to future salvage systemic therapy. Therefore, these patients would have improvement in OS without an improvement in PFS from FSRS + TMZ due to future response to other salvage therapy. One limitation of our cohort is we did not capture response to future salvage therapy.
As seen in other studies that combine radiation therapy with TMZ for GBM and rGBM, acute treatment-related brain inflammation and subacute treatment-related effect (necrosis) can limit a patient’s quality of life.2,3,8,14–16 Although we used very conservative guidelines for adjudicating treatment-related toxicity versus tumor progression, we observed three patients who experienced acute grade 3 brain swelling that required a doubling of dexamethasone dosing followed by a weekly taper. Only one patient required admission to hospital for a grade 4 acute brain swelling, which required bevacizumab therapy in addition to dexamethasone in order to mitigate the effects of the edema. Following a single dose of bevacizumab, the patient clinically improved and was discharged from hospital on a tapering dose of dexamethasone. This improvement after bevacizumab therapy allows us to hypothesize that quality of life may be improved and toxicity may be prevented by the routine use of anti-angiogenic therapy in the setting of FSRS + TMZ. We have developed a randomized protocol evaluating FSRS + TMZ with or without anti-angiogenic therapy, with the primary endpoint being the incidence of acute grade 3 or greater toxicity. rGBM is a very serious diagnosis with a poor prognosis. A focus on both length and quality of life is imperative to maximize the clinical benefit of any prescribed therapy. For this reason, we hypothesize that prophylactic treatment with anti-angiogenic therapy, with the efficacious FSRS + TMZ regimen used in this trial, would maximize the therapeutic ratio of this regimen by minimizing acute toxicity.
Conclusion
FSRS + TMZ is a well tolerated regimen for patients with rGBM and recurrence size <6 cm. The greatest efficacy was apparent in patients with small (<3 cm) recurrences. Although this was a single-arm prospective cohort, this regimen appears to provide superior control to either TMZ or FSRS alone. Further prospective randomized studies would be needed to validate the efficacy of this approach over conventional salvage therapy. The combination of FSRS + TMZ may be more tolerable and therefore more efficacious in the setting of rGBM with the prophylactic use of anti-angiogenic therapy. The results of this study support the continued evaluation of FSRS + TMZ in rGBM.
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Easaw JC, Mason WP, Perry J, et al. Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol. 2011;18:e123–e136. doi: 10.3747/co.v18i3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concominant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concominant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized Phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Hegi M, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 5.Fukushima T, Takeshima H, Kataoka H. Anti-glioma therapy with temozolomide and status of the DNA-repair gene MGMT. Anticancer Res. 2009;29:4845–4854. [PubMed] [Google Scholar]
- 6.Minniti G, Amelio D, Amichetti M, et al. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol. 2010;97:337–381. doi: 10.1016/j.radonc.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiation Oncology Biol Phys. 2008;70:1350–1360. doi: 10.1016/j.ijrobp.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Skeie BS, Enger PO, Brogger J, et al. Gamma knife surgery versus reoperation for recurrent glioblastoma multiforme. World Neurosurg. 2012;78:658–669. doi: 10.1016/j.wneu.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Jaeckle KA, Hess KR, Yung WKA, et al. Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American brain tumor consortium study. J Clin Oncol. 2003;21:2305–2311. doi: 10.1200/JCO.2003.12.097. [DOI] [PubMed] [Google Scholar]
- 10.Brandes AA, Tosoni A, Cavallo G, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from the gruppo italioano cooperative di neuro-oncologia (GICNO) Br J Cancer. 2006;95:1155–1160. doi: 10.1038/sj.bjc.6603376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 12.Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28:2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 13.Combs SE, Thimann C, Edler L, Debus J, Schulz-Ertner D. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23:8863–8869. doi: 10.1200/JCO.2005.03.4157. [DOI] [PubMed] [Google Scholar]
- 14.Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti A, Pontoriero A, Donatella A, et al. Efficacy and toxicity of CyberKnife re-irradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir. 2012;154:203–209. doi: 10.1007/s00701-011-1184-1. [DOI] [PubMed] [Google Scholar]
- 16.Combs SE, Bischof M, Welzel T, et al. Radiochemotherapy with temozolomide as re-irradiation using high precision fractionated stereotactic radiotherapy (FSRT) in patients with recurrent gliomas. J Neurooncol. 2008;89:205–210. doi: 10.1007/s11060-008-9607-4. [DOI] [PubMed] [Google Scholar]
- 17.Park KJ, Kano H, Iyer A, et al. Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: a case-control study. J Neurooncol. 2012;107:323–333. doi: 10.1007/s11060-011-0744-9. [DOI] [PubMed] [Google Scholar]
- 18.Romanelli P, Conti A, Pontoriero A, et al. Rose of stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of recurrent glioblastoma multiforme. Neurosurg Focus. 2009;27(6):E8. doi: 10.3171/2009.9.FOCUS09187. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for Phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 20.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]


